Abstract
Continuous theta burst stimulation (cTBS) is a novel transcranial stimulation technique that causes significant inhibition of synaptic transmission for ≤1 h when applied over the primary motor cortex (M1) in humans. Here we use magnetic resonance spectroscopy to define mechanisms mediating this inhibition by noninvasively measuring local changes in the cortical concentrations of γ-aminobutyric acid (GABA) and glutamate/glutamine (Glx). cTBS to the left M1 led to an increase in GABA compared with stimulation at a control site without significant change in Glx. This direct evidence for increased GABAergic interneuronal activity is framed in terms of a new hypothesis regarding mechanisms underlying cTBS.
INTRODUCTION
Repetitive transcranial magnetic stimulation (rTMS) is an increasingly used noninvasive method of stimulating neural pathways in the awake human brain. rTMS can induce significant excitability changes within the cortex that outlast the period of stimulation (Rossini and Rossi 2007). Of particular interest is the promise of rTMS for clinical applications, such as neurorehabilitation after stroke, when facilitatory stimulation to the affected hemisphere or inhibitory stimulation to the unaffected hemisphere can improve motor function (Di Lazzaro et al. 2008a; Hummel and Cohen 2006; Webster et al. 2006).
However, many of the reported effects of rTMS are short lasting, often highly variable or weak, especially when applied to patients with a range of different neuropathologies (Maeda et al. 2000a; Wassermann 2002). To address this problem, a more novel protocol has been developed that induces more robust and less variable responses across populations of subjects (Huang et al. 2005). Theta burst stimulation (TBS) uses 50-Hz trains of three TMS pulses, repeated every 200 ms. When applied intermittently, TBS leads to a robust increase in cortical excitability for ≤30 min. By contrast, continuous TBS (cTBS) at a frequency of 5 Hz over a period of 40 s (cTBS, 600 stimuli) leads to depression of motor-evoked potential (MEP) amplitudes by ≤50% of the baseline amplitude that can be maintained for ≤60 min (Huang et al. 2005). TBS also has robust effects in stroke patients (Di Lazzaro et al. 2008b).
The mechanisms by which TBS exerts these effects on the cortex are not well defined. There is some evidence that rTMS alters cortical excitability via changes in synaptic strength (Chen et al. 1997; Cooke and Bliss 2006; Fitzgerald et al. 2006; Huang et al. 2005; Maeda et al. 2000b; Pascual-Leone et al. 1994). The TBS protocol is designed to be more similar to paradigms used to induce long-term potentiation (LTP) and long-term depression (LTD) in animal models (Hess et al. 1996; Huemmeke et al. 2002; Larson and Lynch 1986; Vickery et al. 1997) that, in the cortex, depend primarily on the GABAergic system, with some glutamatergic involvement (Komaki et al. 2007; Trepel and Racine 2000). However, in humans, the direct effects of TBS on synaptic transmission are commonly measured indirectly, through administration of neuroactive compounds (Huang et al. 2007).
By contrast, magnetic resonance spectroscopy (MRS) measures γ-aminobutyric acid (GABA) and glutamate levels directly and noninvasively. For example, previous MRS studies have demonstrated reductions of GABA in the human motor cortex while subjects learned a motor skill (Floyer-Lea et al. 2006), after facilitatory stimulation of the motor cortex using an electrical stimulation technique, transcranial DC stimulation (tDCS; CJ Stagg, 2009), after acute deafferentation (Levy et al. 2002), or in a number of neurological conditions (Levy and Hallett 2002).
Here, we sought to test whether the effects of cTBS are mediated by changes in the local activity of inhibitory interneuronal cortical pathways in the primary sensorimotor cortex using GABA-optimized MRS. We measured changes in concentrations of GABA and glutamate/glutamine (Glx) following cTBS over the “hand” area of the motor cortex (verum cTBS) relative to those with stimulation over the vertex of the cranium (control cTBS).
METHODS
Subjects
Sixteen male subjects (mean age 27.5 yr; range: 21–44 yr) gave their informed consent to participate in the study in accordance with local ethics committee approval. All subjects were right-handed as assessed by the Edinburgh Handedness Inventory (Oldfield 1971).
TMS paradigm
All TMS was given using a handheld figure-of-eight coil (70-mm standard coil; Magstim, Whitland, Dyfed, UK), connected to a Magstim Rapid2 stimulator. The cortical representation of the right first dorsal interosseous (FDI) muscle within the left primary motor cortex (M1) was identified using single biphasic pulse TMS and the scalp position overlying this marked (referred to as the “hotspot”). The active motor threshold (AMT) was then established for each subject, defined as the minimum intensity of stimulation required to produce an MEP of >200 μV on 5 of 10 trials from the contralateral FDI when the subject was maintaining a voluntary contraction of about 20% of maximum. Electromyographic responses were recorded using Ag/AgCl electrodes in a belly-tendon montage. The pattern of TBS consisted of bursts containing three pulses at 50 Hz and an intensity of 80% AMT repeated at 200-ms intervals for 40 s (i.e., 600 pulses). Verum stimulation was targeted to the previously identified hotspot, with the coil held tangentially to the skull, orientated at 45° to the midsagittal axis, inducing lateromedial current flow. Control stimulation was given to the control site at the vertex (Cz). Subjects were instructed to relax their hand at all times during the experiment, once the AMT had been recorded, and were blinded as to which TMS condition they received.
GABA MRS
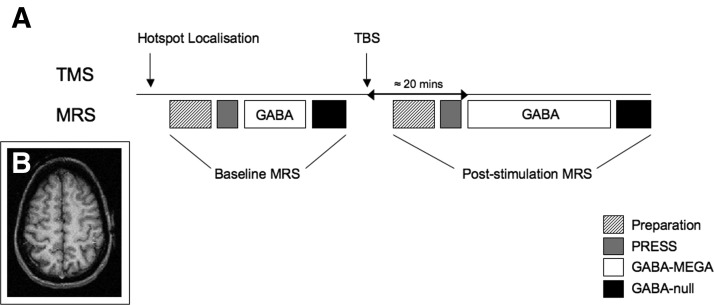
A 3-T Siemens/Varian MRI system was used. Sagittal and axial T1-weighted scout images were acquired and used to place a 2 × 2 × 2-cm voxel of interest by hand over the left precentral knob, a landmark previously described to identify the hand-motor representation in the human brain (Yousry et al. 1997). The placement of a typical voxel is shown in Fig. 1B. To assess the creatine and N-acetylaspartate (NAA) linewidths, a standard PRESS sequence was used to acquire an unedited spectrum with echo time (TE) = 68 ms and 64 averages. Water was suppressed at 4.7 ppm using a technique similar to WET (i.e., water suppression enhanced through T1 effects; Ogg et al. 1994). The MEGA-PRESS (Mescher et al. 1998) sequence was then used to allow simultaneous spectral GABA editing, three-dimensional voxel localization, and water suppression, as reported previously (Floyer-Lea et al. 2006). With the MEGA-PRESS scheme, GABA at 3 ppm was detected through J-difference spectral editing and use of the frequency-selective double-banded 180° pulse.
FIG. 1.
A: experimental protocol. A baseline magnetic resonance spectroscopy (MRS) acquisition was performed after the motor hotspot had been identified in each subject. After this was complete, the subjects were removed from the scanner and a 40-s train of theta burst stimulation (TBS) pulses were applied, either to the motor hotspot (M1; verum stimulation) or to the vertex (Cz; control stimulation). The subjects were then repositioned in the scanner and a poststimulation MRS spectrum was acquired. This commenced after a period of about 20 min of scanner setup. B: typical 2 × 2 × 2-cm voxel placed within the hand region of the left primary motor cortex.
A selective double-banded 180° pulse was created from 20-ms Gaussian pulses. The frequency of the first band of this pulse was set to 4.7 ppm to suppress water. The second band was alternated between 1.9 ppm, the resonance frequency of C3 protons (strongly coupled to the observed C4 GABA protons), and 3.0 ppm (odd-numbered acquisitions) and 7.5 ppm (even-numbered acquisitions), which is symmetrically disposed about the water resonance to equalize off-resonance effects. During the odd-numbered acquisition the GABA C4 (triplet) resonance at 3.0 ppm was fully refocused, whereas during the even-numbered acquisitions this peak was not refocused, but phase-modulated so that the outer triplet signals were inverted at echo time (TE) = 68 ms. The difference between spectra collected during even and odd acquisitions (at TE = 68 ms) revealed the edited GABA spectrum without the larger overlapping creatine resonance.
A metabolite-nulled spectra was acquired using a modified MEGA-PRESS editing sequence with pre-inversion module consisting of inversion recovery, pulse, and recovery time (TI), adjusted to minimize the creatine peak (TI = 0.720 s). In addition, a T1-weighted anatomical image was acquired with each spectral set to allow for correction for varying gray matter volumes within the voxel (3D Turbo Flash, 128 × 2-mm axial slices, repetition time (TR) = 12 ms, TE = 5 ms, TI = 200 ms, flip angle = 8°, field of view = 256 × 256, matrix = 128 × 128).
Experimental protocol
The motor hotspot was identified prior to the baseline scan. A 256- acquisition baseline GABA-optimized MRS was acquired for each subject. The subjects were then removed from the scanner and TBS was performed in a separate room. Following TBS subjects were asked to keep their hand relaxed at all times and interactions with them were kept to a minimum. Each subject was then repositioned into the scanner, with assistance from the experimenters to avoid physical activity of the hand and arm (Huang et al. 2008). A voxel as close as possible to the baseline voxel was then visually identified, from which a 512-acquisition MRS spectrum was obtained. The MR operator was naïve with respect to the TMS stimulation condition. MRS acquisition started about 20 min after TBS was completed (Fig. 1A). Participants watched a nature DVD while in the MR scanner to maintain a constant level of wakefulness.
MRS analysis
To assess the overlap between the pre- and post-stimulation voxels, the voxels were registered in standard space using FLIRT, part of the FMRIB Software Library (www.fmrib.ox.ac.uk/fsl) (Jenkinson et al. 2002; Smith et al. 2004). The overlap between the two voxels was then compared with the volume of the prestimulation voxel to ensure meaningful colocalization. This was confirmed (see results) for all subjects.
Quantitative analyses of the spectra were performed using the jMRUI software package version 2.2 (http://www.mrui.uabes/mrui). The free induction decay (FID) signal was first corrected for any nonzero DC offset and the signal was smoothed using a 2-Hz Lorentzian filter. The residual water signal was then filtered out by fitting and removing Gaussian peaks around the water frequency using singular value decomposition techniques (van den Boogaart 1997; van den Boogaart et al. 1994; Vanhamme et al. 1997). The spectrum was then phased with respect to both the zeroth- and first-order phases.
All spectra were analyzed using AMARES, a nonlinear least-square-fitting algorithm operating in the time domain (Vanhamme et al. 1997). Peak fitting for the GABA and Glx (a combined measure of glutamate and glutamine) spectra was performed using Lorentzian curves with the linewidth constrained to that of the creatine resonance in the nonedited spectrum. The GABA and the Glx resonances were both fitted with two Lorentzian peaks. The linewidth of the inverted NAA resonance was constrained to the linewidth of NAA in the unedited spectrum and a single Lorentzian curve was fitted to this peak. The amplitudes of both GABA peaks were summed to give a signal intensity for GABA; likewise, summing was performed for the Glx peak.
To correct for the expected contribution from mobile brain macromolecules (MMs, which include cytosolic proteins) the GABA-nulled spectrum was analyzed as before and amplitude of the peak resonating at 3 ppm calculated. This was then deducted from the signal intensity of the GABA resonance.
All results presented for both GABA and Glx given here are as expressed as a ratio to NAA, the simultaneously acquired reference peak. Because we are looking for percentage changes within subject, no correction was made for editing efficiency or number of equivalent protons.
RESULTS
Data for the structural scan from one subject were corrupted and spectra from that subject were discarded. All spectral acquisitions were of adequate quality, defined a priori based on an NAA linewidth of <10 Hz. Analyses were performed on the remaining seven subjects in the verum group and eight subjects in the control group. There was no significant difference between the ages of subjects in the two groups (Wilcoxon two-sample test, W = 67.5, P < 0.99). The average active motor threshold was 48.7 ± 5.9% of maximal stimulator output (MSO). The average stimulation intensity was 40 ± 5% MSO, with no significant difference between groups (Wilcoxon two-sample test, W = 55, P < 0.18).
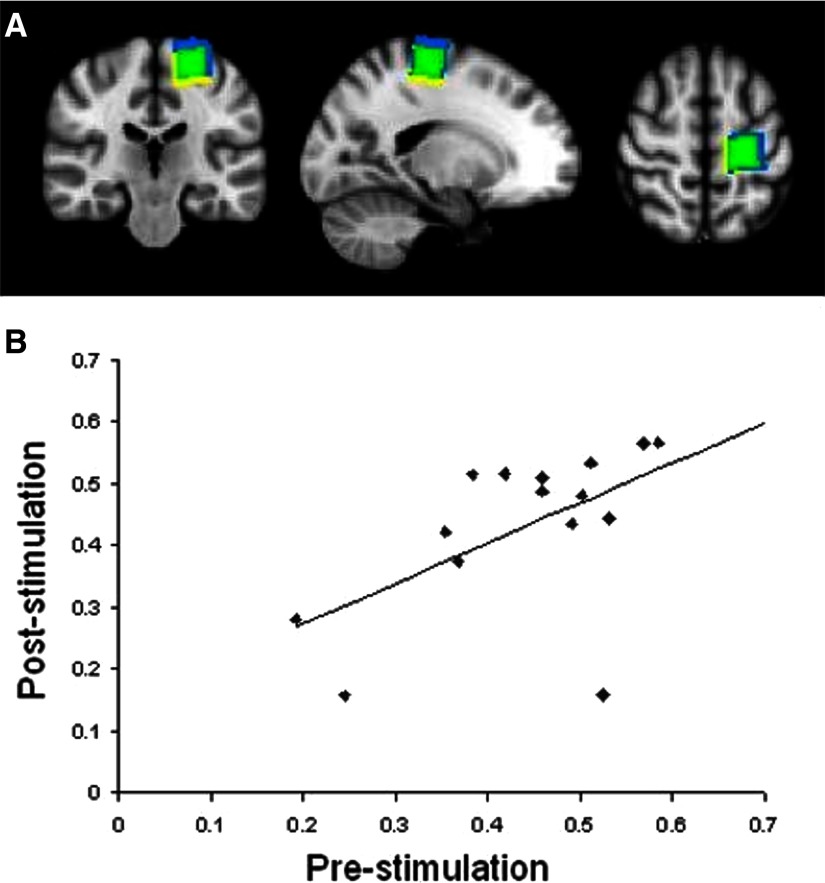
Good registration of voxels was achieved within all subjects. On average the pre- and post-cTBS voxels within individual subjects overlapped in volume by a mean 77%. There were no significant differences between gray matter volume contained within the pre- compared with the post-cTBS voxels (Wilcoxon two-sample test, W = 122, P < 0.95) and the gray matter content for pre- versus post-cTBS voxels was highly correlated across subjects (r = 0.93, P < 0.01). A representative pair of voxels is shown in Fig. 2 A and an overall summary in Fig. 2B.
FIG. 2.
A: typical locations for the prestimulation voxel (yellow) and the poststimulation voxel (blue). The substantial overlap between the 2 is shown in green. B: plot showing the high correlation between the amount of gray matter within the voxel of interest before and after stimulation (r2 = 0.88, P < 0.01).
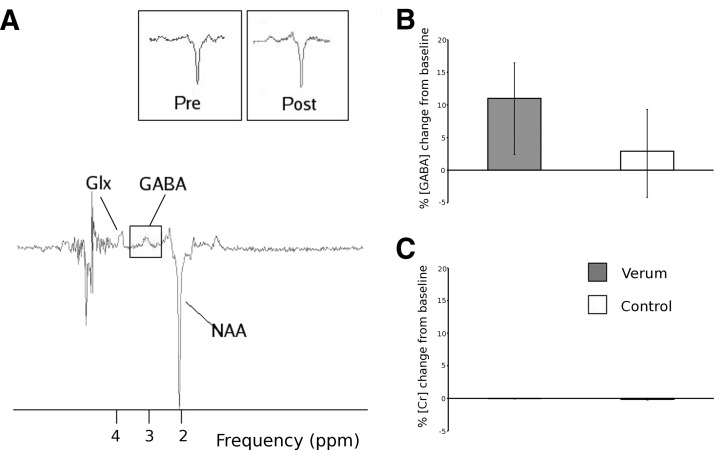
A typical localized spectrum from the sensorimotor cortex is shown in Fig. 3A. A Mann–Whitney U test confirmed that there was a significant increase in GABA concentrations between verum and control stimulations [verum prestimulation corrected GABA:NAA ratio [median (IQ range)] 0.23 (0.16–0.27), poststimulation 0.26 (0.16–0.30); control prestimulation corrected GABA:NAA ratio 0.18 (0.14–0.26), poststimulation 0.17 (0.14–0.19); median change after verum stimulation = 11.0% (2.4–16.44); median change after control stimulation, 2.9% (−4.17–9.33); Wilcoxon two-sample test, W = 46, P < 0.04] (Fig. 3B) and no significant difference in Glx concentrations [verum prestimulation Glx:NAA ratio [median (IQ range)] 0.06 (0.05–0.09), poststimulation 0.08 (0.05–0.08); verum median change = 16.1% (−25.1–39.1); control median change 6.8% (−13.7–87.8); Wilcoxon two-sample test, W = 36, P < 0.68].
FIG. 3.
A: typical γ-aminobutyric acid (GABA)–optimized spectrum acquired. Insets show representative GABA and N-acetylaspartate (NAA) peaks before and after stimulation in the same subject. Glx, composite measure of glutamate and glutamine. B: percentage change in GABA:NAA ratio from baseline. Verum stimulation leads to a significant increase in GABA:NAA ratio, compared with control. C: percentage change in creatine signal for baseline. There is no significant change with either stimulation condition.
To further rule out confounding changes in tissue water, we also measured the absolute concentration of Cr both prestimulation and poststimulation. There was no significant change in the Cr concentration seen after stimulation (Fig. 3C) [verum Cr concentration prestimulation [median (IQ range)] 112 (92–123) [arbitrary units], poststimulation 106 (83–117) median change = −0.02% (−0.1–0.03) control Cr concentration prestimulation 106 (100–114), poststimulation 96 (91–100) median change −0.15% (−0.26–0.04); Wilcoxon two-sample test, W = 33, P < 0.61].
DISCUSSION
Continuous TBS to the M1 increases GABA concentration when compared with control stimulation, without any significant effect on Glx levels. GABAergic activity therefore may be a mechanism by which long-lasting aftereffects of TBS on corticospinal excitability are generated. This is consistent with preclinical data suggesting the importance of the GABAergic mechanisms in LTD-like phenomena within the neocortex in freely moving animals (Hess et al. 1996; Komaki et al. 2007; Trepel and Racine 2000). More indirectly, it may be related to the decreases in local sensorimotor cortex GABA concentrations during successful motor learning in humans (Floyer-Lea et al. 2006).
GABA is produced in neurons by decarboxylation of glutamate by the enzyme glutamic acid decarboxylase (GAD65). The GAD65 isoform is associated with synaptic vesicles and is likely to be involved in synthesizing GABA for neurotransmission (Martin and Rimvall 1993; Martin et al. 1991a,b). In contrast, the GAD67 isoform is distributed more widely in the cytoplasm and is thought to be important in synthesizing GABA for cytosolic use. GAD is the rate-limiting step in production of GABA. In vitro, neuronal activity is associated with increases in the active isoform of GAD65 (de Graaf et al. 2003). In neural stem cell–derived cultures, depolarization of the neurons also leads to an increase in the active isoform of GAD65 (Gakhar-Koppole et al. 2008). Induction of synthesis thus likely enables enhanced GABAergic activity in vivo.
It is important to note that MRS is sensitive only to changes in the overall concentration of a neurotransmitter and cannot inform our understanding of changes within the receptors at the synapses. Specifically, the lack of a change in this measure of Glx does not contradict previous pharmacological studies that show abolition of the effects of cTBS after N-methyl-d-aspartate (NMDA)-receptor antagonism (Huang et al. 2007), but instead strongly suggests that these changes are due to changes in the NMDA receptors themselves.
The aftereffects of cTBS on motor cortex are commonly assessed from their effects on the amplitude of electromyographic responses (MEPs) evoked in a small intrinsic hand muscle by a standard single TMS pulse (Huang et al. 2005). At intensities commonly used for TBS, the effect of TMS is cortical and thus results from stimulation of either excitatory or inhibitory synaptic inputs to layer V pyramidal neurons (Di Lazzaro et al. 1998). A reduced MEP after cTBS therefore indicates either a reduction in the net efficiency of excitatory stimulation by the TMS pulse or an increase in intracortical inhibition.
Our results here—suggesting increased GABAergic inhibition contributes to the aftereffects of TBS—were unexpected. Previous work has shown that the effects of cTBS are abolished by administration of memantine at concentrations sufficient to antagonize NMDA function in the human brain (Huang et al. 2007; Teo et al. 2007). Thus it has usually been assumed that the aftereffects are due to an action on glutamatergic transmission, which leads to a reduction in cortical excitability via an LTD-like action on the excitatory synapses activated by the TMS test pulses.
Integration of the neuropharmacological results with the MRS data suggests a new hypothesis regarding cTBS action. cTBS is applied at a low intensity (80% AMT), which is below the threshold for activating the excitatory inputs to pyramidal neurons (Ziemann et al. 1996). The intensity is the same as that used in a common double-pulse paradigm to assess short-interval intracortical inhibition (SICI) in the motor cortex (Kujirai et al. 1993). To elicit SICI, two TMS pulses are applied through the same coil with the first pulse being subthreshold intensity (usually 80% AMT) and the second pulse large enough on its own to elicit an MEP. If the interval between the pulses is between 1 and 5 ms then the MEP is suppressed. Pharmacological studies suggest the effect is due to activation of the GABAA-ergic neurons by the first pulse (Di Lazzaro et al. 2000, 2008b; Muller-Dahlhaus et al. 2008; Werhahn et al. 1999). Whether the GABAA neurons are activated directly by the TMS pulse or indirectly via an excitatory synaptic input is unclear; there is some evidence that it may be the latter (Bestmann et al. 2003).
Whatever the precise mechanism, these data imply that cTBS (600 stimuli) at 80% AMT activates a population of cortical GABAA-ergic interneurons. The increase in GABAergic activity is then sustained by induction of GAD65 and subsequent increased GABA concentration within the cytoplasm of the GABAA-ergic interneuron.
However, this simple formulation does not account for the decrease in SICI (a TMS measure of relative GABAergic transmission) found with cTBS (Huang et al. 2005, 2008). To account for this, we propose an extension of this simple formulation into a mixed model in which effects are mediated by changes in both glutamatergic and GABAergic signaling within local excitation–inhibition networks (Logothetis 2008). The excitability of the cortical output neurons (as reflected by the MEP) is controlled by associative mechanisms of LTD, whereby both NMDA-receptor–dependent mechanisms and GABA input control excitability (Tsumoto 1992).
There is one more important aspect that may help to explain our present findings. LTD of GABAA synapses is predominantly presynaptic, being mediated through endocannabinoids (Tsumoto 1992). On the other hand, LTD at glutamatergic synapses is more likely to involve postsynaptic changes (Hess and Donoghue 1996). Previous work shows that cTBS reduces the effectiveness of excitatory inputs to MEP generators in motor cortex, as well as the effectiveness of the circuits mediating short-intracortical inhibition (SICI) (Huang et al. 2008). In both cases, cTBS is likely to provoke activity in GABAergic and glutamatergic circuits that may be followed by an increased synthesis of both transmitters. However, on termination of cTBS, presynaptic LTD at GABAergic neurons prevents further GABA release. This leads to increased presynaptic GABA levels that in turn increase the MRS signal. On the other hand, presynaptic release of glutamate is unchanged and no accumulation of glutamate should occur. Consequently, no changes in Glx should occur.
This explanation would make the previous pharmacological studies—suggesting that the aftereffects of cTBS are NMDA-receptor dependent (Huang et al. 2007)—consistent with the results from this study showing an increase in GABA activity. The reduction in SICI can be accounted for because this phasic test of relative GABAA activity in the paired-pulse paradigm would be less effective in the context of a baseline of increased, ongoing inhibition. Although this cannot be proved from these data, this model provides a framework for further testing in future experiments.
There are limitations to our experiment. The measure of total GABA within the voxel gives no information concerning subcellular localization and thus our interpretation can only be speculative. Because sensitivity did not allow a full relaxation time characterization, it remains possible that the increases in apparent concentration arise simply from redistribution of GABA from pools in which it is relatively immobilized, such as by association with MMs, and therefore MR “invisible” (Matthews et al. 1986). However, such large relaxation time changes with short-term changes in functional state would be unprecedented as far as we are aware. It is also possible that the result sreflect a change in the volume of the cells within the voxel. However, we have controlled for this by considering the GABA:NAA ratio rather than using GABA absolute quantification. In addition, there is no significant change in the creatine concentration after stimulation.
There are some questions raised by the current study that should be addressed in future investigations. First, due to the constraints of the technique we have acquired data only from the brain tissue in the stimulated sensorimotor cortex and therefore a future study is needed to clearly distinguish the local and more general effects of tDCS on neurotransmitter levels.
In addition, we are unable to determine the direct relationship between neurotransmitter changes as assessed by MRS and neurophysiological changes as assessed by TMS. However, at this point the experiments required to investigate this relationship remain technically challenging. Subjects were moved out of the scanner for stimulation and there was a delay of about 20 min between the end of stimulation and the start of MRS measurements, which then demanded a further 20 min. Shorter-lived neurochemical changes would not be able to be defined. However, the neurophysiological aftereffects of 600 pulses of cTBS, as applied in the present study, are relatively stable for ≤1 h (Huang et al. 2005, 2008), suggesting that the dynamics of the underlying neurochemical changes also occur over a similar period. In addition it would be of interest to investigate the effects of intermittent TBS in a similar study. There is evidence that iTBS and cTBS affect different intracortical circuits (Di Lazzaro et al. 2005, 2008a), so different effects might be expected.
A significant variability in the Glx measure was seen in both stimulation conditions. In our experience this is often seen in MRS studies and may represent an interaction between the resonance and the neighboring water peak. However, although this interaction would be expected to add variance to the signal, as seen here, no trend toward a significant change in Glx measures with cTBS was seen.
cTBS to the contralesional hemisphere is a promising tool for neurorehabilitation in chronic stroke. In many contexts, inhibitory stimulation to the unaffected hemisphere has led to more robust increases in cortical excitability and motor function on the affected side (Di Lazzaro et al. 2008b; Fregni et al. 2005). TBS, in particular, is a stimulation technique that uses low intensities and is well tolerated in both healthy controls (Huang and Rothwell 2004) and stroke patients (Di Lazzaro et al. 2006; Talelli et al. 2007). This study adds direct evidence that TBS induces LTP- and LTD-like plasticity in the human motor cortex (Huang et al. 2005). It has allowed a more refined hypothesis regarding the mechanism of action that can be tested in future experiments. With a stronger neurophysiological rationale, it can be applied with greater confidence in therapeutic trials (Di Lazzaro et al. 2008a; Fregni et al. 2005), taking advantage of its excellent tolerability (Di Lazzaro et al. 2006; Huang and Rothwell 2004; Talelli et al. 2007).
GRANTS
S. Bestmann was funded by Wellcome Trust Grant 076358/Z/05/Z, J. C. Rothwell was supported by the Medical Research Council, and H. Johansen-Berg was funded by the Wellcome Trust.
Acknowledgments
We thank the Department of Clinical Neurology and the Medical Research Council (MRC) for support through a departmentally allocated MRC Studentship to C. J. Stagg. P. M. Matthews thanks the MRC for personal and laboratory support during most of the period of this study.
Present address of P. M. Matthews: GlaxoSmithKline, 980 Great West Road, Brentford, West London, TW8 9GS, UK.
REFERENCES
- Chen et al. 1997.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48: 1398–1403, 1997. [DOI] [PubMed] [Google Scholar]
- Cooke and Bliss 2006.Cooke SF, Bliss TVP. Plasticity in the human central nervous system. Brain 129: 1659–1673, 2006. [DOI] [PubMed] [Google Scholar]
- de Graaf et al. 2003.de Graaf R, Mason G, Patel A, Behar KL, Rothman DL. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed 16: 339–357, 2003. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro et al. 2006.Di Lazzaro V, Dileone M, Profice P, Pilato F, Cioni B, Meglio M, Capone F, Tonali PA, Rothwell JC. Direct demonstration that repetitive transcranial magnetic stimulation can enhance corticospinal excitability in stroke. Stroke 37: 2850–2853, 2006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro et al. 2000.Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol 111: 794–799, 2000. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro et al. 2008a.Di Lazzaro V, Pilato F, Dileone M, Profice P, Capone F, Ranieri F, Musumeci G, Cianfoni A, Pasqualetti P, Tonali PA. Modulating cortical excitability in acute stroke: a repetitive TMS study. Clin Neurophysiol 119: 715–723, 2008a. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro et al. 2008b.Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Meglio M, Tonali PA, Rothwell JC. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol 586: 3871–3879, 2008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro et al. 2005.Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, Insola A, Tonali PA, Ranieri F, Huang YZ, Rothwell JC. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol 565: 945–950, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro et al. 1998.Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 119: 265–268, 1998. [DOI] [PubMed] [Google Scholar]
- Fitzgerald et al. 2006.Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 117: 2584–2596, 2006. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea et al. 2006.Floyer-Lea A, Wylezinska M, Kincses T, Matthews P. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol 95: 1639–1644, 2006. [DOI] [PubMed] [Google Scholar]
- Fregni et al. 2005.Fregni F, Boggio P, Mansur C, Wagner T, Ferreira M, Lima M, Rigonatti S, Marcolin M, Freedman S, Nitsche M, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 16: 1551–1555, 2005. [DOI] [PubMed] [Google Scholar]
- Gakhar-Koppole et al. 2008.Gakhar-Koppole N, Bengtson CP, Parlato R, Horsch K, Eckstein V, Ciccolini F. Depolarization promotes GAD 65-mediated GABA synthesis by a post-translational mechanism in neural stem cell-derived neurons. Eur J Neurosci 27: 269–283, 2008. [DOI] [PubMed] [Google Scholar]
- Hess et al. 1996.Hess G, Aizenmann C, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol 75: 1765–1778, 1996. [DOI] [PubMed] [Google Scholar]
- Hess and Donoghue 1996.Hess G, Donoghue JP. Long-term depression of horizontal connections in rat motor cortex. Eur J Neurosci 8: 658–665, 1996. [DOI] [PubMed] [Google Scholar]
- Huang et al. 2007.Huang Y-Z, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol 118: 1028–1032, 2007. [DOI] [PubMed] [Google Scholar]
- Huang et al. 2005.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 45: 201–206, 2005. [DOI] [PubMed] [Google Scholar]
- Huang and Rothwell 2004.Huang Y-Z, Rothwell JC. The effect of short-duration bursts of high-frequency, low-intensity transcranial magnetic stimulation on the human motor cortex. Clin Neurophysiol 115: 1069–1075, 2004. [DOI] [PubMed] [Google Scholar]
- Huang et al. 2008.Huang Y-Z, Rothwell JC, Edwards MJ, Chen R-S. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex 18: 563–570, 2008. [DOI] [PubMed] [Google Scholar]
- Huemmeke et al. 2002.Huemmeke M, Eysel U, Mittmann T. Metabotropic glutamate receptors mediate expression of LTP in slices of rat visual cortex. Eur J Neurosci 15: 1641–1645, 2002. [DOI] [PubMed] [Google Scholar]
- Hummel and Cohen 2006.Hummel F, Cohen L. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 5: 708–712, 2006. [DOI] [PubMed] [Google Scholar]
- Jenkinson et al. 2002.Jenkinson M, Bannister P, Brady J, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17: 825–841, 2002. [DOI] [PubMed] [Google Scholar]
- Komaki et al. 2007.Komaki A, Shahidi S, Lashgari R, Haghparast A, Malakouti SM, Noorbakhsh SM. Effects of GABAergic inhibition on neocortical long-term potentiation in the chronically prepared rat. Neurosci Lett 422: 181–186, 2007. [DOI] [PubMed] [Google Scholar]
- Larson and Lynch 1986.Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science 232: 985–988, 1986. [DOI] [PubMed] [Google Scholar]
- Levy and Hallett 2002.Levy L, Hallett M. Impaired brain GABA in focal dystonia. Ann Neurol 51: 93–101, 2002. [PubMed] [Google Scholar]
- Levy et al. 2002.Levy LM, Ziemann U, Chen R, Cohen L. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann Neurol 52: 755–761, 2002. [DOI] [PubMed] [Google Scholar]
- Maeda et al. 2000a.Maeda F, Keenan J, Tormos J, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res 133: 425–430, 2000a. [DOI] [PubMed] [Google Scholar]
- Maeda et al. 2000b.Maeda F, Keenan J, Tormos J, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol 111: 800–805, 2000b. [DOI] [PubMed] [Google Scholar]
- Martin et al. 1991a.Martin DL, Martin SB, Wu SJ, Espina N. Cofactor interactions and the regulation of glutamate decarboxylase activity.Neurochem Res 16: 243–249, 1991a. [DOI] [PubMed] [Google Scholar]
- Martin et al. 1991b.Martin DL, Martin SB, Wu SJ, Espina N. Regulatory properties of brain glutamate decarboxylase (GAD): the apoenzyme of GAD is present principally as the smaller of two molecular forms of GAD in brain. J Neurosci 11: 2725–2731, 1991b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin and Rimvall 1993.Martin DL, Rimvall K. Regulation of γ-aminobutyric acid synthesis in the brain. J Neurochem 60: 395–407, 1993. [DOI] [PubMed] [Google Scholar]
- Mescher et al. 1998.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11: 266–272, 1998. [DOI] [PubMed] [Google Scholar]
- Muller-Dahlhaus et al. 2008.Muller-Dahlhaus MFJ, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex: a pharmacological TMS study. J Physiol 586: 495–514, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg et al. 1994.Ogg RJ, Kingsley RB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B 104: 1–10, 1994. [DOI] [PubMed] [Google Scholar]
- Oldfield 1971.Oldfield RC The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone et al. 1994.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117: 847–858, 1994. [DOI] [PubMed] [Google Scholar]
- Rossini and Rossi 2007.Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology 68: 484–488, 2007. [DOI] [PubMed] [Google Scholar]
- Smith et al. 2004.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23: S208–S219, 2004. [DOI] [PubMed] [Google Scholar]
- Stagg et al. 2009.Stagg C, Best J, Stephenson M, O'Shea J, Wylezinska M, Kincses Z, Morris P, Matthews P, and Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci In press: 2009. [DOI] [PMC free article] [PubMed]
- Talelli et al. 2007.Talelli P, Greenwood R, Rothwell JC. Exploring theta burst stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol 118: 333–342, 2007. [DOI] [PubMed] [Google Scholar]
- Trepel and Racine 2000.Trepel C, Racine RJ. GABAergic modulation of neocortical long-term potentiation in the freely moving rat. Synapse 35: 120–128, 2000. [DOI] [PubMed] [Google Scholar]
- Tsumoto 1992.Tsumoto T Long-term potentiation and long-term depression in the neocortex. Prog Neurobiol 39: 209–228, 1992. [DOI] [PubMed] [Google Scholar]
- van den Boogaart 1997.van den Boogaart A MRUI Manual: A User's Guide to the Magnetic Resonance User Interface Software Package (Version 96.3). Delft, The Netherlands: Delft Technical Univ. Press, 1997.
- van den Boogaart et al. 1994.van den Boogaart A, van Ormondt D, Pijnappel WWF, De Beer R, Ala-Korpela M. Removal of the water resonance from 1H magnetic resonance spectra. In: Mathematics in Signal Processing III, edited by McWhirter JG. Oxford, UK: Clarendon Press, 1994, p. 175–195.
- Vanhamme et al. 1997.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior-knowledge. J Magn Reson 129: 35–43, 1997. [DOI] [PubMed] [Google Scholar]
- Vickery et al. 1997.Vickery R, Morris S, Bindman L. Metabotropic glutamate receptors are involved in long-term potentiation in isolated slices of rat medial frontal cortex. J Neurophysiol 78: 3039–3046, 1997. [DOI] [PubMed] [Google Scholar]
- Wassermann 2002.Wassermann EM Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol 113: 1165–1171, 2002. [DOI] [PubMed] [Google Scholar]
- Webster et al. 2006.Webster BR, Celnik PA, Cohen LG. Noninvasive brain stimulation in stroke rehabilitation. NeuroRX 3: 474–481, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn et al. 1999.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol 517: 591–597, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry et al. 1997.Yousry T, Schmid U, Alkadhi H, Schmidt D, Peraud A, Buettner P, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. Brain 120: 141–157, 1997. [DOI] [PubMed] [Google Scholar]
- Ziemann et al. 1996.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol 496: 873–881, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]