Nanotechnology offers great potential benefits for drug delivery and therapy of respiratory and systemic diseases. Nanoparticles (NPs) have been of significant interest for some time as they can be designed to simultaneously carry a drug payload, specifically target features of diseased tissues, and carry an imaging molecule to track drug accumulation and clearance in tissues. Moreover, they can be engineered to tailor drug delivery and improve pharmacokinetics. A variety of NPs have been investigated in experimental animal models as tools to improve the delivery and therapeutic efficacy of drugs or genes delivered to the lung or other organ systems [1]. The nanotechnology platform for drug delivery contains a number of very different types of nano structures with widely varying properties. Examples of these NPs include dendrimers, fullerenes, carbon nanotubes, and polymeric NPs.
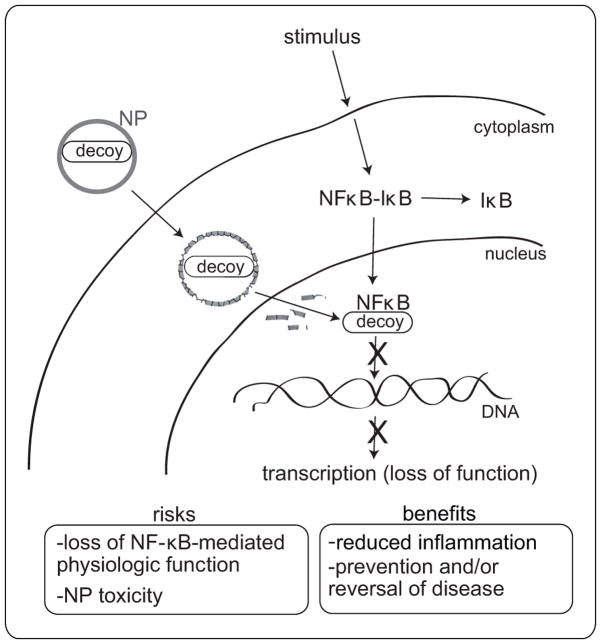
In this issue of Hypertension, Kimura et al [2] report that NF-κB decoy oligodeoxynucleotides (ODNs) encapsulated in poly-(ethylene glycol)-block-lactide/glycolide copolymer (PEG-PLGA) NPs and delivered to the lungs of rats by intratracheal instillation reduced pulmonary arterial hypertension (PAH) induced by monocrotaline (MCT). They showed that NP-encapsulated NF-κB decoy, visualized by FITC labeling, reached the distal regions of the lungs and was present in alveolar macrophages and small pulmonary arteries for up to 14 days after a single instillation. The small pulmonary arteries were also found to be a site of NF-κB activation and NF-κB-dependent inflammatory cytokine production (MCP-1, IL-1, TNF-α) in patients with PAH and in rats with MCT-induced PAH. The decoy ODNs, unlike antisense ODNs which bind specific regions mRNA, bind directly to the transcription factor and inhibit transcription factor binding to target DNA and initiation of gene transcription (Fig. 1). It was speculated by the authors that cellular uptake of the NPs might slowly release encapsulated decoy into the cytoplasm as the polymeric structure of the NP is hydrolyzed, thereby protecting the encapsulated decoy from intracellular degradation before its arrival to the nuclear target and optimizing the inhibitory activity of the decoy. It is noteworthy that the authors of this study showed that treatment of rats with the NF-κB decoy NP 3 weeks after MCT injection led to improved survival [2]. This finding is more clinically relevant than showing prevention of PAH with decoy NP treatment prior to MCT exposure and suggests that individuals with established PAH could potentially benefit from this type of therapy.
Fig. 1.
Schematic representation showing nanoparticle (NP)-mediated delivery of NF-κB decoy oligodeoxynucleotides to block NF-κB-mediated transcription, inflammation, and disease. The possible risks of NP-mediated drug delivery are weighed against the potential benefits.
The NF-κB pathway is one of the most important cellular signal transduction pathways involved in both physiologic processes and disease conditions. It plays important roles in the control of immune function, inflammation, stress response, differentiation, apoptosis, and cell survival [3]. Moreover, NF-κB is involved in cellular processes critical to the development and progression of cancers. NF-κB is a logical choice as a target to reduce lung inflammation after injury as a countless number of inflammatory mediators are regulated by NF-κB. Decoy ODNs for NF-κB have been described previously as a possible strategy for the treatment of numerous diseases including myocardial infarction, glomerulonephritis, arthritis, and cancer [4]. The pathology of these diseases is relatively complicated due to the plethora of cytokines (e.g., IL-1, IL-6, IL-8 and TNF-α) and adhesion molecules (e.g., VCAM and ICAM) that drive the associated inflammatory process. However, an underlying feature of these diseases is that the transcriptional regulation of many of these cytokines and adhesion molecules is controlled by NF-κB. Therefore, blocking NF-κB represents a more efficient strategy for reducing inflammation and disease progression than blocking the action of individual downstream mediators that are regulated by NF-κB. It is recognized that many normal physiologic functions are regulated by NF-κB, and so the efficacy of this strategy in reducing inflammation could come at a high cost. For example, NF-κB is a key regulator of immune function and blocking this signaling pathway could reduce immunity and compromise host defense. Therefore, while NF-κB is an attractive target for the treatment and prevention of a wide spectrum of diseases, some caution should be taken to reduce the risk of developing NF-κB inhibitors that might have the deleterious side effect of dampening the normal physiologic functions of NF-κB.
Targeting NF-κB with an ODN decoy is a relatively novel approach to PAH treatment, especially in the context of combining this therapy with NP-mediated delivery. A previous rat study conducted by Sawada et al. demonstrated that the NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) reduced nuclear localization of NF-κB and VCAM-1 expression on the endothelium of diseased vessels in the lungs and ameliorated MCT-induced PAH [5]. However, PDTC is an antioxidant as well as an NF-κB inhibitor and the authors of this study acknowledged that the beneficial effects observed could have been due to antioxidant properties of PDTC. In addition, they mentioned that there is no evidence from genetically modified animals to demonstrate that NF-κB activation itself is necessary for the development of PAH. Although mice with conditional mutations of the NF-κB system are available, MCT-induced PAH models are unfortunately not reproducible in mice. Despite these limitations, encapsulating PDTC, or other pharmacologic NF-κB inhibitors, within NPs could enhance the beneficial properties of these agents in reducing PAH, in much the same way as was demonstrated by Kimura et al [2]. Other possibilities for enhancing NP delivery platforms include combination therapy (encapsulating two or more inhibitors aimed at the same target within NPs) or packaging inhibitors aimed at two or more transcription factors. Developing an ideal drug delivery platform aimed at NF-κB is not trivial when one considers that there are over 750 inhibitors of the NF-κB pathway, including antioxidants, peptides, small RNA/DNA, microbial and viral proteins, small molecules, and engineered dominant-negative or constitutively active polypeptides [6].
While nano-based drug delivery systems offer the potential for improved therapeutic efficacy, there also are potential risks associated with these novel treatment strategies [7]. Engineered NPs are desirable because they easily enter cells and they can be designed to interact with specific cellular structures (e.g., receptors) to allow for selective accumulation in particular cell types and/or in selected regions of the cell. NPs can also be designed as pH-labile structures so that they degrade within the more acidic microenvironment of the cell to release drug payloads. Biodegradable NPs that are used for drug delivery (e.g., polymeric PEG-PLGA NPs) generally have low toxicity but often do not persist in tissues long enough for sustained drug or gene payload delivery. As a result, more durable NPs are being explored as drug delivery platforms. A primary example is carbon nanotubes (CNTs), whose versatile physicochemical features allow for covalent and noncovalent functionalization to simultaneously carry at least 3 agents: (1) a drug, (2) an imaging agent (to track the course of delivery), and (3) a specific targeting agent (e.g., antibody selective for diseased tissue) [8]. CNTs are durable and persist in biological systems for weeks or longer. Moreover, their tube- and fiber-like structures allow for extensive functionalization and loading of cargo. Despite the potential benefits of CNTs for drug delivery, some of the same unique properties that make CNTs desirable for therapeutic applications also make them potentially toxic. Some studies have shown that CNTs cause inflammation and fibrosis when delivered to the lungs of mice or rats [7]. A recent investigation by Ryman-Rasmussen et al. showed that CNTs cause little adverse pulmonary effects when delivered to the lungs of mice by inhalation, except when the mice were challenged with an allergen [9]. The implications of this study are that CNTs may pose a hazard to individuals with allergic lung inflammatory diseases such as asthma. The durable nature of CNTs along with their fiber-like shape could also lead to persistence in the lung, which might result in asbestos-like complications. In addition to the unique example of CNTs, a number of different types of NPs can stimulate and/or suppress immune responses [10].
While most of the toxicology of NPs is focused on materials that enter the body accidentally, more attention should be given to the toxicology of NPs that are used for biomedical applications, such as drug delivery or imaging, where the materials are deliberately placed in the body. Despite the limitations mentioned above, NPs could be modified or functionalized to reduce toxicity. For example, the compatibility of NPs with the immune system is largely determined by their surface chemistry and modifying this factor alone could significantly reduce their immunotoxicity. Continued research such as that reported by Kimura et al [2] to identify both risks and benefits of NP-mediated drug delivery to the lung should allow for the emergence of safe and effective strategies for the treatment of PAH and other lung diseases including cancer, asthma, fibrosis, and chronic obstructive pulmonary disease.
Acknowledgments
Sources of funding: This work was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES025041) and North Carolina State University College of Agricultural and Life Sciences.
Footnotes
Disclosures: Darryl C. Zeldin and James C. Bonner have no relevant financial, personal or professional relationships with other people or organizations to disclose. Jeffrey W. Card is employed by Cantox Health Sciences International, a scientific consulting company with interests in safety and regulatory aspects of nanotechnology.
References
- 1.De Jong WH, Borm PJ. Drug delivery and nanoparticles:applications and hazards. Int J Nanomedicine. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura S, Egashira K, Chen L, Nakano K, Iwata E, Miyagawa M, Tsujimoto H, Hara I, Morishita R, Sueishi K, Tominaga R, Sunagawa K. Nanoparticle-mediated delivery of NF-κB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension. doi: 10.1161/HYPERTENSIONAHA.108.121418. (In press) [DOI] [PubMed] [Google Scholar]
- 3.Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nature Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 4.Morishita R, Higaki J, Tomita N, Ogihara T. Application of transcription factor “decoy” strategy as means of gene therapy and study of gene expression in cardiovascular disease. Circ Res. 1998;82:1023–1028. doi: 10.1161/01.res.82.10.1023. [DOI] [PubMed] [Google Scholar]
- 5.Sawada H, Mitani Y, Maruyama J, Jiang BH, Ikeyama Y, Dida FA, Yamamoto H, Imanaka-Yoshida K, Shimpo H, Mizoguchi A, Maruyama K, Komada Y. A nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest. 2007;132:1265–1274. doi: 10.1378/chest.06-2243. [DOI] [PubMed] [Google Scholar]
- 6.Gilmore TD, Herscovitch M. Inhibitors of NF-κB signaling: 785 and counting. Oncogene. 2006;25:6887–6899. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- 7.Card JW, Zeldin DC, Bonner JC, Nestmann ER. Pulmonary applications and toxicity of engineered nanoparticles. Am J Physiol Lung Cell Mol Physiol. 2008;295:L400–411. doi: 10.1152/ajplung.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes in drug design and discovery. Acc Chem Res. 2008;41:60–68. doi: 10.1021/ar700089b. [DOI] [PubMed] [Google Scholar]
- 9.Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in a murine model of allergic asthma. Am J Resp Cell Mol Biol. 2009;40:349–58. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]