Abstract
The aminoglycoside, geneticin (G418), was recently shown to have antiviral activity against bovine viral diarrhea virus (BVDV). Since BVDV, dengue virus (DENV) and yellow fever virus (YFV) all belong to the Flaviviridae family, it seemed possible that a common step in their life cycle might be affected by this aminoglycoside. Here it is shown that geneticin prevented the cytopathic effect (CPE) resulting from DENV-2 infection of BHK cells, in a dose-dependent manner with an EC50 value of 3±0.4 μg/ml. Geneticin had no detectable effect on CPE caused byYFV in BHK cells. Geneticin also inhibited DENV-2 viral yield with an EC50 value of 2±0.1 μg/ml and an EC90 value of 20±2 μg/ml. With a CC50 value of 165±5 μg/ml, the selectivity indexof anti-DENV activity of geneticin in BHK cells was established to be 66. Furthermore, 25 μg/ml of geneticin nearly completely blocked plaque formation induced by DENV-2, but not YFV. In addition, geneticin, inhibited DENV-2 viral RNA replication and viral translation. Gentamicin, kanamycin, and the guanidinylated geneticin showed no anti-DENV activity. Neomycin and Paromomycin demonstrated weak antiviral activity at high concentrations. Finally, aminoglycoside-3′-phosphotransferase activity of neomycin-resistant gene abolished antiviral activity of geneticin.
INTRODUCTION
Dengue infection is caused by one of four serotypes of dengue virus (DENV), which is a member of the Flaviviridae family. It occurs in many tropical and subtropical regions and has expanded over the last 30 years to include more than 100 countries (1). There are about 50 million cases of DENV infection annually (Anonymous, 2000).
The epidemiological evidence indicates that immunity to one serotype of DENV increases the chance of a more severe disease upon infection with a second serotype by about ten-fold (a process known as antibody-dependent enhancement of infection (ADE) (Kurane et al., 1994). Although a direct link between ADE and severity of the disease is yet to be established, the concerns that ADE will occur among vaccines, making vaccinated individuals more susceptible to severe disease, have hampered the development of monovalent dengue vaccines. Presently, tetravalent dengue vaccines, targeting all four serotypes, are being developed (Raviprakash et al., 2008), which should potentially abolish ADE, unless virus will continue mutating into new serotypes.
Some aminoglycosides are considered to have antiviral activities. Hygromycin B was shown to inhibit replication of herpes simplex virus (Lacal et al., 1983), mouse hepatitis virus (Macintyre et al., 1991a,b), HIV type 1 (Gatti et al., 1998), influenza virus (Ghendon et al., 1981), and both encephalomyocarditis virus and Semliki forest virus (Lacal et al., 1980). Neomycin and recently developed neomycin analogs were also demonstrated to inhibit HIV replication and viral entry (Zapp et al., 1993; Herold & Spear, 1994; Herold et al., 1994; Hung et al., 2002; Litovchick et al., 2000, 2001; Langeland et al., 1986, 1987).
We previously demonstrated that the aminoglycoside geneticin, although structurally distinct from hygromycin B, inhibited bovine viral diarrhea virus (BVDV), which belongs to the Flaviviridae family (Collett et al., 1988). These results allowed us to hypothesize that geneticin might also have antiviral activity against other members of the Flaviviridae family, such as dengue virus and yellow fever virus.
In this study, we demonstrated that geneticin, a neomycin analog widely used to select for transfected eukaryotic cells (Santerre et al., 1984; Danielson et al., 1989), inhibited DENV-2 induced cytopathology, viral titers, and viral RNA replication and translation. However, surprisingly, geneticin had no effect on cytopathology and proliferation of YFV, demonstrating selectivity of this aminoglycoside to DENV. Close structural analogs of geneticin, such as gentamicin, kanamycin, and guanidilated geneticin at Rings I and II (gG418), had no effect on DENV proliferation, suggesting that the structural specificity of geneticin’s antiviral activity is due to Ring I and II of this aminoglycoside.
EXPERIMENTAL PROCEDURES
Chemicals - All cell culture supplies were obtained from Invitrogen (Carlsbad, CA). Unless specified, all other reagents were supplied by Sigma Aldrich (St. Louis, MO).
Cell culture and virus - Baby Hamster kidney cells (BHK) (ATCC-CCL10), were grown in Dulbecco’s modified Eagle’s media (DMEM) containing 4.5 g of glucose, supplemented with 2 mM glutamine and 5% fetal bovine serum (FBS). Cell media were changed to RPMI supplemented with 1% FBS about 16 h prior to experiments. The stocks of dengue virus serotype 2 (cell culture adapted, mouse brain passaged New Guinea C strain) and vaccine strain YFV (YFV-17D strain) were grown in Vero cells and collected at 4 days post infection (dpi). The viral infectivity was determined using plaque assay in BHK cells.
Antiviral property and drug toxicity assays -BHK cells were seeded at a density of 1–2×103 cells/well in DMEM supplemented with 5% FBS in 96-well plates. Prior to infection, cells were cultured in RPMI supplemented with 1% FBS overnight. Monolayer of cells, at approximately 80% confluency, was infected with DENV-2 at a multiplicity of infection (m.o.i.) of 1 PFU. Mock-infection was carried out as negative control. Following infection with DENV-2 for one hour, cells were washed and medium (RPMI with 1% FBS) containing various concentrations of potential antiviral inhibitors was added to cells and incubated for the desired period of time. Cell viability was determined using the resaruzin (Almar Blue) indicator dye (Mazzio & Soliman, 2004) to assess the antiviral activity and toxicity of the drugs employed in this study. Quantitative analysis of dye conversion (Arbitrary Fluorescence Units --- AFU) was measured using a fluorescent plate reader with excitation/emission = 530/590 nm. For toxicity studies cell viability was expressed as a percent of control (AFUtreated/AFUcontrol). The drug-mediated protection of cell viability was expressed as percent survival as follows: % survival = [(AFUtreated) DENV − (AFUcontrol) DENV]/[(AFUcontrol) mock − (AFUcontrol) DENV] in which (AFUtreated) DENV is the AFU of cells infected with DENV and treated with a certain dilution of geneticin, (AFUcontrol) DENV is the AFU of cells infected with DENV and left untreated, and (AFUcontrol) mock is the AFU of cells mock infected and left untreated. The 50% effective concentration (EC50) was defined as the concentration of compound that offered 50% protection against virus-induced cytopathic effect and was calculated using logarithmic interpolation (Paeshuyse et al., 2006).
Plaque formation assay - BHK cells were infected with DENV-2 at a multiplicity of infection (MOI) of 0.1 and distributed to a 6-well plate. Cells were washed with PBS once after 1 h incubation at 37°C and 5% CO2, followed by addition of 0.5% methyl cellulose in the 5% FBS-containing DMEM media. Crystal violet staining was performed 5 days thereafter.
DENV-2 yield reduction assay - Antiviral activity against DENV-2 was evaluated in a yield reduction assay as previously published (Gu et al., 2007). BHK cells were plated in 96-well plates at a density of 2.5×104 cells/well. Twenty four hours later, the cells were infected with DENV-2 at an MOI of 0.05. After 1h the inoculum was removed and the cells were replenished with DMEM containing the dilutions of geneticin. Cells were incubated at 37°C, 5% CO2 for 72 hours. The supernatants were collected and titrated for DENV-2. For virus titration in 96-well plates, vero cells were plated at 8×103 cells/well and incubated overnight. The Vero cell monolayers were then infected for 1h with various dilutions of the media from virus-infected cells, overlaid with media containing 0.6% tragacanth (ICN, CA) and incubated at 37°C for 72 hours. The culture medium was aspirated; the plate was rinsed, air-dried, and fixed with acetone/methanol (50:50 v/v). Viral foci were detected for enumeration by immunostaining with D1-4G2 (Gu et al., 2007).
Western blot analysis of DENV-2 envelop (E) protein - BHK cells were infected with DENV-2 (MOI of 1) for 1h followed by removal of inoculum and addition of fresh medium (with or without 25 μg/ml of geneticin). At the desired time point, cells were washed with ice-cold PBS and harvested. The cells were lysed on ice for 30 min with 0.15 M NaCl, 10 mM Tris, 5 mM EDTA, 1% Triton X-100, and 1x proteinase inhibitor (Roche, San Francisco, CA). Cell debris was removed by centrifugation and protein concentration was determined by Bio-Rad protein assay (Bio-rad, Hercules, CA). Lysates were quenched with non-reducing sample buffer (final concentration at 50 mM tris pH 6.8, 1% SDS, 5% glycerol and 0.125% bromophenol blue) and 50 μg total protein for each sample was loaded and separated on 10% SDS-PAGE and then transblotted. The blot was incubated overnight with monoclonal antibody D1-4G2 (ATCC, Manassas, VA) diluted 1:5000 at 4°C followed by incubation with anti-mouse IgG-HRP (Sigma, St. Louis, MO) diluted 1:2000 for 60 min room temperature. E protein was probed with Super Signal West Dura (Pierce, Rockford, IL) on EL Logic 1500 imaging system and the protein intensity was analyzed with the Kodak molecular imaging systems software (Carestream Health, Inc. Rochester, NY).
Detection of intracellular viral RNA by RT-qPCR - DENV-2 (MOI of 1) infected BHK cells in the presence and absence of geneticin at 25 μg/ml were washed with ice-cold PBS. RNA was isolated using the RNAeasy kit (Qiagen, Valencia CA) and RNA concentration was determined using the Nanodrop Spectrophotometer (Thermo Scientific, Wilmington, DE) according to the Manufacturer’s instructions. A 25-μl RT-quantitative PCR (qPCR) was performed according to a previous publication (Wang et al., 2002) with forward primer d2C16A (5′-GCTGAAACGCGAGAGAAACC-3′), reverse primer d2C46B (5′-TCCCTGCTCCTGGTIATTTTGAC-3′), and TaqMan probe VICd2C38B (3′-TGTCGACTGTTTCTCTAAGAGTGAACCTTACGA-5′) and the TaqMan one-step RT-PCR master mix reagent kit (Applied Biosystems, Foster City, CA). The amplification conditions were 48°C for 30 min and 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min, in a 7500 fast real time PCR sytem (Applied Biosystems) as recommended by the Manufacturer.
Generation of geneticin-resistant BHK Cells – Resistance to geneticin was conferred to BHK cells (Cabanas et al., 1978) by transfection with pSV3 neo (ATCC, Manassas, VA) (Eustice & Wilhelm, 1984). Mixture of 1.5 μg of pSV3-neo and 8 μl of Lipofectamine LTX (Invitrogen, Carlsbad, CA) in 200 μl Advanced-MEM supplemented with 2mM L-glutamine (A-MEM, Invitrogen, Carlsbad, CA) was incubated for 15 min at room temperature. BHK cells in 10 cm2 wells were rinsed 3x with A-MEM and overlaid with 0.5 ml A-MEM. The transfection mixture was diluted to 2ml with A-MEM and 1.2, 0.6 or 0.2 ml was added to each of three wells. Final volumes were adjusted to 2 ml. The plate was rocked for 45 min at room temperature, 3 ml of A-MEM plus 2% fetal bovine serum (FBS) was added, and the cells were incubated at 37°C, 5% CO2. The cells were released 24 hours later with 0.1 % trypsin, resuspended in A-MEM with 5% FBS, 200 μg/ml G418 and dispersed to 48-well plates. The plates were inspected every two days for colony formation. The most rapidly propagating colony, BHK-neor was subcultured at 15 days and expanded in A-MEM, 5% FBS and 200 μg/ml geneticin.
Data analysis – All experiments for CPE, drug toxicity, yield reduction assay, and viral translation and replication were carried out in triplicates in three or six different days. All data are presented as means ± SE.
RESULTS
Selective antiviral activity of geneticin against DENV-2
The morphology of BHK cells infected with DENV-2 (MOI of 1) was similar to mock-infected control cells up to 48 hrs post-infection (hpi). However, by 72 and 96 hpi, a large number of infected cells were found to shrink and lose their morphological characteristics. By 96 hrs post-infection, about 90% of monolayer was destroyed.
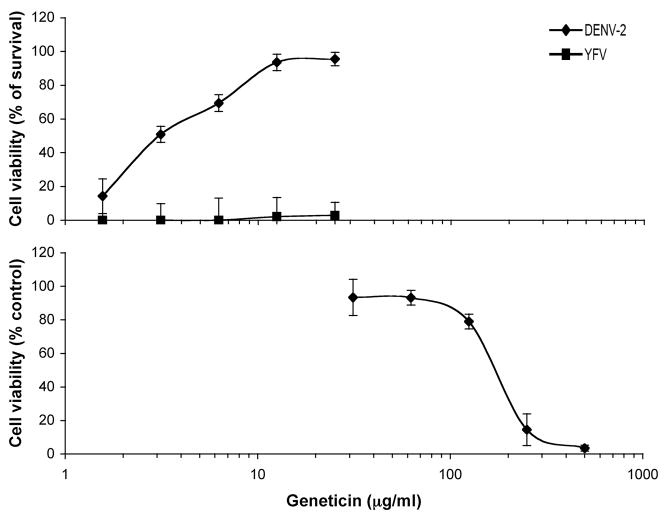
To measure cell viability at 96 hrs in mock-infected and DENV-2-infected BHK cells treated with or without geneticin, we used Almar Blue assay (see Methods). We determined that geneticin reduced viability of BHK cells with a CC50 value of 165±5 μg/ml (Fig. 1, bottom panel). Geneticin prevented DENV-induced CPE (MOI of 1) and increased cell viability of infected cells with an EC50 value of about 3±0.4 μg/ml (Fig. 1, top panel). However, geneticin had no protective activity against CPE induced by YFV (Fig. 1, top panel). Morphologically, cells treated with EC90–100 concentration of geneticin (25 μg/ml) were shown to be indistinguishable from mock-infected controls (data not shown). Interestingly, other aminoglycosides structurally distinct from geneticin, such as hygromycin B, butirosin A, streptozocin, netilmicin, sisomicin, amikacin, tobramycin, ribostamycin, and apramycin had no protective activity against DENV-2 -induced CPE.
Figure 1.
Antiviral activity and cytotoxicity of geneticin. Top) Antiviral property of geneticin (0.3–25 μg/ml) is shown as a compound to protect viability of BHK cells infected with DENV-2 or YFV, represented as percentage of survival at 96 hpi. The viruses were both at an MOI of 1 for infection. Almar Blue assay was performed to measure cell viability. Error bars indicate standard error for each concentration of geneticin (n=4, triplicates for individual experiment). Bottom) Toxicity of geneticin (25–1000 μg/ml) to uninfected BHK cells is expressed as a percent of cell viability of G418-treated cells over control-untreated cells at 120 hrs post treatment. Data are presented as means ± SE for each concentration of geneticin (n=3, triplicates for each experiment).
To further characterize the antiviral property of geneticin, the effect of the drug on virus yield was determined. We demonstrated that geneticin inhibited the yield of viral titers of DENV-2 even after 72 hpi with an EC50 value of 2±0.1 μg/ml and an EC90 value of about 20±2 μg/ml (Fig. 2A), which was consistent with antiviral activity of geneticin in the CPE assay (Fig. 1). We then used the EC90–100 concentration of geneticin (25 μg/ml) to determine the effect of the drug on DENV-induced plaque formation in BHK cells. Thus, we demonstrated (Fig. 2B) that geneticin inhibited the number of plaques produced by the virus, and the sizes the remaining plaques were shown to be significantly reduced, suggesting that the drug inhibited proliferation and spread of the virus. However, in agreement with CPE studies, geneticin had no inhibitory effect on YFV-induced plaque formation (data not shown). This further indicates the difference in antiviral activity of geneticin between these related viruses.
Figure 2.
Effect of geneticin on DENV-2 yield and DENV-2 plaque formation. A) Effect of geneticin at concentrations of 0.3–100 μg/ml on viral yield titers of DENV-2 collected at 72 hpi (see methodology for details). Virus yield is expressed as a percent of the virus titer collected from geneticin-treated infected cells over titer collected from untreated infected cells. Data are presented as means ± SE for each specified concentration of G418 (n=6)B) Representative plaque assay to show viral proliferation in BHK cells in DENV-2 (top panel); infected control and infected cells treated with 25 μg/ml of geneticin (n=3) (bottom panel).
Inhibitory effects of geneticin on DENV-2 RNA and viral E protein synthesis
To understand the mechanism underlying geneticin inhibition of DENV-induced CPE and virus yield reduction, we investigated the effects of geneticin on viral RNA synthesis and protein production. RT-qPCR was employed to measure intracellular accumulation of viral RNA 3, 6, 12, 24, and 48 hpi with DENV-2 (m.o.i. of 1) in the presence or absence of 25 μg/ml geneticin. In the first 6 hpi, viral RNA accumulation did not display significant difference between treatment groups, suggesting that the drug did not block the early events of DENV infection, such as viral entry. Our data show that geneticin inhibited viral RNA synthesis by 40% at 12 hpi, and by 24 and 48 hpi; the addition of geneticin resulted in nearly 90% decrease in viral RNA, compared to infected control without geneticin ( Fig. 3A).
Figure 3.
Effect of geneticin on viral RNA synthesis and viral protein accumulation. A) Quantitative RT-PCR analysis of intracellular viral RNA in BHK cells infected with DENV-2 in the presence of 25 μg/ml of geneticin, at 6, 12, 24, and 48 hpi. Viral RNA expression is defined as a percent of viral RNA collected from geneticin-treated infected cells over RNA detected from untreated infected cells. Data are presented as means ± SE for each specified condition (n=4). B) Western blot representative of DENV-2 E protein expression inhibited by geneticin. BHK cells were infected with DENV-2 (MOI of 1) in the absence or presence of 25μg/ml of geneticin, and at 6,12, 24, and 48 hpi (see Experimental Procedures). Cell lysates were subjected to SDS-PAGE and Western blotting analysis using primary antibody (D1-4G2) for E protein. C) Quantification of Viral E protein expression 48 hpi on Western blots was performed with the Kodak molecular imaging systems software (Carestream Health, Inc. Rochester, NY). The DENV-2-infected control, which was not treated by geneticin, was set as 100% of viral E protein expression. The percentage of inhibition of E protein accumulation was expressed as E protein from geneticin-treated infected cells over viral E protein from untreated infected cells. Data are presented as means ± SE for each specified condition (n=3).
DENV, like other flaviviruses, expresses a polyprotein, which is consequently processed to individual viral proteins. Therefore, to assess the effect of geneticin on translation of viral proteins, we used viral E protein as a marker of DENV-2 translation at 6, 12, 24, and 48 hpi at an MOI of 1. Interestingly, no E protein band was observed until 48 hpi (Fig 3B). Not surprisingly, geneticin treatment (25 μg/ml) inhibited formation of E protein by about 80% (Fig. 3B and C). Moreover, the absence of additional E protein bands in geneticin-treated sample further suggest that geneticin does not affect processing of E protein of DENV-2.
Structural selectivity of geneticin against DENV-2
We also tested the antiviral activity of structurally similar geneticin analogs (Fig 4A), such as gentamicin and kanamycin A. Ring II is structurally similar in all of these aminoglycosides. Geneticin and gentamicin also share structural identity of Ring III. However, among all tested aminoglycosides, only geneticin significantly improved cell protection against DENV-2 (Fig. 1, top and Fig. 4B). Interestingly, neomycin and paromomycin, with high structural homology with geneticin in Rings I and II, showed modest anti-DENV activity at concentrations between 300 and 1000 μg/ml. However, antiviral activity of those aminoglycosides even at 1000 μg/ml never reached full antiviral activity observed with 12 μg/ml of geneticin.
Figure 4.
Geneticin-mediated cytoprotection against DENV-2, compared to its close structural analogs. A) Structural diagrams of geneticin, gentamicin C1, kanamycin B, paromomycin, and neomycin used in this study. B) Antiviral effect of different concentrations of aminoglycosides on viability of BHK cells infected with DENV-2. Cell viability is expressed as a percent survival (see Experimental Procedures). Data are presented as means ± SE for each aminoglycoside and specified concentration (n=3 with triplicates for each experiment).
This modest activity of neomycin and paramomycin suggest that Rings I and II might be responsible for antiviral activity of geneticin. Therefore, we modified geneticin by guanidilating primary amines of Rings I and II of geneticin to test the role of primary amines in the anti-DENV activity of geneticin. Our results showed that guanidinylated geneticin (gG418) (Fig 5A, structure) does not exert any antiviral activity against DENV-2 (Fig. 5A). Likewise, it appears that YFV and BVDV were not blocked by gG418 either (data not shown).
Figure 5.
A) Effect of 25 μg/ml of either guanidinylated analog of geneticin (gG418) or geneticin on viability of cells infected with DENV-2. Cell viability is expressed as percent survival (see Experimental Procedures). Data are presented as means ± SE for each aminoglycoside (n=3, triplicates for individual experiment). B) Antiviral property of geneticin against DENV-2 and cytotoxicity of geneticin in the Neor-containing BHK cells. Top panel, Neor -BHK cells were infected with DENV-2 at an MOI of 1 and 96 hpi cell viability in the presence of geneticin was determined by Almar Blue assay and was presented as percentage of cell survival compared to viral infection without drug (Data are presented as means ± SE; n=6 with triplicates of each experiment). Bottom Panal, Neor-BHK cells were incubated with geneticin at different concentration for 96 hrs and cell viability was measured by Almar Blue assay and was expressed by percentage of survival to control (Data are presented as means ± SE; n=6 with triplicates samples each individual experiments).
It has been well established that cells containing neomycin-resistant gene (neor), encoding aminoglycoside 3′-phosphotransferase, are insensitive to the toxic concentrations of geneticin, due to the 3′-phosphorylation of geneticin (Thompson et al., 1999). To test if 3′-phosphorylated geneticin has antiviral activity, we created neor-containing BHK cells. The neor-containing BHK cells were viable in the presence of geneticin up to 1000 μg/ml (Fig. 5B, bottom). However, the antiviral activity against DENV-2 of geneticin was completely abolished (Fig. 5B, top).
DISCUSSION
In the present study, we demonstrated that geneticin selectively inhibits DENV-2 proliferation by 1) protecting BHK cells against the cytopathic effect of DENV-2, with an EC50 value of about 3±0.1 μg/ml (Fig. 1); 2) reducing the viral yield with an EC50 value of 2±0.1 μg/ml and EC90 value of about 20±2 μg/ml (Fig. 2A ), consistent with the antiviral activity of geneticin in CPE assay; 3) inhibiting DENV-2 plaque formation in both the number and the size of the plaques at its EC90 concentration of 25 μg/ml (Fig. 2B); 4) blocking DENV-2 RNA and protein synthesis (Fig. 3). The selectivity index of anti-DENV activity of geneticin in BHK cells was established to be 66. However, YFV was not inhibited by geneticin in the same cell line, where drug showed clear anti-DENV-2 activity.
The molecular mechanism of antiviral activity of geneticin remains unclear. Geneticin has shown antiviral activity against different RNA viruses in a variety of cell lines, such as MDBK (Birk et al., 2008), BHK, bottle neck dolphin skin cells (data not shown), and rabbit kidney cell (manuscript in preparation), suggesting that geneticin-mediated antiviral mechanism is cell type-independent. Therefore, the antiviral activity of geneticin is more likely dependent on the commonly shared cellular antiviral mechanisms. Alternatively, the drug can selectively affect different viral structures and functions. The other possibility is that geneticin may directly inhibit DENV through specifically interacting with the viral structural or nonstructural proteins or binding to viral RNA. Thus, further exploration of virus selectivity of geneticin and comparison of antiviral mechanisms for different viruses will be required.
Here, we show evidence that geneticin inhibits DENV-2 life cycle at the stages different from that of BVDV (Birk et al., 2008). DENV-2 RNA accumulation was not inhibited by 6 hpi hours of infection (Fig. 3A), suggesting that DENV-2 entry into the cells was not blocked by geneticin, which is similar to the effect of the drug on BVDV infection of MBDK cells. Geneticin inhibited BVDV assembly and release and protected MDBK cells against BVDV-induced CPE and reduced viral yield, without inhibiting BVDV protein and RNA synthesis. Distinctly different from its anti-BVDV activity, geneticin impeded the production of DENV-2 RNA already at 12 hpi by about 40% and prevented the synthesis of viral E protein and viral RNA at 48 hpi by 80% and 90%, respectively (Fig. 3). These data could suggest a possibility that geneticin, being an inhibitor of protein synthesis (Cabanas et al., 1978; Eustice & Wilhelm, 1984), inhibits viral translation, resulting in a decrease of viral RNA synthesis. However, this hypothesis is not supported by the fact that other translational inhibitors, such as kanamycin and gentamycin, had no effect of DENV infection. Moreover, although it was recently demonstrated that both geneticin and gentamicin, and other related aminoglycosides could suppress stop codons and induce frame-shifting (Lai et al., 2004; Konno et al., 2004; Borovinskaya et al., 2007a,b), only geneticin was shown to inhibit DENV-2, suggesting that the antiviral activity of geneticin is different from inhibition or deregulation of translation. Alternatively, it is also possible that geneticin inhibited viral machinery or RNA folding, preventing accumulation of viral RNA, and then resulting in overall decrease in viral proteins.
Structure and function analysis of geneticin and its close structural analogs, including Gentamicin, kanamycin, neomycin and paromomycin, revealed that DENV-2 was responsive to geneticin at an EC50 and an EC90 approximately 2–3 and 20 μg/ml, respectively. Neomycin and paromomycin showed modest anti-DENV-2 activities at 300 through 1000 μg/ml, which did not result in the antiviral effect observed after treatment with geneticin at a 100-fold lower concentration. Both gentamicin and kanamycin did not show antiviral properties (Fig. 4).
As shown in Fig. 4, panel A, Ring II is structurally similar in geneticin, gentamicin and kanamycin, paromomycin and neomycin. Geneticin and gentamicin also share structural identity of Ring III. Furthermore, Ring I of paromomycin and neomycin share the same structure with geneticin, with an exception of functional group at C6, where geneticin has a secondary alcohol, and neomycin and paromomycin have primary amine and primary alcohol, respectively. Based on the fact that only geneticin, neomycin and paromomycin showed anti-DENV activity, we concluded that Ring I is the essential component in geneticin as an anti-DENV-2 agent, and Ring II also play a modest, but important, role in antiviral activity. This is somewhat different from the ability of those aminoglycosides to inhibit protein synthesis via binding to the A site of 30S ribosomes (Benveniste & Davies, 1973) where the ability of geneticin binding to RNA is assigned to its Ring II (Vicens & Westhof, 2003). To confirm the role of geneticin Ring I and II in anti-DENV-2, a novel molecule was generated by guanidinylating the primary amines of geneticin Ring I and II (Fig. 4, panel C). The modified geneticin, gG418, indeed had no antiviral activity, suggesting chemical selectivity of geneticin against DENV-2. Furthermore, geneticin administration to the neor-containing BHK cells showed that phosphorylation of geneticin diminished its antiviral activity, implying that the hydroxyl group in the 3′ –OH of Ring I of geneticin contributed significantly to the anti-DENV activity.
Aminoglycosides are known to be active against HIV (Zapp et al., 1993; Tassew & Thompson, 2003), HCMV (Lobert et al., 1996), HSV (Langeland et al., 1986, 1987; Garcin et al., 1990), and HDV (Rogers et al., 1996; Chia et al., 1997). Thus, the natural and synthesized aminoglycoside-based antibiotics can be a potent tool to explore and develop new specific antiviral drugs against pathogenic RNA viruses. In addition, because geneticin and Gentamicin are the most efficacious therapeutic agents for the treatment of 15% of severe inherited genetic diseases (Yang et al., 2007) are associated with nonsense mutation, it might be foreseeable to develop geneticin or its analog as anti-DENV therapeutics to inhibit viremia and DENV-induced clinical complications. Overall, our data suggest that geneticin represents a novel lead compound for broad-spectrum virus-selective antivirals.
Acknowledgments
This work was supported by NIH/NIDA grant K01 DAO18262 and NIH 5U01AI061441.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anonymous. Dengue/dengue haemorrhagic fever. Wkly Epidemiol Rec. 2000;75:193–196. [Google Scholar]
- Benveniste R, Davies J. Structure-activity relationships among the aminoglycoside antibiotics: role of hydroxyl and amino groups. Antimicrob Agents Chemother. 1973;4:402–409. doi: 10.1128/aac.4.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovinskaya MA, Pai RD, Zhang W, Schuwirth BS, Holton JM, Hirokawa G, Kaji H, Kaji A, Cate JH. Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nat Struct Mol Biol. 2007a;14:727–732. doi: 10.1038/nsmb1271. [DOI] [PubMed] [Google Scholar]
- Borovinskaya MA, Shoji S, Holton JM, Fredrick K, Cate JH. A steric block in translation caused by the antibiotic spectinomycin. ACS Chem Biol. 2007b;2:545–552. doi: 10.1021/cb700100n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk A, Dubovi EJ, Zhang X, Szeto HH. Antiviral activity of geneticin against bovine viral diarrhea virus. Antivir Chem Chemother. 2008;19:33–40. doi: 10.1177/095632020801900105. [DOI] [PubMed] [Google Scholar]
- Cabanas MJ, Vázquez D, Modolell J. Inhibition of ribosomal translocation by aminoglycoside antibiotics. Biochem Biophys Res Commun. 1978;83:991–997. doi: 10.1016/0006-291x(78)91493-6. [DOI] [PubMed] [Google Scholar]
- Chia JS, Wu HL, Wang HW, Chen DS, Chen PJ. Inhibition of hepatitis delta virus genomic ribozyme self-cleavage by aminoglycosides. J Biomed Sci. 1997;4:208–216. doi: 10.1007/BF02253420. [DOI] [PubMed] [Google Scholar]
- Collett MS, Anderson DK, Retzel E. Comparisons of the pestivirus bovine viral diarrhoea virus with members of the flaviviridae. J Gen Virol. 1988;69:2637–2643. doi: 10.1099/0022-1317-69-10-2637. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Knepper JE, Kittrell FS, Butel JS, Medina D, Durban EM. Clonal populations of the mouse mammary cell line, COMMA-D, which retain capability of morphogenesis in vivo. In Vitro Cell Dev Biol. 1989;25:535–543. doi: 10.1007/BF02623566. [DOI] [PubMed] [Google Scholar]
- Eustice DC, Wilhelm JM. Mechanisms of action of aminoglycoside antibiotics in eucaryotic protein synthesis. Antimicrob Agents Chemother. 1984;26:53–60. doi: 10.1128/aac.26.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin D, Massé T, Madjar JJ, Jacquemont B. Herpes simplex virus type-1 immediate-early gene expression and shut off of host protein synthesis are inhibited in neomycin-treated human epidermoid carcinoma 2 cells. Eur J Biochem. 1990;194:279–286. doi: 10.1111/j.1432-1033.1990.tb19454.x. [DOI] [PubMed] [Google Scholar]
- Gatti PJ, Choi B, Haislip AM, Fermin CD, Garry RF. Inhibition of HIV type 1 production by hygromycin B. AIDS Res Hum Retroviruses. 1998;14:885–892. doi: 10.1089/aid.1998.14.885. [DOI] [PubMed] [Google Scholar]
- Ghendon YuZ, Klimov AI. Effects of edeine, hygromycin B and alpha-Sarcin on influenza virus reproduction. Acta Virol. 1981;25:129–137. [PubMed] [Google Scholar]
- Gu B, Mason P, Wang L, Norton P, Bourne N, Moriarty R, Mehta A, Despande M, Shah R, Block T. Antiviral profiles of novel iminocyclitol compounds against bovine viral diarrhea virus, West Nile virus, dengue virus and hepatitis B virus. Antivir Chem Chemother. 2007;18:49–59. doi: 10.1177/095632020701800105. [DOI] [PubMed] [Google Scholar]
- Herold BC, Spear PG. Neomycin inhibits glycoprotein C (gC)-dependent binding of herpes simplex virus type 1 to cells and also inhibits postbinding events in entry. Virology. 1994;203:166–171. doi: 10.1006/viro.1994.1469. [DOI] [PubMed] [Google Scholar]
- Herold BC, Visalli RJ, Susmarski N, Brandt CR, Spear PG. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J Gen Virol. 1994;75:1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- Hung SL, Wang YH, Chen HW, Lee PL, Chen YT. Analysis of herpes simplex virus entering into cells of oral origin. Virus Res. 2002;86:59–69. doi: 10.1016/s0168-1702(02)00055-2. [DOI] [PubMed] [Google Scholar]
- Konno T, Takahashi T, Kurita D, Muto A, Himeno H. A minimum structure of aminoglycosides that causes an initiation shift of trans-translation. Nucleic Acids Res. 2004;32:4119–4126. doi: 10.1093/nar/gkh750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I, Rothman AL, Livingston PG, Green S, Gagnon SJ, Janus J, Innis BL, Nimmannitya S, Nisalak A, Ennis FA. Immunopathologic mechanisms of dengue hemorrhagic fever and dengue shock syndrome. Arch Virol Suppl. 1994;9:59–64. doi: 10.1007/978-3-7091-9326-6_7. [DOI] [PubMed] [Google Scholar]
- Lacal JC, Carrasco L. Antiviral effects of hygromycin B, a translation inhibitor nonpermeant to uninfected cells. Antimicrob Agents Chemother. 1983;24:273–275. doi: 10.1128/aac.24.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal JC, Vázquez D, Fernandez-Sousa JM, Carrasco L. Antibiotics that specifically block translation in virus-infected cells. J Antibiot (Tokyo) 1980;33:441–446. doi: 10.7164/antibiotics.33.441. [DOI] [PubMed] [Google Scholar]
- Lai CH, Chun HH, Nahas SA, Mitui M, Gamo KM, Du L, Gatti RA. Correction of ATM gene function by aminoglycoside-induced read-through of premature termination codons. Proc Natl Acad Sci U S A. 2004;101:15676–15681. doi: 10.1073/pnas.0405155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeland N, Haarr L, Holmsen H. Evidence that neomycin inhibits HSV 1 infection of BHK cells. Biochem Biophys Res Commun. 1986;141:198–203. doi: 10.1016/s0006-291x(86)80354-0. [DOI] [PubMed] [Google Scholar]
- Langeland N, Holmsen H, Lillehaug JR, Haarr L. Evidence that neomycin inhibits binding of herpes simplex virus type 1 to the cellular receptor. J Virol. 1987;61:3388–3393. doi: 10.1128/jvi.61.11.3388-3393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovchick A, Evdokimov AG, Lapidot A. Aminoglycoside-arginine conjugates that bind TAR RNA: synthesis, characterization, and antiviral activity. Biochemistry. 2000;39:2838–2852. doi: 10.1021/bi9917885. [DOI] [PubMed] [Google Scholar]
- Litovchick A, Lapidot A, Eisenstein M, Kalinkovich A, Borkow G. Neomycin B-arginine conjugate, a novel HIV-1 Tat antagonist: synthesis and anti-HIV activities. Biochemistry. 2001;40:15612–15623. doi: 10.1021/bi0108655. [DOI] [PubMed] [Google Scholar]
- Lobert PE, Hober D, Delannoy AS, Wattré P. Evidence that neomycin inhibits human cytomegalovirus infection of fibroblasts. Arch Virol. 1996;141:1453–1462. doi: 10.1007/BF01718247. [DOI] [PubMed] [Google Scholar]
- Macintyre G, Curry B, Wong F, Anderson R. Hygromycin B therapy of a murine coronaviral hepatitis. Antimicrob Agents Chemother. 1991a;35:2125–2127. doi: 10.1128/aac.35.10.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre G, Woods DE, Anderson R. Hygromycin B inhibits synthesis of murine coronavirus RNA. Antimicrob Agents Chemother. 1991b;35:2630–2633. doi: 10.1128/aac.35.12.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzio EA, Soliman KF. Glioma cell antioxidant capacity relative to reactive oxygen species produced by dopamine. J Appl Toxicol. 2004;24:99–106. doi: 10.1002/jat.954. [DOI] [PubMed] [Google Scholar]
- Paeshuyse J, Leyssen P, Mabery E, Boddeker N, Vrancken R, Froeyen M, Ansari IH, Dutartre H, Rozenski J, Gil LH, Letellier C, Lanford R, Canard B, Koenen F, Kerkhofs P, Donis RO, Herdewijn P, Watson J, De Clercq E, Puerstinger G, Neyts J. A novel, highly selective inhibitor of pestivirus replication that targets the viral RNA-dependent RNA polymerase. J Virol. 2006;80:149–160. doi: 10.1128/JVI.80.1.149-160.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviprakash K, Wang D, Ewing D, Holman DH, Block K, Woraratanadharm J, Chen L, Hayes C, Dong JY, Porter K. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J Virol. 2008;82:6927–6934. doi: 10.1128/JVI.02724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Chang AH, von Ahsen U, Schroeder R, Davies J. Inhibition of the self-cleavage reaction of the human hepatitis delta virus ribozyme by antibiotics. J Mol Biol. 1996;259:916–925. doi: 10.1006/jmbi.1996.0369. [DOI] [PubMed] [Google Scholar]
- Santerre RF, Allen NE, Hobbs JN, Jr, Rao RN, Schmidt RJ. Expression of prokaryotic genes for hygromycin B and G418 resistance as dominant-selection markers in mouse L cells. Gene. 1984;30:147–156. doi: 10.1016/0378-1119(84)90115-x. [DOI] [PubMed] [Google Scholar]
- Tassew N, Thompson M. Binding affinity and inhibitory potency of neomycin and streptomycin on the Tat peptide interaction with HIV-1 TAR RNA detected by online acoustic wave sensor. Org Biomol Chem. 2003;1:3268–3270. doi: 10.1039/b307620m. [DOI] [PubMed] [Google Scholar]
- Thompson PR, Schwartzenhauer J, Hughes DW, Berghuis AM, Wright GD. The COOH terminus of aminoglycoside phosphotransferase (3′)-IIIa is critical for antibiotic recognition and resistance. J Biol Chem. 1999;274:30697–30706. doi: 10.1074/jbc.274.43.30697. [DOI] [PubMed] [Google Scholar]
- Vicens Q, Westhof E. Crystal structure of geneticin bound to a bacterial 16S ribosomal RNA A site oligonucleotide. J Mol Biol. 2003;326:1175–1188. doi: 10.1016/s0022-2836(02)01435-3. [DOI] [PubMed] [Google Scholar]
- Wang WK, Sung TL, Tsai YC, Kao CL, Chang SM, King CC. Detection of dengue virus replication in peripheral blood mononuclear cells from dengue virus type 2-infected patients by a reverse transcription-real-time PCR assay. J Clin Microbiol. 2002;40:4472–4478. doi: 10.1128/JCM.40.12.4472-4478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Feng J, Song W, Wang J, Tsai B, Zhang Y, Scaringe WA, Hill KA, Margaritis P, High KA, Sommer SS. A mouse model for nonsense mutation bypass therapy shows a dramatic multiday response to geneticin. Proc Natl Acad Sci USA. 2007;104:15394–15399. doi: 10.1073/pnas.0610878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapp ML, Stern S, Green MR. Small molecules that selectively block RNA binding of HIV-1 Rev protein inhibit Rev function and viral production. Cell. 1993;74:969–978. doi: 10.1016/0092-8674(93)90720-b. [DOI] [PubMed] [Google Scholar]