Abstract
It has been proposed that disruption of normal vitreous humor may permit O2 to travel more easily from the retina to the center of the lens where it may cause nuclear cataract (Barbazetto et al., 2004; Harocopos et al., 2004). In the present study, we injected enzymes intravitreally into guinea pigs (which possess an avascular retina) and rats (which possess a vascular retina) to produce either vitreous humor liquefaction plus a posterior vitreous detachment (PVD) (with use of microplasmin) or vitreous humor liquefaction only (with use of hyaluronidase), and 1–2 weeks later measured lens nuclear pO2 levels in vivo using a platinum-based fluorophore O2 sensor (Oxford-Optronix, Ltd.). Experiments were also conducted in which the animals were allowed to breathe 100% O2 following intravitreal injection with either microplasmin or hyaluronidase in order to investigate possible effects on O2 exchange within the eye. Injection of guinea pigs with either of the two enzymes produced no significant differences in lens pO2 levels 1–2 weeks later, compared to controls. However, for the rat, injection of microplasmin produced a 68% increase in O2 level in the center of the lens, compared to the controls (5.6 mmHg increasing to 9.4 mmHg, p<0.05), with no corresponding effect observed following similar use of hyaluronidase. Treatment of guinea pigs with microplasmin dramatically accelerated movement of O2 across the vitreal space when the animals were later allowed to breathe 100% O2 (for example, O2 traveled to a location directly behind the lens 5 times faster than control; p<0.01); however, the effect following treatment with hyaluronidase was significantly less. When microplasmin-injected rats breathed 100% O2, the time required for O2 to reach the center of the lens was 3 times faster than control (0.4 min compared to 1.4 min, p<0.01). The results have implication with regard to the occurrence of age-related PVD in the human, and a possible acceleration of maturity-onset nuclear cataract. In addition, enzymatic creation of a PVD to increase the rate of O2 exchange within the vitreal space may have potential application for treatment of retinal ischemic disease.
Keywords: guinea pig, lens, microplasmin, nuclear cataract, oxygen, posterior vitreous detachment, rat, vitreous humor
1. Introduction
The past few years have been marked by heightened interest in the measurement of O2 levels in vitreous humor and lens, and the linking of increased levels of O2 in the lens nucleus with formation of cataract. Two groups have recently reported partial pressures of O2 existing in the nucleus of the normal bovine lens in vitro (1.5 mm Hg) (McNulty et al., 2004) and the rabbit lens in vivo (10.4 mm Hg) (Barbazetto et al., 2004). O2 levels have been found to climb in the vitreal space following vitrectomy surgery in both the rabbit (Barbazetto et al., 2004) and human (Holekamp et al., 2005), and vitrectomy has long been known to induce nuclear cataract (Cherfan et al., 1991; Margherio et al., 1985; Michels, 1984). A hypothesis was presented nearly 20 years ago that vitrectomy-induced nuclear opacification is caused by an infusion of O2 into the vitreal space, leading to oxidation of lens proteins (Ogura et al., 1991). It is also known that exposure of experimental animals or human patients to elevated pressures of O2 can induce loss of transparency in the lens nucleus (Gesell and Trott, 2007; Giblin et al., 1995; Palmquist et al., 1984; Schocket et al., 1972), and oxidation of lens proteins is recognized as being strongly associated with nuclear cataract (Simpanya et al., 2005; Truscott, 2005). Recently, an association between age-related vitreous humor liquefaction and human nuclear cataract was demonstrated, and the proposal made that degeneration of the vitreous gel may allow more O2 from the retinal circulation to reach the lens, causing nuclear cataract (Beebe, 2008; Harocopos et al., 2004). Our laboratory has shown that enzymatic liquefaction of vitreous humor, combined with a posterior vitreal detachment (PVD), causes an increase in pO2 levels in the mid vitreous humor of rats and cats (Quiram et al., 2007). However, to our knowledge, experiments have not been conducted previously to determine whether enzymatic liquefaction of vitreous plus a PVD will lead to increased lenticular O2 levels.
There is also considerable interest in comparing rates of O2 exchange and O2 diffusion in normal and liquefied vitreous humor. Vitrectomy surgery is known to improve oxygenation of the inner retina, and thus this surgery can relieve retinal hypoxia in cases of diabetic retinopathy and other ischemic retinopathies (Stefansson et al., 1990; Stefansson and Loftsson, 2006). Experiments conducted by Maurice (1959) and Barton et al., (2007) have indicated that the rate of diffusion of molecules in intact vitreous gel is about the same as that in water. Any observed increase in the rate of redistribution of molecules in liquefied vitreous has been attributed by these investigators to an increase in fluid circulation. However, Stefansson and Loftsson (2006) have suggested that, based on the Stokes-Einstein equation and previously reported measurements of the viscosity of vitreous humor (Lee et al., 1992), rates of diffusion in vitreous should increase many-fold upon liquefaction.
In this study, we have liquefied vitreous humor of rats and guinea pigs by intravitreal injection of either of two enzymes, microplasmin or hyaluronidase. Microplasmin is a recombinant protein, possessing a molecular mass of 28 kDa and containing the catalytic domain of human plasmin (Nagai et al., 2003). Intravitreal injection of microplasmin is known to produce vitreous humor liquefaction, as well as a PVD (Gandorfer et al., 2004), whereas injection of hyaluronidase causes only vitreous liquefaction (Hikichi et al., 2000). Plasmin is a serine protease which hydrolyzes various glycoproteins including laminin and fibronectin which are important for vitreoretinal attachment (Gandorfer et al., 2004). Both microplasmin and hyaluronidase have been shown to be nontoxic to the retina (Gandorfer et al., 2004; Gottlieb et al., 1990; Sakuma et al., 2005). In the current study, we have made in vivo measurements of lens nuclear O2 levels in guinea pigs and rats having either normal or enzymatically liquefied vitreous humor. Guinea pigs, in contrast to rats, possess a naturally avascular retina (Cringle et al., 1996), such that the partial pressure of O2 in the innermost regions of the retina (at the interface of the retina and vitreous humor) is nearly zero, whereas at the same location in the retina of the rat, the pO2 level is about 22 mm Hg (Yu and Cringle, 2001). In this study, we have allowed guinea pigs and rats to breathe 100% O2 and determined whether liquefaction of the vitreous humor, or liquefaction combined with a PVD, can act to accelerate the time required for O2 molecules to reach the center of the lens.
2. Materials and methods
The following species of animals were used in this study: cats (female, 4–5 months old, Liberty Laboratories, Waverly, NY, USA), Brown Norway rats (male, 9–10 months old, 400g, Charles River Laboratories, Wilmington, MA, USA), New Zealand white rabbits (2 kg, Kuiper Rabbit Ranch (Indianapolis, IN, USA) and Hartley guinea pigs (male, 16–17 months old, Kuiper Rabbit Ranch). All procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the Oakland University animal care committee.
2.1 pO2 measurement
Measurement of pO2 in vitreous humor and lens in vivo was accomplished in anesthetized animals (employing ketamine and xylazine as anesthetics) using a fiber optic detection system (Oxylab, Oxford-Optronix, UK), as described previously by us (Quiram et al., 2007) and other vision researchers (Holekamp et al., 2005; McNulty et al., 2004; Shui et al., 2006). The O2-sensing probe consisted of a 250 μm-wide optic fiber with a platinum-based fluorophore at the tip. Since O2 is not consumed by the probe during analysis, it is possible to make accurate measurements of pO2 from 0 to 100mm Hg. The pO2 resolution of the probe is 0.1mm Hg, and the measurements are insensitive to sample viscosity (McNulty et al., 2004). Probes arrived pre-calibrated by the company; however, calibrations were routinely checked before and after use using a 3% solution of sodium sulfite (pO2 = 0mm Hg), as well as water bubbled with 1, 3 and 5% O2 (pO2’s = 7.6, 22.8 and 38.0mm Hg, respectively).
For pO2 measurements made with cats and rabbits, anesthetized animals were intubated and maintained by a ventilator on room air. Measurements with anesthetized guinea pigs and rats were made while the animals breathed room air without use of a ventilator. In certain experiments, measurements were made with guinea pigs and rats while the animals breathed 100% O2 delivered with the use of a face mask. For delivery of the probe into the vitreous space or into the center of the lens, a 25-gauge needle was used to first create an insertion track behind the limbus and through the conjunctiva and sclera. However, for the lens, in order to avoid introducing oxygen into the tissue artifactually, a needle was not used to create an insertion track in the lens tissue itself. Instead, the probe was inserted directly into the lens at the equator, and then advanced to the lens center. No difficulty was experienced with insertion of the probe into the lens centers of any of the species studied. For rabbits, cats, and guinea pigs, pO2 measurements were made in the center of the lens and in two locations in the vitreous humor, including close to the retina over a retinal vessel, and directly behind the lens. For rats, pO2 measurements in the current study were made only in the center of the lens.
2.2. Intravitreal injection of enzymes
The enzymes hyaluronidase (bovine, Sigma-Aldrich, St. Louis, MO, H3631) and microplasmin (human recombinant, obtained as a gift from Thrombogenics, Ltd., Dublin, Ireland) were injected intravitreally into guinea pigs [25 IU hyaluronidase in 20μl; 0.6 units (0.05 mg) microplasmin in 20μl] and rats [12.5 IU hyaluronidase in 10μl; 0.3 units microplasmin (0.025mg) in 10μl]. The injections were carried out under an operating microscope into the mid vitreous cavity of anesthetized animals with use of a 30-gauge needle. The injections were well-tolerated and gave no evidence of anterior segment inflammation, vitritis or increase in intraocular pressure. Experiments conducted with sham intravitreal injections of 20μl saline into guinea pigs showed no increase in vitreal pO2 levels, measured 1–2 weeks later, as compared with vitreous humor of non-injected control animals.
2.3. SEM analysis
Scanning electron microscopy (SEM) analysis was conducted to evaluate the vitreoretinal interface for the presence of a PVD in eyes of intravitreally-injected guinea pigs and rats. Eyes were fixed in 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 moles/L cacodylate buffer, pH 7.4, overnight. Corneas and lenses of the fixed eyes were removed one day later and the whole eye cup with the retina and vitreous humor intact was fixed for one additional day, washed with 7% sucrose, dehydrated in ethanol, dried to the critical point, and sputter-coated in carbon. Analysis was conducted with a Model DS-130 scanning electron microscope manufactured by International Scientific Instruments, Inc., Milpitas, CA.
3. Results
Partial pressures of O2 present in two regions of normal vitreous humor (close to the retina and directly behind the lens), and in the lens center of various species, are shown in Table 1A. For the cat and rabbit, the highest levels of vitreous O2, equal to nearly 30mm Hg, were found close to the retina. For these two species, pO2 levels fell 4 to 7-fold when the pO2 probe was positioned in the vitreous humor directly behind the lens. For the guinea pig, an animal which possesses an avascular retina, pO2 levels were low (<2mm Hg) in the vitreous humor at both locations. For each of the four species, the lowest measured levels of O2 were in the center of the lens. The rabbit showed a 14-fold decrease in pO2 level from a location in the vitreous humor close to the retina, to a position in the center of the lens. The cat and guinea pig each possessed pO2 levels in the lens center of <1mm Hg. The highest level of lens nuclear pO2, nearly 6mm Hg, was found for the rat.
Table 1.
pO2 levels in the vitreous humor and lens center of various species.
| pO2 (mm Hg) | ||||
|---|---|---|---|---|
| Cat | Rabbit | Guinea Pig | * Rat | |
| A. Normal animals: | ||||
| Close to the retina | 31 ± 3 (6) | 28 ± 10 (3) | 1.3 ± 1.1 (3) | |
| Behind the lens | 6.8 ± 1.5 (6) | 4.0 ± 2.0 (3) | 1.3 ± 1.2 (3) | |
| Lens center | 0.5 (1) | 2.0 ± 1.0 (3) | 0.6 ± 0.4 (5) | 5.6 ± 5.0 (6) |
| B. Injected animals: | ||||
| Lens center (MP) | 0.7 ± 0.4 (3) | **9.4 ± 4.0 (10) | ||
| Lens center (HY) | 0.5 ± 0.4 (3) | 4.2 (2) | ||
A: pO2 levels are indicated for two locations in the vitreous humor and in the center of the lens for normal animals.
B: Lens center pO2 analyses were conducted 1–2 weeks after intravitreal injection of either microplasmin (MP) or hyaluronidase (HY).
Results are expressed as means ± S.D. with the number of analyses indicated in parentheses.
For comparison, we have previously reported that the pO2 level of normal rat mid-vitreous humor is 23mm Hg (Quiram et al., 2007).
p<0.05, compared to the lens center value for control rats shown in Table 1A.
Measurements of pO2 levels in the center of the lens were also made for guinea pigs and rats 1–2 weeks after intravitreal injection of either microplasmin or hyaluronidase (Table 1B). For the guinea pig, no significant differences in lens pO2 levels were detected after injection of either of the two enzymes, compared to the control value shown in Table 1A. However, for the rat, intravitreal injection of microplasmin produced a 68% increase in oxygen level in the center of the lens after 1–2 weeks, compared to the control (p<0.05). In contrast, no significant difference was observed for rat lens pO2 levels for hyaluronidase-injected animals, compared to controls (Table 1B compared to Table 1A).
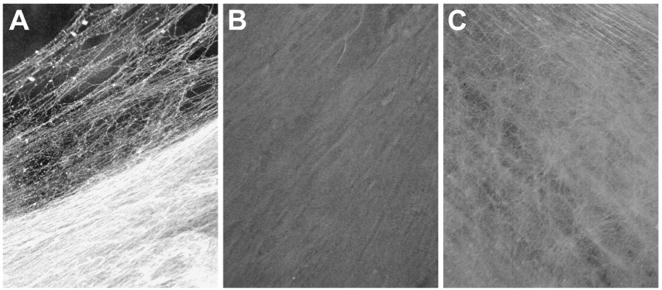
Results of SEM analysis demonstrated that intravitreal injection of microplasmin into the rat eye produced both vitreous humor liquefaction plus a PVD (Fig. 1B), whereas injection of hyaluronidase produced only vitreous humor liquefaction (Fig. 1C). Microplasmin-injected rats showed a vitreoretinal interface free of cortical vitreous (Fig. 1B), compared to hyaluronidase-injected and control animals (Figs. 1C and 1A, respectively). Similar results were obtained for the guinea pig (data not shown).
Figure 1.
Scanning electron micrographs (x500) of the vitreoretinal interface of rat eyes, 1–2 weeks after intravitreal injection of either hyaluronidase or microplasmin. Analysis of the retina was conducted at a location 3.6mm from the optic nerve toward the retinal periphery (7.5mm total distance from the optic nerve to the edge of the retina). The angle of each tissue sample was 15 degrees. A. Control: indicating a dense network of cortical vitreous adhering to the retinal surface. B. Microplasmin: a smooth retinal surface is present, free of cortical vitreous, indicating induction of a posterior vitreous detachment. C. Hyaluronidase: condensed cortical vitreous can be seen at the vitreoretinal interface, indicating vitreous liquefaction without posterior vitreous detachment.
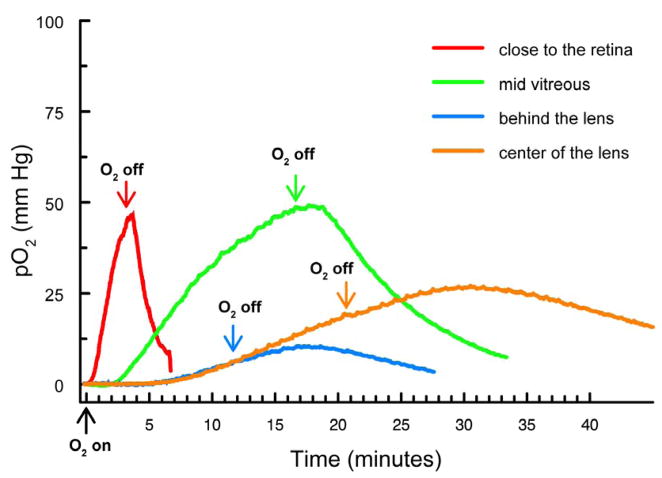
Experiments were conducted in which pO2 levels were monitored in various regions of the vitreous humor and in the lens center of normal guinea pigs after allowing the animals to breathe 100% O2. Representative traces for pO2 changes in various locations of the normal guinea pig eye are shown in Figure 2. With the probe in place in the vitreous humor close to the retina (red trace), a change to 100% O2 produced a rapid (<1 min delay) and steep increase in pO2 level. At this location of the pO2 probe in the vitreous, a change back to room air caused the pO2 level to drop rapidly, presumably as O2 was quickly metabolized by the retina. In contrast, when the probe was located in the vitreous humor directly behind the lens or in the center of the lens (blue and orange traces, respectively), breathing of 100% O2 produced a delayed (>4 min) and gradual increase in pO2 level, followed by a slow decrease in O2 level upon returning to breathing room air. Results obtained when the probe was positioned in the mid vitreous humor (green trace) were intermediate between those for the other locations. The peak increase in pO2 at the mid vitreous location was approximately 50-fold, increasing from about 1 to 50 mm Hg (Fig. 2, green trace).
Figure 2.
Increase in pO2 levels in various regions of vitreous humor and in the lens center of normal guinea pigs after breathing 100% O2. Arrows indicate the start of 100% O2 exposure and a return to breathing room air. red: close to the retina; green: mid-vitreous; blue: directly behind the lens; orange: center of the lens.
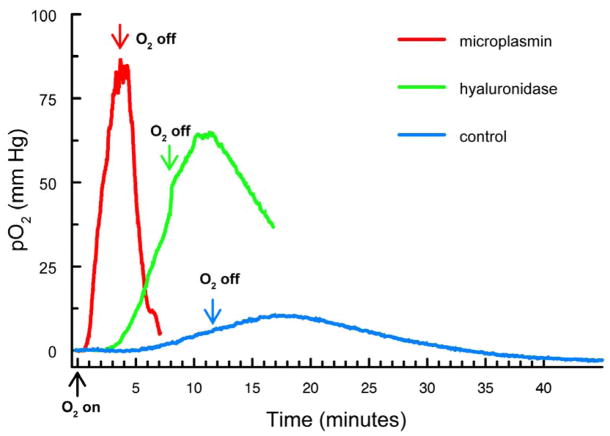
Experiments similar to those for Figure 2 were conducted using guinea pigs that had been intravitreally-injected with either hyaluronidase or microplasmin (Fig. 3). The goal of this study was to determine whether enzymatic liquefaction of vitreous humor, with or without an accompanying PVD, could facilitate O2 exchange within the vitreous space. One to two weeks after intravitreal injection of the animals, vitreal pO2 monitoring studies were conducted with the probe located directly behind the lens, as described above for the data of Figure 2. Representative traces for pO2 changes for the three conditions (microplasmin, hyaluronidase and control) are shown in Figure 3. Following injection of microplasmin (Fig. 3, red trace), a change to 100% O2 was shown to produce a rapid (<1 min delay) and steep increase in pO2 level. Under this condition, a change back to room air caused the pO2 level to drop rapidly. In contrast, for the control condition (Fig. 3, blue trace), breathing 100% O2 produced a delayed (>5 min) and gradual increase in pO2 level, followed by a slow decrease in O2 level upon returning to the breathing of room air. Results following intravitreal injection of hyaluronidase (Fig. 3, green trace) were intermediate between those for microplasmin and control.
Figure 3.
Increase in pO2 levels in the vitreous humor directly behind the lens for intravitreally-injected guinea pigs after breathing 100% O2. Analyses were conducted 1–2 weeks after intravitreal injection of either microplasmin or hyaluronidase. Arrows indicate the start of 100% O2 exposure and a return to breathing room air. red: microplasmin; green: hyaluronidase; blue: control.
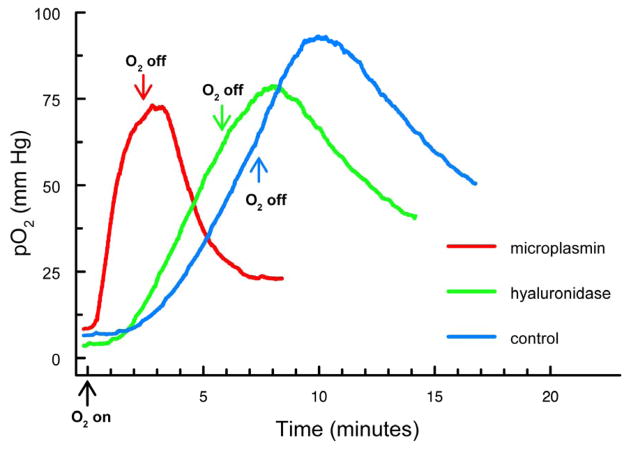
Experiments similar to those of Figure 3 were conducted, except in this case the probe was placed in the center of the rat lens, one to two weeks after intravitreal injection of either microplasmin or hyaluronidase. The goal of this study was to determine whether enzymatic liquefaction of vitreous humor, with or without an accompanying PVD, could facilitate O2 exchange within the center of the rat lens. Representative traces for pO2 changes for the three conditions (microplasmin, hyaluronidase and control) are shown in Figure 4. Following injection of microplasmin (Fig. 4, red trace), a change to 100% O2 produced a relatively rapid (<1 min delay) and steep increase in pO2 level, compared to that for the control rat breathing 100% O2 (Fig. 4, blue trace; >1 min delay). Results following intravitreal injection of hyaluronidase (Fig. 4, green trace) were intermediate between those for microplasmin and control.
Figure 4.
Increase in pO2 levels in the center of the lens for intravitreally-injected rats after breathing 100% O2. Analyses were conducted 1–2 weeks after intravitreal injection of either microplasmin or hyaluronidase. Arrows indicate the start of 100% O2 exposure and a return to breathing room air. Red: microplasmin; green: hyaluronidase; blue: control.
Additional analyses, similar to those conducted for Figures 3 and 4, were conducted for guinea pigs and rats after intravitreal injection of either microplasmin or hyaluronidase (Table 2). For the guinea pig, the pO2 measuring probe was placed in the vitreous humor, either close to the retina or directly behind the lens, or in the lens center. For the rat, the probe was placed in the lens center. Animals were first allowed to breathe room air and then switched to breathing 100% O2. Measurements were made of the time required to first observe an increase in pO2 level (“delay up”, Table 2), as well as the slope of the increase in pO2 level (“slope up”, Table 2). For the guinea pig, with the probe located in the vitreous humor, either close to the retina or behind the lens (Table 2Ai and 2Aii), intravitreal injection with either hyaluronidase or microplasmin produced a faster and steeper response to the breathing of 100% O2 (“delay up” and “slope up”, respectively), compared to the controls. In each case, the response for microplasmin was significantly more pronounced compared to that for either hyaluronidase or the control. For example, for location of the probe in the vitreous humor directly behind the lens (Table 2Aii), the O2 response (”delay up”) for microplasmin was 5x faster than the control (p<0.01) and 4x faster than that for hyaluronidase (p<0.05). Similarly, the slope of the pO2 increase for microplasmin at the location in the vitreous directly behind the lens (Table 2Aii) was 26x higher than the control (p<0.001) and 5x higher than the slope for hyaluronidase (p<0.01).
Table 2.
pO2 trace data at various locations in the eyes of guinea pigs and rats following intravitreal injection of either hyaluronidase or microplasmin, and subsequent exposure to 100% O2.*
| Control | HY | MP | ||
|---|---|---|---|---|
| A. Guinea pig | ||||
| i. Close to retina | ||||
| Delay up (min) | 0.4 ± 0.3 (5) | 0.2 (2) p>0.1 | N.D. | |
| Slope up (mm Hg/min) | 28 ± 36 (5) | 49 (2) p>0.1 | N.D. | |
| ii. Behind the lens | ||||
| Delay up (min) | 4.2 ± 1.7 (4) | 3.2 ± 2.0 (4) p>0.1 | 0.8 ± 0.5 (5) p<0.01 | |
| p<0.05 | ||||
| Slope up (mm Hg/min) | 1.4 ± 0.8 (4) | 7.1 ± 4.5 (4) p<0.05 | 37 ± 10 (4) p<0.001 | |
| p<0.01 | ||||
| iii. Lens center | ||||
| Delay up (min) | 7.9 ± 4.0 (5) | 7.6 ± 3.7 (6) p>0.1 | 8.0 ± 1.2 (4) p>0.1 | |
| p>0.1 | ||||
| Slope up (mm Hg/min) | 2.1 ± 2.7 (5) | 3.5 ± 2.8 (6) p>0.1 | 3.0 ± 0.8 (4) p>0.1 | |
| B. Rat | ||||
| Lens center | ||||
| Delay up (min) | 1.4 ± 0.6 (6) | 0.9 (2) p>0.1 | 0.4 ± 0.3 (9) p<0.01 | |
| p>0.1 | ||||
| Slope up (mm Hg/min) | 15 ± 9 (6) | 18 (2) p>0.1 | 26 ± 9 (9) p<0.05 | |
| p>0.1 | ||||
pO2 levels at various locations in the eyes of guinea pigs and rats were monitored following breathing of 100% O2 (see the pO2 traces of Figures 2, 3, and 4). Analyses were conducted 1–2 weeks after intravitreal injection of either microplasmin (MP) or hyaluronidase (HY). “Delay up” is the time between the change to 100% O2 and the first observable increase in pO2 level. Results are expressed as means ± S.D. with the number of analyses indicated in parentheses. N.D.: not determined (because of the nearly instantaneous O2 response for microplasmin when the probe was positioned in the vitreous humor close to the retina).
For pO2 trace measurements made with the probe located in the guinea pig lens center (Table 2Aiii), the data showed no significant differences between control, hyaluronidase and microplasmin for either the O2 response time (“delay up”) or the slope for the pO2 increase (“slope up”). For example, under each of the three conditions, the time for O2 to reach the center of the guinea pig lens was about 8 min and the slope for pO2 increase was 2–3 mm Hg/min. In contrast, identical studies conducted with the probe positioned in the center of the rat lens (Table 2B) showed that intravitreal injection with microplasmin accelerated both the O2 response time (3.5x faster than the control, p<0.01) and the slope of pO2 increase (nearly 2x steeper than the control, p<0.05). Comparable results for injection with hyaluronidase (Table 2B) showed a slightly faster O2 response time and an elevated slope, compared to controls, but without statistical significance.
4. Discussion
The main finding of this study was that liquefaction of the vitreous humor of the rat combined with a PVD (achieved by intravitreal injection of microplasmin, Fig. 1B) leads to a 68% increase in lens nuclear pO2, 1–2 weeks later (5.6 mm Hg increasing to 9.4 mm Hg, Table 1, p<0.05). Liquefaction of vitreous humor alone in the rat, without a PVD (achieved by intravitreal injection of hyaluronidase, Fig. 1C), did not produce an increase in lens pO2 (Table 1). In contrast, the guinea pig, which possesses an avascular retina, showed no lens pO2 increase with either of the two enzymes (Table 1). We have previously shown a microplasmin-induced increase in mid vitreal pO2 for both the rat and cat, but no similar increase was observed for the guinea pig (Quiram et al., 2007). Thus, it appears that PVD-induced increases in pO2 levels in the lens and vitreous humor arise from the retinal circulation. These results suggest the possibility that liquefaction of vitreous humor plus a PVD in the human might lead to increased levels of O2 in the vitreous humor and lens, increasing the risk for nuclear cataract, but also having potential benefits for ischemic retinopathies, as will be discussed later. Oxygen has been strongly linked with nuclear cataract (Holekamp et al., 2005; Palmquist et al., 1984; Simpanya et al., 2005). Both liquefaction of vitreous humor, as well as PVD, increase with age in the human (Foos and Wheeler, 1982; Harocopos et al., 2004), and a preliminary report has indicated an increased prevalence of human nuclear cataract in eyes with PVD (Nagai et al., 2007). Whether PVD in the human may increase vitreal pO2 levels is not known. The presence of a partial or minor PVD in human patients has been observed not to increase vitreal pO2 levels, compared with patients without a PVD (Y.-B Shui, personal communication), but it is possible that severe or complete PVD in the human might increase vitreal O2 levels.
Our data indicated the presence of a gradient of cat and rabbit vitreal pO2 levels from a location close the retina to directly behind the lens (Table 1A), as has been reported by a number of others (Alder et al., 1983; Barbazetto et al., 2004; Linsenmeier et al., 1981; Ormerod et al., 1987; Sakaue et al., 1989; Shui et al., 2006). Our vitreous pO2 values for the cat (Table 1A) compared favorably with those of others (Alder et al., 1983; Linsenmeier et al., 1981), and as indicated by Buerk et al. (1993), results of vitreal pO2 measurements made close to the retinal surface of the cat depend to a large extent on how close the tip of the probe is to a retinal vessel. Similarly our vitreal pO2 values for the rabbit (Table 1A) were about the same as those reported by others (Ormerod et al., 1987; Sakaue et al., 1989; Shui et al., 2006), although our value of 4.0 mm Hg for a location in the vitreous directly behind the rabbit lens was about 3-fold lower than that obtained by Barbazetto et al. (2004). For the guinea pig, which has an avascular retina, our value of about 1 mm Hg for the vitreal pO2 level close to the retina matched that reported for the guinea pig retina at the vitreal/retinal interface (Yu et al., 1996).
Few in vivo measurements of pO2 levels in the center of the lens have been reported. Our value of 0.5 mm Hg for the lens nucleus of the cat (Table 1A) matches closely with the 1 mm Hg level obtained by Uyama (1973). However, our value of 2.0 mm Hg for the lens nucleus of the rabbit is five times lower than that reported by Barbazetto et al. (2004). The relatively high level of O2 observed in the center of the rat lens, 5.6 mm Hg (Table 1A), may contribute to the high level of intramolecular protein disulfide found to be present in lenses of this species (Hum and Augusteyn, 1987). In contrast to the rat, lenses of most mammals exhibit no increase in protein disulfide concentration as a function of age, until the development of nuclear cataract, at which time nearly all protein –SH groups may eventually become oxidized (Truscott, 2005). For measurements of lens pO2 levels using the fiber optic fluorescence probe, we assumed that the high viscosity of the lens would not affect the results (calibrations of the instrument were conducted with use of aqueous medium). The manufacturer of the probe has stated that pO2 measurements are insensitive to sample viscosity, and McNulty et al. (2004) have also found this to be true for protein concentrations up to at least 30% (the highest value tested by the researchers, and the protein concentration that exists in the lens). Other investigators have used the fiber optic fluorescence probe to measure pO2 levels in solid tissues such as kidney and tumors, and found the results to be comparable with those obtained with other O2-measuring instruments (Leong et al., 2008; Seddon et al., 2001; Wen et al., 2008).
The increase in vitreal pO2 levels observed for the normal guinea pig after the switch from breathing air to breathing 100% O2 (Fig. 2) has also been found to occur for the monkey (Landers, III et al., 1982), cat (Linsenmeier and Yancey, 1989; Quiram et al., 2007), and rat (Quiram et al., 2007; Yu et al., 1990) but, surprisingly, not for the miniature pig (Pournaras et al., 1989). Treatment of guinea pigs with microplasmin to produce both vitreous humor liquefaction plus a PVD dramatically accelerated movement of O2 across the vitreous space when the animals were later allowed to breathe 100% O2 (Tables 2Ai and 2Aii). However, treatment with hyaluronidase which produced only vitreous humor liquefaction had much less of an effect (Tables 2Ai and 2Aii). Thus, it appears that the ability of microplasmin, in contrast to hyaluronidase, to cleanly separate posterior hyaloid membrane from inner limiting membrane (ILM) to produce a bare ILM (Gandorfer et al., 2004) may remove a barrier which inhibits movement of O2 from the retina into the vitreous space. The observed microplasmin-induced acceleration of O2 exchange in the vitreous humor would not appear to be due to an increase in the rate of diffusion. It has been shown previously that the rate of diffusion of molecules in intact vitreous gel is about the same as that in water (Barton et al., 2007; Maurice, 1959). Other researchers have used dynamic light scattering analysis to show that a maximum dose of microplasmin injected into isolated pig eyes increased vitreous humor diffusion coefficients by only 85% (Sebag et al., 2007), whereas in the current study we observed a many-fold increase in the rate of O2 movement across the vitreous space as a result of microplasmin treatment (Tables 2Ai and 2Aii). The slight increase in the rate of O2 movement in the vitreous space observed as a result of hyaluronidase treatment (Tables 2Ai and 2Aii) may have been due mainly to an increase in bulk flow (mixing) of O2 in liquefied vitreous humor.
A surprising result occured when microplasmin-injected guinea pigs were allowed to breathe 100% O2, and the time required for O2 to reach the center of the lens was observed to be the same (8 min) as that for control animals breathing 100% O2 (Table 2Aiii). This result was observed in spite of the fact that identical treatment of guinea pigs with microplasmin caused O2 to reach a location in the vitreous humor directly behind the lens 5 times faster than that for the controls (0.8 min compared to 4.2 min, p<0.01, Table 2Aii). In contrast, when microplasmin-injected rats breathed 100% O2, the time required for O2 to reach the center of the lens was 3 times faster than that for the controls (0.4 min compared to 1.4 min, p<0.01, Table 2B). We had previously shown that when microplasmin-injected rats breathed 100% O2, the time for O2 to reach the mid vitreous location was 4 times faster than controls (0.1 min compared to 0.4 min, p<0.001) (Quiram et al., 2007). Since 100% O2 breathed by both microplasmin-injected guinea pigs and rats was found to travel faster through the vitreous humor to the posterior surface of the lens, why was faster arrival of O2 in the lens center observed only for the rat? One possibility is that O2 may enter the lens of the guinea pig primarily through the anterior surface of the tissue, not the posterior. Shui et al., (2006) have shown that when rabbits breathe either 20% or 60% O2, levels of O2 in the posterior aqueous humor chamber near the vasculature of the ciliary body are substantially higher than those in the vitreous humor directly behind the lens. For the guinea pig, it is possible that the rate at which breathed O2 reaches the center of the lens may depend more on how fast O2 reaches the ciliary body, and not the posterior surface of the lens. Whether O2 enters the lens interior of a certain species primarily through the anterior or posterior surface would also depend to a large extent on the ability of epithelial cells on the anterior surface of the lens to consume O2 via mitochondrial respiration (McNulty et al., 2004).
The ability of microplasmin to induce a PVD and increase O2 exchange in the vitreous space may have clinical applications regarding treatment of retinal ischemia. It has long been known that vitrectomy surgery can aid in the treatment of diabetic retinopathy (Blankenship and Machemer, 1978; Grigorian et al., 2003; Tachi and Ogino, 1996). This phenomenon has been hypothesized to be linked with a PVD-induced relief of hypoxia in ischemic regions of the diabetic inner retina as a result of increased movement of O2 from O2-rich to O2-poor areas of the vitreoretinal surface (Stefansson et al., 1990; Stefansson, 2006). Holekamp et al. (2005) recently demonstrated an increase in pO2 levels in the vitreous space following vitrectomy surgery in humans. Achieving a clean enzymatic separation between the inner limiting membrane of the retina and the posterior hyaloid membrane of the vitreous humor by microplasmin could be an effective treatment for ischemic retinal diseases by increasing vitreal O2 exchange.
In summary, it has been shown that microplasmin-induced formation of a PVD in an animal with a vascular retina (rat) leads to elevated levels of O2 in the lens nucleus. This finding may have implication with regard to the occurrence of age-related PVD in the human and a possible acceleration of maturity-onset nuclear cataract. In addition, the demonstration that an enzymatic creation of a PVD significantly increased the rate of O2 exchange within the vitreal space may have potential application for treatment of retinal ischemic disease.
Acknowledgments
The authors thank Steven Bassnett, Ph.D. of Washington University for use of the fiber optic pO2 measuring instrument, and Janet Schofding for care of the experimental animals, as well as assistance with their handling.
Footnotes
Supported by NIH grants EY 02027 and EY 014803, and funding from ThromboGenics, Ltd.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alder VA, Cringle SJ, Constable IJ. The retinal oxygen profile in cats. Invest Ophthalmol Vis Sci. 1983;24:30–6. [PubMed] [Google Scholar]
- Barbazetto IA, Liang J, Chang S, Zheng L, Spector A, Dillon JP. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res. 2004;78:917–24. doi: 10.1016/j.exer.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Barton KA, Shui YB, Petrash JM, Beebe DC. Comment on: the Stokes-Einstein equation and the physiological effects of vitreous surgery. Acta Ophthalmol Scand. 2007;85:339–40. doi: 10.1111/j.1600-0420.2007.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19:125–33. doi: 10.1016/j.semcdb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship GW, Machemer R. Pars plana vitrectomy for the management of severe diabetic retinopathy: an analysis of results five years following surgery. Ophthalmology. 1978;85:553–9. doi: 10.1016/s0161-6420(78)35639-6. [DOI] [PubMed] [Google Scholar]
- Buerk DG, Shonat RD, Riva CE, Cranstoun SD. O2 gradients and countercurrent exchange in the cat vitreous humor near retinal arterioles and venules. Microvasc Res. 1993;45:134–48. doi: 10.1006/mvre.1993.1013. [DOI] [PubMed] [Google Scholar]
- Cherfan GM, Michels RG, de BS, Enger C, Glaser BM. Nuclear sclerotic cataract after vitrectomy for idiopathic epiretinal membranes causing macular pucker. Am JOphthalmol. 1991;111:434–8. doi: 10.1016/s0002-9394(14)72377-3. [DOI] [PubMed] [Google Scholar]
- Cringle S, Yu DY, Alder V, Su EN, Yu P. Oxygen consumption in the avascular guinea pig retina. Am JPhysiol. 1996;271:H1162–H1165. doi: 10.1152/ajpheart.1996.271.3.H1162. [DOI] [PubMed] [Google Scholar]
- Foos RY, Wheeler NC. Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982;89:1502–12. doi: 10.1016/s0161-6420(82)34610-2. [DOI] [PubMed] [Google Scholar]
- Gandorfer A, Rohleder M, Sethi C, Eckle D, Welge-Lussen U, Kampik A, Luthert P, Charteris D. Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci. 2004;45:641–7. doi: 10.1167/iovs.03-0930. [DOI] [PubMed] [Google Scholar]
- Gesell LB, Trott A. De novo cataract development following a standard course of hyperbaric oxygen therapy. Undersea Hyperb Med. 2007;34:389–92. [PubMed] [Google Scholar]
- Giblin FJ, Padgaonkar VA, Leverenz VR, Lin LR, Lou MF, Unakar NJ, Dang L, Dickerson JE, Jr, Reddy VN. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res. 1995;60:219–35. doi: 10.1016/s0014-4835(05)80105-8. [DOI] [PubMed] [Google Scholar]
- Gottlieb JL, Antoszyk AN, Hatchell DL, Saloupis P. The safety of intravitreal hyaluronidase. A clinical and histologic study. Invest Ophthalmol Vis Sci. 1990;31:2345–52. [PubMed] [Google Scholar]
- Grigorian R, Bhagat N, Lanzetta P, Tutela A, Zarbin M. Pars plana vitrectomy for refractory diabetic macular edema. Semin Ophthalmol. 2003;18:116–20. doi: 10.1076/soph.18.3.116.29813. [DOI] [PubMed] [Google Scholar]
- Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004;45:77–85. doi: 10.1167/iovs.03-0820. [DOI] [PubMed] [Google Scholar]
- Hikichi T, Kado M, Yoshida A. Intravitreal injection of hyaluronidase cannot induce posterior vitreous detachment in the rabbit. Retina. 2000;20:195–8. [PubMed] [Google Scholar]
- Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am JOphthalmol. 2005;139:302–10. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Hum TP, Augusteyn RC. The nature of disulphide bonds in rat lens proteins. Curr Eye Res. 1987;6:1103–8. doi: 10.3109/02713688709034882. [DOI] [PubMed] [Google Scholar]
- Landers MB, III, Stefansson E, Wolbarsht ML. Panretinal photocoagulation and retinal oxygenation. Retina. 1982;2:167–75. doi: 10.1097/00006982-198200230-00007. [DOI] [PubMed] [Google Scholar]
- Lee B, Litt M, Buchsbaum G. Rheology of the vitreous body. Part I: Viscoelasticity of human vitreous. Biorheology. 1992;29:521–33. doi: 10.3233/bir-1992-295-612. [DOI] [PubMed] [Google Scholar]
- Leong CL, O’Connor PM, Eppel GA, Anderson WP, Evans RG. Measurement of renal tissue oxygen tension: systematic differences between fluorescence optode and microelectrode recordings in anaesthetized rabbits. Nephron Physiol. 2008;108:11–7. doi: 10.1159/000114203. [DOI] [PubMed] [Google Scholar]
- Linsenmeier RA, Goldstick TK, Blum RS, Enroth-Cugell C. Estimation of retinal oxygen transients from measurements made in the vitreous humor. Exp Eye Res. 1981;32:369–79. doi: 10.1016/s0014-4835(81)80016-4. [DOI] [PubMed] [Google Scholar]
- Linsenmeier RA, Yancey CM. Effects of hyperoxia on the oxygen distribution in the intact cat retina. Invest Ophthalmol Vis Sci. 1989;30:612–8. [PubMed] [Google Scholar]
- Margherio RR, Cox MS, Jr, Trese MT, Murphy PL, Johnson J, Minor LA. Removal of epimacular membranes. Ophthalmology. 1985;92:1075–83. doi: 10.1016/s0161-6420(85)33902-7. [DOI] [PubMed] [Google Scholar]
- Maurice DM. Protein dynamics in the eye studied with labelled proteins. Am JOphthalmol. 1959;47:361–8. doi: 10.1016/s0002-9394(14)78042-0. [DOI] [PubMed] [Google Scholar]
- McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. JPhysiol. 2004;559:883–98. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels RG. Vitrectomy for macular pucker. Ophthalmology. 1984;91:1384–8. doi: 10.1016/s0161-6420(84)34136-7. [DOI] [PubMed] [Google Scholar]
- Nagai K, Sasaki H, Honda R, Kanazawa Y, Sakamoto Y, Qu J, Sasaki K. Change of light scattering intensity in eyes with posterior vitreous detachment: Monzon Eye Study. Abstracts, US-Japan Cooperative Cataract Research Group Meeting. 2007:HT-5. [Google Scholar]
- Nagai N, Demarsin E, Van HB, Wouters S, Cingolani D, Laroche Y, Collen D. Recombinant human microplasmin: production and potential therapeutic properties. JThromb Haemost. 2003;1:307–13. doi: 10.1046/j.1538-7836.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Takanashi T, Ishigooka H, Ogino N. Quantitative analysis of lens changes after vitrectomy by fluorophotometry. Am JOphthalmol. 1991;111:179–83. doi: 10.1016/s0002-9394(14)72256-1. [DOI] [PubMed] [Google Scholar]
- Ormerod LD, Edelstein MA, Schmidt GJ, Juarez RS, Finegold SM, Smith RE. The intraocular environment and experimental anaerobic bacterial endophthalmitis. Arch Ophthalmol. 1987;105:1571–5. doi: 10.1001/archopht.1987.01060110117044. [DOI] [PubMed] [Google Scholar]
- Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br JOphthalmol. 1984;68:113–7. doi: 10.1136/bjo.68.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournaras CJ, Riva CE, Tsacopoulos M, Strommer K. Diffusion of O2 in the retina of anesthetized miniature pigs in normoxia and hyperoxia. Exp Eye Res. 1989;49:347–60. doi: 10.1016/0014-4835(89)90045-6. [DOI] [PubMed] [Google Scholar]
- Quiram PA, Leverenz VR, Baker RM, Dang L, Giblin FJ, Trese MT. Microplasmin-induced posterior vitreous detachment affects vitreous oxygen levels. Retina. 2007;27:1090–6. doi: 10.1097/IAE.0b013e3180654229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue H, Negi A, Honda Y. Comparative study of vitreous oxygen tension in human and rabbit eyes. Invest Ophthalmol Vis Sci. 1989;30:1933–7. [PubMed] [Google Scholar]
- Sakuma T, Tanaka M, Mizota A, Inoue J, Pakola S. Safety of in vivo pharmacologic vitreolysis with recombinant microplasmin in rabbit eyes. Invest Ophthalmol Vis Sci. 2005;46:3295–9. doi: 10.1167/iovs.04-1517. [DOI] [PubMed] [Google Scholar]
- Schocket SS, Esterson J, Bradford B, Michaelis M, Richards RD. Induction of cataracts in mice by exposure to oxygen. Isr JMed Sci. 1972;8:1596–601. [PubMed] [Google Scholar]
- Sebag J, Ansari RR, Suh KI. Pharmacologic vitreolysis with microplasmin increases vitreous diffusion coefficients. Graefes Arch Clin Exp Ophthalmol. 2007;245:576–80. doi: 10.1007/s00417-006-0394-3. [DOI] [PubMed] [Google Scholar]
- Seddon BM, Honess DJ, Vojnovic B, Tozer GM, Workman P. Measurement of tumor oxygenation: in vivo comparison of a luminescence fiber-optic sensor and a polarographic electrode in the p22 tumor. Radiat Res. 2001;155:837–46. doi: 10.1667/0033-7587(2001)155[0837:motoiv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Shui YB, Fu JJ, Garcia C, Dattilo LK, Rajagopal R, McMillan S, Mak G, Holekamp NM, Lewis A, Beebe DC. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci. 2006;47:1571–80. doi: 10.1167/iovs.05-1475. [DOI] [PubMed] [Google Scholar]
- Simpanya MF, Ansari RR, Suh KI, Leverenz VR, Giblin FJ. Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis. Invest Ophthalmol Vis Sci. 2005;46:4641–51. doi: 10.1167/iovs.05-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006;51:364–80. doi: 10.1016/j.survophthal.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Stefansson E, Loftsson T. The Stokes-Einstein equation and the physiological effects of vitreous surgery. Acta Ophthalmol Scand. 2006;84:718–9. doi: 10.1111/j.1600-0420.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- Stefansson E, Novack RL, Hatchell DL. Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 1990;31:284–9. [PubMed] [Google Scholar]
- Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am JOphthalmol. 1996;122:258–60. doi: 10.1016/s0002-9394(14)72018-5. [DOI] [PubMed] [Google Scholar]
- Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–25. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Uyama C. Diffusion model of a cat eye. In: Kessler M, editor. Oxygen supply: theoretical and practical aspects of oxygen supply and microcirculation of tissue. University Park Place; Baltimore: 1973. pp. 64–66. [Google Scholar]
- Wen B, Urano M, Humm JL, Seshan VE, Li GC, Ling CC. Comparison of Helzel and OxyLite systems in the measurements of tumor partial oxygen pressure (pO2) Radiat Res. 2008;169:67–75. doi: 10.1667/RR0888.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208. doi: 10.1016/s1350-9462(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Alder VA. The response of rat vitreal oxygen tension to stepwise increases in inspired percentage oxygen. Invest Ophthalmol Vis Sci. 1990;31:2493–9. [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Alder VA, Su EN, Yu PK. Intraretinal oxygen distribution and choroidal regulation in the avascular retina of guinea pigs. Am JPhysiol. 1996;270:H965–H973. doi: 10.1152/ajpheart.1996.270.3.H965. [DOI] [PubMed] [Google Scholar]