Summary
Vcsa1 plays an important role in the erectile physiology of the rat. We conducted experiments to determine if erectile function, testosterone levels and Vcsa1 expression were correlated. In orchiectomized rats, total testosterone in blood fell from an average of 4ng/ml to <0.04ng/ml. Erectile function was significantly lower compared to controls and Vcsa1 expression was significantly (>6-fold) decreased. Injection of orchiectomized animals with testosterone (2mg in 100ml sesame oil every 4 days for two weeks) restored average levels of testosterone to 2ng/ml, increased erectile function and significantly increased Vcsa1 expression. In isolated corporal cells there was testosterone dependent Vcsa1 expression. However, intracorporal injection of orchiectomized animals with a plasmid expressing Vcsa1 or its gene product Sialorphin (previously demonstrated to improve erectile function in old animals) gave no significant improvement in erectile function. Also, the ability of Sialorphin to reduce tension in corporal smooth muscle strips isolated from orchiectomized animals was impaired compared to controls.
Keywords: Vcsa1, testosterone, erectile function, corpora, submandibular gland
1. Introduction
The Vcsa1 gene encodes a pentapetide called Sialorphin, which belongs to a recently identified family of peptides which act as endogenous neutral endopeptidase (NEP) inhibitors. Vcsa1 is expressed in a tissue specific manner in the rat. The primary organs where the transcript is significantly expressed are the penile corpora, submandibular gland (SMG) and prostate gland (18, 20). It has been reported that in the rat SMG Vcsa1 expression is regulated by androgens (16). This is believed to explain the marked gender-specific expression of Vcsa1 in the SMG, with 1000-fold greater expression in male rats than in female rats (16). In addition circulating plasma levels of Sialorphin are approximately 100-fold greater in the male compared to the female rat (18). Androgenic regulation of Vcsa1 in penile corporal tissue has not been reported. However, corporal tissue has been shown to express androgen receptors and therefore regulation of Vcsa1 may potentially be androgen-regulated (5, 6).
Sialorphin has been demonstrated to be involved in many physiological processes, such as pain perception, antidepressant effects, sexual behavior, and erectile function (4, 14, 17, 20). Both Vcsa1 and its human homologues (ProL1 and hSMR3A/B) have been shown to modify erectile physiology of the rat. This was demonstrated by the ability of gene transfer of plasmids (pVAX-Vcsa1, pVAX-Prol1 or pVAX-SMR3A/B) to improve erectile physiology in retired breeder rats one week after transfer (20, 21). In addition, Sialorphin was able to significantly improve erectile function in retired breeder rats with erectile dysfunction (ED) within 45 minutes following intracorporal injection (4).
Testosterone has been shown in animal models to have a role in modulating central and peripheral regulation of erectile function (23). Testosterone deprivation has a strong negative impact on penile tissue causing a diverse set of changes at the structural, biochemical and physiological level (23). Some of the biochemical changes that are believed to have a major impact on erectile physiology are a reduction in nitric oxide synthase, phosphodiesterase, reduced MaxiK potassium channel expression, and an increase in apoptosis resulting in lowered smooth muscle content (8, 15, 28). These effects can be reversed by administration of testosterone (25).
Given evidence in rats that both testosterone and Vcsa1 play roles in erectile physiology, and that testosterone could potentially regulate Vcsa1 expression in the corpora, we have investigated the relationship between testosterone and Vcsa1 levels and how these may relate to erectile physiology.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats weighing 300–350g were used in these studies and obtained from Charles River Breeding Laboratories (Wilmington, MA, USA). One group of rats underwent a sham operation with a perineal skin incision only and served as control. The other group underwent bilateral orchiectomy and was divided into two subgroups. Immediately after orchiectomy, one subgroup was injected subcutaneously with testosterone (2mg testosterone propionate/100μl sesame oil; Sigma-Aldrich, St. Louis, MO, USA) every four days for two weeks according to a previous study (1) and another subgroup was treated with vehicle only. All surgical procedures were performed under anesthesia by intraperitoneal injection of sodium pentobarbital (35mg/kg; Abbott Laboratory, Chicago, IL, USA). Total testosterone levels were assayed by radioimmunoassay, as described in section 2.2. All animal protocols were approved by the Animal Use Committee at the Albert Einstein College of Medicine.
2.2. Testosterone assay
Immediately after euthanasia of animals blood was drawn by cardiac puncture for the determination of testosterone levels. Whole blood was allowed to clot for 30 minutes at room temperature and centrifuged at 5000rpm for 15 minutes at 4°C. The serum was collected, aliquotted and stored at -80°C. Testosterone levels were determined by radioimmunoassay using the Coat-a-Count Total Testosterone Assay Plate from Diagnostic Products Corp. (Los Angeles, CA, USA). The assays were performed by the Animal Health Diagnostic Center, College of Veterinary Medicine, Cornell University.
2.3. ICP/BP measurement
Two weeks after orchiectomy or sham surgery, animals underwent erectile function measurement as previously described (4, 20, 21). Rats were first anesthetized via intraperitoneal injection of sodium pentobarbital (35mg/kg). A cannula was inserted into the carotid artery for systemic pressure (BP) monitoring throughout the experiment. Next an incision was made in the perineum, the ischiocavernosus muscle removed to expose a penile crus, and a 23-gauge needle inserted to measure intracavernosal pressure (ICP). The cavernous nerves were identified in a position ventrolateral to the prostate gland and carefully isolated. Direct electrostimulation of the cavernous nerve was performed using a delicate stainless steel bipolar hook electrode attached to a multijointed clamp. Each probe was 0.2mm in diameter with a 1mm separation between the 2 poles. Monophasic rectangular pulses were delivered by a signal generator (custom-made and with built-in constant current amplifier). Stimulation parameters are described in the figure legends (Figures 1, 4). The changes in ICP and systemic BP were recorded at each level of neurostimulation. The mean ICP/BP and standard error of the mean were calculated for each of the treatment groups. A one way analysis of variance (ANOVA) was used to determine treatment effects.
Figure 1.
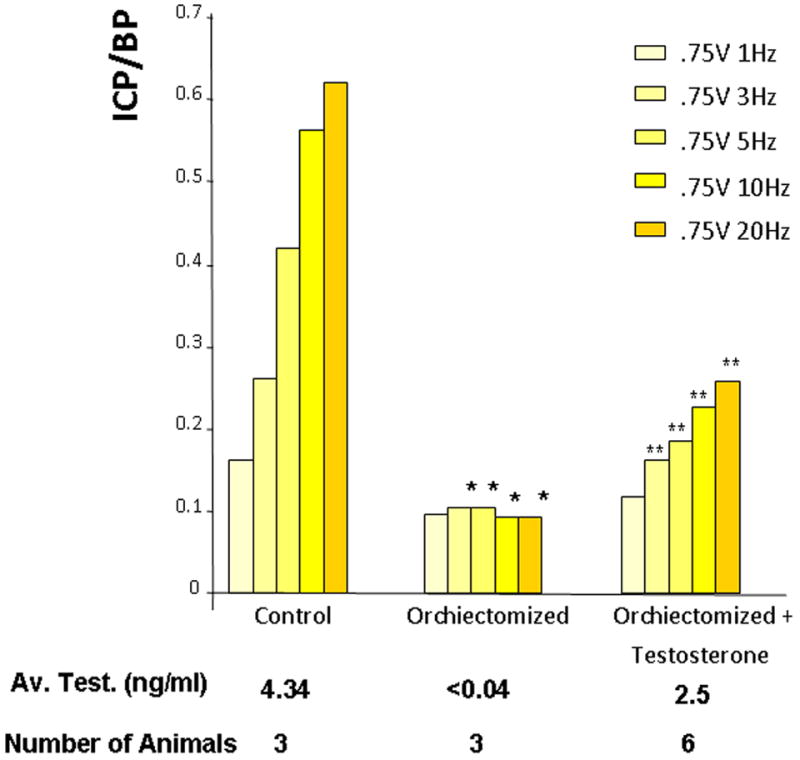
The effect of orchiectomy on erectile function, as determined by intracorporal pressure/blood pressure (ICP/BP) with increasing levels of electrical stimulation of the cavernous nerve. A group of animals was orchiectomized and a subset of orchiectomized animals was treated with testosterone (2mg testosterone propionate/100μl sesame oil). Control animals were sham-operated. The number of animals and the average testosterone level are shown for each group. *=Significantly different ICP/BP ratio of orchiectomized compared to the control animals (P < 0.05), **=Significantly different ICP/BP ratio of orchiectomized with testosterone treatment compared to the orchiectomized group of animals, (P < 0.05).
Figure 4.
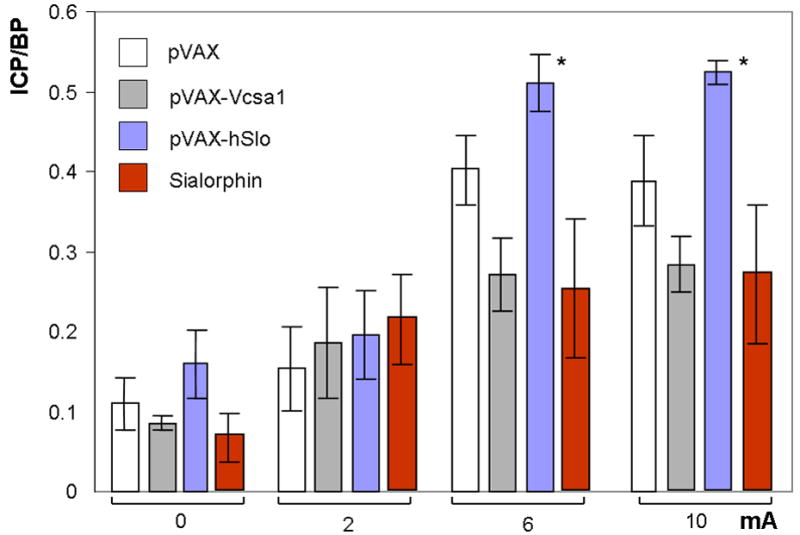
The effect of gene transfer of plasmids expressing Vcsa1 or hSlo, or direct intracorporal injection of Sialorphin on erectile function in orchiectomized animals as determined by intracorporal pressure/blood pressure (ICP/BP) response following stimulation of the cavernous nerve. Four groups of five animals each were orchiectomized and injected intracorporally with either 100 μg pVAX-hSlo or pVAX-Vcsa1 or pVAX (control) for one week, or 100 μg Sialorphin for 2 hours prior to the determination of ICP/BP. The ICP/BP was determined at 4 levels of cavernous nerve stimulation (0, 2, 6 and 10 mA). Bars represent the average ICP/BP measurement from 5 animals, and the error bars the standard error of the mean. *=Significantly different ICP/BP ratio of treated to the control animals (P < 0.05).
2.4. Gene transfer of plasmids or Sialorphin injection into animals
Microinjection of pVAX, pVAX-hSlo, and pVAX-Vcsa1, or the peptide Sialorphin (Sigma, St Louis, MO, USA) into rat corporal tissue was performed essentially as previously described (2-4, 13, 20, 21). Briefly, animals were anesthetized by an intraperitoneal injection of sodium pentobarbital (35mg/kg). An incision was made in the perineum and the left penile crura exposed. All microinjections were a single bolus injection consisting of either 100μg plasmid or 100μg Sialorphin in a 150μl solution of phosphate-buffered saline and 20% sucrose, and were injected into the corporal tissue using an insulin syringe. The ICP/BP response to cavernous nerve electrostimulation was determined one week after gene transfer of plasmids or 2 hours after intracorporal injection of Sialorphin (as described above).
2.5. Testosterone on corporal cells in vitro
Corporal smooth muscle (CSM) cells were isolated from a normal Sprague-Dawley rats as previously described (9) and grown for three passages in DMEM containing 10% fetal bovine serum at a constant environment of 37°C and 5% CO2. Prior to experimental treatment, cells were split into 6-well plates and maintained at 80% confluency in serum-free DMEM containing 5% antibiotics. Cells were then treated with various doses (from 0-16ng/ml, as shown in Figure 3) of testosterone propionate (Sigma-Aldrich, St. Louis, MO, USA) for 24 hours. At the end of the incubation period, RNA was isolated from CCSM cells and changes in gene expression were determined by quantitative RT-PCR.
Figure 3.
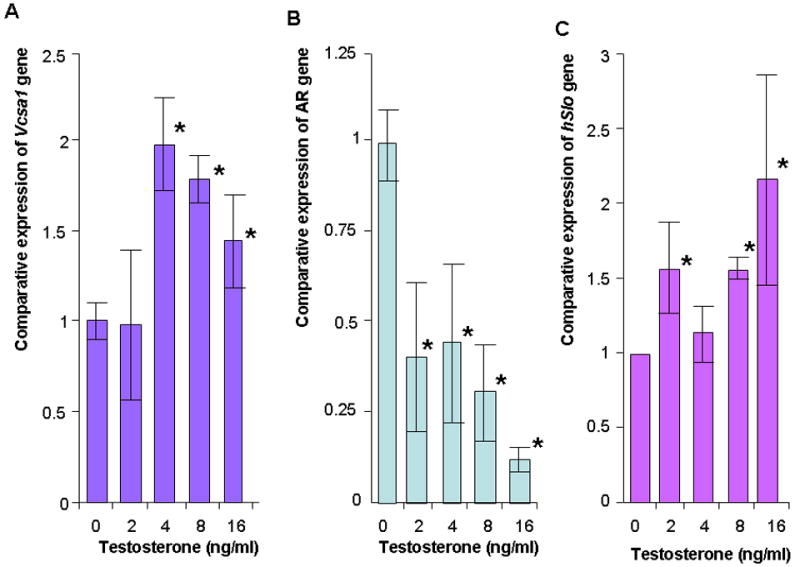
A, B and C: The expression of Vcsa1, AR and Slo in corporal smooth muscle cells in vitro with increasing concentrations of testosterone. Expression of gene transcripts normalized to RPL24 was analyzed using the comparative crossing threshold (Ct) method. The untreated cells were used as calibrator (set as 1). Each quantitative RT-PCR measurement was performed more than four times for each concentration of testosterone. The bars represent the mean comparative expression of the gene, and the error bars the standard deviation. *=Significantly different expression of Vcsa1, AR or Slo compared to cells in the absence of testosterone (P < 0.05).
2.6. RNA isolation from corporal tissue and cells
Following the ICP/BP measurement, animals were euthanized, and blood collected via cardiac puncture for serum testosterone measurement via radioimmunoassay. Denuded corporal tissue was isolated and flash frozen in liquid nitrogen. Total RNA was extracted from frozen tissue with TRIzol according to the manufacturer’s instructions. Briefly, 1 ml TRIzol reagent was added to 100 mg pulverized corpora tissue and the mixture homogenized using a polytron homogenizer (Brinkman, Westbury, NY) for 30 seconds. For the CSM cells, 1mL TRIzol was added to each 35mm well and the lysates transferred to polypropylene tubes. The homogenized samples were incubated for 5 min at room temperature followed by addition of 200 μl chloroform/1mL TRIzol. After mixing, the aqueous phases were separated by centrifugation at 12000×g and 4°C for 15 minutes and then were transferred to clean tubes. The RNA was precipitated from the aqueous phase by addition of isopropyl alcohol and pelleted by centrifugation at 12000×g for 15 min at 4°C, washed once with 75% ethanol, and again pelleted at 7500×g and 4°C for 10 minutes. The RNA pellet was air-dried and dissolved in sterile, nuclease-free water.
2.7. Quantitative RT-PCR
RNA extracted from corporal tissue and CSM cells was used to analyze gene expression by quantitative RT-PCR, essentially as previously described (20-22). The quantity and purity of RNA were determined using the ND-1000 NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). RNA was converted to cDNA using the SuperScript™ First-Strand Synthesis System (Invitrogen Corporation, Carlsbad, CA). In a 20 μL reaction volume, 1 μg total RNA was combined with 0.5 μg Oligo(dT)12-18 primer and 1 μl of 10mM dNTP mix and heated for 5 minutes at 65°C, immediately followed by incubation on ice for 5 minutes. The sample was then pre-incubated at 42°C for 2 minutes in a mixture consisting of 40 units RNaseOUT™ recombinant ribonuclease inhibitor, 10mM dithiothreitol, 50mM Tris-HCl, 75mM KCl, and 3mM MgCl2 200 units of SuperScript™ reverse transcriptase was then added, the contents mixed gently, and the reaction carried out for 50 minutes at 42°C and inactivated at 70°C for 15 minutes. Real-time quantitative PCR analysis was then performed on the RT products using the 7300 real-time PCR system (Applied Biosystems, Foster City, CA). The primers used to quantify expression levels of the genes are shown in Table 1.
Table 1.
Primers used in quantitative RT-PCR
| Gene | Primer | Sequence |
|---|---|---|
| Vcsa1 | forward primer | 5’-GAGGGTGTCAGAGGCCC-3’ |
| reverse primer | 5’-GAGCAGTTAGCTGCCACTGATA-3’ | |
| AR | forward primer | 5’-TGACCAGATGGCAGTCATTCA-3’ |
| reverse primer | 5’-TGAAAACCAGGTCAGGTGCA-3’ | |
| Slo | forward primer | 5’-TACTTCAATGACAATATCCTCACCCT -3’ |
| reverse primer | 5’-ACCATAACAACCACCATCCCCTAAG-3’ | |
| RPL24 | forward primer | 5’-TCGAGCTGTGCAGTTTTAGTGG-3’ |
| reverse primer | 5’-GCGGACTCACATTTGGCATTA-3’ | |
| GAPDH | forward primer | 5’-CCGAGGGCCCACTAAAGG-3’ |
| reverse primer | 5’-GCATCAAAGGTGGAAGAATGG-3’ |
The PCR amplification and real-time detection was performed in a 96-well plate, with 5 μl of 1:100 diluted cDNA, 300 nM each upstream and downstream primer, and 2x Sybr Green MasterMix in a 25 μl reaction volume. The cycling conditions were as follows: activation of Sybr Green DNA polymerase at 95°C for 10 minutes, 40 cycles of denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute. Melting curve analysis was performed after the last cycle to confirm amplification of a single product.
Results from real-time RT-PCR are presented as fold change in expression relative to control, using target gene crossing thresholds normalized to that of a house-keeping gene (GAPDH or RPL24) crossing thresholds based on the 2− ΔΔCt method as previously described (11). This method was applicable because the amplification efficiency of the primers was found to be the same as that of the house keeping genes, GAPDH and RPL24 (as described in the figure legends). The standard deviation was calculated and the significance determined by Student’s t test for unpaired samples. A p-value of <0.05 was considered significant.
2.8. Contractility studies on isolated corporal smooth muscle tissue
Studies of contractility of isolated CSM tissue were carried out as described previously (4). Longitudinal strips of cavernous tissue from the crura of three control and three orchiectomized animals (4 strips each animal) were dissected free from the tunica albuginea and were suspended between two small surgical hooks in a tension-measuring device (Multimyograph Model 610M, Copenhagen, Denmark) that allows simultaneous monitoring of four muscle strips. Tissue was equilibrated for 90 minuntes in 5ml Krebs-Henseleit buffer composed of 110mM NaCl, 4.8mM KCl, 2.5mM CaCl2, 1.2mM MgSO4, 1.2mM KH2PO4, 25mM NaHCO3, and 11mM glucose in distilled water. Organ chambers were maintained at 37°C and continuously bubbled with 95% O2 and 5% CO2 to maintain a mean pH of 7.4 ± 0.1. Continuous recording of tension developed by the muscle strips was carried out using Powerlab software (Chart version 4.2.4, ADinstruments, CO) on a dedicated computer. Strips of corpus cavernosum were pre-contracted with 1μM phenylephrine. Then, relaxation was induced using 1μM C-type natriuretic peptide (CNP) (Sigma, St Louis, MO, USA) dissolved in DMSO, the vehicle alone (DMSO) or 1μg/ml Sialorphin (Sigma, St Louis, MO, USA). Results from four separate strips from six animals were averaged and the significance determined by Student’s t test for unpaired samples. A p-value of <0.05 was considered significant.
Results
3.1. Circulating Testosterone levels influences erectile function and Vcsa1 expression
As shown in Figure 1, control (sham-operated) animals had average testosterone levels of 4.34ng/ml, as determined by radioimmunoassay, which is in the range of previously reported values (27). Orchiectomized animals which received no testosterone supplementation had dramatically lower, approximately 100-fold-less, circulating testosterone. Animals supplemented with testosterone injections had significantly higher levels; however, the average did not reach the levels seen in the control animals. Control animals had an ICP/BP ratio of about 0.6 at the highest level of stimulation (0.75V, 20Hz) (Figure 1) which was associated with a visible erection. Orchiectomized animals showed very poor erectile response to cavernous nerve stimulation (an ICP/BP ratio of <0.1). However, orchiectomized animals treated with testosterone propionate had significantly increased ICP/BP response to cavernous nerve stimulation compared to the untreated orchiectomized animal, though the ICP/BP ratio did not return to the levels of the control animals.
After the ICP/BP determination we isolated RNA from the corpora and performed quantitative-RT-PCR and normalized Vcsa1 expression to GAPDH. As shown in Figure 2A in the orchiectomized animals there was a significant (>6-fold) decrease in Vcsa1 expression in the corpora. In the testosterone-supplemented animals there was a significant (approximately 2-fold) increase in the Vcsa1 expression compared to the orchiectomized animals. In contrast, expression of the androgen receptor transcript was significantly up-regulated (approximately 2.5-fold) in the castrated animals, but levels of expression were normalized in testosterone-supplemented animals (Figure 2B). We also looked at the expression of another gene, Slo (encoding the MaxiK potassium channel), that plays a role in the regulation of smooth muscle tone and therefore erectile function (Figure 2C)(3, 26). Its expression was significantly down-regulated in orchiectomized animals, and the expression was reversed to about 80% that of control when orchiectomized animals were treated with testosterone.
Figure 2.
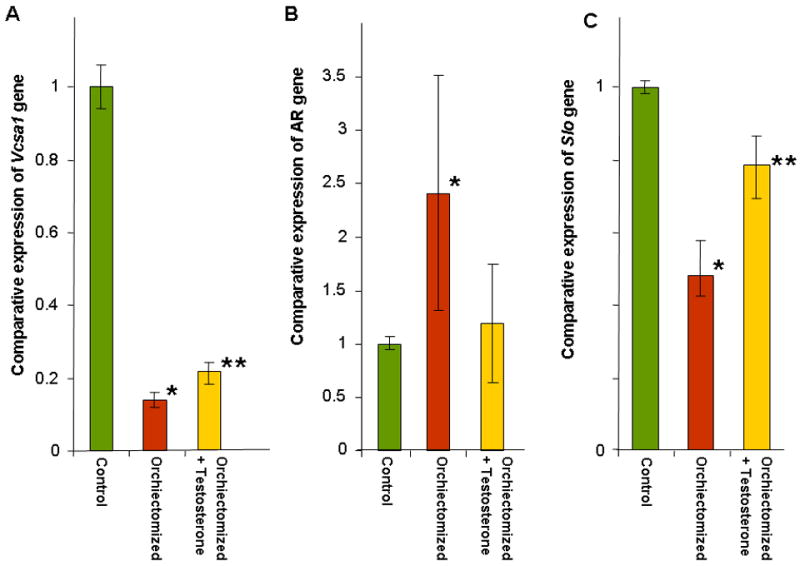
A, B and C: The expressions of Vcsa1, AR and Slo in the corpora of the same experimental groups described in Figure 1 are shown. The number of animals in each group was as follows; control group N=3 animals, orchiectomized N=3 animals and orchiectomized plus testosterone, N=6 animals. Expression of transcripts were normalized to GAPDH and analyzed using the comparative crossing threshold (Ct) method. The control group corporal tissue was used as the calibrator tissue (set as 1). Each quantitative RT-PCR measurement was performed in duplicate for each sample. The bars represent the mean comparative expression of the gene, and the error bars the standard deviation. *=Significantly different expression of Vcsa1, AR or Slo in the corpora of orchiectomized compared to control animals (P < 0.05), **=Significantly different expression of transcripts in the corpora of orchiectomized treated with testosterone compared to the orchiectomized group of animals, (P < 0.05).
3.2. The effect of testosterone on Vcsa1 expression in corporal cells in vitro
Vcsa1 expression is both a marker of erectile function and plays a direct role in erectile physiology (20). The effect of testosterone on Vcsa1 expression could therefore be indirect, being a response to the recovery of erectile function, rather than a direct effect of testosterone on Vcsa1 expression. Therefore we investigated the effect of testosterone on corporal smooth muscle (CSM) cells in vitro. CSM cells were treated with various concentrations of testosterone for 24 hours prior to the determination of Vcsa1 and androgen receptor transcript expression (Figure 3A). As testosterone levels increased in the normal physiologic range (from 0ng/mL to 4ng/mL) there was a 2-fold up-regulation of Vcsa1 expression. The levels of expression decreased with supraphysiologic levels (> 4ng/ml) of testosterone (higher levels, > 16ng/ml, of testosterone not shown). Interestingly there was a down-regulation of the AR gene (Figure 3B) and an increase in Slo expression (Figure 3C) as testosterone levels increased.
3.3. The physiological response in orchiectomized animals to gene transfer of plasmids expressing Vcsa1 or Slo
We have previously documented that intracorporal injection of Sialorphin or gene transfer of plasmids expressing Vcsa1 and hSlo into an aging model of ED improves erectile function (4, 20, 21). Given that testosterone has a direct effect on Vcsa1 expression, and the previous demonstration that both Sialorphin and Vcsa1 can directly improve erectile function in aged rats, we performed experiments to determine if intracorporal injection of Sialorphin or gene transfer of plasmids expressing Vcsa1 into an orchiectomized animal can improve erectile function. As shown in Figure 4, one week following intracorporal gene transfer of pVAX-Vcsa1 there was no significant difference in the ICP/BP ratio of animals following electrostimulation of the cavernous nerve compared to controls. In addition, injection of Sialorphin into animals two hours prior to the determination of ICP/BP also did not result in an improved erectile function (Figure 4). In contrast orchiectomized animals treated with pVAX-hSlo showed a significant improvement in erectile function at the higher levels of cavernous nerve stimulation (6 and 10 mA) and had visible erections.
3.4. Orchiectomy impairs the ability of sialorphin to induce relaxation of corporal smooth muscle strips
We have previously demonstrated that in isolated rat corporal smooth muscle (CSM) strips Sialorphin can enhance relaxation induced by C-type natriuretic peptide (CNP)(4). We determined the ability of Sialorphin to relax isolated corporal smooth muscle strips in orchiectomized compared to control animals. Typical myograph recordings are shown in Figure 5A-D, and the average change in tone in response to treatment is shown graphically in Figure 5E. Addition of phenylephrine to both CSM strips of orchiectomized and control animals resulted in increased force generation, which was partly reversed by the addition of CNP. There was no significant difference in CNP-induced relaxation between orchiectomized and control animals (Figure 5B, D and E). However, the % relaxation resulting from the addition of Sialorphin was significantly reduced (>50%) in CSM strips isolated from the orchiectomized animals in the presence or absence of CNP compared to the control animals.
Figure 5.
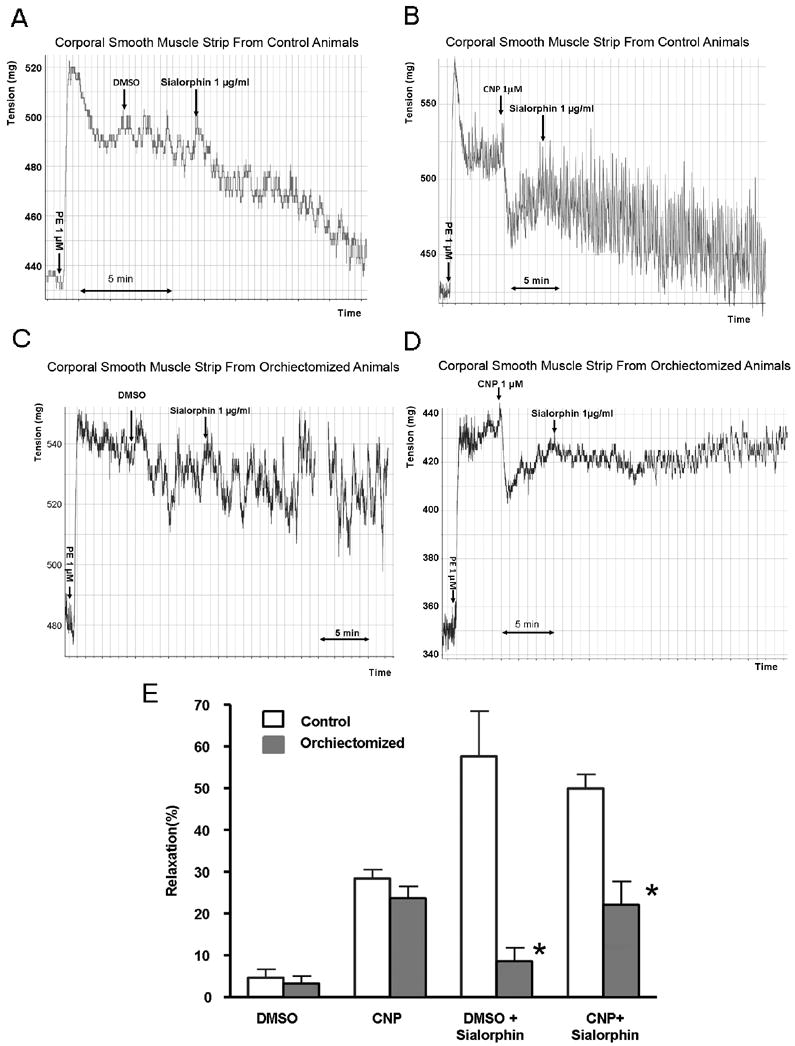
A-E. The ability of sialorphin to induce relaxation of corporal smooth muscle strips from control and orchiectomized animals. A-D representative myograph tracings of CSM strips from control animals (5A and B) and orchiectomized animals (5C and D). Arrows indicate the addition of 1 μM phenylephrine (PE), 1 μM C-type natriuretic peptide (CNP) or its vehicle dimethyl sulfoxide (DMSO), or 1 μg/ml sialorphin. In Figure 5E the percentage relaxation (from the PE contracted state) is shown. Bars represent the average from 4 strips from three animals (a total of 12 determinations). Error bars represent the standard deviation. *=Significantly different relaxation of CSM strips between control and orchiectomized animals (P < 0.05).
Discussion and Conclusion
We demonstrate that the expression of the Vcsa1 gene is down-regulated in the corpora of animals with orchiectomy-induced ED. Supplementation of orchiectomized animals with testosterone partially reverses the effect on erectile function, with a concomitant increase in Vcsa1 expression. We also investigated the effects of testosterone on Vcsa1 expression in vitro. Testosterone induces Vcsa1 expression in vitro in corporal smooth muscle cells at physiologic levels. Therefore our results demonstrate that transcription of Vcsa1 in corporal tissue is regulated by testosterone. However, the regulation of Vcsa1 expression may be complicated by the down-regulation of the androgen receptor (AR) in corporal tissue that occurs with increasing testosterone levels, which we observed both in animals and in vitro, and has been previously reported by Lin et al. (10). The change in expression of the AR may be a compensatory response to maintain a normal level of signaling pathway activity in response to changes in testosterone levels.
In orchiectomized animals Vcsa1 expression decreased approximately 6-fold in the corporal tissue. Although this is a significant decrease, in the sub-mandibular gland of the rat there is approximately 1000-fold greater gene expression in the males, mainly ascribed to the effect of androgens (16). In male mice, castration leads to an almost complete loss of detection of the Vcsa1 transcript in the sub-mandibular gland (19). There may be different levels of expression of the AR in the corpora compared to the sub-mandibular gland, or a greater down-regulation of its expression in response to androgens, thereby limiting their effect. Transcription of the Slo gene, encoding the Maxi-K potassium channel which is an important mediator of CSM tone and has a direct involvement in erectile function (3, 26) was also decreased in expression in the orchiectomized animals. The activity of the channel in rat CSM was recently demonstrated to be androgen regulated (8). The expression of the Slo gene also correlated with the restoration of erectile function when orchiectomized animals were supplemented with testosterone.
Previous studies have demonstrated that Vcsa1 can play a direct role in erectile function. Animals that have erectile dysfunction resulting from aging can have erectile function normalized by gene transfer of plasmids expressing Vcsa1 (or direct administration of its protein product, Sialorphin)(4, 20, 21). However, in the studies presented here, gene transfer of plasmids expressing Vcsa1 was not sufficient to restore erectile function in orchiectomized animals. Sialorphin was also unable to improve orchiectomy-induced ED or induce relaxation of CSM strips isolated from orchiectomized animals. In contrast, gene transfer of pVAX-hSlo was able to restore erectile function in the orchiectomized animals. In previous studies pVAX-hSlo has been shown to restore erectile function in two animal models (diabetes and aging) and is currently undergoing clinical trials for the treatment of ED (2, 3, 12). Testosterone effects multiple pathways involved in the regulation of CSM tone such as nitric oxide synthase and phosphodiesterase, as well as the smooth muscle content of corporal tissue (8, 15, 28). The expression of hSlo from plasmids may represent a site of intervention in the biochemical pathways leading to smooth muscle relaxation (and thereby tumescence) that are downstream of those activated by Vcsa1.
The role of testosterone in human erectile function remains controversial (7, 23, 24). Most studies suggest that only extremely low testosterone levels have a physiologic impact on human erectile function. Testosterone supplementation, prescribed for hypogonadal men, is believed to exert its action primarily through an increase in the libido. Gene transfer of Vcsa1 and measuring the ICP/BP ratio as a result of cavernous nerve stimulation, only determine the physiologic effect on erectile function. However, the peptide product of Vcsa1 (Sialorphin) plays a role in the sexual behavior of rats, acting on the central nervous system (14). Therefore, although not determined in this study, it is possible that the changes in expression of Vcsa1, and therefore the circulating levels of Sialorphin, may not play a role in restoring erectile physiology, but could act as a mediator of the effect of testosterone on libido.
We therefore conclude that Vcsa1 expression in the corporal smooth muscle tissue is regulated by testosterone. Although Vcsa1 acts as a marker of testosterone-modulated erectile function, gene transfer of plasmids expressing Vcsa1 is insufficient to restore erectile function. Vcsa1 has highly specific tissue expression (in the corpora, submandibular gland and prostate), and we now show that in two of these tissues (the corpora and submandibular gland) expression is regulated by testosterone. However, the protein product of Vcsa1 (Sialorphin) is secreted into the bloodstream, where it may affect other tissues distal to the corpora. Therefore, testosterone may exert some of its physiological effects from its role in modulating secondary effects through the regulation of circulating sialorphin levels.
Acknowledgments
This work was supported by grants awarded by the NIH/NIDDK to Kelvin P. Davies (R21DK079594 and R01DK077665) and Michael DiSanto (R01DK077116). We thank Dwarka Kuppam for technical assistance in some of the animal experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banu KS, Govindarajulu P, Aruldhas MM. Testosterone and estradiol modulate TSH-binding in the thyrocytes of Wistar rats: influence of age and sex. J Steroid Biochem Mol Biol. 2001;78:329–342. doi: 10.1016/s0960-0760(01)00107-8. [DOI] [PubMed] [Google Scholar]
- 2.Christ GJ, Day N, Santizo C, Sato Y, Zhao W, Sclafani T, Bakal R, Salman M, Davies K, Melman A. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H1544–1553. doi: 10.1152/ajpheart.00792.2003. [DOI] [PubMed] [Google Scholar]
- 3.Christ GJ, Rehman J, Day N, Salkoff L, Valcic M, Melman A, Geliebter J. Intracorporal injection of hSlo cDNA in rats produces physiologically relevant alterations in penile function. Am J Physiol. 1998;275:H600–608. doi: 10.1152/ajpheart.1998.275.2.H600. [DOI] [PubMed] [Google Scholar]
- 4.Davies KP, Tar M, Rougeot C, Melman A. Sialorphin (the mature peptide product of Vcsa1) relaxes corporal smooth muscle tissue and increases erectile function in the ageing rat. BJU Int. 2007;99:431–435. doi: 10.1111/j.1464-410X.2006.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehm SM, Tindall DJ. Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol. 2007;21:2855–2863. doi: 10.1210/me.2007-0223. [DOI] [PubMed] [Google Scholar]
- 6.DiSanto ME. Corpus cavernosum smooth muscle physiology: a role for sex hormones? J Androl. 2003;24:S6–16. doi: 10.1002/j.1939-4640.2003.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 7.Guay AT. Testosterone and erectile physiology. Aging Male. 2006;9:201–206. doi: 10.1080/13685530601051155. [DOI] [PubMed] [Google Scholar]
- 8.Han DH, Chae MR, Jung JH, So I, Park JK, Lee SW. Effect of testosterone on potassium channel opening in human corporal smooth muscle cells. J Sex Med. 2008;5:822–832. doi: 10.1111/j.1743-6109.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- 9.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation. 1996;3:313–328. doi: 10.3109/10739689609148305. [DOI] [PubMed] [Google Scholar]
- 10.Lin MC, Rajfer J, Swerdloff RS, Gonzalez-Cadavid NF. Testosterone down-regulates the levels of androgen receptor mRNA in smooth muscle cells from the rat corpora cavernosa via aromatization to estrogens. J Steroid Biochem Mol Biol. 1993;45:333–343. doi: 10.1016/0960-0760(93)90002-e. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. hMaxi-K gene transfer in males with erectile dysfunction: results of the first human trial. Hum Gene Ther. 2006;17:1165–1176. doi: 10.1089/hum.2006.17.1165. [DOI] [PubMed] [Google Scholar]
- 13.Melman A, Biggs G, Davies K, Zhao W, Tar MT, Christ GJ. Gene transfer with a vector expressing Maxi-K from a smooth muscle-specific promoter restores erectile function in the aging rat. Gene Ther. 2008;15:364–370. doi: 10.1038/sj.gt.3303093. [DOI] [PubMed] [Google Scholar]
- 14.Messaoudi M, Desor D, Nejdi A, Rougeot C. The endogenous androgen-regulated sialorphin modulates male rat sexual behavior. Horm Behav. 2004;46:684–691. doi: 10.1016/j.yhbeh.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Morelli A, Filippi S, Zhang XH, Luconi M, Vignozzi L, Mancina R, Maggi M. Peripheral regulatory mechanisms in erection. Int J Androl. 2005;28(Suppl 2):23–27. doi: 10.1111/j.1365-2605.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- 16.Rosinski-Chupin I, Huaulme JF, Rougeot C, Rougeon F. The transcriptional response to androgens of the rat VCSA1 gene is amplified by both binary and graded mechanisms. Endocrinology. 2001;142:4550–4559. doi: 10.1210/endo.142.10.8428. [DOI] [PubMed] [Google Scholar]
- 17.Rougeot C, Messaoudi M, Hermitte V, Rigault AG, Blisnick T, Dugave C, Desor D, Rougeon F. Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc Natl Acad Sci U S A. 2003;100:8549–8554. doi: 10.1073/pnas.1431850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rougeot C, Rosinski-Chupin I, Njamkepo E, Rougeon F. Selective processing of submandibular rat 1 protein at dibasic cleavage sites. Salivary and bloodstream secretion products. Eur J Biochem. 1994;219:765–773. doi: 10.1111/j.1432-1033.1994.tb18556.x. [DOI] [PubMed] [Google Scholar]
- 19.Senorale-Pose M, Jacqueson A, Rougeon F, Rosinski-Chupin I. Acinar cells are target cells for androgens in mouse submandibular glands. J Histochem Cytochem. 1998;46:669–678. doi: 10.1177/002215549804600512. [DOI] [PubMed] [Google Scholar]
- 20.Tong Y, Tar M, Davelman F, Christ G, Melman A, Davies KP. Variable coding sequence protein A1 as a marker for erectile dysfunction. BJU Int. 2006;98:396–401. doi: 10.1111/j.1464-410X.2006.06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong Y, Tar M, Melman A, Davies K. The opiorphin gene (ProL1) and its homologues function in erectile physiology. BJU Int. 2008 doi: 10.1111/j.1464-410X.2008.07631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong Y, Tiplitsky SI, Tar M, Melman A, Davies KP. Transcription of G-Protein Coupled Receptors in Corporeal Smooth Muscle is Regulated by the Endogenous Neutral Endopeptidase Inhibitor Sialorphin. J Urol. 2008 doi: 10.1016/j.juro.2008.03.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol. 2007;52:54–70. doi: 10.1016/j.eururo.2007.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traish AM, Guay AT. Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidence. J Sex Med. 2006;3:382–404. doi: 10.1111/j.1743-6109.2006.00245.x. discussion 404-387. [DOI] [PubMed] [Google Scholar]
- 25.Traish AM, Park K, Dhir V, Kim NN, Moreland RB, Goldstein I. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology. 1999;140:1861–1868. doi: 10.1210/endo.140.4.6655. [DOI] [PubMed] [Google Scholar]
- 26.Werner ME, Zvara P, Meredith AL, Aldrich RW, Nelson MT. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J Physiol. 2005;567:545–556. doi: 10.1113/jphysiol.2005.093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabka AG, Mitchell GS, Behan M. Ageing and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol. 2005;563:557–568. doi: 10.1113/jphysiol.2004.077511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang XH, Morelli A, Luconi M, Vignozzi L, Filippi S, Marini M, Vannelli GB, Mancina R, Forti G, Maggi M. Testosterone regulates PDE5 expression and in vivo responsiveness to tadalafil in rat corpus cavernosum. Eur Urol. 2005;47:409–416. doi: 10.1016/j.eururo.2004.10.021. discussion 416. [DOI] [PubMed] [Google Scholar]


