Abstract
Objective
To define the magnitude and mechanism of the effect of brain infarcts on the odds of dementia in a prospective study.
Methods
We examined the effects of brain infarcts and Alzheimer’s disease (AD) pathology on the risk for dementia in 179 subjects from the Baltimore Longitudinal Study of Aging Autopsy Program. All subjects had longitudinal clinical and cognitive evaluations, and underwent postmortem examination of the brain.
Results
Brain infarcts were common in our cohort, and both symptomatic and asymptomatic infarcts conferred a significant increase in the odds of dementia. Risk factors for stroke in the absence of an infarct did not increase the odds of dementia, which was quantitatively related to the number but not the size of hemispheral infarcts; deep subcortical infarcts conferred no increased risk for dementia. The contribution of microscopic infarcts to dementia was significant and equivalent to that of macroscopic infarcts. In subjects with intermediate AD pathology scores, a single macroscopic hemispheral infarct was sufficient to cause dementia. A logistic regression model of the effect of infarcts and AD pathology on dementia indicated that AD pathology alone accounts for 50% of the dementia seen in this cohort, and that hemispheral infarcts alone or in conjunction with AD pathology account for 35%.
Interpretation
Cerebrovascular disease is a significant and potentially preventable cause of dementia in the Baltimore Longitudinal Study of Aging. Burden and location of infarcts are significantly associated with cognitive decline.
In epidemiological studies of the incidence and prevalence of dementia, Alzheimer’s disease (AD) is the most common cause, followed by vascular dementia.1–4 Because these studies rely on controversial and inconsistent clinical criteria for distinguishing between vascular dementia and AD,5,6 pathological studies have become the more reliable indicator of the relative impact of brain infarcts on dementia. Several prospective autopsy studies7–14 have reported on the role of vascular pathology in the cause of dementia. Although all agree on the importance of cerebral infarcts as a cause of dementia, the results conflict on the importance of vascular risk factors, asymptomatic infarcts, infarct size, and infarct location in the cause of dementia. There has been more agreement among the studies on how AD and vascular pathologies interact, with most studies showing they act in an additive fashion, although the quantitative nature of this association has not been documented.
We report here the results of the Baltimore Longitudinal Study of Aging Autopsy Program, a prospective study of the effects of aging on cognition and dementia. The intensity of the prospective evaluations of these subjects, the large number of autopsies, and the surprisingly large number of subjects with brain infarcts make this cohort unique for elucidating the contribution of cerebrovascular pathology to dementia.
Subjects and Methods
Cohort
A total of 579 participants from the Baltimore Longitudinal Study of Aging (total enrollment, 3,006) have agreed to postmortem brain examinations. The rate of dementia and clinical stroke in the autopsy cohort is no different from the cohort as a whole.15 As of December 2006, 194 cases have died and had a brain autopsy (86% autopsy rate). Of this group, we excluded 14 cases who had other pathological explanations for cognitive impairment; 9 had both a clinical and pathological diagnosis of Parkinson’s disease, 1 had a primary brain tumor, 1 had an inflammatory leukoencepha-lopathy, 1 had metastatic brain lesions, and 1 had hippocampal sclerosis/frontotemporal dementia. An additional case was excluded because language deficits from a clinical stroke compromised assessment of cognition. These exclusions left 179 cases for this analysis; 122 were men, and 57 were women. They were predominantly white (92%) with 17.5 ± 3.7 (± standard deviation) years of education. The mean age at death was 86.9 ± 8.2 years.
All subjects were cognitively and neurologically normal at the time of entry into the Baltimore Longitudinal Study of Aging. They were assessed at baseline, within 18 months of death (mean of 8.9 ± 6.7 months before death), and periodically in between. The majority was seen annually after age 70, although approximately 25% of the cohort had gaps in their follow-up of several years’ duration. The percentage of subjects with dementia ( p = 0.9), symptomatic stroke ( p = 0.5), or pathological infarcts ( p = 0.8) was not different between the groups with annual evaluations (n = 140) and those with less intense follow-up (n = 39) using Fisher’s Exact test. Studies of this cohort are conducted under the auspices of the Johns Hopkins and MedStar Research Institute institutional review boards, and all subjects provided written informed consent.
Neuropsychological and Risk Factor Evaluation
Annual evaluations included a series of neuropsychological tests,16 neurological examination, interval medical history, medication review, and a structured informant and subject interview as described previously.15 A diagnosis of diabetes or hypertension required both a documented history and the use of one medication for that condition. Severe hypertension was defined as that lasting 10 years, requiring two or more medications simultaneously, and a documented systolic blood pressure greater than 160mm Hg while on medication at least once. A diagnosis of coronary artery disease required a history of myocardial infarction or coronary artery disease plus medication prescribed to treat coronary artery disease. Apolipoprotein E genotype was obtained on 133 of the 179 subjects. Fasting total cholesterol levels were obtained, off medication, on entry into the study.
Diagnosis of Dementia and Clinical Stroke
All subjects were reviewed at a consensus conference at time of death or during life if their Blessed Information Memory Concentration score17 was 3 or greater, if their informant or subject Clinical Dementia Rating18 score was 0.5 or greater, or if the Dementia Questionaire19 was abnormal. Diagnoses of dementia were based on Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised criteria20 and required evidence of a progressive cognitive syndrome, including memory decline. All 54 demented subjects in this study with a brain infarct showed significant deterioration over at least one follow-up evaluation after an initial diagnosis of dementia or mild cognitive impairment. A history of clinical stroke was determined as described previously.15
Brain Pathology
Postmortem examination of all brains was performed at Johns Hopkins by a pathologist with access to clinical data related to the patient but not to the final conference diagnosis. Both hemispheres were cut in 1cm coronal slices. Any vascular lesions were measured and photographed. Tissue blocks of 1.5cm were dissected from all observed vascular lesions and from the following regions of the left brain: middle frontal, superior and middle temporal, inferior parietal, occipital, cingulate, orbitofrontal, amygdala and entorhinal cortex, hippocampus, basal ganglia, basal forebrain, thalamus, midbrain, pons, medulla, and cerebellum. Paraffin sections from all blocks were stained with hematoxylin and eosin; selected sections were stained with the Hirano silver method.21 Hippocampal sclerosis is an uncommon diagnosis in our study compared with others,22 likely because of interpretations of the disease-appropriate degree of neuronal loss in CA1 caused by AD pathology.
Infarcts judged acute or subacute, based on macroscopic and microscopic features,23 were not included in this analysis. Brain infarcts were divided into hemispheral and deep subcortical. Hemispheral infarcts included those in the cortical gray matter or underlying white matter as long as the lesion was above the level of the basal ganglia/internal capsule. Deep subcortical infarcts were those involving the basal ganglia, cerebellum, brainstem, or internal capsule. Old hemorrhages (of which there were only three) were treated similarly to infarcts. The volume of hemispheral infarcts was calculated by measuring the maximal size in three dimensions and then modeling it as a cone (1/3πr2h) or ellipsoid (4/3πabc). Both methods yielded the same results. Of note, the number and volume of hemispheral infarcts were not significantly related (correlation, 0.06; p = 0.4). Microscopic infarcts were defined as lesions not visible on macroscopic inspection but observed during the examination of the histological sections prepared from the brain regions described earlier.
AD pathology was examined on silver stains and graded according to Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)24 and Braak criteria.25 In addition, we generated a composite AD pathology score by summing the CERAD and Braak scores in equal measure. CERAD scores were assigned to three groups: 1 = zero or mild neuritic plaques; 2 = moderate neuritic plaques; and 3 = frequent neuritic plaques. Braak scores were divided into three groups: 1 = Braak stages 0, 1, and 2; 2 = Braak stages 3 and 4; and 3 = Braak stages 5 and 6. The sum of the modified Braak and CERAD scores yielded a composite score ranging from 2 to 6.
Statistics
Potential predictors of dementia were analyzed using univariate and stepwise multivariable logistic regression. All models included age at death and sex as covariates except for Table 2. Age was examined as both a continuous variable and stratified in quartiles or tertiles without any difference in the results. Comparisons between groups were performed using analysis of variance. All reported p values are two sided. Statistical analyses were performed using NCSS software (Kaysville, UT).
Table 2.
Odds Ratios and 95% Confidence Intervals for Dementia, Infarcts, and Microscopic Infarcts
| Risk Factor | Dementia (n = 89) |
Any Infarct (n = 79) |
Microscopic Infarct (n = 39) |
|---|---|---|---|
| Hypertension (n = 89) | 1.2 (0.6–2.1) | 1.5 (0.8–2.7) | 1.9 (0.9–4.1) |
| Severe hypertension (n = 38) | 1.1 (0.5–2.5) | 3.2a (1.5–7.0) | 4.5a (2.0–10) |
| CAD (n = 51) | 1.2 (0.6–2.3) | 2.2a (1.1–4.1) | 2.6a (1.2–5.4) |
| Diabetes (n = 42) | 0.6 (0.3–1.1) | 1.1 (0.5–2.3) | 1.1 (0.5–2.6) |
| Cholesterol (quartile 4 vs 1) | 0.5 (0.2–1.5) | 0.6 (0.2–1.3) | 0.9 (0.3–2.6) |
| Female sex (n = 57) | 1.8 (0.9–3.6) | 1.7 (0.9–3.4) | 2.0 (0.9–4.3) |
| Apolipoprotein E4 (33/133) | 3.0a (1.2–7.4) | 1.0 (0.4–2.1) | 1.1 (0.4–2.7) |
| Apolipoprotein E2 (25/133) | 1.4 (0.6–3.4) | 1.3 (0.6–3.2) | 1.6 (0.6–4.4) |
| Macroscopic infarct (n = 72) | 3.6a (1.9–6.9) | — | 10.8a (4.2–26) |
| CERAD score | 2.6a (1.9–3.6) | 1.1 (0.9–1.3) | 1.1 (0.9–1.4) |
| Braak score | 2.0a (1.5–2.7) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) |
| Composite AD score | 2.7a (2.0–3.6) | 1.1 (0.9–1.3) | 1.0 (0.8–1.3) |
For Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), Braak, and Composite Alzheimer’s disease (AD) pathology scores, the odds ratio indicates the effect of a single-step increase in the relevant pathology score on the odds of clinical dementia or pathological infarct.
p < 0.05.
CAD = coronary artery disease.
Results
Role of Brain Infarcts and Stroke Risk Factors in the Cause of Dementia
Brain infarcts were common in this cohort, being present in 79 of the 179 autopsy results. Of these, 72 had a total of 128 macroscopic infarcts, whereas 7 had only microscopic infarcts (32 had macroscopic plus microscopic lesions). The distribution of those infarcts is detailed in Table 1. The odds ratio (OR) for dementia (adjusted for sex and age at death) was significantly increased by the presence of an infarct (4.0; 95% confidence interval [CI], 2.1–7.8; n = 79), independent of whether it was symptomatic (4.5; 95% CI, 1.9 –11; n = 35) or asymptomatic (3.6; 95% CI, 1.6–8.1; n = 44).
Table 1.
Number of Subjects with Macroscopic and Microscopic Infarcts at Autopsy Are Listed by the Location of Those Infarcts
| Infarct Location | Macroscopic | Microscopic |
|---|---|---|
| Total | 72 | 39 |
| Hemispheral | 37 | 31 |
| Deep subcortical | 54 | 19 |
| Hemispheral and deep subcortical | 19 | 11 |
Although brain infarcts significantly increased the odds of dementia, clinical risk factors for stroke such as coronary artery disease and severe hypertension did not (Table 2), implying that the infarct itself was the cause of the dementia. Of particular importance, neither increasing AD pathology nor apolipoprotein E genotype were risk factors for brain infarcts (see Table 2). More-over, brain infarcts and vascular risk factors were not associated with increasing AD pathology, and all the interaction terms such as infarct × CERAD score or hemispheral infarcts × composite AD pathology were not significant ( p = 0.12, β = −0.6, OR = 0.6; and p = 0.4, β = 0.45, OR, 1.5, respectively).
Role of Infarct Size and Location on the Odds of Dementia
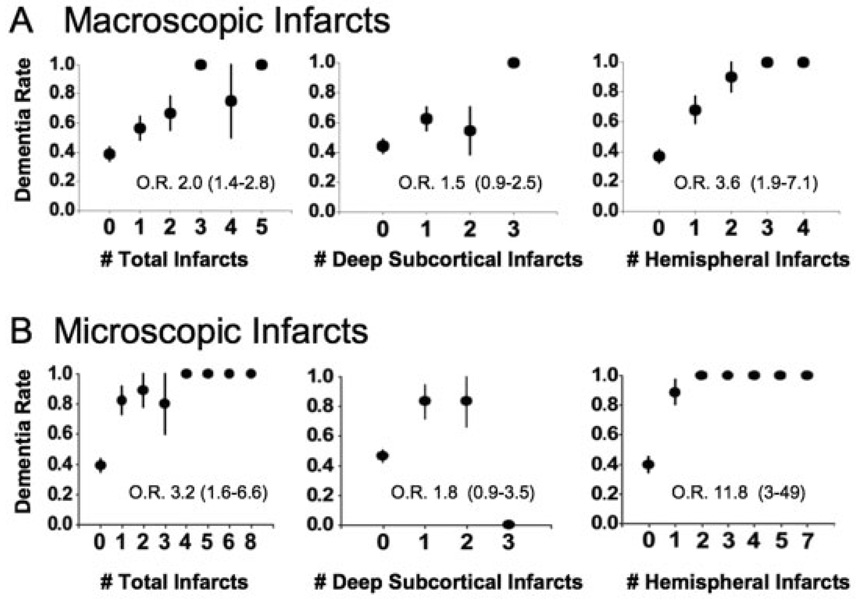
Age- and sex-adjusted odds of dementia were strongly related to the number of total macroscopic infarcts and to the number of macroscopic hemispheral infarcts using logistic regression but not to the number of deep subcortical infarcts (Fig 1A). To assess whether the volume of hemispheral infarcts contributed additional information to the number of hemispheral infarcts in determining the odds of dementia, we ranked total hemispheral infarct size by increasing volume or by quartiles of infarct volume. Each was significant in univariate analysis, but neither volume measure was significant when the number of macroscopic hemispheral infarcts was included in the analysis (Table 3). In contrast, the number of hemispheral infarcts remained a significant cause of dementia in all multivariate analyses involving infarct volume (see Table 3). Multivariate analyses of total infarct volume and total infarct number yielded the same result (not shown).
Fig 1.
Effect of the number of infarcts on the odds of dementia. (A) The relation between the number of macroscopic infarcts and dementia is shown stratified by location. The listed odds ratio (O.R.) refers to the age- and sex-adjusted odds for a single-step increase in the number of relevant infarcts. (B) The same analysis is applied to microscopic infarcts. All data are plotted as standard error plots.
Table 3.
Effect of Infarct Volume and Number on the Odds of Dementia
| Risk Factor | Adjustment Factors | |||
|---|---|---|---|---|
| Age and Sex | Age, Sex, and Number of Hemispheral Macroinfarcts | Adjusted for Sex, Age, and Hemispheral Infarct Volume | ||
| Rank | Quartiles | |||
| Hemispheral infarct volume (rank)a | 1.2b(1.1–1.3) | 1.0 (0.9–1.1) | — | — |
| Hemispheral infarct volume (quartile)a | 2.5b(1.4–4.5) | 1.0 (0.8–1.2) | — | — |
| Number of macroscopic hemispheral infarctsc | — | — | 4.6b(1.6–13) | 5.0b(1.6–17) |
Thirty-seven subjects with macroscopic hemispheral infarcts were compared with 100 subjects without infarcts using logistic regression. Age refers to age at death.
The odds of developing dementia based on the total hemispheral infarct volume (ranked in two separate ways) was determined controlling for age, sex, and the number of macroscopic hemispheral infarcts. The odds ratio indicates the effect of a single-step increase in the relevant volume measurement on the odds of clinical dementia.
Significant values, p < 0.05.
The effect of infarct volume (ranked by quartile or rank order) on the role of hemispheral infarct number in the odds of dementia was determined.
Relation of Deep Subcortical Infarcts to Dementia
In our cohort, 54 participants had a total of 71 macroscopic infarcts involving the basal ganglia, cerebellum, internal capsule, and brainstem. In an age- and sex-adjusted logistic regression analysis, the presence of these deep subcortical infarcts increased the odds of dementia (Table 4), although there was not a significant quantitative relation between the number of deep subcortical infarcts and dementia. The effect of deep subcortical infarcts on dementia was no longer significant after adjusting for the number of hemispheral infarcts. In contrast, hemispheral infarcts remained highly related to the odds of dementia after controlling for the number of deep subcortical infarcts (see Table 4A). The same results were obtained when we corrected for the presence, rather than the number, of hemispheral or deep subcortical infarcts. Additional evidence against the role of deep subcortical infarcts in the cause of dementia was derived from subjects with macroscopic deep subcortical infarcts who were free of macroscopic hemispheral lesions (n = 35). The OR for dementia in this group was not significantly increased compared with subjects with no infarcts (1.7; 95% CI, 0.7–3.4). In contrast, in 18 subjects with only macroscopic hemispheral infarcts, the OR for dementia was significantly increased (4.8; 95% CI, 1.6–14). The distinction between hemispheral and deep subcortical infarcts was not a white matter versus gray matter issue, because subjects with hemispheral infarcts that did not involve gray matter (n = 10) had an odds of dementia (4.7; 95% CI, 1.2–19) similar to that of the total group of subjects with hemispheral infarcts. Comparing dementia rates in subjects with deep subcortical infarcts in different locations (thalamus, caudate, among others), demonstrated no high-risk regions (data not shown).
Table 4.
Odds Ratios (95% Confidence Intervals) for Dementia Associated with Macroscopic or Microscopic Infarcts Adjusted for Age, Sex, and the Presence, Number, or Location of Other Infarct Types
| Infarcts | Adjusted for Factors | ||||
|---|---|---|---|---|---|
| Sex, Age | Sex, Age, and Number of Deep Subcortical Macroscopic Infarcts | Sex, Age, and Number of Hemispheral Macroscopic Infarcts | Sex, Age, and Presence of Any Microscopic Hemispheral Infarct | Sex, Age, and Presence of Any Deep Subcortical Microscopic Infarct | |
| Macroscopic infarcts | |||||
| Number of total macroscopic infarcts | 2.0a(1.4–2.8) | 3.4a(1.7–6.7) | 1.2 (0.7–2.2) | 1.7a(1.1–2.7) | 1.9a(1.3–2.7) |
| Number of hemispheral macroscopic infarcts | 3.6a(1.9–7.1) | 3.4a(1.7–6.7) | 2.9a(1.4–6.0) | 3.3a(1.7–6.4) | |
| Number of deep subcortical macroscopic infarcts | 1.5 (0.9–2.5) | 1.2 (0.7–2.1) | 1.2 (0.6–2.2) | 1.4 (0.8–2.4) | |
| Any hemispheral macroscopic infarct | 5.6a(3.0–12) | 5.0a(2.0–12) | 3.5a(1.4–9.6) | 5.5a(2.2–13) | |
| Any deep subcortical macroscopic infarct | 2.0a(1.1–4.1) | 1.5 (0.7–3.3) | 1.5 (0.6–3.2) | 2.0a(1.0–4.1) | |
| Microscopic infarcts | |||||
| Any microscopic infarct | 9.3a(3.4–25) | 6.0a(2.1–17) | 1.3 (0.3–5.7) | 25a(3.0–200) | |
| Any hemispheral microscopic infarct | 40a(5–312) | 6.8a(2.2–21) | — | 37a(4.7–290) | |
| Any deep subcortical microscopic infarct | 3.8a(1.2–12) | 2.2 (0.6–7.8) 2.4 (0.7–8.2) |
1.5 (0.4–6.2) | – | |
The odds ratio refers to that associated with a single-step increase in the independent variable. Age refers to age at death.
Significant values, p < 0.05.
Effect of Microscopic Infarcts on the Odds of Dementia
Microscopic lesions, independent of macroscopic infarcts, were also a significant cause of dementia (see Table 4). In 39 participants, we found a total of 89 microscopic infarcts, and most had macroscopic lesions as well. Microscopic infarcts shared the same risk factors as macroscopic infarcts, and the primary risk factor for a microscopic infarct was the presence of a macroscopic infarct (see Table 2).
The presence of microscopic infarcts at autopsy significantly increased the odds of dementia even when adjusting for macroscopic hemispheral lesions (see Table 4). The effect appeared to be a threshold phenomenon (see Fig 1B), potentially because of the incomplete nature of our sampling procedure. Like macroscopic infarcts, only the presence of hemispheral microscopic infarcts increased the odds of dementia in multivariate analyses (see Table 4B). Consistent with this distinction, a comparison of the rate of dementia in subjects with deep subcortical microscopic infarcts versus subjects with hemispheral microscopic infarcts showed a significant difference ( p = 0.02, Fisher’s exact test).
Interaction of Cerebrovascular and Alzheimer’s Disease Pathology in the Cause of Dementia
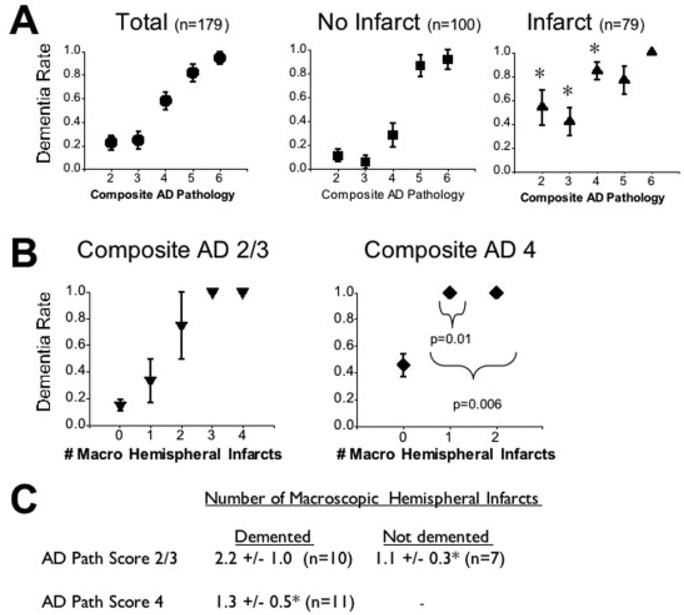
As shown in many other studies, AD pathology, quantified by Braak and CERAD scores, is an important predictor of dementia. In our cohort, a more complete estimate of the contribution of AD pathology to dementia was a composite AD pathology score, derived from equal contributions of Braak and CERAD scores. Using logistic regression, we found the composite AD pathology score to be significantly better at predicting dementia than the Braak or CERAD scores, either alone or together (data not shown). In the presence of any brain infarct (Fig 2A), dementia was more likely at low or intermediate AD pathology scores. The number of macroscopic hemispheral infarcts associated with dementia at low AD pathology scores was different from that associated with dementia at intermediate AD pathology scores (see Figs 2B, C). In subjects with low composite AD pathology scores, the mean number of macroscopic hemispheral infarcts in the demented group was 2.2. Those with a single macroscopic hemispheral infarct were most often not demented. In subjects with an intermediate AD pathology score of 4, however, a single macroscopic hemispheral infarct was always associated with dementia.
Fig 2.
Interaction between cerebrovascular disease and Alzheimer’s disease (AD) pathology in the cause of dementia. (A) Standard error plots of the dementia rate in subjects with increasing amounts of composite AD pathology are shown, stratified by the presence or absence of any infarct. Asterisks indicate significant differences between the infarct and no infarct groups. (B) Standard error plots of the dementia rate in subjects with increasing numbers of macroscopic hemispheral infarcts are shown, stratified by the amount of composite AD pathology. The indicated p values compare the composite AD pathology score 4 group with the composite AD pathology 2/3 group at the indicated number of hemispheral macroscopic infarcts. (C) The number of macroscopic hemispheral infarcts in demented and nondemented subjects at the indicated amount of composite AD pathology is shown. *p = 0.02 compared with the demented group with an AD pathology score of 2 or 3.
Quantitative Contributions of Hemispheral Infarcts and Alzheimer’s Disease Pathology to the Cause of Dementia
To determine the quantitative contribution of hemi-spheral infarcts to dementia, we developed a logistic regression model including AD pathology, the number of hemispheral macroscopic infarcts, and the presence of hemispheral microscopic infarcts as covariates. These variables accounted for 87% of the diagnostic classifications in this cohort, and all three were independently related to dementia. For example, when adjusted for composite AD pathology, the OR for the effect of a single-step increase in the number of hemispheral infarcts on dementia remained significant (5.6; 95% CI, 2.5–11.9). Using the reduction from the model r2 as the indication of the relative contribution of each variable to the odds of dementia, we found that composite AD pathology alone accounted for 50% of the dementia cases in the cohort, whereas hemispheral infarcts were an important factor in an additional 35% of the dementia cases. Microscopic and macroscopic infarcts contributed equally to the vascular risk. We used the composite AD pathology score as the surrogate for AD in this analysis, rather than the Braak or CERAD scores, because it resulted in the largest effect attributable to AD pathology. To test this result, we determined the number of cases of dementia in the group of subjects with infarcts (see Fig 2A, right) that might be attributable to their composite AD pathology score alone, based on the results from the no infarct group (see Fig 2A, center). This left 29 excess dementia cases in the infarct group that was not explained by their AD pathology. Given that there were 89 demented individuals in the cohort, the percentage attributable to infarcts was 33%, a close agreement with our regression model.
Discussion
We have found that hemispheral infarcts are a common cause of dementia, regardless of whether they are silent or clinically manifest, contributing to 35% of the dementia cases in this prospective cohort. Given that our participants had access to excellent medical care, the large contribution of vascular disease to dementia in this group is quite sobering. The observation that cerebrovascular disease is an important cause of dementia is supported by several large prospective pathological studies.7–14 Our results also agree with three prospective imaging studies,26–28 which showed an increase in dementia risk in subjects with asymptomatic infarcts defined by magnetic resonance imaging or computed tomography.
Importantly, our data suggest that it is the infarct itself and not the associated clinical risk factors for stroke such as coronary artery disease or severe hypertension that is responsible for the excess dementia in these cases. The fact that hemispheral infarcts are related to dementia and that deep subcortical infarcts are not would also argue against stroke risk factors being the important determinant in vascular dementia. Although epidemiological studies have identified a relation between cognitive impairment and vascular risk factors,29–31 none of these could correct for asymptomatic infarcts, either macroscopic or microscopic. However, the advantages of large epidemiological studies over small autopsy cohorts suggest that this should be an area of continued investigation.
All the prospective pathological studies that have examined the effect of cerebral infarcts on dementia have found a relation between the number of vascular lesions and dementia.9,10,12 The relation has always been defined qualitatively, with multiple lesions having a greater effect than a single lesion. We found that there is a direct relation between the number of macroscopic hemispheral infarcts and the odds of dementia with no additional effect conferred by the size of the lesion. The fact that microscopic infarcts were a powerful cause of dementia in our cohort, as demonstrated previously in the Honolulu-Asia Aging Study12 and, to a lesser extent, in the Religious Orders Study,8,9 also argues against the importance of infarct size in dementia.
We found a strong relation between infarct location and dementia, with hemispheral infarcts having a strong effect on the odds of dementia, whereas deep subcortical infarcts, as we have defined them, did not. These results conflict with the results of the Nun’s Study,7 which found that basal ganglia infarcts were more important than hemispheral lesions for dementia. Although the number and extent of basal ganglia infarcts (which make up most of our “deep subcortical” infarcts) were virtually identical in the two studies, the Nun’s Study had far fewer hemispheral infarcts and had insufficient power to detect the significance of these lesions. In a recent publication from the Rush Memory and Aging Project,32 “subcortical” infarcts were found to be correlated with dementia and cognitive dysfunction. However, in this study, most of the subcortical infarcts actually involved the hemispheral white matter, lesions that would have been included in the hemispheral group in our study. In contrast with our findings, the Geneva autopsy database33 found that deep subcortical infarcts were capable of causing dementia in subjects with no hemispheral infarcts, but only in those with a large number of basal ganglia infarcts, a group that is epidemiologically small. However, given that our cohort is not epidemiologically rigorous, a role for deep subcortical infarcts in dementia cannot be excluded. The mechanism by which hemispheral infarcts, independent of size but related to number and coexisting AD pathology, contribute to dementia may be related simply to the widespread nature of the individual or combined pathologies. Infarcts in deep subcortical areas may, for the most part, affect functions unrelated to a dementia diagnosis in the elderly, such as psychomotor speed, reward, or motor processing.34
Two other important issues are whether cerebrovascular disease and AD pathology are synergistic (ie, whether one causes the other), and how they combine to cause dementia. About the latter, our study agrees closely with the Religious Orders Study,9 which showed a relatively uniform relation between vascular lesions and dementia across the spectrum of AD pathology. The only differences between our findings and those from the Religious Orders Study is that at lower grades of AD pathology more hemispheral infarcts are required to cause dementia than at intermediate grades of AD pathology. Similar results have also been found in a community-based but retrospective study35 and in the Medical Research Council Cognitive Function and Ageing Study.11
The only study with results different from those cited earlier is the Nun’s Study,9 which found no effect of vascular lesions on dementia at the lowest level of AD pathology. It is likely that they were underpowered (only 39 autopsies had cerebral infarcts). Moreover, only a few of their subjects had hemispheral infarcts, the group we found most significant in causing dementia.
We found no evidence that AD pathology caused brain infarcts or that brain infarcts increased AD pathology, a finding consistent with other reports.7,9 However, several pathological studies have suggested a synergistic relation between AD and vascular pathology. A retrospective review of the National Alzheimer’s Coordinating Center database showed a correlation between cerebral atherosclerosis and neuritic plaques,36 whereas two other studies have found a relation between severe amyloid angiopathy and infarcts.37,38 These disparate results underline the need for further work on the causative relation between atherosclerosis and AD pathology.
Our participants comprise a sample of convenience. They are nearly all white and well educated. This makes our study unrepresentative and less likely to generalize to the whole population. However, the relative uniformity of the sample lends strength in isolating particular interactions. Despite all the material advantages enjoyed by our participants, hemispheral infarcts are still a considerable cause of dementia, which is a sobering finding given the more impressive burden of cerebrovascular lesions in other populations. 39 Additional limitations of this study include the lack of full accounting for all microscopic infarcts and a lack of analysis of amyloid angiopathy. Moreover, it is possible that a more quantitative assessment of Alzheimer’s pathology could increase the contribution of AD to the overall dementia burden. Nevertheless, our study makes clear that cerebrovascular disease, which is potentially preventable, is a substantial contributor to the burden of dementia in the United States.
Acknowledgments
This work was supported by NIH (National Institute on Aging), P50 AG05146 (R.J.O, J.C.T.); Intramural Research Program, National Institute on Aging (A.B.Z., S.M.R.) and the Burroughs Well-come Fund for Translational Research (1005227, R.J.O.).
References
- 1.von SE, Viitanen M, De RD, et al. Aging and the occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Arch Neurol. 1999;56:587–592. doi: 10.1001/archneur.56.5.587. [DOI] [PubMed] [Google Scholar]
- 2.Lobo A, Launer LJ, Fratiglioni L Neurologic Diseases in the Elderly Research Group. Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurology. 2000;54:S4–S9. [PubMed] [Google Scholar]
- 3.Andersen K, Lolk A, Nielsen H, Kragh-Sorensen P. Prevalence and incidence of dementia in Denmark. The Odense study. Ugeskr Laeger. 2000;162:4386–4390. [PubMed] [Google Scholar]
- 4.Roman GC. Stroke, cognitive decline and vascular dementia: the silent epidemic of the 21st century. Neuroepidemiology. 2003;22:161–164. doi: 10.1159/000069885. [DOI] [PubMed] [Google Scholar]
- 5.Chui HC, Mack W, Jackson JE, et al. Clinical criteria for the diagnosis of vascular dementia: a multicenter study of comparability and interrater reliability. Arch Neurol. 2000;57:191–196. doi: 10.1001/archneur.57.2.191. [DOI] [PubMed] [Google Scholar]
- 6.Gold G, Bouras C, Canuto A, et al. Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psychiatry. 2002;159:82–87. doi: 10.1176/appi.ajp.159.1.82. [DOI] [PubMed] [Google Scholar]
- 7.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 8.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 9.Schneider JA, Wilson RS, Bienias JL, et al. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 10.Esiri MM, Nagy Z, Smith MZ, et al. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 11.Neuropathology Group. Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late onset dementia in a muticentre community based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 12.White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 13.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 14.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 15.Gamaldo A, Resnick S, Kilada S, et al. The interaction between stroke and alzheimer pathology on dementia in the Baltimore Longitudinal Study of Aging. Neurology. 2006;67:1363–1369. [Google Scholar]
- 16.Kawas C, Gray S, Brookmeyer R, et al. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 17.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 9(suppl 1):173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- 19.Kawas C, Segal J, Stewart WF, et al. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 21.Yamamoto T, Hirano A. A comparative study of modified Bielschowsky, Bodian and thioflavin S stains on Alzheimer’s neurofibrillary tangles. Neuropathol Appl Neurobiol. 1986;12:3–9. doi: 10.1111/j.1365-2990.1986.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 22.Chui HC, Zarow C, Mack WJ, et al. Cognitive impact of sub-cortical vascular and Alzheimer’s disease pathology. Ann Neurol. 2006;60:677–687. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chuaqui R, Tapia J. Histologic assessment of the age of recent brain infarcts in man. J Neuropathol Exp Neurol. 1993;52:481–489. doi: 10.1097/00005072-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 25.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 26.Vermeer SE, Prins ND, den HT, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 27.Kuller LH, Lopez OL, Jagust WJ, et al. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology. 2005;64:1548–1552. doi: 10.1212/01.WNL.0000160115.55756.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebetrau M, Steen B, Hamann GF, Skoog I. Silent and symptomatic infarcts on cranial computerized tomography in relation to dementia and mortality: a population-based study in 85-year-old subjects. Stroke. 2004;35:1816–1820. doi: 10.1161/01.STR.0000131928.47478.44. [DOI] [PubMed] [Google Scholar]
- 29.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 30.Elkins JS, O’Meara ES, Longstreth WT, et al. Stroke risk factors and loss of high cognitive function. Neurology. 2004;63:793–799. doi: 10.1212/01.wnl.0000137014.36689.7f. [DOI] [PubMed] [Google Scholar]
- 31.Van Oijen M, Jan de Jong F, Witteman JC, et al. Atherosclerosis and risk for dementia. Ann Neurol. 2007;61:403–410. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 32.Schneider JA, Boyle PA, Arvanitakis Z, et al. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol. 2007;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- 33.Gold G, Kövari E, Herrmann FR, et al. Cognitive conse-quences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005;36:1184–1188. doi: 10.1161/01.STR.0000166052.89772.b5. [DOI] [PubMed] [Google Scholar]
- 34.Tisch S, Silberstein P, Limousin-Dowsey P, Jahanshahi M. The basal ganglia: anatomy, physiology, and pharmacology. Psychiatr Clin North Am. 2004;27:757–799. doi: 10.1016/j.psc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Riekse RG, Leverenz JB, McCormick W, et al. Effect of vascular lesions on cognition in Alzheimer’s disease: a community-based study. J Am Geriatr Soc. 2004;52:1442–1448. doi: 10.1111/j.1532-5415.2004.52405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer’s Coordinating Center. Neurology. 2005;64:494–500. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- 37.Suter OC, Sunthorn T, Kraftsik R, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33:1986–1992. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- 38.Olichney JM, Hansen LA, Hofstetter CR, et al. Association between severe cerebral amyloid angiopathy and cerebrovascular lesions in Alzheimer disease is not a spurious one attributable to apolipoprotein E4. Arch Neurol. 2000;57:869–874. doi: 10.1001/archneur.57.6.869. [DOI] [PubMed] [Google Scholar]
- 39.White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]