Abstract
Objectives
To describe and define changes in the infant DP-gram during an age continuum from the preterm period through the first 6 mo of postnatal life. This information provides normative guidelines for audiologists or hearing screeners using DPOAEs to monitor infant hearing status.
Design
In this retrospective study, 2f1 − f2 DP-grams (DPOAE level × f2) were recorded with primary tones at 65/55 dB SPL, f2/f1 = 1.2, and f2 frequencies ranging from 1500 to 9000 Hz. Results from one ear of 290 healthy infants ranging in age from 31 wks postconceptional age to 6-mo-old were examined. Data were collected using both longitudinal design (repeated tests on the same infant over time) and cross-sectional methodology (a different group of subjects representing each age category). Subjects were divided into three groups according to age and experimental design. The effects of age and frequency on DPOAE level were analyzed in the three groups separately.
Results
The combined results from the three databases indicate that (1) DPOAE level increased for mid-frequencies throughout the preterm period, from 31 to 33 wks until the time period associated with term birth. This change was significant for 4500 and 6000 Hz; (2) DPOAE level decreased as f2 frequency increased. In many infants, a shallow trough was observed with peak amplitude at 1500 Hz, a reduction in response amplitude through 4500 Hz, and a second peak around 6000 Hz; (3) during the postnatal period from birth through 6 mo, DPOAE level did not change significantly as a function of age and the DP-gram was relatively flat across f2 frequency; and (4) infants showed mean DPOAE levels that were 4 to 12 dB higher than adult levels.
Conclusions
The results indicate a frequency-dependent increase in DPOAE level during the preterm period in human infants. After birth, there is little change in amplitude through 6 mo. The infant DPOAE remains larger than adult amplitude at all ages tested, as shown in other reports, well into childhood, suggesting continued changes in DPOAE level during the first decade of life. Recent research suggests that immaturities of the conductive pathway may account for infant–adult differences in DPOAE level; however, it is not yet clear whether other sources contribute.
Introduction
During the early 1990s, initial experiments were conducted to describe infant distortion product otoacoustic emissions (DPOAEs). This work followed earlier reports describing the basic properties and characteristics of the DPOAE in normal-hearing adults (Lonsbury-Martin, et al., 1990; Probst, et al., 1986). Many of these early studies described adult–infant age differences in the context of a “DP-Gram,” which is a plot of DPOAE level as a function of primary tone frequency. Results from these early studies generally found that human infants have 2f1 − f2 DPOAE levels that are on average between 2 and 10 dB higher than adult levels (Abdala, 1996; Bonfils, et al., 1992; Lafreniere, et al., 1991; Lasky, et al., 1992; Smurzynski, et al., 1993). These age differences vary with f2 frequency. Both Lafreniere et al. and Lasky reported that the average DPOAE level for newborns was higher than that of adults at low-to mid-frequencies but lesser or equal to adult levels from 4800 to 8000 Hz. Similarly, Abdala reported the largest age-related level difference between newborn and adult DPOAEs at f2 = 1500 Hz (7 dB), compared with 6000 Hz (3 dB). In early childhood, this pattern shifts and the greatest age differences are observed at higher frequencies. Prieve et al. (1997) found that infants younger than 1 yr had higher mean levels than all other pediatric age groups studied (1–3, 4–5, and 6–8 yr olds) in the 2000 to 3000 Hz range but by 4 to 8 yrs, children had higher DPOAE levels than adults at 6000 Hz only.
Newborn DP-grams have often been described as showing a “dip” (i.e., reduced levels) in the 3400 to 4000 Hz frequency region (Lafreniere, et al., 1991; Smurzynski, et al., 1993). Marco et al. (1995) noted two “peaks” of maximum response level, one at 2000 Hz and one at 5000 to 6000 Hz with a reduction in response level at mid to high frequencies. This trough in the DP-gram is observed in adults as well, but typically at slightly lower frequencies (Lasky, et al., 1992; Smurzynski, et al., 1993). The infant DP-gram appears to be generally flatter than adult DP-grams, showing less pronounced variation across f2 frequency (Lasky, et al., 1992).
Other aspects of the clinical DP-gram are unique to newborns as well (Note: the clinical DP-gram is considered a graph with a limited number of discrete f2 test frequencies, typically between 4 and 10, recorded in a clinical setting for the assessment of hearing. This is in contrast to a DP-gram recorded for research purposes at finely spaced frequency intervals). For example, the mean noise floor is generally higher in infants than adults, with mean noise levels elevated by 5 to 15 dB in the low-frequency range (Bonfils, et al., 1992; Lasky, 1998; Marco, et al., 1995). Additionally, although not well detailed, infant DPOAE level seems to be more variable than adult DPOAEs as test–retest reliability is reported to be “slightly reduced” in infants relative to adults (Lasky, 1998; Lasky, et al., 1992).
A few studies have investigated and reported differences between DPOAE level recorded from prematurely born infants tested during the preterm period and term-born infants (Abdala, 1996; Bergman, et al., 1995; Brown, et al., 2000; Smurzynski, 1994; Smurzynski, et al., 1993). Smurzynski et al. found that a third of their premature subjects had DPOAE levels above the 90th percentile of levels observed in term-born infants, for at least two f2 frequencies. Preterm DPOAE levels were highest at 2800 and 4000 Hz. Smurzynski also conducted a longitudinal study of the DP-gram (f2 = 850–8000 Hz) in seven prematurely born infants initially tested at 33 to 39 wks postconceptional age (PCA) and then once a week for at least three sessions. In general, DPOAE level increased with increasing PCA and changes were most prominent in the 2800 to 4000 Hz frequency range where 77% to 85% of preterm ears showed growth of DPOAE level over time. Similarly, Brown et al. recorded DP-grams in both preterm and term-born infants and found the magnitude of the emission was largest in full-term infants and prematurely born infants who had reached the equivalence of term, compared with prematurely born infants tested at 30 to 33 wks PCA.
Cumulatively, this early work indicates that there are differences between newborn and adult DP-grams, and additionally suggests there may be changes in DPOAE level during the preterm period, although the time course of these changes has not been fully specified. The age effects described vary somewhat among studies, most likely due to differences in the methodology, including definition of age categories, transducers and stimulus level, and frequency. Several of the studies had small numbers of subjects and many of the results were qualitative and descriptive in nature.
The purpose of this experiment is to quantify and describe changes in DPOAE level during an age continuum that spans the early preterm period through the first half-year of postnatal life. In doing so, we hope to provide audiologists who use the DP-gram as their main tool of assessment in the pediatric audiology clinic or hearing screening program, with a normative framework. The present study extends earlier work by combining both cross-sectional and longitudinal design in nearly 300 infants and by describing changes in the clinical DP-gram spanning an 8-mo period that includes both preterm and postnatal maturation.
Materials and Methods
Subjects
The subjects described in this retrospective study participated in various experiments in the first author’s laboratory during a 5-yr period. For the purposes of this report, DP-grams from one ear of 290 healthy infants and 48 normal hearing adults were separated into three databases based on common range of f2 frequencies, age categorization, and time frame during which tests were conducted. There was no subject overlap between groups and each of the 290 infants was only used in one experiment.
Infant subjects were born at Women’s and Children’s Hospital, Los Angeles County, University of Southern California Medical Center. All infants passed a click-evoked ABR (35 dB HL) and a DP-gram hearing screening at birth (DPOAE present with >5 dB SNR at four of five frequencies from 1500 to 6000 Hz). None of the infants included in this study had high-risk factors for hearing loss with the exception of prematurely born infants, some of whom had NICU stays of >48 hrs for observation (JCIH, 2000). Adult subjects ranged in age between 18 and 35 yrs (mean age = 26.8 yrs), had audiometric thresholds ≤15 dB HL for the frequencies from 500 to 8000 Hz and a negative history of otologic disease or noise exposure.
Group 1: Cross-sectional database
The first group included 261 infants. One hundred twenty-five were born prematurely and are categorized by PCA at test: P1 = 31–33 wks PCA, P2 = 34–36 wks PCA, and P3 = 37–41 wks PCA. PCA is defined as the gestational age at birth plus weeks between birth and test. One hundred eighteen infants were term-born and tested within 48 to 96 hrs after birth and 18 infants were term-born and tested at 1 mo (mean = 4.5 wks). Each subject was tested only once at a targeted age and a different group of infants comprised each age group; thus, age was treated as a cross-sectional variable. There was no overlap in age among groups. A breakdown of age categories is provided in Table 1.
TABLE 1.
Infant characteristics
| Group | Number | Test ages | Gender (F, M) | Ear (L, R) |
|---|---|---|---|---|
| Group 1 | ||||
| Cross-sectional | 35 | P1 = 31–33 wks PCA | 20, 15 | 13, 22 |
| 28 | P2 = 34–36 wks PCA | 13, 15 | 19, 9 | |
| 62 | P3 = 37–40 wks PCA | 35, 27 | 38, 24 | |
| 118 | Term | 48, 70 | 70, 48 | |
| 18 | 4.5 wks of age | 9, 9 | 7, 11 | |
| Group 2 | ||||
| Longitudinal preterm | 9 | 33–40 wks PCA (testing at weekly intervals) | 4, 5 | 6, 3 |
| Group 3 | ||||
| Longitudinal postnatal | 20 | Birth, 3, 4, 5, and 6 mo | 9, 11 | 11, 9 |
| Total | 290 | |||
Group 2: Preterm longitudinal database
For the second group of subjects, data were collected using longitudinal methodology. Nine prematurely born infants were tested repeatedly during a 6- to 8-wk period. They were tested initially at 31–33 wks PCA, and then at weekly intervals until they reached the equivalence of term status between 37 and 40 wks PCA. Four adults were tested weekly for 5 wks as a comparison and these data were used to assess normal test–retest reliability of DPOAE amplitude in adults.
Group 3: Postnatal longitudinal database
In the third data set, a longitudinal design was also used as 20 infants were tested repeatedly during a 6-mo period. Of the 20 infant subjects, 11 were term-born and tested initially within 72 hrs of birth and nine were born prematurely and tested initially once they reached the equivalence of term status (37–40 wks PCA). All infants were then tested again at 3, 4, 5, and 6 mos.
Instrumentation and Signal Analysis
All data collected for this study, with the exception of data in group 3 (Postnatal Longitudinal Database) were collected with the same custom-designed 16-bit D/A acquisition system used in the Infant Auditory Research Laboratory. Group 3 infant subjects comprise the most recently collected data recorded using an upgraded custom-designed 24-bit data acquisition system (SupprDP) developed at the House Ear Institute by the same systems programmer who developed the legacy system.
An Ariel DSP16+ signal processing and acquisition board housed within a Compaq Prolinea 590 personal computer with Pentium processor was used to generate stimuli and acquire data. The Ariel board was connected to an Etymotic Research ER-10C probe system and to an analog high-pass filter (12 dB/oct; 710 Hz high-pass cutoff). The ER-10C probe contains two output transducers and a low-noise microphone. The two primary tones were generated by the DSP board; the primary tone at f1 was generated by one D/A-converter and delivered via one transducer and the primary tone at f2 was produced by the second D/A-converter and output through the second transducer. The signal at the probe microphone was high-pass filtered and sampled at a rate of 50 kHz with a sweep length of 4096 samples, giving a frequency resolution of 12.2 Hz.
Twenty-five sweeps of the microphone signal were added and comprised one block for f2 = 3000 to 9000 Hz. Because of elevated noise in the low frequencies, 50 sweeps were added to make up one block at f2 frequencies <3000 Hz. Sweeps were accepted into a block only when the estimated root mean-squared level in that sweep did not exceed a user-controlled artifact rejection threshold. A minimum of six and a maximum of 12 acceptable blocks of data were averaged to compute the DPOAE grand average, that is, final DPOAE level, which met the following criteria: (1) the measured DPOAE level was at least 5 dB above the average noise in the six bins around 2f1 − f2 and (2) averaged noise measurements for the six frequency bins was not greater than 0 dB SPL. If the maximum number of blocks was collected (12 blocks or 240 sweeps) without the grand average meeting these criteria, no DPOAE response was collected and the program moved on to the next test condition.
The upgraded custom-designed DPOAE acquisition system (SupprDP) used a 48,000 Hz sampling rate. The data acquisition hardware was based on an Audio Processor developed by House Ear Institute Engineering Department including 2-channel D/A, 2-channel A/D, and a DSP processor (all 24-bit) as well as an analog high-pass filter (12 dB/oct.; 700 Hz high-pass cutoff). The A/D converter received the electrical output signal from the same probe used in the earlier system, the Etymotic Research ER-10C probe microphone. The microphone signal was high-pass filtered before being sampled by the A/D converter. Data acceptance criteria were the same for both systems and as described in the previous paragraph.
Calibration
Intermodulation distortion produced by the recording system at 2f1 − f2 was measured with the probe in a Zwislocki coupler for all test conditions. The mean level of distortion was −21 dB SPL for the legacy system and −25 dB SPL for SupprDP. The recording system noise floor was determined using a similar method with no tones present. The level of system noise floor ranged between −22 and −30 dB SPL (both systems). An in situ calibration procedure was conducted on both output transducers before each subject was tested. Individual frequencies were swept from 250 to 10,000 Hz in the older system. A chirp tone (swept-frequency signal from 10 to 10,000 Hz) with fixed voltage was presented to the transducer in the newer system. The resulting SPL was recorded in the ear canal and based on this information, an equalization of output levels was performed for each subject to achieve target stimulus levels across test frequencies.
Procedure
All procedures in this study were approved by the Institutional Review Boards of the University of Southern California Health Sciences and St. Vincent Medical Center. Following the acquisition of informed parental consent, infants were tested at Women’s and Children’s Hospital, Los Angeles County + USC Medical Center in a quiet room. They were swaddled and fed if necessary, then placed in a hospital isolette for testing. For the longitudinal studies, older infants were tested either in the isolette, in the parent’s arms or sleeping in an infant seat. If tested in the isolette, the infant was positioned either on his/her back or slightly angled onto their side with blankets for support at their back. The positioning was conducted to ensure optimal accessibility to the external auditory meatus for placement of the probe. When the probe was placed at the meatus snugly and the cable fixed to the isolette or the blankets with surgical tape to minimize movement, the test was initiated. If the probe tip slipped out minimally during testing (as noted by a 2–3 dB drop in stimulus and DPOAE levels), it was nudged back into place until appropriate levels were achieved and the test continued. If the probe tip came out of the ear completely during the recording of a DPOAE, it was refit and levels were recalibrated before continuing. Adult subjects were tested within an IAC sound attenuated booth at the House Ear Institute while sitting comfortably in a lounge chair.
The 2f1 − f2 DP-grams were generated as part of a larger research protocol conducted during various experiments in the Infant Auditory Research Laboratory (USC Medical Center) over 5 yrs. They were recorded with fixed primary tones of L1 = 65, L2 = 55 dB SPL, and a fixed f2/f1 ratio of 1.2. Mid-level primary tones were chosen because they have been found to be most diagnostic for hearing loss (Stover, et al., 1996). The f2 frequency was presented at 1500, 2000, 3000, 4500, 6000, and 8000 (or 9000 Hz).
Data Analysis
Alpha level was set at p = 0.05 for all analyses. When multiple analyses were conducted on the same database, a Bonferroni correction was applied to adjust the alpha level appropriately (0.05 divided by the number of comparisons).
Adult
Although this study focused on DPOAE changes during infancy, two separate analyses were conducted on adult data to provide a standard of the fully mature auditory system: (1) the effect of f2 frequency on adult DPOAE level was assessed with a one-way ANOVA and (2) test–retest reliability was assessed in four adult subjects who were tested at weekly intervals for 5 wks to characterize stability of DPOAE level in adults.
Infant
Group 1: Cross-Sectional Database
An omnibus two-way ANOVA (age × frequency) including 261 infant subjects was conducted to assess the effects of age and f2 frequency on DPOAE level. Post hoc one-way ANOVAs were conducted on age and frequency separately to further scrutinize effects.
Group 2: Preterm longitudinal database
Too few infant subjects comprised this data set to perform statistical analyses (N = 9); therefore, central tendencies were shown and data were qualitatively described. Infant DPOAE data at two discrete ages were graphically compared with adult (N = 25) data: (a) adult versus 33 wks PCA and (b) adult versus 38 wks PCA. This adult grouping comprised 25 adult subjects with common f2 frequencies who had been tested using the same DPOAE system (as used with these premature subjects), during the same time frame.
Group 3: Postnatal longitudinal database
One-way ANOVAs with repeated measures on f2 frequency were conducted at each age/test session separately (birth, 3, 4, 5, and 6 mos) to assess frequency effects on DPOAE level. One-way ANOVAs with repeated measures on age/test session were conducted at each f2 frequency to assess age effects on DPOAE level. Additionally, infant data collected at two ages were graphically compared with adult data (N = 10) as follows: (a) adult versus infant at birth and (b) adult versus infant at 6 mo. The 10 adults used for this comparison were selected because they were tested as part of the same experiment as the infants in this database (during the same time period), they shared common f2 frequencies and both were tested with an upgraded DPOAE data collection system (SupprDP).
RESULTS
Adults
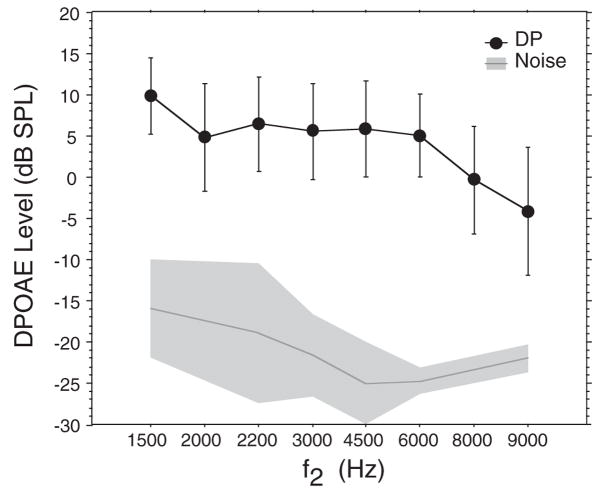
Adult DPOAEs are presented here only as a comparison with infant data and to provide the standard of a fully mature DP-gram. Adults (N = 48) showed decreasing DPOAE level as a function of frequency (Fig. 1; p < 0.001: note that Fig. 1 includes 2200 Hz because these data were available for adults only). Peak DPOAE level occurred at the lowest f2 frequency (1500 Hz) with a mean of 10.5 dB SPL. Mean DPOAE level plateaued and was relatively flat between 2000 and 6000 Hz, averaging 5 to 6 dB SPL, then dropped markedly at 8000 and 9000 Hz to levels near 0 dB SPL or into negative values. Forty-two percent of adult subjects showed a shallow trough (at least 3 dB) of reduced DPOAE level between 1500 and 4000 Hz with recovery at higher frequencies.
Fig. 1.
Mean DP-gram (DPOAE level × f2 frequency) for 48 adult subjects. The frequency axis in Figure 1 (and in figures throughout the manuscript) represents discrete values of f2 rather than a continuum of frequency. The spacing between these values is shown as equal although the actual frequency interval between them varies. Mean noise levels are indicated by the dark gray line. Error bars and shaded area of noise floor = ±1 SD of the mean.
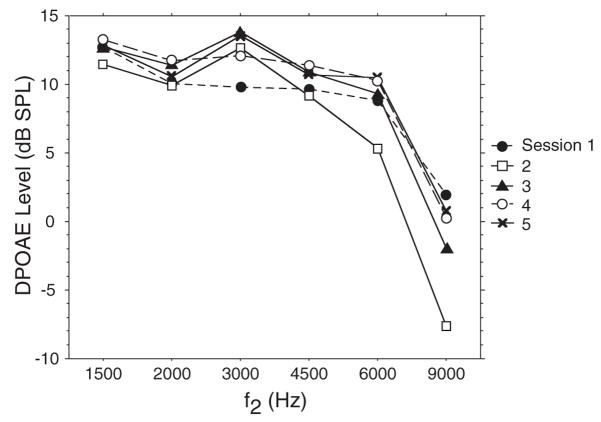
Test–retest reliability was assessed in four adult subjects tested weekly for five sessions. Measures of intrasubject variability were as follows: 1500 Hz = ±2.7 dB; 2000 Hz = ±2.5; 3000 Hz = ±2.7 dB; 4500 Hz = ±3.3 dB; 6000 Hz = ±2.2 dB (95th percentile confidence intervals). Figure 2 shows the DP-gram of one adult subject during the 5-wk test period. DPOAEs within an adult subject were repeatable over time and showed level variability that was less than between-subject variability as indicated by adult standard deviations, which ranged from ±4.6 (1500 Hz) to ±7.7 dB SPL (9000 Hz).
Fig. 2.
Five superimposed DP-grams measured in one adult subject tested once a week for 5 wks.
Infants
General
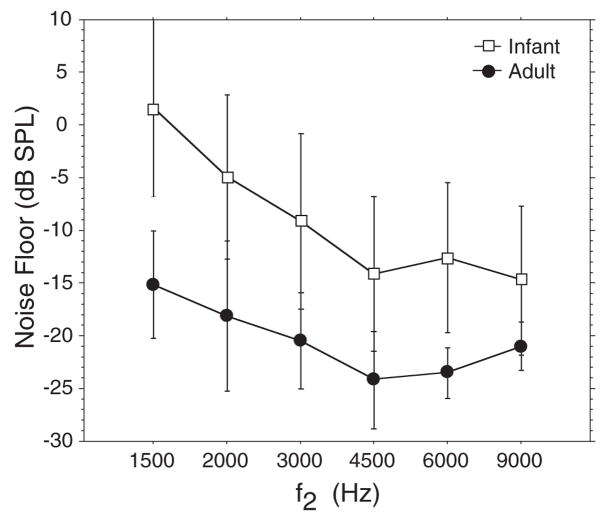
Although the minimal acceptable signal to noise ratio (SNR) was 5 dB, the actual SNRs were much higher in most cases. If considering all f2 frequencies presented, 85% of the DPOAE level values from infants showed >10 dB SNR. If the lowest two f2 frequencies (1500 and 2000 Hz) are eliminated, just over 90% of all infant DPOAE level values had SNR >10 dB. The mean noise floor was between 10 and 17 dB higher in infants than adults (Fig. 3) and ranged from 1.7 dB to −14.7 dB.
Fig. 3.
Mean noise levels measured in 261 infant and 48 adult subjects. Error bars = ±1 SD of the mean.
Group 1: Cross-sectional database
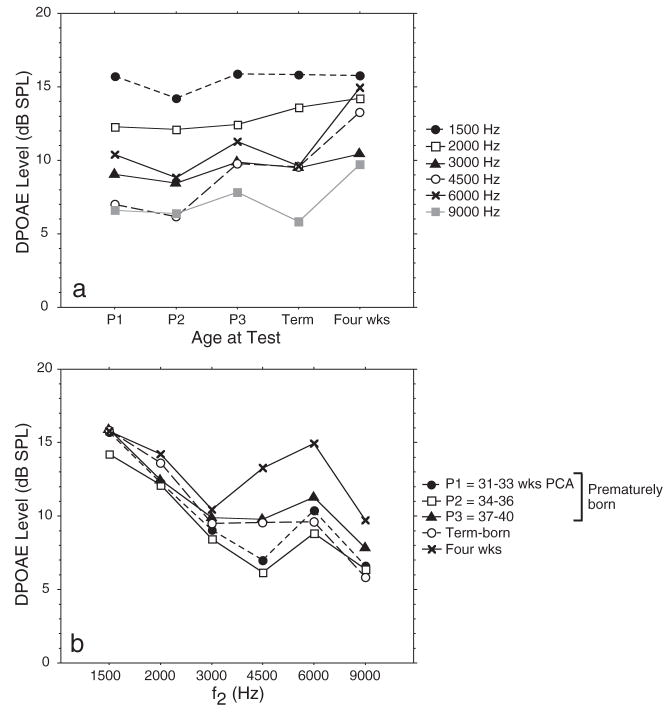
Table 2 presents mean DPOAE level and variability for subjects in this database. DPOAEs recorded in infants ranging from 31–33 wks PCA to 1 mo generally increased in level as infant age increased (Fig. 4a), and decreased in level as f2 frequency increased (Fig. 4b). Infants (N = 261) showed main effects for both age (p < 0.041) and frequency (p < 0.001) on DPOAE level and an interaction between age and frequency (p < 0.001). Post hoc analyses were conducted of age effects at each frequency separately and showed significant effects of age on DPOAE level in the mid-to high-frequency range (4500 Hz: p < 0.001 and 6000 Hz: p = 0.002). At the youngest PCAs (P1, P2), DPOAE level was lowest, increasing throughout the preterm period and showing peak level around 1 mo after term birth. Post hoc analyses conducted at each age separately to assess frequency effects, confirmed that DPOAE level decreased as f2 frequency increased for all ages except the oldest infants, as shown in Figure 4b (P1, P2, P3, and term: p < 0.001 for all groups).
TABLE 2.
Mean DPOAE level (dB SPL) and SD for cross-sectional database
| F2(Hz) | P1 | P2 | P3 | Term | 4.5 wks | Adult |
|---|---|---|---|---|---|---|
| 1500 | ||||||
| Mean | 15.7 | 14.2 | 15.9 | 15.8 | 15.8 | 9.9 |
| SD | 6.7 | 6.7 | 4.1 | 5.8 | 7.1 | 4.6 |
| 2000 | ||||||
| Mean | 12.3 | 12.1 | 12.3 | 13.6 | 14.2 | 6.0 |
| SD | 6.1 | 5.7 | 5.6 | 5.5 | 5.6 | 6.0 |
| 3000 | ||||||
| Mean | 9.1 | 8.4 | 9.9 | 9.5 | 10.5 | 5.6 |
| SD | 6.2 | 5.9 | 5.9 | 5.9 | 9.1 | 5.8 |
| 4500 | ||||||
| Mean | 7.0 | 6.2 | 9.8 | 9.5 | 13.3 | 5.9 |
| SD | 8.0 | 7.5 | 5.8 | 6.4 | 6.7 | 5.9 |
| 6000 | ||||||
| Mean | 10.4 | 8.8 | 11.3 | 9.6 | 15.0 | 5.1 |
| SD | 4.6 | 7.4 | 5.5 | 5.8 | 6.4 | 5.0 |
| 8000 | ||||||
| Mean | — | — | — | 5.8 | 9.7 | −0.3 |
| SD | — | — | — | 6.8 | 7.5 | 6.5 |
| 9000 | ||||||
| Mean | 6.6 | 6.4 | 7.9 | 5.9 | — | −4.1 |
| SD | 7.1 | 7.4 | 6.8 | 6.6 | — | 7.7 |
P1, 31–33 wks PCA; P2, 34–36 wks PCA; P3, 37–40 wks PCA.
Fig. 4.
(a) Mean DPOAE level as a function of age for 261 infants comprising group 1: cross-sectional database; (b) DP-gram (DPOAE level × f2) for the same 261 infants in five age categories. SDs were not included in these figures because their presence makes it difficult to view and interpret the data. However, all means and SDs are provided in Table 2.
An additional analysis was conducted between the two infant groups that had been tested at the same PCA, but differed only in terms of their maturational status at birth. Both P3 and term-born infant groups were tested between 37 and 40 wks PCA, but P3 infants had been born prematurely. A two-way ANOVA (age × frequency) with repeated measures on frequency was conducted to test whether premature birth affected the DP-gram. Results indicate that both infant groups had comparable DP-grams. Neither an age nor age × frequency interaction was noted. This finding suggests that a premature birth, alone, does not alter DPOAE level across frequency and PCA at test is the relevant variable determining response level.
Group 2: Preterm longitudinal database
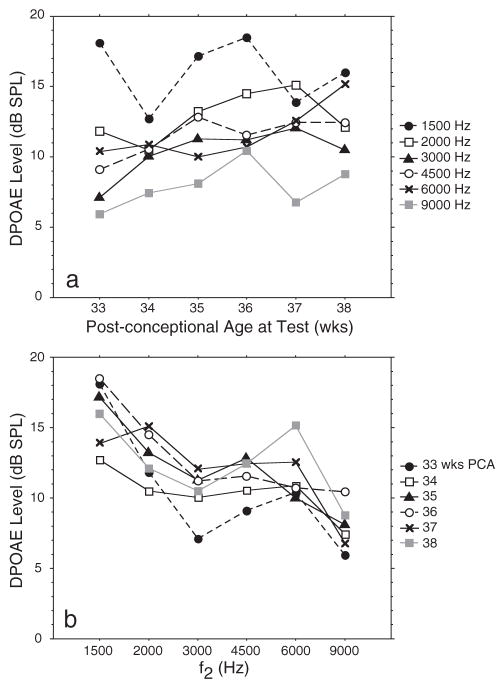
The first six columns of Table 3 include the mean DPOAE values and standard deviations from subjects in group 2. Mean DPOAE data from nine preterm infants tested at weekly intervals from 31–33 wks PCA through term equivalence (38–40 wks PCA) are shown in Figure 5a. Except for 1500 Hz, DPOAEs show a trend for increasing response level with increasing age from 33 through 38 wks PCA. This is consistent with age effects detected in group 1 (cross-sectional database) that showed increasing DPOAE level from 31–33 wks PCA through 1 mo in the mid to high frequency range. Figure 5b shows DPOAE level for the same group of infants as a function of frequency, with age as the parameter. DPOAE level was highest at 1500 Hz (mean levels = 12–18.5 dB SPL), showed a shallow trough between 2000 and 4500 Hz (mean levels = 7–15 dB SPL) then peaked again at 6000 Hz. DPOAE level decreased for all ages at 9000 Hz.
TABLE 3.
Mean DPOAE level (dB SPL) and SD for preterm and postnatal longitudinal databases
| Preterm database |
Postnatal database |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F2(Hz) | 33 wks PCA | 34 wks PCA | 35 wks PCA | 36 wks PCA | 37 wks PCA | Term* (38–40 wks) | 3 mo | 4 mo | 5 mo | 6 mo |
| 1500 | ||||||||||
| Mean | 18.1 | 12.7 | 17.2 | 18.5 | 13.9 | 13.8 | 11.4 | 13.3 | 11.8 | 11.5 |
| SD | 7.5 | 0.9 | 4.9 | 4.7 | 2.2 | 5.7 | 9.3 | 5.2 | 6.3 | 6.9 |
| 2000 | ||||||||||
| Mean | 11.8 | 10.5 | 13.2 | 14.5 | 15.1 | 11.5 | 11.9 | 11.8 | 10.5 | 11.4 |
| SD | 6.1 | 3.5 | 4.1 | 2.1 | 5.8 | 5.3 | 7.2 | 5.5 | 5.9 | 6.7 |
| 3000 | ||||||||||
| Mean | 7.1 | 10.0 | 11.3 | 11.2 | 12.1 | 9.2 | 10.3 | 8.5 | 11.1 | 10.1 |
| SD | 4.9 | 6.1 | 6.1 | 5.0 | 4.8 | 4.0 | 5.6 | 6.3 | 4.3 | 4.3 |
| 4500 | ||||||||||
| Mean | 9.1 | 10.6 | 12.9 | 11.6 | 12.4 | 10.1 | 13.9 | 10.4 | 13.6 | 10.8 |
| SD | 5.0 | 5.7 | 7.1 | 5.2 | 3.7 | 4.9 | 5.1 | 8.4 | 3.7 | 7.7 |
| 6000 | ||||||||||
| Mean | 10.4 | 10.9 | 10.0 | 10.7 | 12.6 | 12.8 | 10.4 | 10.2 | 12.0 | 9.9 |
| SD | 5.8 | 5.6 | 6.9 | 6.4 | 5.5 | 5.1 | 7.4 | 6.9 | 4.3 | 7.6 |
| 8000 | ||||||||||
| Mean | — | — | — | — | — | 8.2 | 9.8 | 10.0 | 11.2 | 9.4 |
| SD | — | — | — | — | — | 4.4 | 9.6 | 4.5 | 5.3 | 6.7 |
| 9000 | ||||||||||
| Mean | 5.9 | 7.4 | 8.1 | 10.4 | 6.8 | 10.1 | — | — | — | — |
| SD | 5.5 | 6.1 | 5.5 | 5.0 | 5.6 | 8.1 | — | — | — | — |
Combined data from both databases and includes between 12 and 33 observations at each F2.
Fig. 5.
(a) Mean DPOAE level as a function of six postconceptional ages at test for nine infants comprising group 2: premature longitudinal database. The f2 frequency is the parameter. These infants were initially tested between 31 and 33 wks PCA and then weekly for between 6 and 8 sessions; (b) mean DP-gram (DPOAE level × f2) for the same nine infants with postconceptional age as the parameter. See Table 3 for SDs of the mean.
Infant–adult comparisons show that at the youngest age tested, 33 wks PCA, infant DPOAE levels overlap with adult levels in the mid to high frequency range (3000–6000 Hz) (Fig. 6). Adult-like Infant DPOAEs in this frequency range are consistent with group 1 results showing that DPOAE level has not yet peaked early in the preterm period. Once these same infants reached 38 wks PCA, they showed higher DPOAE levels than adults at all f2 frequencies (Fig. 6). These combined findings suggest a general increase in DPOAE level (in the mid to high frequency range) with increasing PCA during the preterm period and are consistent with the statistical analyses of group 1 data.
Fig. 6.
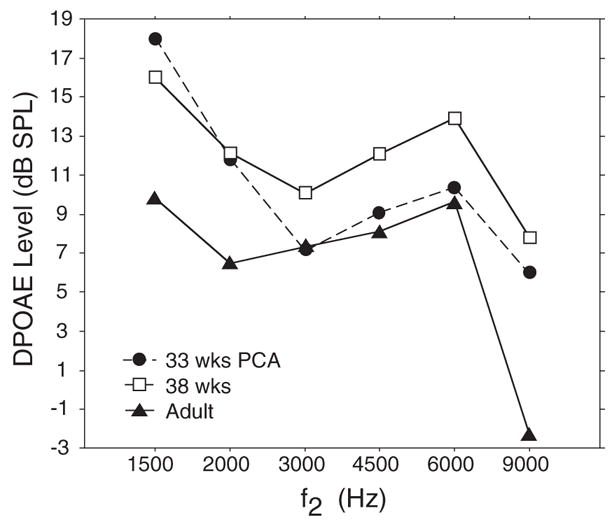
Mean DP-gram (DPOAE level × f2) for preterm infants (N = 9) at two ages: 33 and 38 wks PCA, and for a group of adults (N = 25).
Group 3: Postnatal longitudinal database
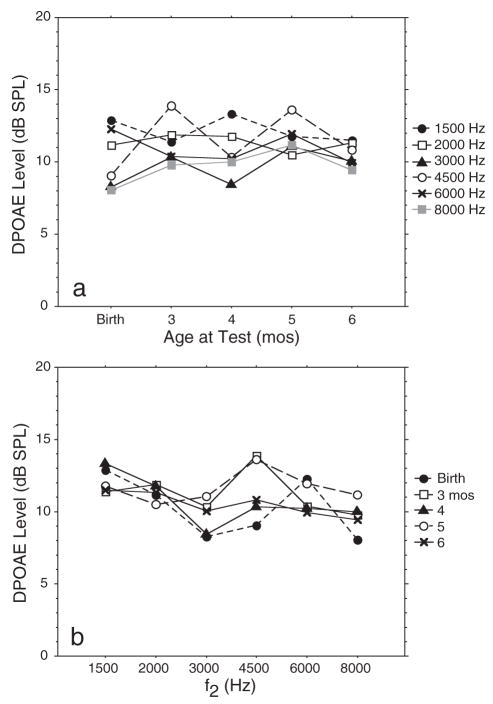
The last five columns of data in Table 3 present the means and standard deviations for this data set. There were no effects of age on DPOAE level at any f2 frequency (1500–8000 Hz) in the postnatal period. Thus, response level did not change significantly from birth through the first 6 postnatal months (Fig. 7a). There was a significant effect of frequency on DPOAE level only at birth (p = 0.016) and at 4 mo (p = 0.015; Fig. 7b). This was due to the typical trough configuration previously described.
Fig. 7.
(a) Mean DPOAE level as a function of age for 20 infants comprising group 3: postnatal longitudinal database. Infants were tested initially at birth (or equivalence) then repeatedly at 3, 4, 5, and 6 mos. The f2 frequency is the parameter; (b) mean DP-gram (DPOAE level × f2) for the same 20 infants with age as the parameter. See Table 3 for SDs of the mean.
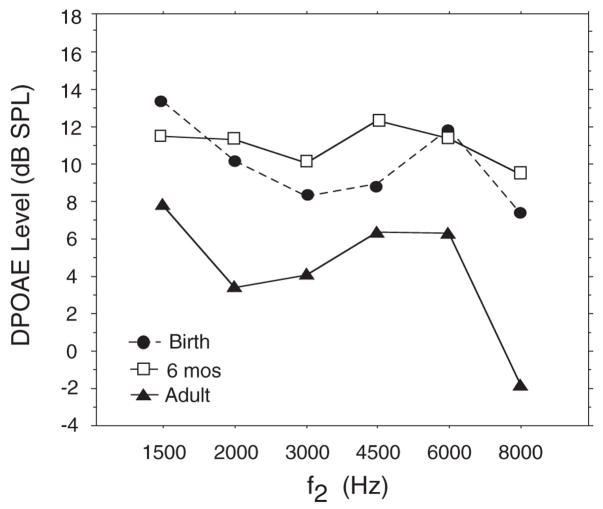
Infant–adult comparisons indicate that at both birth and 6 mos, DPOAE level was larger in infants than adults (Fig. 8). Infant mean values ranged from 7.2 to 13 dB SPL across frequency whereas adult mean values ranged from −1 to 7.7 dB SPL.
Fig. 8.
Mean DP-gram (DPOAE level × f2) for postnatal infants (N = 20) at two ages: birth and 6 mos and for a group of adults (N = 10).
Summary of Results
In general, DPOAE response level increases in infants throughout the preterm period from 31–33 wks PCA through the first month after birth, as per both cross-sectional and longitudinal experiments. After birth, during the postnatal period, DPOAE level does not change significantly with age through the first 6 postnatal months. Even so, infant DPOAE level is higher than the adult response at all ages.
In addition to the age effects, during the preterm period DPOAE level is reduced as f2 frequency increases. A typical DP-gram configuration shows peak level at 1500 Hz, a steady decrease from 1500 through 4500 Hz, a second peak between 4500 and 6000 Hz, followed by a marked decline at higher f2 frequencies. In the postterm period, DPOAE level did not vary significantly among f2 frequencies at most ages and the DP-grams appeared relatively flat.
Discussion
The clinical “DP-Gram,” defined as DPOAE level recorded as a function between 4 and 10 audiometrically relevant primary tone frequencies, has been applied successfully to the assessment of hearing status for many years. DPOAE level shows a moderate association with audiometric threshold (Martin, et al., 1990; Gorga, et al., 1993, 1997). In general, DPOAEs measured from ears with hearing loss tend to be smaller than DPOAEs recorded in ears with normal audiometric thresholds, which produce relatively robust responses (Gorga, et al., 1997). The DPOAE has proven useful in separating hearing-impaired from normal-hearing ears particularly in the mid to high frequency ranges, with relatively poor performance at low and very high frequencies (Gorga, et al., 1997). Diagnostic accuracy of DPOAEs in categorizing ears as normal hearing or hearing impaired is optimal when using mid-level primary tones. Including additional levels or recording an entire DPOAE input/output function does not seem to improve diagnostic precision (Stover, et al., 1996). Thus, DP-grams recorded at 65–55 dB SPL, the levels used in the present study, have become the conventional DPOAE tool of choice in many audiology clinics.
Using the DPOAE Age Continuum as a Normative Guide
The present study provides a guideline for audiologists recording DP-grams from infant patients as part of a hearing assessment battery, hearing screening program, or to monitor changes in cochlear status throughout an ototoxic regimen, for example. The results describe a continuum of normal, expected changes in DPOAE level from early in the preterm period through the first postnatal months. Consistent with previous work (Brown, 2000; Smurzynski, 1994), results suggest that a young prematurely born infant in the NICU might show baseline DPOAE levels (recorded at 31–33 wks PCA) that are lower by 3 to 5 dB than those observed even 1 mo later. The increase in response level during these 4 to 6 wks will be most apparent in the mid to high frequency ranges (3000–6000 Hz). At frequencies either lower than 3000 Hz or higher than 6000 Hz, DPOAE levels at 33 wks seem to be similar to levels recorded from term-born infants. Thus, DPOAEs from very young prematurely born infants have not yet peaked in response level in the mid to high frequency range, the very range that has shown to be most diagnostic for hearing loss (Gorga, et al., 1997). If infants of this age are being monitored for hearing status because they are receiving ototoxic medication, knowing of this natural, age-related increase in DPOAE level should help a clinician assess normal maturation versus medication-related changes that might prompt additional testing.
Once an infant reaches the equivalence of term status or a month beyond, DPOAE levels have peaked and an audiologist might expect little change in response level over the next 6 mo of life. Again, if an infant is being tracked for progressive hearing loss, marked deviations from this pattern, such as diminishing level during the postnatal months may raise a red flag and suggest the need for more detailed diagnostic tests to rule out deterioration in cochlear function or middle ear pathology. In infants, it is not clearly established what constitutes a “marked deviation” from test to test because detailed test–retest stability figures are not available. Our repeated measures on infant subjects cannot be used to generate estimates of intrasubject variability, because DPOAE changes due to maturation preclude this. The range of DPOAE level test–retest variability for adults presented here may help guide the decision of what is acceptable, normal intrasubject variability in infants.
In regards to DP-gram configuration, our results suggest that most preterm patients will have peak DPOAE level in the low frequencies, around 1500 Hz, decreasing (“dip” or trough) through the mid-frequency region and a second peak at around 5000 to 6000 Hz. A similar pattern of infant DPOAE level across f2 frequency has been reported in previous work as well (Lafreniere, et al., 1991; Lasky, et al., 1998; Smurzynski, et al., 1993) and thus, should be considered normal, rather than indicative of hearing loss at the trough frequencies. This pattern of DPOAE level variation across frequency during the preterm period is in contrast to the relatively flat configuration noted during the postnatal period, and in contrast to an adult configuration that shows decreasing level with increasing frequency. An atypical configuration across f2 frequency when testing an infant might prompt an audiologist to refit the probe, retest or perform an additional auditory evaluation to further assess hearing.
The Preterm Period
Consistent with previous work (Brown, et al., 2000; Gorga, et al., 2000; Lasky, 1998; Smurzynski, 1994), DPOAE level increased during the preterm period as a function of PCA. Although it is likely that DPOAE level increases somewhat as residual debris in the ear canal and fluid in the conductive pathway is reabsorbed following the birth process, this is an insufficient explanation. The increase in DPOAE level with age was only observed in subjects tested during the preterm period. Infants born after a term-birth and tested repeatedly through 6 mo did not show any significant effect of age on DPOAE level. Thus, aeration of the conductive auditory pathway following birth does not provide an adequate explanation. It is possible that outer and middle ear maturation is taking place during the developmental period when increases in DPOAE level are observed. Anatomical immaturities in the outer and middle ear and corresponding functional changes have been documented in human infants (Anson & Donaldson, 1981; Keefe, et al., 1993, 1994; Saunders, et al., 1983) but not specifically examined during the preterm period. Thus, the extent of outer and middle ear changes from 31–33 wks through the equivalence of term has not been documented.
It is also possible that subtle cochlear immaturities remain during the preterm period producing the change in DPOAE level. However, experiments applying DPOAE suppression techniques in infants tested from 33 to 40 wks PCA have noted minimal change in suppression tuning during this developmental period (Abdala, 1998; Abdala, et al., 1996). Another possible source of the level changes in prematurely born infants is the medial olivocochlear (MOC) system and its influence on cochlear function. Research has shown that functional immaturities exist in the MOC system during the preterm period and through term birth (Abdala, et al., 1999; Lasky, 1998; Morlet, et al., 1993). It is possible that MOC immaturities and the subsequent maturational process influence OAE generation and specifically, DPOAE level. At present, the developmental processes producing an increase in DPOAE level during the preterm period are not known, but likely reflect a combination of the aforementioned factors.
Adult–Infant Differences
Infant subjects, at all ages tested, had higher DPOAE level than adults. These infant–adult age differences might initially be interpreted as cochlear in nature, because the DPOAE is a cochlear-based response; however, there may be a more parsimonious explanation. Studies of human cochlear maturation using postmortem fetal tissue have shown that cochlear membranes and sensory cells are morphologically mature very early in gestation, sometime around 14 to 21 fetal weeks (Lavigne-Rebillard & Pujol, 1987, 1988). This makes it unlikely that functional immaturities in the cochlea exist beyond 6 postnatal months. In contrast, outer and middle ear maturation extends at least into early preadolescence (Okabe, 1988). The DPOAE, although it reflects integrity of the outer hair cells, is highly dependent on integrity of the conductive pathway, as are all OAEs. Primary tones evoking the DPOAE must travel this pathway to stimulate a response. Conversely, the DPOAE must travel from its points of origin, during reverse transmission, through the conductive pathway and into the ear canal for measurement. Any immaturity in outer and middle ear transmission will, thus, influence the measurement of DPOAEs in the ear canal.
Recent experiments (Abdala & Keefe, 2006; Keefe & Abdala, 2007) strongly suggest that immaturities in transmission through the infant outer and middle ear are present beyond 6 mo. The newborn middle ear attenuates the stimulus level during forward transmission to the cochlea by an average of 15 dB while, more relevant to the current experiment, the reduced ear canal area in infants boosts DPOAE level during reverse transmission from cochlear origin to microphone. Keefe and Abdala found that amplification of the DPOAE during reverse transmission varied with ear canal area and estimated that reverse transmittance level was on average 7 dB higher in newborns. This factor, along with other reflectance-related effects, may account for the higher DPOAE level noted in infants and children. These estimates and predictions are all based on data from one very limited set of f1, f2 primary tones (5000 and 6000 Hz) and one DPOAE frequency (4000 Hz); therefore, it is not possible to predict the impact of ear canal area at all frequencies. Further work is needed to define the impact of outer and middle ear immaturities on DPOAE level throughout the audiometric frequency range.
Although this recent research clearly suggests that characteristics of the immature conductive pathway can account for some of the adult–infant level differences observed, we cannot rule out contributions from other processes or segments of the auditory system that also show a postnatal developmental time course. We are currently investigating various potential sources, such as immaturity of the MOC system and its regulatory effect on cochlear function, and immaturity of DPOAE fine structure that reflects the relative contribution of multiple DPOAE components to the infant DPOAE (Dhar & Abdala, 2007).
In summary, these systematic and significant changes in DPOAE level and DP-gram configuration during the preterm period can be used to guide clinical expectations. Decisions about hearing status and changes in hearing status are made regularly by pediatric audiologists and hearing screeners based on OAE results although a paucity of information is available to guide decision-making with preterm infants. One of the principal objectives of this study was to provide firm guidelines for the clinician who evaluates, monitors, tracks, or screens infants using DPOAE methodology during the preterm and early postnatal period. Although various publications have provided fragments of this picture, the current study sought to present a cohesive continuum of change in a large cross-sectional group of infants and in smaller groups of infants tested longitudinally. The changes in DPOAE level during the preterm period and the persistent infant–adult differences in DPOAE level reported consistently in the literature, may be due to cochlear or non-cochlear factors or a combination; regardless, they provide a normative framework against which to compare neonatal DP-grams and enhance accuracy of clinical decision-making in our youngest patients.
Acknowledgments
The authors thank Dr. Ellen Ma and Leslie Visser-Dumont for collection and management of infant data.
This research was supported by the National Institutes of Health, NIDCD R01 DC003552 and the House Ear Institute.
References
- Abdala C. Distortion product otoacoustic emission (2f1 − f2) amplitude as a function of f2/f1 frequency ratio and primary tone level separation in human adults and neonates. J Acoust Soc Am. 1996;100:3726–3740. doi: 10.1121/1.417234. [DOI] [PubMed] [Google Scholar]
- Abdala C. A developmental study of DPOAE (2f1 − f2) suppression in humans. Hear Res. 1998;121:125–138. doi: 10.1016/s0378-5955(98)00073-2. [DOI] [PubMed] [Google Scholar]
- Abdala C, Keefe DH. Effects of middle-ear immaturity on distortion product otoacoustic emission suppression tuning in infant ears. J Acoust Soc Am. 2006;120:3832–3842. doi: 10.1121/1.2359237. [DOI] [PubMed] [Google Scholar]
- Abdala C, Ma E, Sininger Y. Maturation of medial efferent system function in humans. J Acoust Soc Am. 1999;105:2392–2402. doi: 10.1121/1.426844. [DOI] [PubMed] [Google Scholar]
- Abdala C, Sininger Y, Ekelid M, et al. Distortion product otoacoustic emission suppression tuning curves in human adults and neonates. Hear Res. 1996;98:38–53. doi: 10.1016/0378-5955(96)00056-1. [DOI] [PubMed] [Google Scholar]
- Anson B, Donaldson J. Surgical anatomy of the temporal bone and ear. Philadelphia: Saunders; 1981. [Google Scholar]
- Bergman BM, Gorga MP, Neely ST, et al. Preliminary descriptions of transient-evoked and distortion-product otoacoustic emissions from graduates of an intensive care nursery. J Am Acad Audiol. 1995;6:150–162. [PubMed] [Google Scholar]
- Bonfils P, Avan P, Francois M, et al. Distortion-product otoacoustic emissions in neonates: normative data. Acta Otolaryngol (Stockh) 1992;112:739–744. doi: 10.3109/00016489209137468. [DOI] [PubMed] [Google Scholar]
- Brown DK, Bowman DM, Kimberley BP. The effects of maturation and stimulus parameters on the optimal f2/f1 ratio of the 2f1 − f2 distortion product otoacoustic emission in neonates. Hear Res. 2000;145:17–24. doi: 10.1016/s0378-5955(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Dhar S, Abdala C. A preliminary study of DPOAE fine structure in human infants, adolescents and young adults. J Acoust Soc Am. 2007;122:2191–2202. doi: 10.1121/1.2770544. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Bergman B, et al. Otoacoustic emissions from normal-hearing and hearing-impaired subjects: distortion product responses. J Acoust Soc Am. 1993;93:2050–2060. doi: 10.1121/1.406691. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Ohlrich B, et al. From laboratory to clinic: a large-scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear. 1997;18:440–455. doi: 10.1097/00003446-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Norton SJ, Sininger YS, et al. Identification of neonatal hearing impairment: distortion product otoacoustic emissions during the perinatal period. Ear Hear. 2000;21:400–424. doi: 10.1097/00003446-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Joint Committee on Infant Hearing. Joint Committee on Infant Hearing Year 2000 Position Statement. Principles and guidelines for early hearing detection and intervention programs. Audiol Today. 2000 August;12:6–27. [PubMed] [Google Scholar]
- Keefe DH, Abdala C. Theory of forward and reverse middle ear transmission applied to otoacoustic emissions in infant and adult ears. J Acoust Soc Am. 2007;121:978–993. doi: 10.1121/1.2427128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Bulen JC, Hoberg Arehart K, et al. Ear-canal impedance and reflection coefficient of human infants and adults. J Acoust Soc Am. 1993;94:2617–2638. doi: 10.1121/1.407347. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Campbell S, Burns EM. Pressure transfer function and absorption cross-section from the diffuse field to the human infant ear canal. J Acoust Soc Am. 1994;95:355–371. doi: 10.1121/1.408380. [DOI] [PubMed] [Google Scholar]
- Lafreniere D, Jung MD, Smurzynski J, et al. Distortion-product and click-evoked otoacoustic emissions in healthy newborns. Arch Otolaryngol Head Neck Surg. 1991;117:1382–1389. doi: 10.1001/archotol.1991.01870240074012. [DOI] [PubMed] [Google Scholar]
- Lasky R. Distortion product otoacoustic emissions in human newborns and adults: I. Frequency effects. J Acoust Soc Am. 1998;103:981–991. doi: 10.1121/1.421215. [DOI] [PubMed] [Google Scholar]
- Lasky R, Perlman J, Hecox K. Distortion-product otoacoustic emissions in human newborns and adults. Ear Hear. 1992;13:430–441. doi: 10.1097/00003446-199212000-00009. [DOI] [PubMed] [Google Scholar]
- Lavigne-Rebillard M, Pujol R. Surface aspects of the developing human organ of Corti. Acta Otolaryngol Suppl. 1987;436:43–50. doi: 10.3109/00016488709124975. [DOI] [PubMed] [Google Scholar]
- Lavigne-Rebillard M, Pujol R. Hair cell innervation in the fetal human cochlea. Acta Otolaryngol. 1988;105:398–402. doi: 10.3109/00016488809119492. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Harris FP, Stagner BB, et al. Distortion product emissions in humans. I. Basic properties in normally hearing subjects. Ann Otol Rhinol Laryngol Suppl. 1990;147:3–13. [PubMed] [Google Scholar]
- Marco J, Morant A, Caballero J, et al. Distortion product otoacoustic emissions in healthy newborns: normative data. Acta Otolaryngol (Stockh) 1995;115:187–189. doi: 10.3109/00016489509139288. [DOI] [PubMed] [Google Scholar]
- Martin GK, Ohlms IA, Franklin DJ, et al. Distortion product emissions in humans. III. Influence of sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1990;99:30–42. [PubMed] [Google Scholar]
- Morlet T, Collet L, Salle B, et al. Functional maturation of cochlear active mechanisms and of the medial olivocochlear system in humans. Acta Otolaryngol. 1993;113:271–277. doi: 10.3109/00016489309135808. [DOI] [PubMed] [Google Scholar]
- Okabe K, Tanaka S, Hamada H, et al. Acoustic impedance measurement on normal ears of children. J Acoust Soc Japan. 1988;9:287–294. [Google Scholar]
- Prieve BA, Fitzgerald TS, Schulte LE, et al. Basic characteristics of distortion product otoacoustic emissions in infants and children. J Acoust Soc Am. 1997;102:2871–2879. doi: 10.1121/1.420342. [DOI] [PubMed] [Google Scholar]
- Probst R, Lonsbury-Martin BL, Martin GK, et al. Otoacoustic emissions in ears with hearing loss. Am J Otolaryngol. 1986;8:73–81. doi: 10.1016/s0196-0709(87)80027-3. [DOI] [PubMed] [Google Scholar]
- Saunders J, Kaltenbach J, Relkin E. The structural and functional development of the outer and middle ear. In: Romand R, Romand M, editors. Development of auditory and vestibular systems. New York: Academic Press; 1983. pp. 3–25. [Google Scholar]
- Smurzynski J. Longitudinal measurements of distortion-product and click-evoked otoacoustic emissions of preterm infants: preliminary results. Ear Hear. 1994;15:210–223. doi: 10.1097/00003446-199406000-00002. [DOI] [PubMed] [Google Scholar]
- Smurzynski J, Jung MD, Lafreniere D, et al. Distortion-product and click-evoked otoacoustic emissions of preterm and full-term infants. Ear Hear. 1993;14:258–274. doi: 10.1097/00003446-199308000-00005. [DOI] [PubMed] [Google Scholar]
- Stover L, Gorga M, Neely S, et al. Toward optimizing the clinical utility of distortion product otoacoustic emission measurements. J Acoust Soc Am. 1996;100:956–967. doi: 10.1121/1.416207. [DOI] [PubMed] [Google Scholar]