Abstract
Fast forming hydrogels prepared by crosslinking a poly(ethylene glycol) (PEG)-based copolymer containing multiple thiol (SH) groups were evaluated for the controlled ocular delivery of pilocarpine and subsequent pupillary constriction. Physical properties of the hydrogels were characterized using UV-Vis spectrophotometry, transmission electron microscopy (TEM), rheometry, and swelling kinetics. Pilocarpine loading efficiency and release properties were measured in simulated tear fluid. The hydrogel formulations exhibited high drug loading efficiency (~74%). Pilocarpine release was found to be biphasic with release half times of ~2 and 94 h, respectively, and 85–100% of the drug was released over 8-days. Pilocarpine-loaded (2% w/v) hydrogels were evaluated in a rabbit model and compared to a similar dose of drug in aqueous solution. The hydrogels were retained in the eye for the entire period of the study with no observed irritation. Pilocarpine-loaded hydrogels sustained pupillary constriction for 24 h after administration as compared to 3 h for the solution, an 8-fold increase in duration of action. A strong correlation between pilocarpine release and pupillary response was observed. In conclusion, the current studies demonstrate that in situ forming PEG hydrogels possess the viscoelastic, retention, and sustained delivery properties required for an efficient ocular drug delivery system.
Keywords: Hydrogel, Ocular drug delivery, Pilocarpine, Poly(ethylene glycol), Pupil diameter, Sustained release
Introduction
Controlled drug delivery to the eye remains a challenging task due to normal ocular protective mechanisms such as blinking and tear drainage that promote rapid clearance and reduced bioavailability resulting in a short duration of pharmacological response [1]. The residence time of most conventional ocular solutions ranges between 5–25 minutes [2, 3]. Only 1–10% of topically applied drug is absorbed [4], which also includes absorption from the gastrointestinal tract due to drainage through the nasal-lacrimal duct [5]. As a result, a frequent dosing regimen is typically necessary to achieve therapeutic efficacy [6]. However, frequent instillation of eye drops results in local side effects such as headaches due to ciliary muscle spasm, decreased vision in poor illumination due to miosis, and accommodative myopia [7]. Ocular delivery systems with prolonged ocular residence time such as ointments and suspensions have been developed to overcome these challenges and increase ocular drug bioavailability [8, 9]. There are, however, several limitations that reduce patient compliance. For example, ointments are greasy and produce blurred vision [10, 11]. Similarly, non-erodible inserts such as Ocusert also have limitations. These are due, in part, to the fact that most glaucoma patients are geriatric and find weekly insertion and removal of Ocusert difficult. Furthermore, when pilocarpine is administered continuously, tachyphylaxis occurs, further diminishing the potential of this technology. Other limitations are difficulty with retention, unnoticed loss of the unit from the eye and rupture of its membrane causing excessive bolus drug release [12, 13]. Ocusert, however, is still considered a technical breakthrough, even though it has achieved only limited success in the market due to these limitations [14].
Current research efforts are focused towards the design and evaluation of ocular delivery systems that are easy to administer, require decreased administration frequency, and provide controlled and possibly sustained drug release in order to increase therapeutic efficacy and patient compliance. Hydrogels are a crosslinked network of hydrophilic polymers that have the ability to absorb large amounts of water and swell, while maintaining their three-dimensional structure. Molecules of different sizes can diffuse into and out of the hydrogel network, which allows their possible use as a drug-depot for controlled release applications. Hydrogels show minimum tendency to adsorb protein from body fluids due to their low interfacial tension. They closely resemble living tissue due to their high water content, and soft/rubbery characteristics. As a result of these favorable properties, hydrogels are increasingly being used as scaffolds in tissue engineering [15] and drug delivery systems in various biomedical [16] and pharmaceutical [17–20] applications. As ocular drug delivery systems, hydrogels are expected to provide prolonged corneal contact time, reduced precorneal drug loss, and convenience in administration as compared to eye drops, suspensions or ointments. The viscoelastic properties of hydrogels should allow for sufficient mechanical strength to resist clearance due to blinking resulting in prolonged ocular residence time.
Hydrogels can be preformed [21, 22] or prepared in situ [23–25]. Preformed hydrogels are simple viscous solutions [21] or hydrogel films [22], which gel outside of the eye and do not undergo any modification after administration. Viscous solutions do not have the mechanical strength to resist ocular clearance mechanisms and only offer a transient improvement in ocular residence time. In situ forming hydrogels are liquids upon instillation and undergo a phase transition to form a viscoelastic gel in response to environmental changes like temperature [23, 25], pH [26] and electrolyte composition [27]. In situ forming hydrogels are attractive as ocular drug delivery systems because of facile dosing as a liquid, which insures rapid and complete ocular coverage. They also allow for accurate and reproducible quantities to be administered to the eye in contrast to pre-gelled formulations. The above-mentioned in situ hydrogels are often unstable and reversible. The currently examined hydrogels are produced in situ at physiological pH by covalent intermolecular crosslinking of polymer chains through irreversible thioether bonds resulting in stable viscoelastic hydrogels.
Pilocarpine has been widely used topically in the eye for controlling elevated intraocular pressure associated with glaucoma [28, 29]. When administered as a solution, it has good water solubility but suffers from low ocular bioavailability of 0.1–3% [3, 30] due to its low lipophilicity and the short residence time of aqueous solutions in the eye. In the present work, pilocarpine-loaded hydrogels were prepared at physiological pH by the intermolecular crosslinking of a thiol-containing copolymer with the bifunctional crosslinker, HBVS (1, 6-hexane-bis-vinylsulfone). The hydrogels were prepared using different copolymer/crosslinker concentrations and pH to optimize hydrogel formation, drug loading, and release properties. It was further evaluated in rabbits for controlled ocular delivery of pilocarpine and pupillary constriction.
2. Materials and methods
2.1. Materials
Polyoxyethylene bis(amine) [PEO, MW ~3350 Da], pilocarpine hydrochloride (hereafter referred to as pilocarpine), Ellman’s reagent [5,5′-dithiobis(2-nitrobenzoic acid)], mercaptosuccinic acid, trityl chloride, 4-dimethylaminopyridine, p-toluenesulfonic acid monohydrate, trifluoroacetic acid, and triisopropyl silane were obtained from Sigma-Aldrich (St. Louis, MO). N, N-dimethylformamide, dichloromethane, diethyl ether, acetonitrile and HPLC grade solvents were obtained from Fisher Scientific (Pittsburgh, PA). HBVS was obtained from Pierce (Rockford, IL). The Spectra/Por RC 7 dialysis membrane (MWCO: 50,000 Da) was obtained from Spectrum Laboratories Inc (Rancho Dominguez, CA). Gel permeation chromatography (GPC) was carried on a Waters Breeze GPC system (Milford, MA) equipped with dual-absorbance UV and refractive index detectors using an Ultrahydrogel 1000 size-exclusion column (7.8 × 300 mm). PEO/PEG molecular weight standards kit was obtained from Polymer Standards Service-USA Inc. (Warwick, RI). A DSC Q100 (TA Instruments, New Castle, DE) was used for differential scanning calorimetry (DSC). The optical transmission (OT) of the hydrogel was determined using a UV 1201 UV-Vis spectrophotometer (Shimadzu, Columbia, MD). Rheological data were obtained on a SR-2000 rheometer from Rheometric Scientific Inc (Piscataway, NJ) and analyzed by RSI orchestrator software. TEM studies were carried out on a Philips CM12 transmission electron microscope at 100Kv using an AMT digital camera. Waters HPLC system equipped with a UV detector and reverse phase (RP) C18 column (Waters, Novapak, 3.9 × 150mm) was used to analyze pilocarpine concentration.
2.2. Copolymer synthesis and characterization
The copolymer was synthesized using a solution polymerization technique as previously reported by our group [31]. PEO-bis-amine (1 g, 0.298 mM) was dissolved in anhydrous dichloromethane (20.0 mL). S-tritylmercaptosuccinic acid (1 equiv., 116.9 mg), 4-dimethylaminopyridine (1 equiv., 36.4 mg), and p-toluenesulfonic acid monohydrate (1 equiv., 56.6 mg) were added to the solution under anhydrous conditions. The solution was cooled to 0°C and 1, 3-diisopropyl carbodiimide (4 equiv., 184 μL) was added. The reaction mixture was then stirred at RT for 24 h. Crude copolymer was obtained by precipitation from cold ether (4 × 25 mL) and subsequent drying. Dialysis was performed to remove low molecular weight impurities. The copolymer was dissolved in water (~50.0 mL), transferred to a dialysis membrane and suspended in stirring water (2.0 L). Dialysis was performed for 24 h. After dialysis, the copolymer solution was transferred to polypropylene tubes and freeze-dried (Yield. 835 mg). Copolymer was finally treated with acid to remove the trityl groups. Copolymer (800 mg) was suspended into the trifluoroacetic acid solution (10.0 mL) containing 5% each of triisopropyl silane and water. The reaction mixture was placed on a shaker for 5 h and free thiol-containing copolymer was obtained after precipitation from cold ether. The copolymer was obtained as white flakes after vacuum drying for 1–2 h (Yield. 510 mg).
The weight-average (Mw) and number-average molecular weight (Mn) and polydispersity index of the copolymer were estimated by GPC. Polymer standards (50μL, 2mg/mL) with molecular weight (Mw) of 1490, 4270, 6950, 12400, 20100, 41300 and 70400 respectively were injected to generate a calibration curve. Finally, an aqueous solution of copolymer was injected and molecular weights were estimated by using the GPC software. To measure glass transition temperature (Tg) by DSC, copolymer samples were weighed into aluminum pans that were sealed and perforated. The samples were heated from 0–200 °C under a nitrogen atmosphere, cooled to −50 °C, and re-heated to 200 °C at a sweep rate of 10 °C/min. Tg was determined by Universal Analysis software from the first heating cycle and Tm was determined from the average of the melting points calculated from the first and second heating and the cooling cycles.
2.3. Hydrogel formation
Hydrogels were prepared by mixing the copolymer and crosslinker solutions in sodium phosphate buffer (20 mM, pH = 7.4) at RT. Hydrogels were prepared from 3, 4, 5 and 6% (w/v) copolymer solutions using copolymer to crosslinker stoichiometries of 1:1 or 1:2. Pilocarpine (2%, w/v) was mixed with the copolymer solution prior to mixing of copolymer and crosslinker solutions. Hydrogel formation was determined by the “inverted tube method” and hydrogels were considered to have formed once the solution ceased to flow from the inverted tube. Hydrogels used for optical transmission, degree of swelling, drug loading and in vitro release studies were 300 μL in volume, 10 mm in diameter, and 0.3 mm in thickness.
2.4. Physicochemical characteristics of the hydrogel
2.4.1. pH titrations
4% hydrogels (w/v) as described above were prepared in aqueous sodium phosphate buffer solutions (20 mM) with pH values of 6.5, 6.9, 7.4 and 7.9 to investigate the influence of pH on hydrogel formation.
2.4.2. Optical transmission
Hydrogels (300 μL) were placed in a quartz cuvette containing distilled water and transmission of light was measured at 480 nm [32]. Cuvette containing only distilled water was used as reference. Eight hydrogels were used for the OT measurements. All OT studies were done in triplicate and the mean±standard error (SEM) is reported. The statistical significance between groups was measured using student’s t-test.
2.4.3. Rheological studies
Rheological measurements were performed using a rheometer with a cone plate geometry at 37 °C (plate diameter: 25 mm, gap: 3 mm, 2° angle) [33, 34]. Hydrogels were prepared from 3% and 5% (w/v) copolymer solutions using a copolymer to crosslinker stoichiometry of 1:1. The hydrogel samples were equilibrated on the plate for 5 min to reach the running temperature before each measurement. Rheological test parameters, storage/elasticity (G′) and loss (G″) moduli were obtained under dynamic conditions of non-destructive oscillatory tests. All rheological studies were performed in triplicate and the mean±SEM is reported.
Dynamic strain sweep test
Experiment was performed at a constant frequency of 1 Hz with percent strain ranging from 10−1 to 103.
Dynamic frequency sweep test
Experiment was carried out at a constant strain of 1% (linear viscoeleastic region) with frequency ranging from 10−1 to 101 Hz.
2.4.4. Transmission Electron Microscopy (TEM)
Hydrogel samples (3% and 5% w/v, 1:1 stoichiometry) were prepared by placing the solution on a 400 mesh copper grid coated with carbon mica [35]. The excess hydrogel was removed after 1 min. The grids were stained negatively with 1% phosphotungstic acid solution (PTA) and electroscoped.
2.4.5. Degree of swelling
The effect of the copolymer concentration (3, 4, & 6%, w/v) and copolymer to crosslinker ratios (1:1 and 1:2) on the degree of hydrogel swelling were evaluated. Hydrogels were placed in a vial and weighed to obtain the initial weight. They were next immersed in simulated tear fluid (4.0 mL) and placed in a shaking incubator (37 °C, 60 rpm). The tear fluid was prepared by dissolving sodium chloride (0.67%, w/v), sodium bicarbonate (0.2% w/v) and calcium chloride dihydrate (0.008% w/v) in water and pH adjusted to 7.4 [23]. The tear fluid was removed at predetermined time intervals and the weights of vial with swollen hydrogels were recorded. The simulated tear fluid was replaced after every measurement and swelling experiments were continued until equilibrium hydration has been reached. The increase in the weight of the hydrogel in the vial was correlated to the tear fluid uptake by the hydrogel. All measurements were made in triplicate for each hydrogel and the mean±SEM of the values is reported.
2.5. In vitro release studies
2.5.1. Drug loading efficiency
The pilocarpine-loaded hydrogels containing 2% (w/v) of pilocarpine were dissected into small pieces (300 μL) and suspended into the simulated tear fluid (4.0 mL). The suspension was sonicated for 30 min to completely extract pilocarpine from the hydrogel. The amount of pilocarpine extracted was quantified by RP HPLC analysis. After extraction, the suspension containing the hydrogel was stored for several days at 37 °C and then re-analyzed to ensure the complete extraction of pilocarpine from the hydrogel. Pilocarpine was stable under the conditions used (37 °C), as determined by HPLC analysis.
2.5.2. In vitro release
The hydrogels (300 μL) loaded with 2% (w/v) of pilocarpine were suspended in vials containing simulated tear fluid (4.0 mL). The vials containing the tear fluid were placed in a shaking incubator (37 °C, 60 rpm) during the duration of the study (8-days). Aliquots (200 μL) were collected from the vials at predetermined intervals and replaced with equal volume of tear fluid medium to maintain sink conditions through out the study. The concentration of pilocarpine in the release medium was determined using RP HPLC at a wavelength of 220 nm. Water containing 0.1 % trifluoroacetic acid (solvent A) and 100% acetonitrile (solvent B) were used as mobile phase. The retention time for pilocarpine was 15 min. The cumulative amount of pilocarpine released from the hydrogel was determined using a calibration curve. All release experiments were done in triplicate and the results are reported as mean±SEM.
2.6. In vivo studies
Four female New Zealand albino rabbits weighing 3.5–4.0 kg (Charles River Laboratories, Wilmington, MA) were obtained and acclimatized for 3-days prior to use. They were fed a normal diet and exposed to alternating 12 h light and dark cycles. Animals were treated according to the Principles of Animal Care by National Institutes of Health and animal protocol approved by the Rutgers University Institutional Animal Care and Use Committee.
2.6.1. Eye irritation test
Acute eye irritation/corrosion [36] and ocular irritation potential of the hydrogel or its components (copolymer and HBVS) was tested prior to the approval of the protocol by the Rutgers University Institutional Animal Care and Use Committee. The irritation test was performed according to the Organization for Economic Cooperation and Development (OECD/OCED) test guideline 405. Hydrogels (25 μl) were prepared from 4% (w/v) copolymer solutions (copolymer/crosslinker: 1:2) with no drug and placed in the conjunctival sac of one of the rabbit’s eye; the untreated eye served as control. The ocular irritation potential was evaluated for 21-days by scoring lesions of conjunctiva, cornea and iris at specific time intervals. Experiments were done in triplicate.
2.6.2. In vivo efficacy
All rabbits were acclimatized to laboratory testing conditions for 30 min before the study. The right and left pupil diameters were alternatively measured four times with in 30 min, using a standard pupillary diameter gauge held at a fixed distance from each rabbit to establish baseline values for both eyes. For each pair of readings, the differences in pupil diameter (control minus test eye) were determined. These predosing differences were averaged, and the mean was used to convert post administration data to the baseline-corrected values. This process minimized both animal and day variation.
Both the aqueous and hydrogel formulations were tested in each rabbit. A minimum of 1 week elapsed between tests in the same rabbit. Hydrogel prepared from 4% (w/v) copolymer solutions using copolymer to crosslinker stoichiometry of 1:2 was chosen for this study. 25 μl of a hydrogel solution containing 2% pilocarpine was placed in the lower conjunctival sac of the right eye. To avoid experimental bias, the left eyes received 25 μl of a hydrogel solution with no drug (control). The eyes were checked frequently and no inflammation was observed in any experiment. After one week, the above procedure was repeated using 2% pilocarpine solution in phosphate buffered saline (PBS, pH = 7.4), hereafter referred to as drops. 25 μl of pilocarpine drops were placed in the lower conjunctival sac of the right eye and 25 μl of PBS with no drug was placed in the left eye of the rabbits and pupil diameter was measured.
After administration of both the control vehicle and the pilocarpine containing copolymer solutions, the pupil diameters of both the eyes were measured at predetermined time intervals (0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 12 and 24 h). For each time point, the difference in pupil diameter (control minus test eye) was calculated and data was converted to a baseline-corrected value (i.e., the pharmacological response of pilocarpine) by subtracting the average baseline difference in pupil diameter for each experiment on the basis of the readings obtained before dosing. To assess the extent of total pharmacological response of the various formulations, areas under the decrease in pupil diameter vs. time profiles in 24 h (AUC0–24) were calculated using the GraphPad Prism 4 software. The mean±SEM of four determinations is reported. Two way analysis of variance (ANOVA) was used to determine the overall statistical significance, while student’s t-test was used for individual comparison between groups.
Results and Discussion
Copolymer synthesis and characterization
The synthesis of the copolymer poly[poly(ethylene oxide)-alt-poly(mercaptosuccinic acid)] has been reported earlier by our group [31]. Briefly, a polycondensation reaction was carried out between polyoxyethylene bis(amine) 1 and S-tritylmercaptosuccinic acid 2 to obtain copolymer 3 containing alternating units of poly(oxyethylene glycol) and S-tritylmercaptosuccinic acid. The thiol-containing copolymer 4 was obtained after removing the trityl group with acid (Scheme. 1). The copolymer was characterized by GPC and DSC analysis. The GPC profile of crude copolymer showed the presence of a predominantly high molecular weight peak at 6.91 min (copolymer) along with peaks at 11.77 and 16.05 min, which correspond to low molecular weight impurities (monomeric PEG/other reagents). The relative proportion of copolymer in the crude reaction mixture was calculated as ≥50% from AUC. Low molecular weight impurities were removed by dialysis (MWCO = 50,000). The Mw and Mn for purified copolymer were estimated as 64435 and 57289 Da, respectively and the polydispersity index of the copolymer was calculated as 1.15. The Tg of copolymer as measured by DSC, was found below −40 °C whereas the Tm was found as 45±1 °C. The extrapolated on set and end set of melting points were found to be 37±1 °C and 49±1 °C respectively. The entropy/heat of fusion (ΔH), which is defined as the heat required for melting the copolymer was calculated to be 110±5 J/g. The thiol contents of copolymer were estimated as 183 μM/1.2 × 10−5 mM by the Ellman’s assay.
Scheme 1.
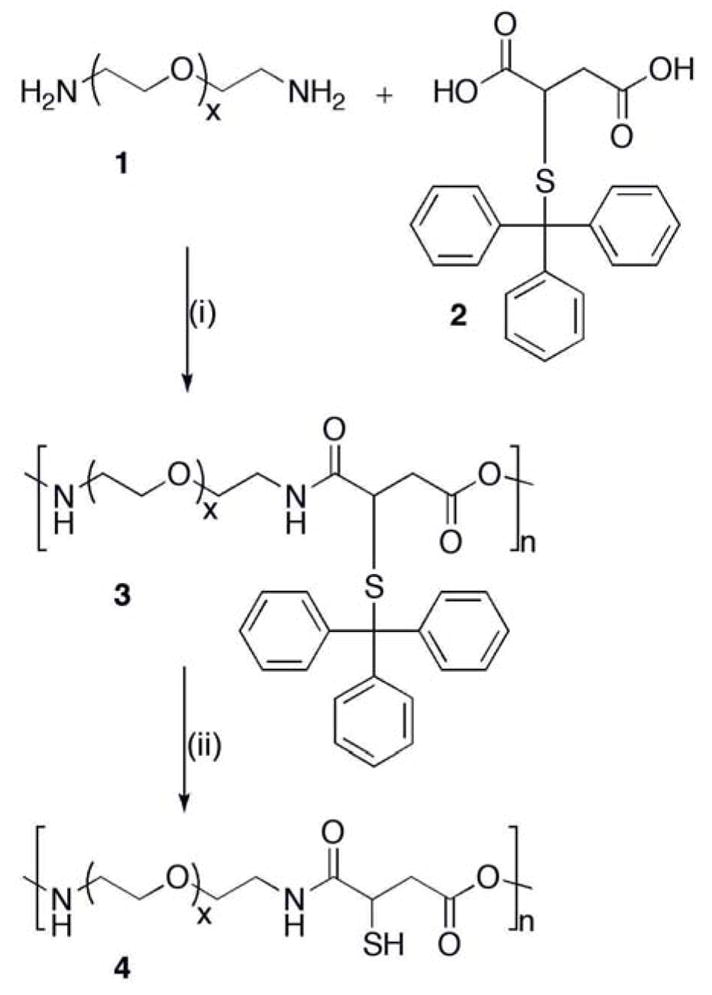
Copolymer synthesis, (i) N, N-dimethylaminopyridine, p-toluene sulfonic acid monohydrate, N, N-diisopropylcarbodiimide/dichloromethane, RT, 24 h; (ii) trifluoroacetic acid, triisopropyl silane/water, RT, 5 h.
The advantage of using this copolymer is that it contains PEG units, which are water soluble, non-toxic and biocompatible [37]. They have been widely used in various pharmaceutical products administered by parenteral, topical and oral routes [38, 39]. The advantage of using mercaptosuccinic acid is that it provides reactive thiol functionalities that can be utilized for intermolecular crosslinking of copolymer or to anchor drugs, targeting and/or signaling moieties on to the copolymer.
Hydrogel studies
A thiol-containing copolymer was crosslinked with the bifunctional crosslinker HBVS resulting in the formation of a hydrogel network through thioether bonds. The thiol groups are known to react with mutually reactive groups such as thiol, maleimide, and vinyl sulfone to form disulfide and thioether bonds [40]. The disulfide bonds are cleavable (reversible) under reducing conditions whereas the thioether bonds are highly stable (irreversible) in the biological environment.
Different copolymer concentrations (w/v) and stoichiometric amounts of crosslinker (1:1) were used to prepare the hydrogels. It was observed that 2% copolymer solution (w/v) did not form a hydrogel whereas 3, 4, 5 and 6% copolymer solutions resulted into hydrogels in 4.0, 2.5, 2.0 and 1.8 min respectively. Copolymer solutions beyond 6% (w/v) were too viscous and hence not investigated. When prepared using 1:2 stoichiometries, the 3–6% hydrogels were formed in 3.0, 2.0, 1.4 and 1.2 min respectively. Thus, an increase in copolymer/crosslinker concentration decreased the time required for hydrogel formation by producing more rapid and intense crosslinking. Hydrogels were further characterized using TEM (Fig. 1) [35]. The micrographs of hydrogels prepared from 3 and 5% copolymer solutions clearly showed that an increase in copolymer concentration leads to the formation of denser crosslinked polymer networks.
Fig. 1.
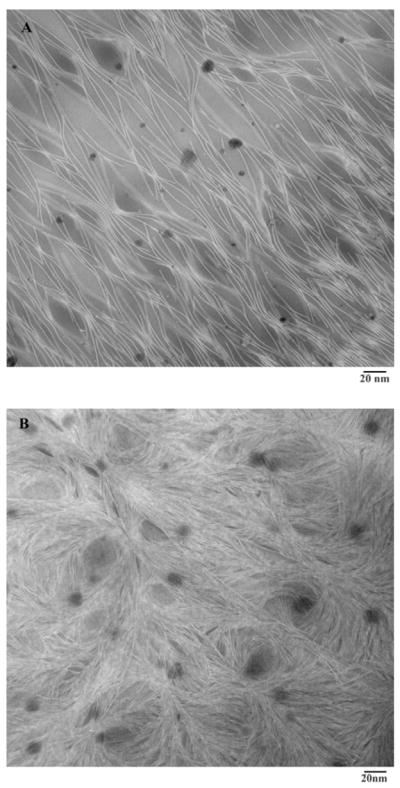
TEM images of 3% (A) and 5% (B) hydrogels prepared using copolymer and crosslinker in 1:1 stoichiometry. The crosslinking networks are clearly visible; the network density increases with increase in copolymer concentration.
The influence of buffer pH on hydrogel formation was evaluated and 4% copolymer solution was found to form hydrogels in 5.2, 2.8, 2.5 and 1.0 min at pH 6.5, 6.9, 7.4 and 7.9. The decrease in gelation time is due to enhanced thiol reactivity at elevated pH [41].
Delivery systems for the eye should ideally be transparent. Optical Transmission (OT) is defined as the ratio of the amount of light passing through the hydrogel to the amount of light incident on it (expressed as percentage). Since human eyes are most responsive around 480 nm, the OT of hydrogels was measured at this wavelength [32]. Hydrogels with optical transmission ≥90% are classified as transparent, ≤90% but ≥10% as translucent, and ≤10% as opaque [32]. Fig 2 shows the percentage of copolymer (w/v) containing crosslinker in the ratio of 1:1 or 1:2 plotted vs. %OT. The groups that showed a statistically significant difference (p<0.05 or p<0.01) are indicated by * and **, respectively. All hydrogels used in the present study were either transparent (OT > 90%) or nearly transparent (OT > 78%). An increase in copolymer concentration decreased the transparency of the hydrogel whereas an increase in crosslinker concentration has little influence. These results indicate that the optical properties of the hydrogels make them suitable as ocular drug delivery systems.
Fig. 2.
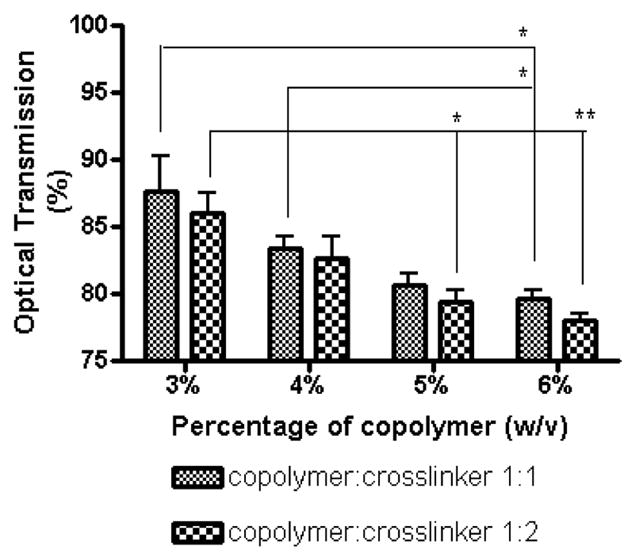
Optical transmissivities of 3, 4, 5 and 6% hydrogels prepared using copolymer and crosslinker in 1:1 or 1:2 stoichiometry. The statistically significant different groups are denoted by * (p<0.05) and ** (p<0.01). The optical transmissivities of the hydrogel decreases with increase in copolymer and crosslinker concentration. All the hydrogels were found to be transparent or close to transparent.
The mechanical strength and viscoelastic properties of the hydrogels were investigated using rheological measurements [33, 34] to assess their retention behavior and physical integrity in vivo. Viscoelastic properties were investigated because hydrogels with good mechanical strength are expected to maintain their integrity and help to prevent physical drug loss from blinking [42]. The strain sweep test (Fig. 3A) was performed on 3% and 5% hydrogels in order to establish the regime of linear viscoelasticity (LVE) and to determine if the elasticity of the formulations differed, as expressed by the storage/elastic modulus (G′). The strain sweep test results suggest that G′ dominates in both the formulations and this is supported by the results obtained from the frequency sweep test (Fig. 3B). Since G′ was one order of magnitude greater than G″ (loss modulus), this suggests that the hydrogel system is more elastic than viscous in the investigated frequency range. This is in direct contrast to reversible gels, which have poor mechanical strength leading to reduced corneal residence time. Fig’s 3A and 3B also show that the G′ is independent of frequency and strain whereas the G″ is weakly dependent on them. The hydrogels containing higher copolymer concentrations (5%) showed a higher and constant storage modulus under increasing frequency, suggesting that the hydrogels have the ability to resist structural changes under strain. The loss tangent (tanδ) values calculated as G″/G′ indicate that the storage modulus is the dominant feature in all of the hydrogels, as a small tanδ indicates an elastic material. There is little variation in the tanδ values, and the curves are similar for the different hydrogels, which further supports the strain sweep test results suggesting that the variations in hydrogel formulations in the current study do not result in extreme variations in rheological parameters. The rheological data show that the hydrogels have good mechanical properties, which might help prolong their ocular contact time. An increased contact time in turn may lead to an increased duration of pharmacological response.
Fig. 3.
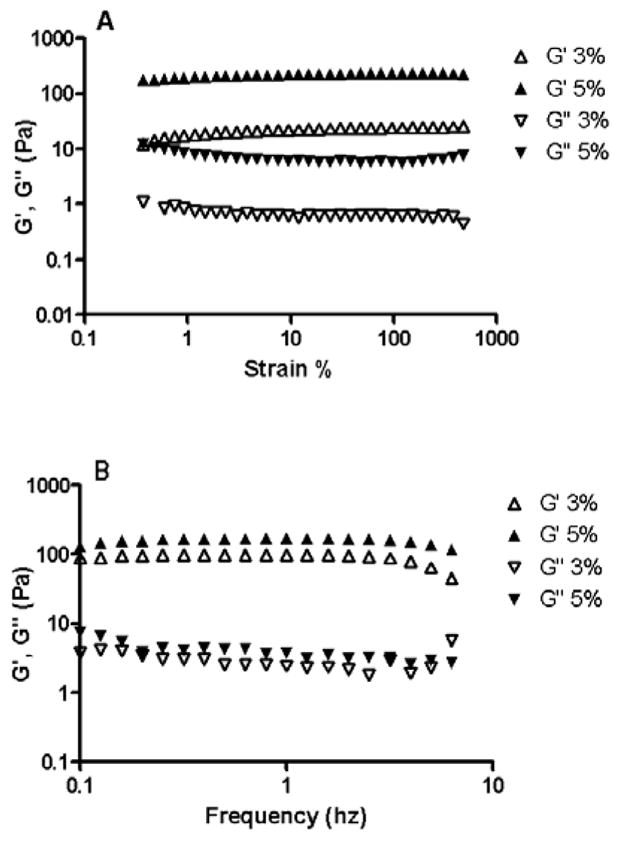
Influence of strain (A) and frequency (B) on G′ and G″ of hydrogels. The rheological measurements were carried out on 3% and 5% (w/v) hydrogels prepared using copolymer and crosslinker in 1:1 stoichiometry. The strain sweep test establishes the regime of linear viscoelasticity (LVE). The frequency sweep test shows that the hydrogels are more elastic than viscous and that they have the ability to resist structural changes under strain.
The swelling behavior of hydrogel influences its surface and mechanical properties as well as the drug release kinetics (solute diffusion coefficients). Also since hydrogels are swelling controlled systems, the degree of swelling is a measure of the crosslinking density in the hydrogel and is important for regulating the pore size of the hydrogel. The effect of copolymer concentration and the copolymer to crosslinker ratios on the degree of swelling was determined. The degree of swelling for each hydrogel was determined by using the equation below:
| (1) |
Where Ws is the weight of the swollen hydrogel at time t and W0 is the initial weight. Fig. 4 shows the degree of swelling expressed as percent swelling plotted against time for 3, 4 and 6% hydrogels prepared using 1:1 and 1:2 copolymer/crosslinker ratios. Initially, the hydrogels swelled rapidly and then gradually reached the equilibrium. Compared to hydrophilic hydrogels reported in the literature [43, 44], a relatively lower degree of swelling (<8%) was observed in this case. Furthermore, the degree of swelling decreased with an increase in copolymer (3–6%, w/v) and crosslinker (1–2) concentrations. Hydrogel swelling is caused by the presence of hydrophilic groups in the network, due to which polymer chains are hydrated to different degrees (sometimes, more than 90% wt), depending on the nature of the aqueous environment and polymer composition [45]. Although PEG is highly hydrophilic and is expected to swell significantly, the controlled degree of hydrogel swelling in the present case is due to the incorporation of the less hydrophilic mercaptosuccinic acid in the copolymer chain. Further, the decrease in hydrogel swelling with increasing crosslinker concentration is due to the smaller pore size and the fact that higher crosslinking reduces the exposure of copolymer chains to water [46, 47].
Fig. 4.
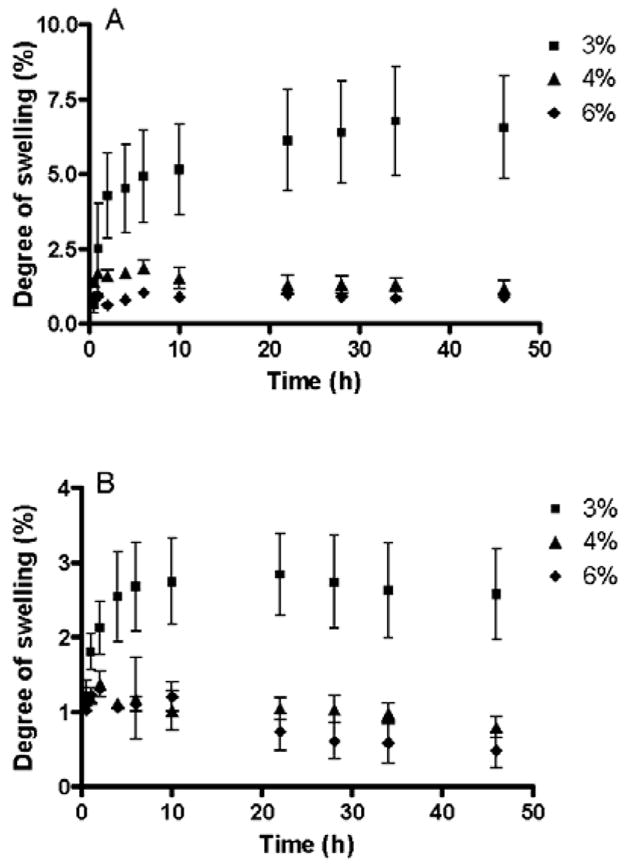
Swelling kinetics of the hydrogel as a function of time. 3, 4 and 6% (w/v) hydrogels were prepared using 1:1 (A) and 1:2 (B) stoichiometries. All measurements were performed in triplicate and plotted as mean±SEM. The degree of hydrogel swelling decreases with increasing copolymer and crosslinker concentrations.
In vitro pilocarpine loading and release
Pilocarpine-loading efficiency results show that 3, 4, 5 and 6% (w/v) hydrogels with 1:1 stoichiometries entrapped 70.0, 72.5, 66.5, and 68.0% of loaded pilocarpine. Similarly, the 3–6% (w/v) hydrogels prepared using 1:2 stoichiometries resulted in pilocarpine loading efficiencies of 73.6, 72.9, 71.6, and 70.8%. The hydrogels prepared using 1:2 stoichiometry showed up to 5% higher pilocarpine loading compared to hydrogels prepared using 1:1 stoichiometry, which might be due to an increase in crosslinking density between the copolymer chains.
The pilocarpine release profiles from different hydrogels were studied in vitro using simulated tear fluid as dissolution medium. The plot of fractional release of pilocarpine (Mt/M∞) as a function of square root of time (Fig. 5) demonstrates that pilocarpine entrapment in the hydrogel slows down the drug release and there is a sustained release for about 192 h (8-days). The percentage of pilocarpine released from 3, 4, 5 and 6% hydrogel formulation prepared using 1:1 stoichiometry decreased from 100 to 86% whereas same hydrogels prepared using 1:2 stoichiometry released 98 to 85 % of pilocarpine. Neither difference was considered significant. Thus, most of the loaded pilocarpine was released in all of the hydrogel formulations evaluated. It is also evident from the plot that increase in copolymer/crosslinker concentration results in slower pilocarpine release, which might be due to the formation of a tighter hydrogel network and decreased pore size.
Fig. 5.
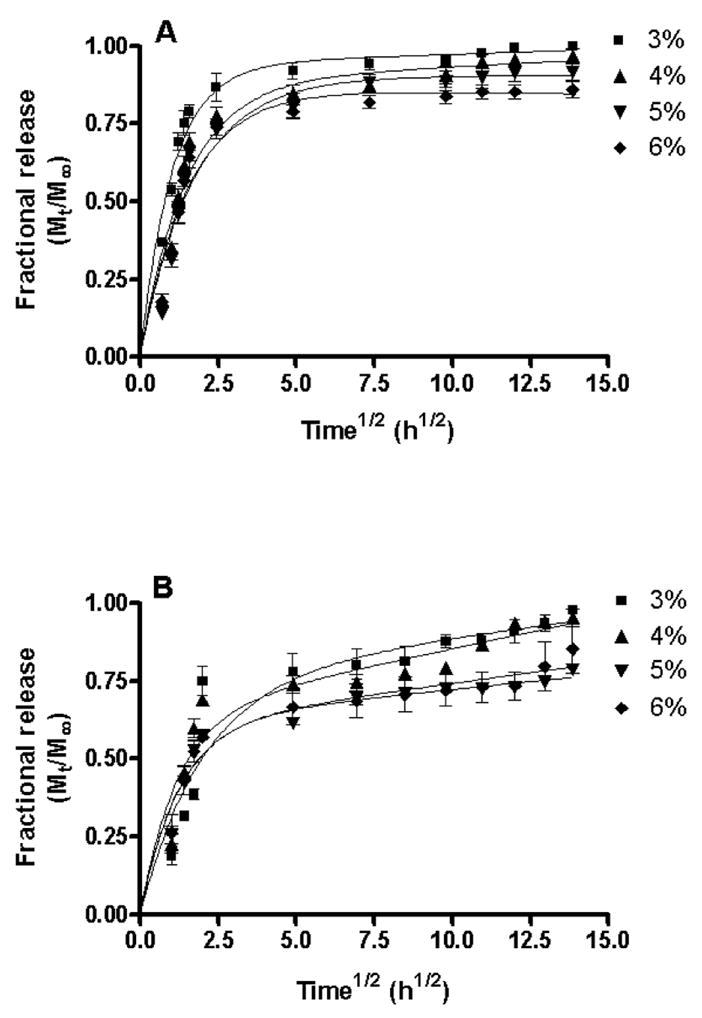
Fractional release of pilocarpine as a function of time for 3, 4, 5 and 6% (w/v) hydrogels prepared using 1:1 (A) and 1:2 (B) stoichiometries. All measurements were performed in triplicate and plotted as mean±SEM. The release data were fitted using a two-phase exponential association equation in GraphPad Prism 4 software. The goodness of fit (R2) for the different hydrogels varied from 0.76 to 0.93. The initial burst release of pilocarpine appears to correlate well with the swelling phase (~0–3h) as shown in Figure 4. Once swelling terminates, a sustained release phase begins. The higher burst phase appears to also be affected by pore size with the largest pore size hydrogel (3%) having the largest burst effect. The release mechanism is non-fickian or anomalous involving both diffusion and polymer relaxation (0.5<n<1). An increase in copolymer/crosslinker concentration results in a slower pilocarpine release.
The relative influence of diffusion and polymer relaxation on the mechanism of pilocarpine release was determined by fitting the experimental data (first 60% of the total amount released) to the Ritger-Peppas equation [48].
| (2) |
In Equation 2, Mt/M∞ is the fractional release of the drug, k is the proportionality constant, n is the diffusional exponent and t is the time. The diffusional exponent was calculated from the slope of the natural logarithmic values (ln) of the fractional release as a function of time (Table 1). The release mechanism for all of the hydrogels was found to be non-fickian or anomalous involving both diffusion and polymer relaxation (0.5<n<1). Pilocarpine release was dependent on two simultaneous processes, water migration into the hydrogel and drug diffusion through continuously swelling hydrogels. The apparent diffusion coefficients at the early stage of controlled release (Mt/M∞ < 0.6) were determined using an equation derived from Fick’s second law of diffusion [49].
Table 1.
Estimation of diffusional exponent (n) and apparent diffusion coefficient (D) for various hydrogel formulations.
| % Hydrogels (copolymer:crosslinker) | Diffusional exponent (n) | Apparent diffusion coefficient (cm2 sec−1) × 10−6 |
|---|---|---|
| 3% (1:1) | 0.98 | 7.44 |
| 4% (1:1) | 0.88 | 7.10 |
| 5% (1:1) | 0.81 | 6.23 |
| 6% (1:1) | 0.63 | 5.87 |
| 3% (1:2) | 0.89 | 5.05 |
| 4% (1:2) | 0.81 | 4.19 |
| 5% (1:2) | 0.74 | 2.91 |
| 6% (1:2) | 0.57 | 1.91 |
| (3) |
In Equation 3, Mt/M∞ is the fractional release of the drug, D is the diffusion coefficient, t is the time, and h is the thickness of the hydrogel. The apparent diffusion coefficient was calculated from the slope of the fractional release as a function of square root of time/thickness of the hydrogel (Table 1). The diffusional exponents and the apparent diffusion coefficients decreased with an increase in copolymer and crosslinker concentrations. This indicates a decrease in swelling and pore size of the hydrogels. The in vitro release studies show that by changing the copolymer/crosslinker concentrations, drug release from hydrogels can be optimized.
In vivo studies
The possibility of eye irritation due to the hydrogel administration was evaluated in rabbits. Three veterinarians independently graded the rabbits for ocular lesions and no symptoms of ocular irritation such as tearing, redness, inflammation or swelling were observed after hydrogel administration. Furthermore fluorescein staining did not indicate corneal or conjunctival epithelial defects.
The pharmacological response (i.e., decrease in pupil diameter) vs. time profiles of a 4% (w/v) hydrogel formulation prepared using copolymer and crosslinker in a 1:2 ratio and drops containing 2% pilocarpine were compared next (Fig 6). The miotic effect of the hydrogels is significantly greater than drops (p<0.001) as shown by ANOVA. The time points that showed a statistically significant difference (p<0.05 or p<0.01) were indicated by * and **, respectively. At all times post administration, the decrease in pupil diameter was greater for the hydrogel formulation compared to drops, although the pharmacological profile was similar for up to 3 h. Five indicators of pharmacological efficacy are summarized in Table 2: (1) Imax, the peak miotic intensity (2) Tmax, the time to reach the peak miotic intensity (3) AUC, the area under the decrease in pupil diameter vs. time curve (4) the duration of miotic response and (5) the slope of the linear portion of the decrease in pupil diameter vs. time curve. Imax, Tmax and duration of miotic responses were determined by linear interpolation between the data points, while AUC was calculated using Graph Pad Prism 4 software.
Fig. 6.
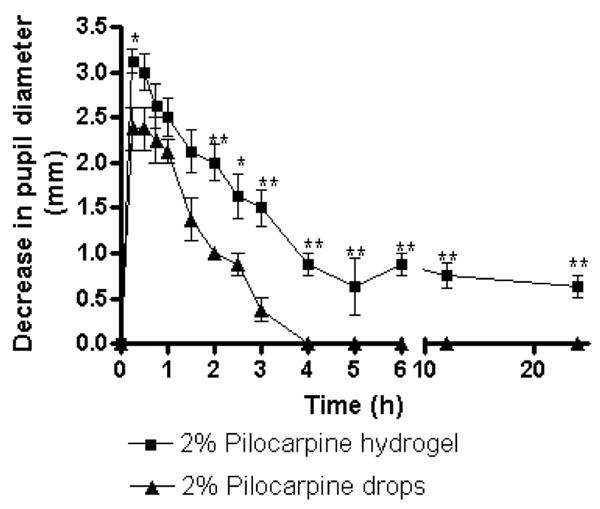
Decrease in pupil diameter vs. time for pilocarpine-loaded hydrogel (4%, w/v, copolymer and crosslinker in 1:2 stoichiometry) and aqueous pilocarpine (2%, w/v) solution in PBS. Mean±SEM of four determinations is reported. The statistically significant differences in pupil diameter changes between both the groups is denoted by * (p<0.05) and ** (p<0.01). The hydrogel shows sustained pharmacological response for a period of 24 h.
Table 2.
Comparison of pharmacological efficacy of 2% pilocarpine administered ocularly as hydrogel and aqueous solution.
| Ocular Delivery System | Imax (mm) | Tmax (h) | AUC (mm/h) | Duration (h) | Slope |
|---|---|---|---|---|---|
| Hydrogel | 3.125 | 0.25 | 22.19 | 24 | −0.26 |
| Drops | 2.375 | 0.25 | 4.45 | 3 | −0.44 |
It can be seen from Table 2 that Imax for the hydrogel formulation is 1.3 times higher than the drops even though Tmax for both formulations is similar (15 min). Also, the duration of miotic response is 8-fold greater for the hydrogel formulation (24 h) than the drops (3 h), indicating a more sustained release due to greater diffusional resistance. The AUC0–24h values indicate approximately a 5-fold increase in total miotic response for the hydrogel formulation relative to drops. In addition, pupillary response is greater for hydrogel formulation than drops, shown by a 1.7-fold higher slope for the hydrogel formulation. The higher efficacy of pilocarpine loaded hydrogel formulation compared to drops, is possibly due to the increased residence time in cul-de-sac, sustained drug release, and decreased clearance by tears.
Fig. 7 shows a linear correlation between the in vitro release of pilocarpine and in vivo pharmacological response in the eye. As the amount of pilocarpine available for absorption increases, there is a corresponding decrease in pupil diameter. The in vivo results coupled with the in vitro results suggest that the hydrogel formulation significantly prolongs the pilocarpine contact time and pharmacological response, which makes it a superior formulation for sustained ocular pilocarpine delivery compared to conventional drops.
Fig. 7.
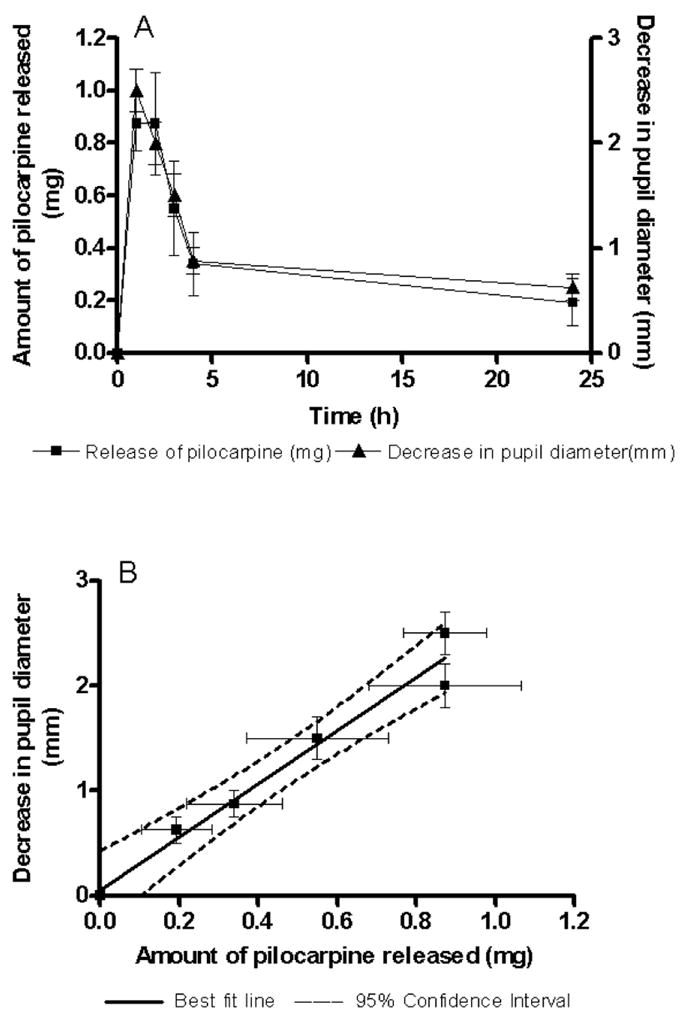
Correlation between in vitro pilocarpine release and pupillary constriction obtained in vivo. A linear correlation is evident with an R2 of 0.97. As the amount of pilocarpine available for absorption decreases, a corresponding increase in pupil diameter is observed. Data are reported as mean±SEM. Solid line indicates the best-fit line and dashed line indicates the 95% confidence interval.
4. Conclusion
The in situ PEG hydrogels prepared and evaluated in the current study are stable, show resistance to external forces, give high pilocarpine loading, and provide sustained pilocarpine release. These hydrogel formulations are administered into the eye as a solution, rapidly forming a hydrogel that is able to withstand the shear forces in the cul-de-sac of the eye. The in vivo results show that compared to drops, the hydrogel formulations provide prolonged pharmacological response as measured by a decrease in pupil diameter with no visible irritation. Overall, the results support the rationale behind using PEG-based hydrogels as ocular drug delivery systems.
Acknowledgments
This work was funded by the National Institutes of Health CounterACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Award #U54AR055073). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maurice DM. In: Ophthalmic drug delivery: biopharmaceutical, technological, and clinical aspects. Saettone MF, Bucci M, Speiser P, editors. Vol. 11. Liviana Press; Padova: 1987. pp. 19–26. [Google Scholar]
- 2.Vandamme TF, Brobeck L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J Control Release. 2005;102(1):23–38. doi: 10.1016/j.jconrel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Chrai SS, Robinson JR. Corneal permeation of topical pilocarpine nitrate in the rabbit. Am J Ophthalmol. 1974;77(5):735–739. doi: 10.1016/0002-9394(74)90541-8. [DOI] [PubMed] [Google Scholar]
- 4.Nanjawade BK, Manvi FV, Manjappa AS. In situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122(2):119–134. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Sieg JW, Robinson JR. Vehicle effects on ocular drug bioavailability II: Evaluation of pilocarpine. J Pharm Sci. 1977;66(9):1222–1228. doi: 10.1002/jps.2600660905. [DOI] [PubMed] [Google Scholar]
- 6.Chien YW, Cabana BE, Mares SE. Novel Drug Delivery Systems: Fundamentals, Development Concepts and Biomedical Assessments. Marcel Dekker Inc; NY: 1982. [Google Scholar]
- 7.Kass MA, Meltzer DW, Gordon M, Cooper D, Goldberg J. Compliance with topical pilocarpine treatment. Am J Ophthalmol. 1986;101(5):515–523. doi: 10.1016/0002-9394(86)90939-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee VH, Robinson JR. Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol. 1986;2(1):67–108. doi: 10.1089/jop.1986.2.67. [DOI] [PubMed] [Google Scholar]
- 9.Sieg JW, Robinson JR. Vehicle effects on ocular drug bioavailability i: evaluation of fluorometholone. J Pharm Sci. 1975;64(6):931–936. doi: 10.1002/jps.2600640606. [DOI] [PubMed] [Google Scholar]
- 10.Zimmer A, Kreuter J. Microspheres and nanoparticles used in ocular delivery systems. Adv Drug Deliv Rev. 1995;16:61–73. [Google Scholar]
- 11.Simamora P, Nadkarni SR, Lee YC, Yalkowsky SH. Controlled delivery of pilocarpine. 2. In vivo evaluation of gelfoam device. Int J Pharm. 1998;170:209–214. [Google Scholar]
- 12.Banker G, Rhodes C. Modern Pharmaceutics. Marcel Dekker Inc; NY: 2002. [Google Scholar]
- 13.Gupta D. Glaucoma Diagnosis and Management: Diagnosis and Management. Lippincott Williams & Wilkins; Baltimore, MD: 2005. [Google Scholar]
- 14.Ali Y, Lehmussaari K. Industrial perspective in ocular delivery. Adv Drug Deliv Rev. 2006;58:1258–1268. doi: 10.1016/j.addr.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54(1):3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 17.Gupta P, Vermani K, Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today. 2002;7(10):569–579. doi: 10.1016/s1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- 18.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem Rev. 1999;99(11):3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 19.Natu MV, Sardinha JP, Correia IJ, Gil MH. Controlled release gelatin hydrogels and lyophilisates with potential application as ocular inserts. Biomed Mater. 2007;2(4):241–249. doi: 10.1088/1748-6041/2/4/006. [DOI] [PubMed] [Google Scholar]
- 20.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50(1):27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 21.Kecik T, Szlaski J, Portacha L, Lewandowski P, Marchlewska B, Pacak I. Studies of releasing rate of pilocarpine hydrochloride from hydrogel eye ointments. Klin Oczna. 1993;95(7):263–264. [PubMed] [Google Scholar]
- 22.Hsiue GH, Guu JA, Cheng CC. Poly(2-hydroxyethyl methacrylate) film as a drug delivery system for pilocarpine. Biomaterials. 2001;22(13):1763–1769. doi: 10.1016/s0142-9612(00)00336-7. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki S, Suzuki S, Kawasaki N, Endo K, Takahashi A, Attwood D. In situ gelling xyloglucan formulations for sustained release ocular delivery of pilocarpine hydrochloride. Int J Pharm. 2001;229(1–2):29–36. doi: 10.1016/s0378-5173(01)00825-0. [DOI] [PubMed] [Google Scholar]
- 24.Lin HR, Sung KC, Vong WJ. In situ gelling of alginate/pluronic solutions for ophthalmic delivery of pilocarpine. Biomacromolecules. 2004;5(6):2358–2365. doi: 10.1021/bm0496965. [DOI] [PubMed] [Google Scholar]
- 25.Desai SD, Blanchard J. In vitro evaluation of pluronic F127-based controlled-release ocular delivery systems for pilocarpine. J Pharm Sci. 1998;87(2):226–230. doi: 10.1021/js970090e. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Haglund BO, Himmelstein KJ. In situ-forming gels for ophthalmic drug delivery. J Ocul Pharmacol. 1994;10(1):47–56. doi: 10.1089/jop.1994.10.47. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, Lobel E, Trevgoda A, Peled Y. A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J Control Release. 1997;44(2–3):201–208. [Google Scholar]
- 28.Katzung B. Basic and clinical pharmacology. McGraw Hill Professional; Chicago, IL: 2004. [Google Scholar]
- 29.Gupta SK, Agarwal R, Galpalli ND, Srivastava S, Agrawal SS, Saxena R. Comparative efficacy of pilocarpine, timolol and latanoprost in experimental models of glaucoma. Methods Find Exp Clin Pharmacol. 2007;29(10):665–671. doi: 10.1358/mf.2007.29.10.1147765. [DOI] [PubMed] [Google Scholar]
- 30.Lazare R, Horlington M. Pilocarpine levels in the eyes of rabbits following topical application. Exp Eye Res. 1975;21(3):281–287. doi: 10.1016/0014-4835(75)90099-8. [DOI] [PubMed] [Google Scholar]
- 31.Qiu B, Stefanos S, Ma J, Lalloo A, Perry BA, Leibowitz MJ, Sinko PJ, Stein S. A hydrogel prepared by in situ cross-linking of a thiol-containing poly(ethylene glycol)-based copolymer: a new biomaterial for protein drug delivery. Biomaterials. 2003;24(1):11–18. doi: 10.1016/s0142-9612(02)00227-2. [DOI] [PubMed] [Google Scholar]
- 32.Murthy SK, Ravi N. Hydrogels as potential probes for investigating the mechanism of lenticular presbyopia. Curr Eye Res. 2001;22(5):384–393. doi: 10.1076/ceyr.22.5.384.5493. [DOI] [PubMed] [Google Scholar]
- 33.Rudraraju VS, Wyandt CM. Rheology of Microcrystalline Cellulose and Sodiumcarboxymethyl Cellulose hydrogels using a controlled stress rheometer: part II. Int J Pharm. 2005;292(1–2):63–73. doi: 10.1016/j.ijpharm.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Xu Z. Physical characterization of a chitosan-based hydrogel delivery system. J Pharm Sci. 2002;91(7):1669–1677. doi: 10.1002/jps.10157. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Yumoto M, Kimura M, Shirai H, Hanabusa K. A family of low-molecular-weight hydrogelators based on L-lysine derivatives with a positively charged terminal group. Chemistry. 2003;9(1):348–354. doi: 10.1002/chem.200390030. [DOI] [PubMed] [Google Scholar]
- 36.OECD, Test No. 405: Acute Eye Irritation/Corrosion. vol 1, no 4 (2002) 1–14.
- 37.Shalaby SW, Shalaby M. In: Polyethylene glycol-based copolyesters: Absorbable and biodegradable polymers. Shalaby SW, Burg KJL, editors. CRC Press; Boca Raton, FL: 2004. pp. 39–58. [Google Scholar]
- 38.Greenwald R. PEG drugs: an overview. J Control Release. 2001;74:159–171. doi: 10.1016/s0168-3659(01)00331-5. [DOI] [PubMed] [Google Scholar]
- 39.Singh Y, Palombo M, Sinko P. Recent trends in targeted anticancer prodrug and conjugate design. Curr Med Chem. 2008;15:1802–1826. doi: 10.2174/092986708785132997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh Y, Spinelli N, Defrancq E. Chemical strategies for oligonucleotide-conjugate synthesis. Curr Org Chem. 2008;12:263–290. [Google Scholar]
- 41.Morpurgo M, Veronese F, Kachensky D, Harris J. Preparation and characterization of poly(ethylene glycol) vinyl sulfone. Bioconj Chem. 1996;7:363–368. doi: 10.1021/bc9600224. [DOI] [PubMed] [Google Scholar]
- 42.Carlfors J, Edsman K, Petersson R, Jornving K. Rheological evaluation of Gelrite in situ gels for ophthalmic use. Eur J Pharm Sci. 1998;6(2):113–119. doi: 10.1016/s0928-0987(97)00074-2. [DOI] [PubMed] [Google Scholar]
- 43.de Souza Costa-Junior E, Pereira MM, Mansur HS. Properties and biocompatibility of chitosan films modified by blending with PVA and chemically crosslinked. J Mater Sci Mater Med. 2009;20(2):553–561. doi: 10.1007/s10856-008-3627-7. [DOI] [PubMed] [Google Scholar]
- 44.Liu SQ, Ee PL, Ke CY, Hedrick JL, Yang YY. Biodegradable poly(ethylene glycol)-peptide hydrogels with well-defined structure and properties for cell delivery. Biomaterials. 2009;30(8):1453–1461. doi: 10.1016/j.biomaterials.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 45.Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60(15):1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Park H, Park K, Kim D. Preparation and swelling behavior of chitosan-based superporous hydrogels for gastric retention application. J Biomed Mater Res A. 2006;76(1):144–150. doi: 10.1002/jbm.a.30533. [DOI] [PubMed] [Google Scholar]
- 47.Tan H, Chu CR, Payne KA, Marra KG. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2008.12.080. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serra L, Domenech J, Peppas NA. Drug transport mechanisms and release kinetics from molecularly designed poly(acrylic acid-g-ethylene glycol) hydrogels. Biomaterials. 2006;27(31):5440–5451. doi: 10.1016/j.biomaterials.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 49.Crank J. The Mathematics of Diffusion. Oxford University Press; New York, NY: 1975. [Google Scholar]


