Abstract
Saccades are rapid eye movements that change the direction of gaze, although the full-field image motion associated with these movements is rarely perceived. The attenuation of visual perception during saccades is referred to as saccadic suppression. The mechanisms that produce saccadic suppression are not well understood. We recorded from neurons in the dorsal medial superior temporal area (MSTd) of alert macaque monkeys and compared the neural responses produced by the retinal slip associated with saccades (active motion) to responses evoked by identical motion presented during fixation (passive motion). We provide evidence for a neural correlate of saccadic suppression and expand on two contentious results from previous studies. First, we confirm the finding that some neurons in MSTd reverse their preferred direction during saccades. We quantify this effect by calculating changes in direction tuning index for a large cell population. Second, it has been noted that neural activity associated with saccades can arrive in the parietal cortex ≤30 ms earlier than activity produced by similar visual stimulation during fixation. This led to the question of whether the saccade-related responses were visual in origin or were motor signals arising from saccade-planning areas of the brain. By comparing the responses to saccades made over textured backgrounds of different contrasts, we provide strong evidence that saccade-related responses were visual in origin. Refinements of the possible models of saccadic suppression are discussed.
INTRODUCTION
Saccadic eye movements are used to change the direction of gaze. Although saccades generate rapid image motion across the retina, this motion is rarely perceived. The imperceptibility of wide-field motion during saccades has been dubbed saccadic suppression. This phenomenon is not due to poor sensitivity for fast motion because stimuli with saccade-like speed and duration produce a powerful sense of movement during fixation (Ross et al. 2001) and drive neurons at various stages in the visual system (e.g., subcortical: Price and Ibbotson 2001; cortical: Price et al. 2005; Thiele et al. 2002). It has been theorized that the loss of visual sensitivity during saccades is due to active attenuation of components within the visual system. For human observers saccadic suppression is observed as reduced contrast sensitivity in the period from −80 to +100 ms relative to saccade onset (Fig. 3 from Diamond et al. 2000). The magnocellular pathway appears to be most affected by saccadic suppression, with powerful suppression observed in the motion domain (Burr et al. 1982, 1994; Ilg and Hoffmann 1993; Shioiri and Cavanagh 1989; but see Fischer et al. 1996). However, the perception of motion direction for low spatial frequency stimuli appears to be maintained during saccades (Castet and Masson 2000). An everyday example of visual perception maintained during saccades can occur while looking out the side window of a car traveling along a highway or bridge: the guardrails that appear to zip past in a blur can suddenly become visible if one makes a saccade in the opposite direction the car is moving.
Electrophysiological studies have provided some insights into the mechanisms underlying saccadic suppression. Early in the visual pathway, neurons in the dorsal lateral geniculate nucleus (dLGN) are suppressed during saccades but enhanced afterward (Lee and Malpeli 1998; Reppas et al. 2002) and the pregeniculate complex contains multiple classes of neurons that change their activity around the time of saccades (Livingston and Fedder 2003). Importantly, these effects outlast any modulation that could be solely attributed to changes in the visual input. Although one might expect strong modulation during saccades in motion-processing regions of the cortex, such as middle temporal (MT) and dorsal medial superior temporal (MSTd) areas, because of the retinal slip the saccades produce, the findings are not so simple. Recent studies have provided evidence for suppression before and during saccades followed by postsaccadic enhancement (Ibbotson et al. 2007, 2008; Rajkai et al. 2008). Interestingly, about a third of direction-selective cells in MT and MSTd were found to reverse their direction selectivity, and it has been proposed that signals from reversed and unchanged cells could annul each other to produce saccadic suppression (Thiele et al. 2002).
Another perplexing finding that has arisen from studies of saccadic suppression in MT and MSTd is that response latencies are reduced around the time of saccades (Ibbotson et al. 2006, 2007, 2008; Price et al. 2005; Thiele et al. 2002). The response latencies to image motion generated during saccades can be as short as 30 ms, compared with around 70 ms in the absence of saccades (median values; Ibbotson et al. 2007; Thiele et al. 2002). If activity along the visual pathway were being attenuated during saccades, one would expect longer latencies for the responses to saccade-induced retinal slip due to an iceberg effect, where weak responses take longer to reach spiking threshold (e.g., Raiguel et al. 1999). The paradoxically short latencies have raised the question of whether the responses during saccades were visual in origin or were motor signals arising from saccade-planning areas of the brain.
The aim of this study was to address two aspects of saccadic suppression that deserve further attention. First, the observation that cells in MT and MSTd reverse their direction selectivity was based on responses that developed some time (30–190 ms) after the saccades had taken place (Thiele et al. 2002), which may have incorporated after-responses generated by motion cessation. In the present study we analyzed the direction tuning of MSTd neurons in awake macaques around the time of saccades, taking care to prevent the inclusion of after-responses, and found evidence of direction reversals. Second, we tested whether the short-latency responses that occur during saccades were of visual origin by altering the contrast of the textured background commonly used in directed-saccade tasks. We found that neural latency increased as background contrast was decreased, providing strong evidence that short-latency responses that occur in MSTd around the time of saccades are visual in origin. Interestingly, we found that saccadic suppression was relatively more powerful for low-contrast backgrounds (∼10%) that are typical of natural scenes (e.g., Chirimuta et al. 2003).
METHODS
Surgical procedures
Behavioral and single-unit data were collected from three normal rhesus monkeys (Macaca mulatta). All surgical and experimental procedures were performed in strict compliance with National Institutes of Health guidelines and the protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Emory University. Sterile surgical procedures were carried out under aseptic conditions using isoflurane anesthesia (1.25–2.0%) to stereotaxically implant an magnetic resonance imaging (MRI)–compatible head-stabilization system (Crist Instruments, Hagerstown, MD) and a recording chamber centered above the superior temporal sulcus (lateral: 15 mm; posterior: 5 mm). A scleral search coil for measuring eye movements was implanted underneath the conjunctiva of one eye.
Our recording locations in MSTd were verified using MRI as described in detail previously (Ibbotson et al. 2007, 2008). Briefly, monkeys were initially sedated (ketamine/telezol) and anesthetized (isoflurane 1.0–1.5%) and subsequently placed in an MRI-compatible stereotaxic frame. Three-dimensional T1-weighted images were collected at 1-mm intervals along the anteroposterior axis around the region of the recording chambers using a Siemens 3-T MRI. Neurolens software was used to identify regions of interest below our Cilux recording chambers (Crist Instruments). Guide tubes and electrodes were positioned using an adjustable radius-and-angle holder that attached to the recording chamber. The radius-and-angle device could also hold a saline-filled guide tube made of fused silica (Plastics One, Roanoke, VA) for visualization during MRI sessions. The small internal diameter (0.15 mm) of the fused silica probe facilitated accurate localization.
Behavioral paradigms and visual stimulation
During all experiments, monkeys were comfortably seated facing a tangent screen placed 61 cm from the eyes, with the head stabilized in the horizontal stereotaxic plane. Visual stimuli comprised random texture patterns formed of 0.8° dark or light squares spanning 77 × 77°. They were rear-projected using a Mirage 2000 digital light projector (DLP; Christie Digital Systems, Cypress, CA) with resolution of 1,024 × 1,024 pixels, frame rate of 96 Hz, and mean luminance 170 cd/m2 (for issues relating to the DLP, see Price et al. 2005). Texture contrast is defined as: Michelson contrast = ([Luminancemax − Luminancemin]/[Luminancemax + Luminancemin]) × 100. All neurons were tested with 100% background contrast and a subset was also tested with background contrasts of 0, 10, 20, and 50%.
After isolating a neuron, its preferred direction was determined by recording the responses to motion of a wide-field texture stimulus in eight directions, separated by 45°. During stimulus presentation, the animals were rewarded with fruit juice every 0.5–1 s for maintaining fixation on a red spot presented in the center of the screen. Although we tested only planar motion, we expected that most MSTd neurons including the single-, double-, and triple-component neurons described by Duffy and Wurtz (1995) would provide fairly directional responses to this stimulus. While the monkey was fixating, we mapped the borders of the receptive fields with a small mouse-controlled patch of dots to ensure the edges were within the bounds of the screen borders. Subsequently, we characterized MSTd responses to 1) saccade-induced retinal slip, which we call active motion; and 2) simulated saccades during fixation, which we call passive motion. For active motion stimuli monkeys made rewarded saccades back and forth between two alternately presented fixation points separated by 10°. The fixation points were each offset by 5° from the center of the screen. After fixation was maintained for ≥500 ms, the fixation target disappeared and reappeared on the opposite side of the visual field. After fixation was maintained for 500 ms on this new target, it disappeared and reappeared in the original location. The axis of the saccades was always aligned with the preferred direction of the recorded neuron. In addition, the texture pattern was rotated such that the edges of the squares were aligned parallel and perpendicular to the preferred direction of the neuron. For passive motion stimuli, monkeys fixated a central red spot while the textured background was moved in the cell's preferred and antipreferred directions with a speed profile that simulated a 10° saccade. The speed profile was predetermined from the averaged eye movements during 20 saccades. Texture motion and the orientation of the texture squares were aligned with the preferred axis of the recorded neuron.
Data collection and analysis
Eye position was monitored in two dimensions using a magnetic coil system (CNC Electronics, Seattle, WA) and sampled at 1 kHz with 16-bit precision using a Power 1401 (CED, Cambridge, UK). Eye velocity v(t) was calculated off-line by differentiation of the eye position traces p(t) using a central difference algorithm: v(t) = [p(t + δt) − p(t − δt)]/2δt, with δt = 2 ms. Eye acceleration was calculated using the same algorithm by differentiating the velocity traces. Saccades were initially identified from peaks in the eye velocity traces that were >10°/s. Resting pre- and postsaccadic eye positions were calculated by averaging eye position in the windows 200–50 ms prior to and 100–200 ms after the velocity peak. If the eye position in these two periods exceeded 0.25° in range, or 0.2° in SD, saccades were rejected. Saccadic eye movements and the associated neural responses were aligned on the time of saccade onset, identified as the time when eye acceleration first exceeded a 100°/s2 threshold. On average, the responses to 16–64 saccades (active motion) or simulated saccades (passive motion) were recorded for each neuron.
Unit activity was sampled at 25 kHz using iron-tipped, epoxy-coated tungsten electrodes with impedance 1–4 MΩ prior to iron-tipping (FHC, Brunswick, ME). Single-unit spikes were detected on-line with a hardware window discriminator or software template-matching algorithm (Alpha-Omega, Nazareth, Israel). In addition, we checked spike shape and detection off-line using the Wavemark template matching provided in Spike2 (CED). For off-line analysis, neuronal responses were represented as spike density functions (SDFs) with 1-kHz resolution generated by initially convolving a delta function at each spike arrival time with a Gaussian window with σ = 3 ms. SDFs for stimulus-evoked responses were calculated by averaging responses to individual stimulus presentations, including only portions of the response during which the eye position was within ±1° of the desired fixation point.
Response latencies for passive motion stimuli were calculated relative to a synchronization pulse provided by the stimulus-generation computer at the time of simulated saccade onset. Response latencies for active motion stimuli were calculated relative to the time of real saccade onset (see preceding text). In most cases, response latencies were calculated using an automated Poisson-statistic algorithm described previously (Price et al. 2006). First, the spontaneous neuronal activity in the 500 ms of stationary pattern presentation before passive motion or saccade start was fit with a Poisson distribution. We then calculated a response threshold based on the 99% cutoff from the Poisson distribution. The response latency was taken as the first time after motion onset when the SDF exceeded this threshold and stayed above the threshold for the subsequent 25 ms. This procedure worked very well for the majority of our data. For cells with a robust spontaneous rate and inhibitory responses to motion, latency of the inhibitory response was assigned as the time when firing rate dropped below the 1% Poisson threshold and remained below it for ≥25 ms. Finally, for cells that gave strong responses to preferred motion, but had antipreferred responses that were too weak to establish a Poisson threshold, we made the antipreferred latency equal to the preferred latency. This procedure was based on the observation that neurons excited by both preferred and antipreferred motion had similar latencies for both directions. This subset of assigned latencies was checked by eye, but was not used for latency analysis, only to obtain response amplitudes (see following text). Relative delays in the stimulus trigger, window discriminator, and software template-matching system used to identify action potentials were always <1.6 ms; thus they did not significantly affect the latency calculations. There is always the opportunity for error in the measurement of latency for eye motion as well as neuronal responses. For eye movements, having tried several eye speeds and accelerations, the acceleration threshold of 100°/s2 was found to be optimal in reducing errors in a subset of manually checked exemplar neurons. For neuronal responses, the ease of separating responses from spontaneous firing depended on both inherent spiking variability in the recorded cell and response amplitude. Nevertheless, by measuring changes in calculated latencies as the Poisson threshold was altered, we estimate that errors were never >5 ms for cells with low spike rates and were significantly lower for cells with robust responses.
Response amplitude to active and passive motion was quantified in a time window that started at response onset (i.e., the neuronal latency) and ended at a time equal to the motion (or saccade) duration (usually close to 30 ms for the 10° saccades that were used in these experiments). Direction selectivity was quantified using a difference over sum calculation: passive preferred direction response (Rpref) minus the passive antipreferred direction (Ranti) response divided by the sum of those values
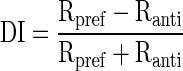 |
When active and passive responses were measured using background contrasts of 0, 10, 20, 50, and 100% we used the least-squares method to fit sigmoid curves to the normalized responses (Albrecht and Hamilton 1982)
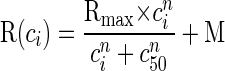 |
where R(ci) is the amplitude of the evoked response at contrast ci, M is the spontaneous rate, n is the exponent that determines the steepness of the curve, Rmax is the maximum elevation in response above the spontaneous rate, and c50 is the contrast that generates a response elevation of half Rmax.
RESULTS
We refer to the retinal slip produced by saccades as active motion and the retinal slip produced by saccade-like stimulus motion during fixation as passive motion (Thiele et al. 2002). Likewise, responses to these types of motion are referred to as active and passive responses, respectively.
Saccadic influence on directionality
First, we examined the influence of saccades on direction tuning of MSTd neurons to planar motion. For a population of 79 MSTd neurons (41, 16, and 22 from monkeys QP, HZ, and UJ, respectively), we compared the mean response amplitude for passive antipreferred direction motion as a function of the response to passive preferred motion (Fig. 1A). In this and subsequent plots, the responses are represented as the mean spike rate in the selected time window minus the spontaneous activity. We termed the direction producing the larger response the preferred direction and thus by definition, all points on the graph fall below the diagonal line of equality in Fig. 1A. However, it is noteworthy that many cells were not strongly modulated by the brief presentations of planar motion (median direction index: 0.25). It was previously noted that phasic responses of MSTd neurons are less selective for stimulus motion than tonic responses and that many cells generate sizable excitatory responses to motion in the antipreferred direction (Duffy and Wurtz 1991). Furthermore, 10° saccades induce retinal-slip velocities of 300–400°/s and these fast speeds may have reduced the direction selectivity of MSTd neurons (although this does not appear to be the case for area MT; Price et al. 2005).
FIG. 1.
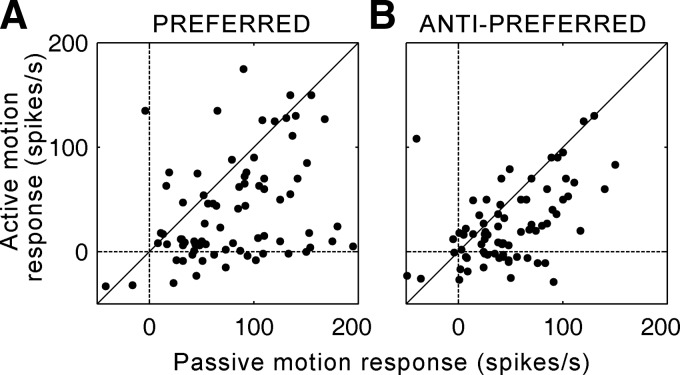
Direction selectivity of dorsal medial superior temporal area (MSTd) neurons during saccades. A and B: plots of response amplitudes generated by preferred direction motion (abscissa) against antipreferred motion (ordinate) during passive and active viewing, respectively. In both conditions the preferred direction is defined by the passive viewing case. Firing rates (in spikes/s) have had the spontaneous rate subtracted. Diagonal lines show the lines of equality. C and D: histograms of the direction-selectivity indexes (DIs; see methods) obtained from MSTd neurons under passive and active viewing conditions, respectively.
Figure 1B plots the preferred motion response against the antipreferred response for active motion. For this plot, preferred and antipreferred motions refer to the directions obtained with passive motion (see online supplement for an alternate analysis where this is not the case).1 Two differences between active and passive responses are evident. First, the cells show considerably higher levels of direction selectivity—i.e., the difference in amplitude between the responses to opposing directions of motion was greater, causing points in Fig. 1B to lay further from the diagonal line of equality. This is most noticeable in cells where motion in the antipreferred direction actually produces inhibition in the active case, whereas in the passive case both directions of motion produce excitation. Second, many cells actually reverse their directionality, with motion in what had been the antipreferred direction producing the largest excitation (dots above the line of equality, Fig. 1B).
Next, we calculated direction selectivity indices (DIs; see methods) for each cell and created population histograms (Fig. 1, C and D). The index we used gives a value of zero if the responses are nondirectional. An index of 1 shows strong direction selectivity, whereas values >1 indicate that motion in the antipreferred direction led to inhibition below the spontaneous rate. The latter case is an indication of very strong direction selectivity. With passive stimulation, the majority of cells have low DI indices: the median was 0.25 (Fig. 1C) and the first and third quartiles were 0.10 and 0.41, respectively. Twelve cells have DIs >0.6 and, of these, only two cells exhibit inhibition for antipreferred direction motion. Note that three cells have negative-direction indices: these are cells in which motion in all directions produced firing that was lower than the spontaneous rate (Fig. 1C). When stimulus motion is generated by real saccades (active case), the directionality indices were more widely distributed (first quartile = −0.67; median = 0.14; third quartile = 0.83). All DI calculations were made using the preferred direction indicated by the passive condition (see online supplement for an alternate analysis). Fifty cells retained positive DI indices, whereas 29 had negative DI indices. That is, 29/79 cells (37%) showed directional reversals in the active case. Therefore about a third of cells reverse their directional-tuning properties and most cells show a dramatic increase in directional tuning in the active case. The latter observation applies to cells that retain or reverse their directional-tuning properties during saccades.
Directionality of saccadic suppression
We have shown that the responses to active motion generated by saccades and similar passive motion presented during fixation usually have different directional tuning. We now ask whether the absolute response amplitudes are influenced by saccades. The data presented are derived entirely from stimuli with 100% contrasts. Figure 2A plots the results derived from retinal slip in the preferred directions of the cells (as defined during passive stimulation), whereas Fig. 2B shows the results from the antipreferred direction. For the preferred and antipreferred directions of motion, 83 and 81% of cells show reduced amplitudes in the active case, respectively. A two-way ANOVA comparing type of motion (active vs. passive) and direction (preferred vs. antipreferred) revealed that regardless of direction active responses were significantly lower than the passive responses across the population [F(1,315) = 44, P ≪ 0.01], and a trend toward significance for the interaction between motion type and direction suggests that during saccades firing rates in the preferred direction may be reduced more than firing rates in the antipreferred direction [F(1,312) = 3.2, P < 0.08]. These data provide a direct neural correlate of saccadic suppression.
FIG. 2.
Saccadic suppression in MSTd. A: plot of the passive motion response to preferred motion (abscissa) against the active motion response (ordinate). B: plot of the passive motion response to antipreferred motion (abscissa) against the active motion response (ordinate). Note that for both preferred and antipreferred motions the response is smaller during saccades (active case). Firing rates (in spikes/s) have had the spontaneous rate subtracted and diagonal lines show the lines of equality.
Contrast sensitivity
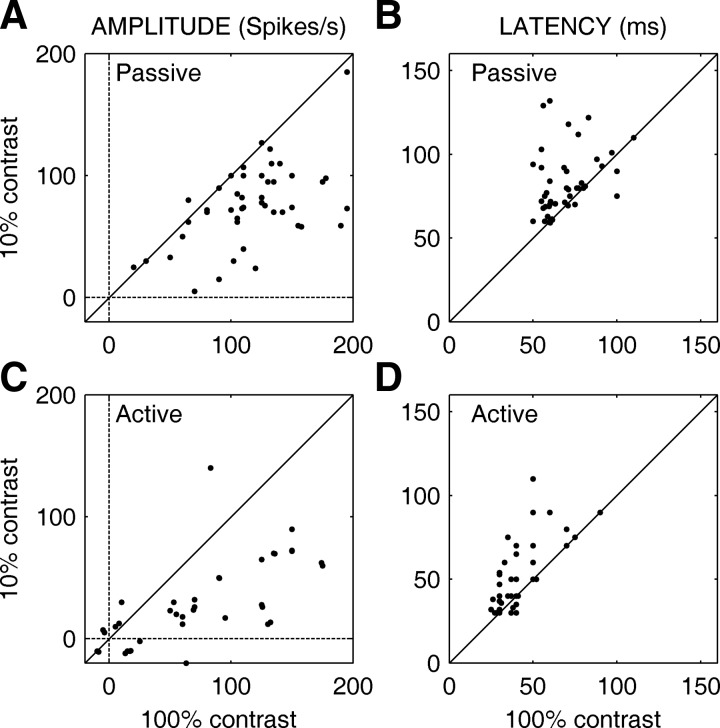
As outlined in the introduction, saccadic suppression is associated with very short latency responses. The passive latency using a 100% contrast background has been shown to be ≤30 ms shorter than the active latency (Ibbotson et al. 2007, 2008; also see Table 1). Do the short-latency responses we report have visual origins or did they arise from motor commands? To test this, we varied the contrast of the background stimulus during real and simulated saccades. Three contrasts were used: 0, 10, and 100%. Saccades during the 0% background contrast condition did not evoke significant responses in any MSTd neurons. This lack of evoked spiking provided strong evidence that short-latency responses are visual in origin and also showed that the visual edge created by the border of the screen did not modulate MSTd neurons during saccades. However, to measure the effect of eye movements and contrast on latency, we also used a background with 10% contrast to elicit sufficiently large responses for latency to be calculated. Response amplitudes and latencies were compared for 10 and 100% backgrounds for a subset of 45 MSTd neurons, of which 37 cells gave sufficiently strong responses in the active low-contrast condition to obtain latencies. Figure 3 summarizes the effects of contrast on active and passive responses. Figure 3A plots the passive response amplitude of 45 MSTd cells during stimulation at 100% contrast as a function of passive response amplitude using 10% contrast and Fig. 3C plots the active response amplitudes in the same manner. For both graphs nearly all points lie below the dashed line of equality, indicating that the spike rate was higher when the background was 100% contrast than when it was 10%. An unbalanced two-way ANOVA comparing type of motion (active vs. passive) and background contrast revealed that response amplitudes were significantly larger when stimulus contrast was higher [F(1,163) = 47, P ≪ 0.01] and that response amplitudes were significantly larger during passive motion [F(1,163) = 56, P ≪ 0.01]. The lack of interaction between motion type and contrast [F(1,163) = 0.1, P > 0.79] indicates that contrast alters a neuron's firing rate during saccades in much the same way it does during fixation. Mean spike rates for each condition are reported in Table 1.
TABLE 1.
Response amplitudes and latencies for active saccade-induced motion and passive saccade stimulations in which the visual stimulus had a contrast of 10 or 100%
| Parameter | 10% Contrast | 100% Contrast |
|---|---|---|
| Passive response amplitude | 76.6 ± 5.0 spikes/s | 117.3 ± 5.8 spikes/s |
| Active response amplitude | 28.7 ± 4.8 spikes/s | 72.9 ± 8.6 spikes/s |
| Passive response latency | 83.1 ± 2.9 ms | 69.3 ± 2.2 ms |
| Active response latency | 51.0 ± 3.1 ms | 41.1 ± 2.2 ms |
Values are means ± SE.
FIG. 3.
Effect of contrast on saccadic suppression in MSTd neurons. A: plot of mean response amplitudes (in spikes/s) generated by passive motion of a stimulus of 100% contrast (abscissa) against responses generated by passive motion of a stimulus of 10% contrast (ordinate). B: plot of the latencies (in milliseconds) of the passive responses shown in A. Note that latencies are longer for the lower-contrast stimulus. C: plot of mean response amplitudes generated by saccades over a background of 100% contrast (abscissa) against responses generated by saccades over a background of 10% contrast (ordinate). D: plot of the latencies (in milliseconds) of the active responses shown in C and, once again, latencies are longer for the lower contrasts. Diagonal lines show the lines of equality.
Figure 3B plots the passive response latency of 45 MSTd cells during stimulation at 100% contrast as a function of passive response latency using 10% contrast and Fig. 3D plots the active response latencies in the same format. For the latency graphs, almost all points are above the dashed line of equality, indicating that latencies were longer for low contrasts during active and passive viewing. Again, we used an unbalanced two-way ANOVA to compare the effect of motion (active vs. passive) and background contrast on latencies. We found that latencies were significantly longer for 10% background contrast [F(1,163) = 21, P ≪ 0.01] and that latencies were significantly longer during passive viewing regardless of contrast [F(1,163) = 132, P ≪ 0.01]. Once again, there was no interaction between motion type and contrast [F(1,163) = 0.56, P > 0.45], which suggests that the short-latency responses that occur during saccades are visual in origin and can be altered by contrast. The mean latencies for each condition are summarized in Table 1.
We collected active and passive responses for a population of 44 MSTd neurons using background contrasts of 0, 10, 20, 50, and 100%. Figure 4 plots the mean contrast response functions for this population after their responses were normalized to the passive response to the 100% contrast background. Three curves are shown: the top solid curve shows the fitted contrast response function for the passive condition (see methods); the bottom dotted curve shows the fitted function in the active condition; and the middle curve (dashed line) shows the fitted curve from the active case scaled up to the same maximum fitted value (Rmax) as the curve for the passive case. It is obvious that the contrast response function in the active case is suppressed at all contrasts relative to the passive case (sometimes called response gain control). The normalized response to the 100% contrast background in the active case is 54% lower than that in the passive case. Importantly, relative reductions in response amplitude were greater at low contrasts. For the 10% contrast background, normalized active responses were reduced by 74%. The semisaturation contrast (c50) changes from 4.8% during passive viewing to 21.3% during active viewing, indicating that the contrast response function in the active case is shifted to the right relative to the passive case, which is an example of contrast gain control (cf. Crowder et al. 2006; Ibbotson 2005).
FIG. 4.
Contrast response functions during active and passive motions. The least-squared fits to the mean of normalized contrast response functions during active (dotted lines) and passive motion (solid lines) are shown for a subset of MSTd neurons (n = 44). The dashed line shows the fit to active motion scaled up to the passive condition for comparative purposes. Error bars denote SE.
DISCUSSION
In this study we investigated the neural processing of visual motion that is produced by rapid eye movements called saccades. Typically, this saccade-induced motion is not perceived—a phenomenon referred to as saccadic suppression. Here, we show that the response amplitude, direction tuning, and latency of neurons in area MSTd of macaques are altered during saccades. Neurons in MSTd have been proposed to participate in the decoding of the visual consequences of self-motion (i.e., optic flow; Duffy and Wurtz 1991, 1995) and have been shown to play a role in motion perception (Celebrini and Newsome 1994, 1995). Therefore saccadic suppression in MSTd may be important to ensure that retinal slip associated with saccades is not interpreted and/or perceived as self-motion.
Directional reversals
Earlier work on motion processing during saccades indicated that about a third of neurons in MT and MSTd exhibit reversals in direction tuning during saccades (Thiele et al. 2002). Although we previously found no directional reversals in MT neurons (Price et al. 2005), in the present work 37% of our MSTd cell population reversed preferred directions during saccades. The conflicting observations for MT cells may be caused by differing analysis, but this is not likely to be an issue for observations in MSTd. The two studies mentioned earlier and the current work required that monkeys make 10° saccades, which take about 30 ms to complete. The analysis by Thiele et al. (2002) measured responses of MT/MSTd neurons in a time window that outlasted the saccade-induced motion by around 160 ms and may have included after-responses produced by saccade cessation. Conversely, the analysis by Price et al. (2005) measured response amplitudes 25–75 ms after saccade start and were less likely to include after-responses. In the present study we measured response amplitude in a manner similar to that of Price et al. (2005; see methods), yet we observed direction reversals like Thiele et al. (2002). In addition to direction reversals, we also showed that directional tuning can become enhanced during saccades. This effect was not observed in other investigations of saccadic suppression, perhaps because neurons with a direction index of <0.5 had been excluded from analysis (Price et al. 2005; Thiele et al. 2002), which may have created a ceiling effect for their data. Recent work has shown that in response to smooth-pursuit eye movements firing of MT neurons was most correlated with motion of the retinal image, whereas firing of MST neurons was most correlated with motion of the stimulus independent of retinal slip (Inaba et al. 2007). It was also shown, for smooth-pursuit stimuli at least, that MT neurons code for image motion more precisely than MST neurons, perhaps indicating that the conversion from retina- to stimulus-centered coordinates is imprecise (Inaba et al. 2007). If this imperfect coordinate transformation occurs during all types of eye movements, it could contribute to both the enhancement and reversals of direction index we observed.
One last important observation to consider is that the time course of direction-tuning changes in MT and MSTd appears to peak around 150 ms after saccade start (Thiele et al. 2002), whereas both psychophysical and physiological studies indicate that saccadic suppression is evident before the saccade even begins (Diamond et al. 2000; Ibbotson et al. 2008). This timing is inconsistent with a scenario where saccadic suppression in the parietal cortex arises because neurons with reversed direction tuning sum their responses with neurons whose direction tuning remains unchanged to cancel out all evidence of saccade-induced retinal slip. Instead, the long-latency changes in direction tuning that Thiele et al. (2002) observed, which we had referred to as after-responses, may be the physiological correlate of backward masking from the textured background. Masking stimuli presented ≤100 ms before or after saccades have been shown to produce psychophysical and physiological suppression (Wurtz 2008). Masking effects are known to be separate from suppression produced by corollary discharge (Volkmann 1986; Wurtz 2008), but they almost certainly interact during natural vision. Examining the relationship between these two processes should provide a better understanding of the physiological basis of saccadic suppression.
Latency
Thiele et al. (2002) showed that response latencies were shorter for active motion (63.5 ms) than for passive motion (73.5 ms) in MSTd neurons and our results show an even more dramatic difference in latencies (active: 41.1 ms vs. passive: 69.3 ms). These results are similar to the latencies for active and passive motion observed in MT (Price et al. 2005; Thiele et al. 2002). Although considered very short, latencies of 30–40 ms have been previously reported for flashed and moving stimuli in cortical areas V1 (Maunsell and Gibson 1992; Mazer et al. 2002) and MT/MST (Ibbotson et al. 2007; Kawano 1999; Petersen et al. 1985; Raiguel et al. 1989, 1999).
The saccade-related data we present reveal two effects that are difficult to reconcile. On the one hand we found that retinal motion generated by the saccadic movements of the eyes (active motion) generated relatively small responses in MSTd neurons compared with nearly identical image motion while the animal's eyes were stationary (passive motion). This decrease in response amplitude around the time of saccades is thought to parallel the decrease in visual perception around the time of saccades (i.e., saccadic suppression). On the other hand, response latencies to active motion were significantly shorter than latencies to passive motion. Normally, reduced response amplitudes would lead to increased response latencies (e.g., Ibbotson et al. 1994; Raiguel et al. 1999). This phenomenon has been most studied in the realm of contrast coding. As the contrast—and thus response amplitude—is reduced the response latencies tend to increase (Gawne et al. 1996; Kapadia et al. 1999; Reich et al. 2001). Indeed, in the present study we clearly show that reductions in stimulus contrast led to decreases in response amplitude and increases in latency even for stimuli presented during saccades (Fig. 3; Table 1).
There is evidence that early stages of the visual pathway change their activity around the time of saccades. About 25% of magnocellular, parvocellular, and koniocellular neurons in the dLGN are suppressed during saccades and enhanced afterward (Lee and Malpeli 1998; Reppas et al. 2002; Royal et al. 2006). Furthermore, around two thirds of neurons in the superficial layers of the superior colliculus (SC) show striking suppression during saccades (Richmond and Wurtz 1980; Robinson and Wurtz 1976). There are at least two ways that MSTd neurons could inherit this suppressed activity from earlier visual areas, yet have a counterintuitive decrease in latency. First, suppression in dLGN or SC may cause a release from inhibition in neurons that project directly to the parietal cortex but have relatively weak feedforward drive. For example, there is a direct route from the dLGN to MT via the koniocellular layers of the dLGN (Sincich et al. 2004) and the pulvinar has been shown to project directly to the cortex (Cusick et al. 1993; Stepniewska et al. 1999). A second possibility is that the nonvisual mechanisms that act to prime the visual system for the onset of fixation could actually shorten neuronal latencies for activity that arrives in the parietal cortex after the saccade has occurred, including the responses to saccade-induced retinal slip. In the primary visual cortex, the onset of fixation has been associated with a phase concentration of neuronal oscillations that produces maximal excitability (Rajkai et al. 2008). If something similar happens in the parietal cortex, there may be a delicate balance of saccadic suppression and postsaccadic enhancement where the resting potentials of MT/MST neurons are close to their spiking thresholds so that the suppressed inputs are slightly amplified, but neurons will reach their spiking thresholds sooner, thus resulting in shorter latencies. This scenario would require enhancement to occur locally in MST (and earlier as well), but suppression to originate early in the visual pathway. The observation that spontaneous rates of dLGN neurons showed saccadic suppression and postsaccadic enhancement when saccades were made in darkness, but MSTd neurons showed only postsaccadic enhancement, indicates that this may be the case (Ibbotson et al. 2008; Ramcharan et al. 2001; Reppas et al. 2002; Royal et al. 2006). The purpose of perisaccadic modulation appears to be twofold: 1) to minimize the disturbing effects of saccade-induced retinal slip and 2) to prime the visual system to facilitate transfer of information to cortex once the eyes have landed at their new location (Ibbotson et al. 2007, 2008; Rajkai et al. 2008; Royal et al. 2006). However, the distinct characteristics of suppression and enhancement are only beginning to be uncovered.
Contrast sensitivity
Electrophysiological studies show that neurons in various areas of the brain respond to retinal slip during saccades (dLGN: Ramcharan et al. 2001; Reppas et al. 2002; V1/V2: Battaglini et al. 1986; Toyama et al. 1984; Wurtz 1969; V4: Tolias et al. 2001; and MT/MSTd: Price et al. 2005; Thiele et al. 2002). Thus total suppression of neural activity is rare. Our experiments show that the strength of suppression is not equal across all contrasts: we found relatively more suppression to lower contrasts. This finding is interesting because natural scenes are dominated by lower contrasts (0–25%; Balboa and Grzywacz 2003; Brady and Field 2000; Chirimuta et al. 2003; Durant et al. 2007; Ruderman and Bialek 1994; Tadmor and Tolhurst 2000; Vu et al. 1997), which suggests saccadic suppression would be most effective under natural viewing conditions. Our electrophysiological data showing contrast gain control in MSTd during saccades agree with psychophysical predictions: Burr and Morrone (1996) suggested that saccadic suppression could be mediated by contrast gain control mechanisms early in the visual pathway, particularly magnocellular components. However, even though saccadic suppression appears to share some characteristics with contrast gain control, caution should be used in assigning a locus to the altered contrast coding because contrast tuning appears to be malleable at nearly every stage on the way to MSTd (LGN: Solomon et al. 2004; V1: Sclar et al. 1989; MT: Thiele et al. 2004).
GRANTS
This work was funded by Australian Research Council Centre of Excellence in Vision Science Grant CE0561903 to M. R. Ibbotson, National Institutes of Health Grants EY-06069 and RR-0165 to M. J. Mustari, and a Natural Sciences and Engineering Research Council of Canada grant to N. A. Crowder.
Supplementary Material
Acknowledgments
We thank Drs. Markus Hietanen and Seiji Ono and T. Broznya, K. Peixoto, and A. Gazy for help with experiments and animal care.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Albrecht 1982.Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol 48: 217–237, 1982. [DOI] [PubMed] [Google Scholar]
- Balboa 2003.Balboa RM, Grzywacz NM. Power spectra and distribution of contrasts of natural images from different habitats. Vision Res 43: 2527–2537, 2003. [DOI] [PubMed] [Google Scholar]
- Battaglini 1986.Battaglini PP, Galletti C, Aicardi G, Squatrito S, Maioli MG. Effect of fast moving stimuli and saccadic eye movements on cell activity in visual areas V1 and V2 of behaving monkeys. Arch Ital Biol 124: 111–119, 1986. [PubMed] [Google Scholar]
- Brady 2000.Brady N, Field DJ. Local contrast in natural images: normalisation and coding efficiency. Perception 29: 1041–1055, 2000. [DOI] [PubMed] [Google Scholar]
- Burr 1982.Burr DC, Holt J, Johnstone JR, Ross J. Selective depression of motion sensitivity during saccades. J Physiol 333: 1–15, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr 1996.Burr DC, Morrone MC. Temporal impulse response functions for luminance and colour during saccades. Vision Res 36: 2069–2078, 1996. [DOI] [PubMed] [Google Scholar]
- Burr 1994.Burr DC, Morrone MC, Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature 371: 511–513, 1994. [DOI] [PubMed] [Google Scholar]
- Castet 2001.Castet E, Jeanjean S, Masson GS. “Saccadic suppression”—no need for an active extra-retinal mechanism. Trends Neurosci 24: 316–318, 2001. [DOI] [PubMed] [Google Scholar]
- Castet 2000.Castet E, Masson GS. Motion perception during saccadic eye movements. Nat Neurosci 3: 177–183, 2000. [DOI] [PubMed] [Google Scholar]
- Celebrini 1994.Celebrini S, Newsome WT. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. J Neurosci 14: 4109–4124, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celebrini 1995.Celebrini S, Newsome WT. Microstimulation of extrastriate area MST influences performance on a direction discrimination task. J Neurophysiol 73: 437–448, 1995. [DOI] [PubMed] [Google Scholar]
- Chirimuuta 2003.Chirimuuta M, Clatworthy PL, Tolhurst DJ. Coding of the contrast in natural images by visual cortex (V1) neurons: a Bayesian approach. J Opt Soc Am A 20: 1253–1260, 2003. [DOI] [PubMed] [Google Scholar]
- Crowder 2006.Crowder NA, Price NSC, Hietanen MA, Dreher B, Clifford CWG, Ibbotson MR. Relationship between contrast adaptation and orientation tuning in areas V1 and V2 of cat visual cortex. J Neurophysiol 95: 271–283, 2006. [DOI] [PubMed] [Google Scholar]
- Cusick 1993.Cusick CG, Scripter JL, Darensbourg JG, Weber JT. Chemoarchitectonic subdivisions of the visual pulvinar in monkeys and their connectional relations with the middle temporal and rostral dorsolateral visual areas, MT and DLr. J Comp Neurol 336: 1–30, 1993. [DOI] [PubMed] [Google Scholar]
- Diamond 2000.Diamond MR, Ross J, Morrone MC. Extraretinal control of saccadic suppression. J Neurosci 20: 3449–3455, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy 1991.Duffy CJ, Wurtz RH. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large-field stimuli. J Neurophysiol 65: 1329–1345, 1991. [DOI] [PubMed] [Google Scholar]
- Duffy 1995.Duffy CJ, Wurtz RH. Response of monkey MST neurons to optic flow stimuli with shifted centers of motion. J Neurosci 15: 5192–5208, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant 2007.Durant S, Clifford CW, Crowder NA, Price NS, Ibbotson MR. Characterizing contrast adaptation in a population of cat primary visual cortical neurons using Fisher information. J Opt Soc Am A Opt Image Sci Vis 24: 1529–1537, 2007. [DOI] [PubMed] [Google Scholar]
- Fischer 1996.Fischer WH, Schmidt M, Stuphorn V, Hoffmann KP. Response properties of relay cells in the A-laminae of the cat's dorsal lateral geniculate nucleus after saccades. Exp Brain Res 110: 435–445, 1996. [DOI] [PubMed] [Google Scholar]
- Gawne 1996.Gawne TJ, Kjaer TW, Richmond BJ. Latency: another potential code for feature binding in striate cortex. J Neurophysiol 76: 1356–1360, 1996. [DOI] [PubMed] [Google Scholar]
- Ibbotson 2005.Ibbotson MR Physiological mechanisms of adaptation in the visual system. In: Fitting the Mind to the World: Adaptation and After-Effects in High-Level Vision, edited by Clifford CWG, Rhodes G. New York: Oxford Univ. Press, 2005, p. 150–46.
- Ibbotson 2008.Ibbotson MR, Crowder NA, Cloherty S, Price NSC, Mustari MJ. Saccadic modulation of neural responses: possible roles in saccadic suppression, enhancement and time compression. J Neurosci 28: 10952–10960, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibbotson 2007.Ibbotson MR, Price NSC, Crowder NA, Ono S, Mustari MJ. Enhanced motion sensitivity follows saccadic suppression in the superior temporal sulcus of the macaque cortex. Cereb Cortex 17: 1129–1138, 2007. [DOI] [PubMed] [Google Scholar]
- Ilg 1993.Ilg UJ, Hoffmann KP. Motion perception during saccades. Vision Res 33: 211–220, 1993. [DOI] [PubMed] [Google Scholar]
- Inaba 2007.Inaba N, Shinomoto S, Yamane S, Takemura A, Kawano K. MST neurons code for visual motion in space independent of pursuit eye movements. J Neurophysiol 97: 3473–3483, 2007. [DOI] [PubMed] [Google Scholar]
- Kapadia 1999.Kapadia MK, Westheimer G, Gilbert CD. Dynamics of spatial summation in primary visual cortex of alert monkeys. Proc Natl Acad Sci USA 96: 1273–1278, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano 1999.Kawano K Ocular tracking: behavior and neurophysiology. Curr Opin Neurobiol 9: 467–473, 1999. [DOI] [PubMed] [Google Scholar]
- Lee 1998.Lee D, Malpeli JG. Effects of saccades on the activity of neurons in the cat lateral geniculate nucleus. J Neurophysiol 79: 922–936, 1998. [DOI] [PubMed] [Google Scholar]
- Livingston 2003.Livingston CA, Fedder SR. Visual-ocular motor activity in the macaque pregeniculate complex. J Neurophysiol 90: 226–244, 2003. [DOI] [PubMed] [Google Scholar]
- Maunsell 1992.Maunsell JH, Gibson JR. Visual response latencies in striate cortex of the macaque monkey. J Neurophysiol 68: 1332–1344, 1992. [DOI] [PubMed] [Google Scholar]
- Mazer 2002.Mazer JA, Vinje WE, McDermott J, Schiller PH, Gallant JL. Spatial frequency and orientation tuning dynamics in area V1. Proc Natl Acad Sci USA 99: 1645–1650, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen 1985.Petersen SE, Baker JF, Allman JM. Direction-specific adaptation in area MT of the owl monkey. Brain Res 346: 146–150, 1985. [DOI] [PubMed] [Google Scholar]
- Price 2006.Price NS, Crowder NA, Hietanen MA, Ibbotson MR. Neurons in V1, V2, and PMLS of cat cortex are speed tuned but not acceleration tuned: the influence of motion adaptation. J Neurophysiol 95: 660–673, 2006. [DOI] [PubMed] [Google Scholar]
- Price 2005.Price NS, Ibbotson MR, Ono S, Mustari MJ. Rapid processing of retinal slip during saccades in macaque area MT. J Neurophysiol 94: 235–246, 2005. [DOI] [PubMed] [Google Scholar]
- Price 2001.Price NSC, Ibbotson MR. Pretectal neurons optimized for the detection of saccade-like movements of the visual image. J Neurophysiol 85: 1512–1521, 2001. [DOI] [PubMed] [Google Scholar]
- Raiguel 1989.Raiguel SE, Lagae L, Gulyas B, Orban GA. Response latencies of visual cells in macaque areas V1, V2 and V5. Brain Res 493: 155–159, 1989. [DOI] [PubMed] [Google Scholar]
- Raiguel 1999.Raiguel SE, Xiao DK, Marcar VL, Orban GA. Response latency of macaque area MT/V5 neurons and its relationship to stimulus parameters. J Neurophysiol 82: 1944–1956, 1999. [DOI] [PubMed] [Google Scholar]
- Rajkai 2008.Rajkai C, Lakatos P, Chen CM, Pincze Z, Karmos G, Schroeder CE. Transient cortical excitation at the onset of visual fixation. Cereb Cortex 18: 200–209, 2008. [DOI] [PubMed] [Google Scholar]
- Ramcharan 2001.Ramcharan EJ, Gnadt JW, Sherman SM. The effects of saccadic eye movements on the activity of geniculate relay neurons in the monkey. Vis Neurosci 18: 253–258, 2001. [DOI] [PubMed] [Google Scholar]
- Reich 2001.Reich DS, Mechler F, Victor JD. Temporal coding of contrast in primary visual cortex: when, what, and why. J Neurophysiol 85: 1039–1050, 2001. [DOI] [PubMed] [Google Scholar]
- Reppas 2002.Reppas JB, Usrey WM, Reid RC. Saccadic eye movements modulate visual responses in the lateral geniculate nucleus. Neuron 35: 961–974, 2002. [DOI] [PubMed] [Google Scholar]
- Richards 1969.Richards W Saccadic suppression. J Opt Soc Am 59: 617–623, 1969. [DOI] [PubMed] [Google Scholar]
- Richmond 1980.Richmond BJ, Wurtz RH. Vision during saccadic eye movements. II. A corollary discharge to monkey superior colliculus. J Neurophysiol 43: 1156–1167, 1980. [DOI] [PubMed] [Google Scholar]
- Robinson 1976.Robinson DL, Wurtz RH. Use of an extraretinal signal by monkey superior colliculus neurons to distinguish real from self-induced stimulus movement. J Neurophysiol 39: 852–1870, 1976. [DOI] [PubMed] [Google Scholar]
- Ross 2001.Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends Neurosci 24: 113–121, 2001. [DOI] [PubMed] [Google Scholar]
- Royal 2006.Royal DW, Sary G, Schall JD, Casagrande VA. Correlates of motor planning and postsaccadic fixation in the macaque monkey lateral geniculate nucleus. Exp Brain Res 168: 62–75, 2006. [DOI] [PubMed] [Google Scholar]
- Ruderman 1994.Ruderman DL, Bialek W. Statistics of natural images: scaling the woods. Phys Rev Lett 74: 814–818, 1994. [DOI] [PubMed] [Google Scholar]
- Sclar 1989.Sclar G, Lennie P, DePriest DD. Contrast adaptation in striate cortex of macaque. Vision Res 29: 747–755, 1989. [DOI] [PubMed] [Google Scholar]
- Shioiri 1989.Shioiri S, Cavanagh P. Saccadic suppression of low-level motion. Vision Res 29: 915–928, 1989. [DOI] [PubMed] [Google Scholar]
- Sincich 2004.Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct geniculate input to area MT. Nat Neurosci 7: 1123–1128, 2004. [DOI] [PubMed] [Google Scholar]
- Solomon 2004.Solomon SG, Peirce JW, Dhruv NT, Lennie P. Profound contrast adaptation early in the visual pathway. Neuron 42: 155–162, 2004. [DOI] [PubMed] [Google Scholar]
- Stepniewska 1999.Stepniewska I, Qi HX, Kaas JH. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? Eur J Neurosci 11: 469–480, 1999. [DOI] [PubMed] [Google Scholar]
- Tadmor 2000.Tadmor Y, Tolhurst DJ. Calculating the contrasts that retinal ganglion cells and LGN neurones encounter in natural scenes. Vision Res 40: 3145–3157, 2000. [DOI] [PubMed] [Google Scholar]
- Thiele 2004.Thiele A, Distler C, Korbmacher H, Hoffmann KP. Contribution of inhibitory mechanisms to direction selectivity and response normalization in macaque middle temporal area. Proc Natl Acad Sci USA 101: 9810–9815, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele 2002.Thiele A, Henning P, Kubischik M, Hoffmann KP. Neural mechanisms of saccadic suppression. Science 295: 2460–2462, 2002. [DOI] [PubMed] [Google Scholar]
- Tolias 2001.Tolias AS, Moore T, Smirnakis SM, Tehovnik EJ, Siapas AG, Schiller PH. Eye movements modulate visual receptive fields of V4 neurons. Neuron 29: 757–767, 2001. [DOI] [PubMed] [Google Scholar]
- Toyama 1984.Toyama K, Kimura M, Komatsu Y. Activity of the striate cortex cells during saccadic eye movements of the alert cat. Neurosci Res 1: 207–222, 1984. [DOI] [PubMed] [Google Scholar]
- Volkmann 1986.Volkmann FC Human visual suppression. Vision Res 26: 1401–1416, 1986. [DOI] [PubMed] [Google Scholar]
- Vu 1997.Vu TQ, McCarthy ST, Owen GW. Linear transduction of natural stimuli by dark adapted rods of the salamander, Ambystoma tirgrinum. J Physiol 505: 193–204, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz 1969.Wurtz RH Comparison of effects of eye movements and stimulus movements on striate cortex neurons of the monkey. J Neurophysiol 32: 987–994, 1969. [DOI] [PubMed] [Google Scholar]
- Wurtz 2008.Wurtz RH Neuronal mechanisms of visual stability. Vision Res 48: 2070–2089, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.