Abstract
Biodegradable polymeric scaffolds are of interest for delivering antibiotics to local sites of infection in orthopaedic applications, such as bone and diarthrodial joints. The objective of this study was to develop a biodegradable scaffold with ease of drug loading in aqueous solution, while providing for drug depot delivery via syringe injection. Elastin-like polypeptides (ELPs) were used for this application, biopolymers of repeating pentapeptide sequences that were thermally triggered to undergo in situ depot formation at body temperature. ELPs were modified to enable loading with the antibiotics, cefazolin and vancomycin, followed by induction of the phase transition in vitro. Cefazolin and vancomycin concentrations were monitored, as well as bioactivity of the released antibiotics, to test an ability of the ELP depot to provide for prolonged release of bioactive drugs. Further tests of formulation viscosity were conducted to test suitability as an injectable drug carrier. Results demonstrate sustained release of therapeutic concentrations of bioactive antibiotics by the ELP, with first-order time constants for drug release of ~ 25h for cefazolin and ~ 500h for vancomycin. These findings illustrate that an injectable, in situ forming ELP depot can provide for sustained release of antibiotics with an effect that varies across antibiotic formulation. ELPs have important advantages for drug delivery, as they are known to be biocompatible, biodegradable and elicit no known immune response. These benefits suggest distinct advantages over currently used carriers for antibiotic drug delivery in orthopaedic applications.
Keywords: Elastin-like polypeptide, antibiotic, drug delivery, orthopaedics, polymer
Introduction
Infections associated with open fractures and implanted prosthetic devices present a significant clinical and economical challenge in orthopaedic surgery1. Current treatment options include long-term intravenous antibiotic administration as the septic areas are frequently avascular with little access to parenteral antibiotic routes. Local antibiotic delivery systems have been of great interest for the treatment and prophylaxis of orthopaedic infections in order to improve patient compliance over long-term oral or intravenous administration, and to decrease overall drug dosing and associated side effects as compared to systemic administration. Local antibiotic delivery in orthopaedic surgery is widely practiced 2 through the use of antibiotic impregnated polymethylmethacrylate (PMMA) beads that are implanted for the treatment of orthopaedic infections. This practice has several recognized disadvantages that include the need to administer the PMMA depot through an open surgical procedure, lack of PMMA degradability, the need for its removal through a secondary surgery, poor drug release characteristics from PMMA, and its poor utility for infection prophylaxis.3 Over the past decade, researchers have developed biodegradable polymeric scaffolds for use in bone infections made from poly(glycolic/lactic) acid, fibrin, chitosan, collagen, hydroxyapatite, and other polymeric carriers4,5,6,7. While these approaches are generally successful for providing long-term sustained release of therapeutic antibiotic concentrations, drug loading is often accomplished via drug mixing with polymers dissolved in harsh solvents. Furthermore, few of these scaffolds are deliverable via syringe injection in a minimally invasive manner, although subcutaneous injection of a polymeric paste has recently been demonstrated for sustained release of gentamycin8. There remains a need for a polymeric local drug delivery system in the treatment and prophylaxis of orthopaedic infections, with capabilities of both minimally invasive delivery and ease of antibiotic drug loading.
Elastin-like polypeptides (ELPs) have been developed as injectable and in situ-forming drug depots for local delivery to the joint space9, perineural cavity10, and solid tumors11, and have demonstrated efficacy for transdermal drug delivery from crosslinked bioelastic membranes12. ELPs are biopolymers constructed of repeating pentapeptide sequences from native human elastin (V-P-G-X-G, where X may be any amino acid except proline).13,14. ELPs are attractive for drug delivery applications as they undergo a thermally triggered phase transition at a temperature (Tt) associated with a hydrophobic intra-molecular collapse that t may serve as an effective drug depot15. The environmental sensitivity of ELPs enables the biopolymer to be soluble in aqueous solution below Tt, yet to form micron-size particles at body temperature when higher than Tt. ELPs may be genetically engineered, enabling gene-level control of the molecular weight, peptide sequence, and coupling to bioactive peptides that may include drugs, reactive peptides, or cell-binding sequences16,17,18-20. When coupled to the ELP, or entrapped within a collapsed ELP matrix, a therapeutic agent may be released as molecules in the depot re-solubilize, thus reducing the size of the aggregated mass over time. Additionally, ELP sequences are biocompatible, biodegradable, and non-immunogenic21,22 all of which make ELPs well suited for biomedical applications.
The objective of this study was to develop an injectable, in situ forming ELP that could provide for sustained antibiotic release in orthopaedic infections. Genetically engineered ELPs were modified to enable loading with the antibiotics, cefazolin and vancomycin, and evaluated in vitro for their ability to provide for prolonged release of biological active drugs, and to evaluate their formulation viscosity to test suitability as an injectable drug carrier.
Materials and Methods
Synthesis and Preparation of Elastin-Like Polypeptides Constructs
The gene encoding the amino acid sequence VPGKG(VPGVG)16-102 was obtained from Chilkoti and co-workers23. This ELP sequence is termed KV16-102 where the subscript 16 denotes the repeats of the corresponding pentapeptide, and 102 denotes the repeats of the 17 pentapeptide unit (MW 42.7 kDa). The ELP was expressed in Escherichia coli BLR(DE3) (Novagen, Madison, WI) and purified as described previously.16,23 Purified ELPs were processed by crosslinking to enable a high degree of drug entrapment, while enabling preservation of the thermally triggered phase transition, as follows. A trifunctional, water-soluble crosslinker, β-[Tris(hydroxymethyl) phosphino] proprionic acid (THPP) (Pierce Biotechnology, Rockford, IL) was solubilized in PBS to a final concentration of 250mg/ml24. THPP is an organophosphorous crosslinker that links phosphines to the primary and secondary amines on the ELP. ELP constructs formed from THPP crosslinking have been described for ELP sequences with a much higher density of amine-reactive sites than KV16-102 sequences used here24. Based on this prior work, the range of pore sizes for the crosslinked ELP constructs was expected to exceed 10-30 microns. For each sample (Group I, n=35), 3 μl of the THPP solution were added to 400μl of KV16-102 at a concentration of 150mg/ml via manual pipetting. This resulted in a 1:1 molar ratio of THPP reactive sites to primary amines on the ELP molecules that could participate in crosslinking. In order to test for an effect of ELP concentration on drug entrapment and release kinetics, ELP constructs were also prepared at 225 mg/ml in a subset of samples (Group II, n=15) with a volume of THPP for a 1:1 molar ratio of THPP:ELP. The resultant mixtures were agitated for 30 minutes at 4°C to provide for thorough mixing and heated to 37°C, initiating a phase transition that was observed as a change in solution turbidity. After incubating for 24hrs, the mixture was frozen at -80°C for 30 minutes, lyophilized for 24 hours, and rehydrated for 24 hours in a glycine-containing solution to quench the crosslinking process (4:1 molar excess glycine: THPP reactive sites). This process was performed to insure against inadvertent covalent cross-linking of the antibiotic to THPP. The mixture was again frozen at -80°C for 30 minutes, lyophilized for 24 hours and the water weight loss and associated volume was recorded.
Antibiotic Loading
In order to achieve a targeted and uniform mass concentration of antibiotic in each construct, all crosslinked ELP constructs were rehydrated in antibiotic solutions prepared in PBS as described. For Group I experiments, constructs were rehydrated with a PBS solution (volume ~ 90% of water weight loss) containing: (1) 5mg, 10mg, or 20mg of vancomycin hydrochloride (MW=1,449 Da, Sigma-Aldrich, Inc, St. Louis, MO; (2) 5mg, 10mg, or 20mg of cefazolin sodium salt (MW=454 Da, Sigma-Aldrich); or (3) no antibiotic (control). Five replicates of each drug loading were used. These conditions gave rise to drug-to-peptide molar ratios of 2.4:1, 4.8:1, 9.8:1 for vancomycin, and 7.8:1, 15.6:1, 31.1:1, respectively for cefazolin.
In Group II samples, constructs were rehydrated with a PBS solution containing: (1) 7.5mg of vancomycin hydrochloride; (2) 7.5mg of cefazolin sodium salt; or (3) no antibiotic (control). The drug mass was chosen to compare constructs prepared with ELP at 225mg/ml against those with ELP at 150mg/ml.
Antibiotic Release Assay
In order to evaluate the kinetics of antibiotic release, each antibiotic-loaded ELP gel was placed into a reaction vessel (2 × 1.5 mm) with only one surface available for diffusion. A volume of PBS (3 ml) was added to each reaction vessel and samples were placed at 37°C. At 37°C, it has been shown that crosslinked ELPs will undergo a transition to a gel-like phase within five minutes 25, that was observed here as a change in opacity of the ELP constructs. The supernatant was removed at the start of the experiment, and replaced with warmed PBS over time to insure maintenance of dilute bath conditions. For vancomycin-loaded samples, the supernatant was completely exchanged on days 1-14, 21, and 28, and for cefazolin-loaded samples, the supernatant was completely replaced on days 1-10 (due to faster drug release). At the end of the experiment, 2 ml of PBS was added to each construct at 4°C in order to resolubilize the material, and aliquots taken to record total antibiotic remaining. Absorbance readings at 280nm and 270nm were recorded for vancomycin and cefazolin, respectively (UVmini-1240, Shimadzu Corp., Kyoto, Japan), and converted to antibiotic concentrations using a standard curve. All absorbance measurements were adjusted for mean values of PBS-loaded ELP controls prior to determining antibiotic concentration.
Measured vancomycin and cefazolin concentrations released from the ELP constructs were numerically matched to a model of one-dimensional, unsteady diffusion to determine a time constant for drug release for comparison across formulations. The mass fraction of drug released to the overlying bath, x(t), [x(t) = m(t)/mi] is given by (adapted from 26),
where m(t) is the mass of drug released to the supernatant at time t, xo is the mass fraction of drug released instantaneously into the supernatant at time zero (normalized to mi), and mi is the total mass of drug loaded into the ELP gel. The time constants of release, τ, and mass fraction undergoing immediate burst release, xo, were determined by numerical fitting of cumulative antibiotic release to this model using non-linear least-squares regression (fmincon, constrained optimization, MATLAB, MathWorks, Natick MA).
Antibiotic Bioassay
To determine if the eluted antibiotics remained bioactive, disk diffusion susceptibility bioassays were performed for a subset of samples using Bacillus subtilis (ATCC 6633). Bacterial suspensions were prepared in tryptic soy broth following manufacturer recommendations (Lyfo Disk, MicroBiologics, St. Cloud, MN), then swabbed onto petri dishes containing Mueller-Hinton agar (n=3 replicates per condition). Twenty microliters of calibrating antibiotic solutions over the range of 0.05-2 mg/ml were pipetted onto 6mm filter paper disks applied to the surface of the agar, and the plates incubated for 18 hours at 37°C. Zones of inhibition (ZOI) were measured optically27, with a lower ZOI limit of 0.05mg/ml for vancomycin and 0.5mg/ml for cefazolin. Supernatants from vancomycin-laden ELP samples (150mg/ml ELP, 20 mg vancomycin, n=5) were collected at days 1, 7, 14, 21, and 28, as well as a sample of the final re-solubilized construct, and similarly tested. Supernatants from five cefazolin-laden samples (150mg/ml ELP, 20 mg cefazolin) were collected at days 1, 2, and 3, and from the final homogenized construct, and similarly tested. ZOI were compared to those obtained for the calibrating standards in order to estimate the bioactivity of the drug released into the supernatant from the ELP-loaded construct.
Rheology of ELP Preparation
In order to determine the suitability of the resultant preparation for delivery via syringe injections, the viscosity of the ELP constructs were tested in a dynamic shearing experiment. Volumes of uncrosslinked and THPP-crosslinked ELPs at 150 mg/ml (~ 400 μl) were placed upon the lower platen of a low-force displacement-controlled rheometer (ARES, TA Instruments, New Castle DE) at 4°C to insure a temperature below Tt. The upper cone was brought into close contact for testing, and a strain-controlled dynamic frequency sweep test was performed, as described previously (γ0=1, ω = 1-100 rad/s28) in order to determine the dynamic viscosity |η*|. The dynamic viscosity, |η*|, represents the frequency-dependent viscous drag of a viscoelastic material.
Statistical Analysis
Time constants of drug release (τ) and burst release mass fraction (xo) from different formulations were compared using one-factor ANOVA with post-hoc Dunn’s test, at the α = 0.05 level of significance. JMP 6 statistical software (SAS Institute, Cary, NC, USA) was used to determine differences amongst three antibiotic doses (5mg, 10mg, 20mg) for Group I ELP samples (150 mg/ml ELP). Additionally, one-factor ANOVA was used to test for an effect of ELP concentration between Group I (150 mg/ml ELP) and Group II samples (225 mg/ml ELP) for vancomycin or cefazolin-loaded samples.
Results
Antibiotic Release Profiles
Values for the time constants (τ) and mass fraction initially released (x0) are summarized in Table 1. For cefazolin-loaded ELP constructs, there was very little instantaneous, or burst release upon introduction of the PBS supernatant in the reaction vessel, with values for all Group I samples that were not appreciably different from control samples (average x0 = -0.01) and did not vary across drug loading doses. The time constant of cefazolin release varied with drug loading dose (p<0.05, ANOVA), however, with values between τ = 20-32h, corresponding to approximately 95% drug release in less than 4 days. Slower release rates were observed for the ELP samples loaded with 5 mg cefazolin, as compared to 10 mg or 20 mg samples (p < 0.002, Dunn’s test). While the longer-term release profile appeared flat, cefazolin concentration in the supernatant continued to decrease over time to a final minimum value of 4 μg/ml at day 10. The reported minimal inhibitory concentration (MIC) for cefazolin against E. coli (ATCC 25922) and S. aureus (ATCC 29213) is 2μg/ml, demonstrating that the antibiotic concentrations released from these ELP constructs of 400 μl volume were always above the MIC29.
Table 1.
Parameters of Drug Release Model from ELP Constructs
| drug dose (mg) | time constant τ (hours) | burst release fraction (mo) | |
|---|---|---|---|
| Cefazolin | |||
| Group I (150 mg/ml ELP) | 5 | 32 ± 3 | -0.006 ± 0.007 |
| 10 | 25 ± 3 * | -0.013 ± 0.001 | |
| 20 | 24 ± 2 * | -0.013 ± 0.002 | |
|
| |||
| Group II (225 mg/ml ELP) | 7.5 | 32 ± 2 | -0.018 ± 0.002 |
|
| |||
| Vancomycin | |||
| Group I (150 mg/ml ELP) | 5 | 480 ± 180 | 0.06 ± 0.03 |
| 10 | 550 ± 30 | 0.30 ± 0.04 | |
| 20 | 530 ± 180 | 0.55 ± 0.10 | |
|
| |||
| Group II (225 mg/ml ELP) | 7.5 | 1170 ± 90 ** | 0.13 ± 0.05 |
n=5 per group unless otherwise indicated
p<0.05, significantly different from 5 mg drug construct, Dunn’s test
p<0.05, significantly different from 5 mg drug : 150 mg ELP construct, ANOVA
An effect of ELP concentration on drug release was evaluated by increasing the ELP concentration to 225 mg/ml for Group II samples, while keeping the drug to ELP molar ratio constant with Group I samples. The time constants and burst release fraction for Group II samples were not significantly different from corresponding Group I samples (p >0.5, ANOVA, Table 1), however, suggesting that ELP concentration did not have an effect on drug release kinetics for cefazolin.
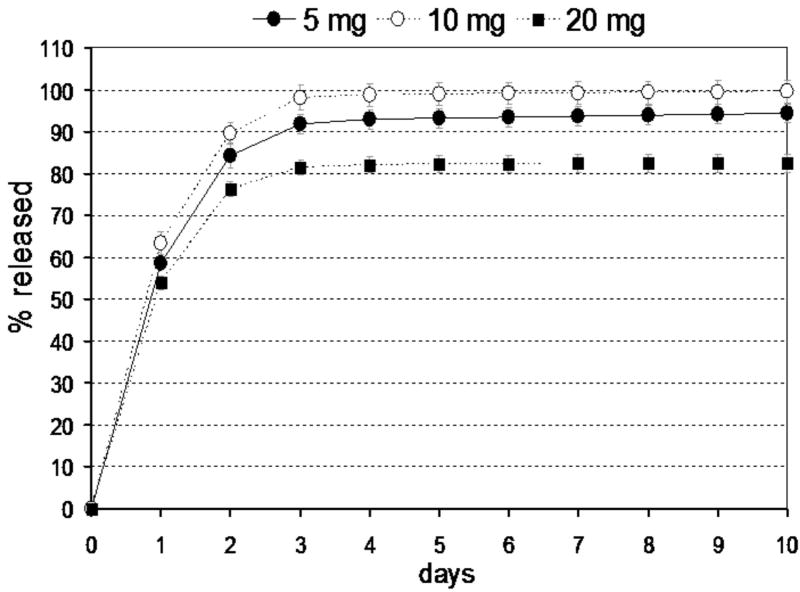
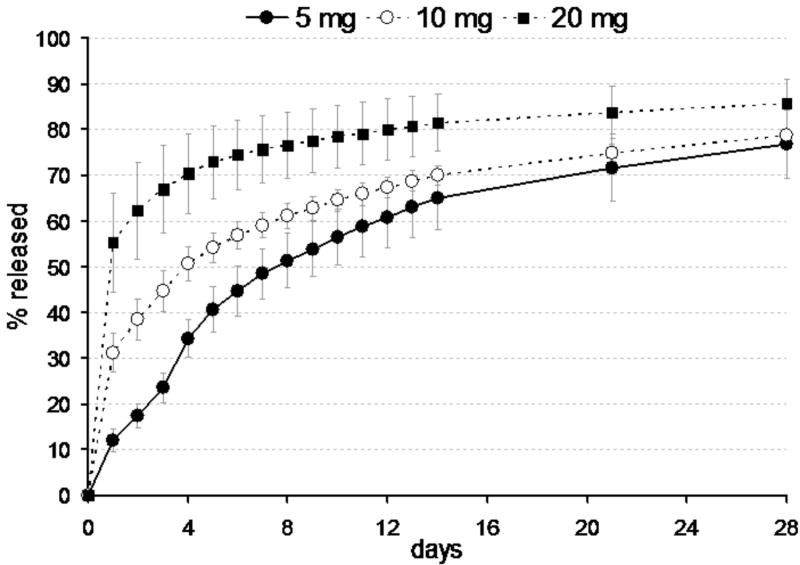
Burst release fraction (x0) from vancomycin-loaded ELP constructs was much greater than for cefazolin-loaded ELP constructs, with values that varied with drug loading dose (p<0.01, see Table 1, Group I samples). This burst release fraction reflects drug that was not fully entrapped within the construct at the start of the experiment, suggesting that the ELP was more effective at entrapping cefazolin than vancomycin at these concentrations. The time constant of vancomycin release was dramatically higher than that for cefazolin, and exhibited no trend with differences in drug loading dose (p>0.5, ANOVA). Average values for τ and x0 across all samples were 520 hours and 0.3, corresponding to approximately 95% drug release within 60 days. The greatest mass fraction of vancomycin released from all Group I samples occurred for the 20 mg drug-loaded group (85% at 28 days). As for the cefazolin-loaded constructs, the concentration of vancomycin in the supernatant across all testing conditions and times was above the reported MIC (0.5-3μg/ml) for vancomcyin against S. aureus.29-31, again demonstrating that this drug-loaded ELP construct would release therapeutic concentrations of vancomycin at all times.
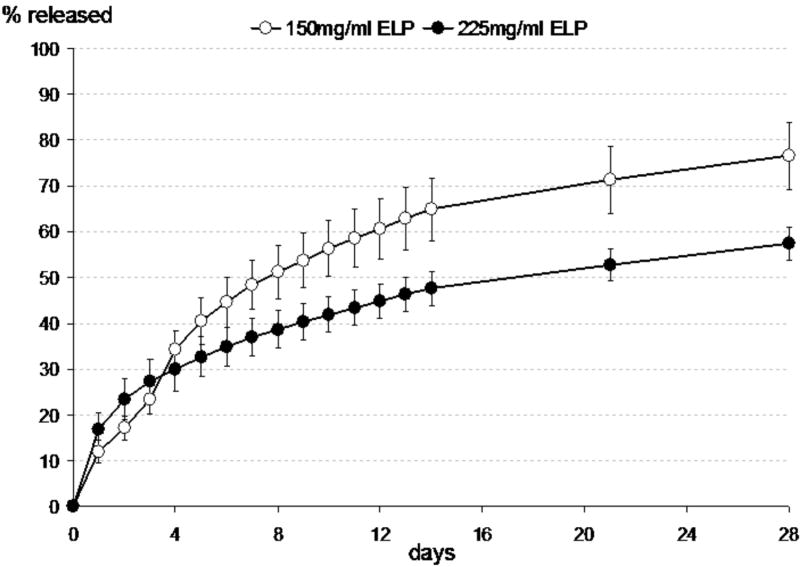
In Group II samples, the time constant, but not burst release fraction, was significantly different from corresponding Group I samples (p <0.05, ANOVA, Table 1, Figure 3). By increasing the concentration of ELP from 150mg/ml to 225mg/ml, the average time constant increased from ~480 hours to ~1100 hours, with values indicating that 95% of entrapped drug would be released only after 120 days.
Figure 3.
Release of vancomycin from 5 mg drug-loaded ELP constructs prepared at 150mg/ml ELP (Group I samples) and 225 mg/ml ELP. Data presented as averages and standard deviations for n=5 per group.
Bioactivity of Released Antibiotics
Vancomycin and cefazolin released into the supernatant from 20 mg drug-loaded ELP constructs were tested for bioactivity against B. subtilis using the disk diffusion susceptibility method (Group I samples only, Figure 1 for vancomycin only). All collected supernatants tested demonstrated bioactivity. The ZOIs for all cefazolin samples on days 1-3 were estimated to fall between 0.05 – 0.5 mg/ml as determined from experiments with calibrating standards. These estimated concentrations corresponded to supernatant concentrations separately measured using UV-Vis spectrophotometry, suggesting excellent bioactivity of the released antibiotic. Additionally, the ZOIs for antibiotic remaining in the sample after resolubilization at day 10 similarly corresponded to the remaining amount of cefazolin calculated from x0 and cumulative release measures.
Figure 1.
Release of cefazolin from ELP constructs prepared at 150mg/ml ELP (Group I samples). Data presented as averages and standard deviations for n=5 per group.
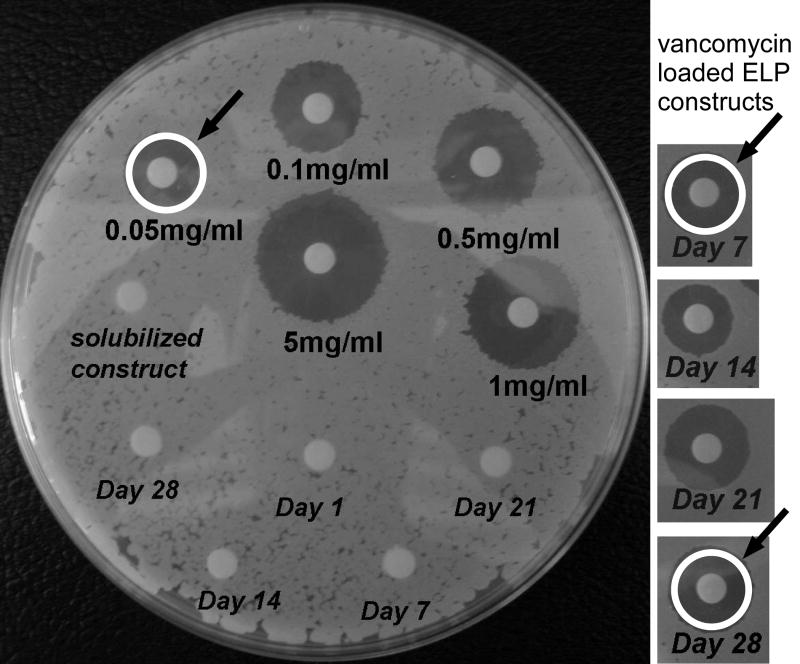
The ZOIs in supernatants from all vancomycin loaded samples on all days were estimated to fall between 0.05 - 2 mg/ml, and also compared well to supernatant concentrations measured via spectrophotometry as determined from experiments with calibrating standards (Figure 4). As for cefazolin, the ZOIs for vancomycin in the sample after resolubilization at day 28 corresponded to that calculated from x0 and cumulative release measures.
Figure 4.
Representative agar plate assay showing method for determining the zone of inhibition (ZOI) following antibiotic placement upon substrates cultured with B. subtillis. Plate shown on left illustrates ZOI for calibrating solutions of vancomycin, along with ELP control samples cultured for periods of 1 to 28 days (italic labels). The white ring (with arrow) illustrates a typical measurement of diameter for ZOI calculation. Panels on right illustrate B. subtillis cultures after exposure to supernatant from vancomycin-loaded ELP constructs after 7 to 28 days of culture. Comparison of white ring sizes demonstrates that activity of supernatants was equivalent to 0.05-0.1 mg/ml vancomycin.
Rheology of ELP Preparation
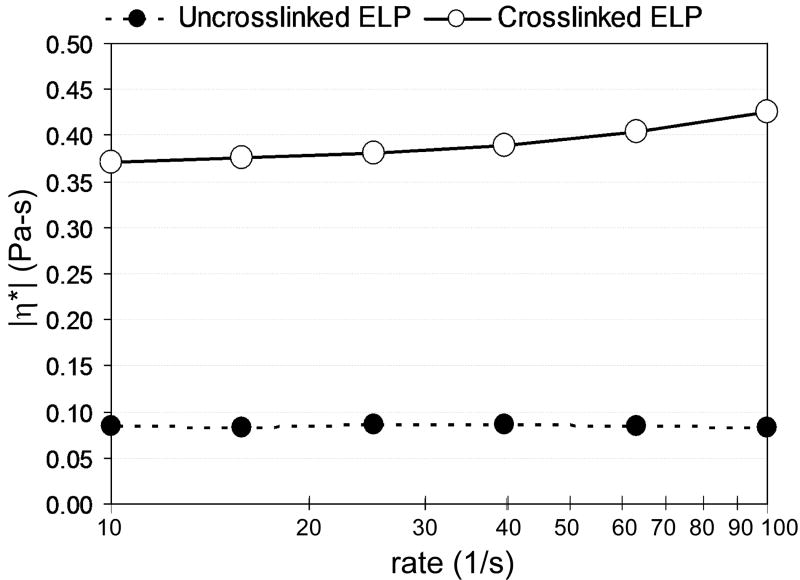
Viscosity of the uncrosslinked ELP (150 mg/ml) appeared to be independent of frequency of oscillation with an average value for |η*| of ~0.08 Pa-s (Figure 5). After THPP crosslinking to prepare the construct for drug loading, the viscosity of the ELP increased dramatically, with an |η*| of ~0.37 Pa-s at 10-1 seconds (for comparison, the dynamic viscosity of synovial fluid is ~ 1 Pa-s32).
Figure 5.
Dynamic viscosity (|η*|) measures for a representative ELP construct, before and after preparation by THPP crosslinking (150 mg/ml). A frequency dependence was observed for the crosslinked constructs, but not the uncrosslinked ELP.
Discussion
The objective of this study was to develop an injectable, drug containing ELP depot from which bioactive antibiotics could be slowly released over time. Results of this study demonstrate that a thermally triggered ELP can successfully entrap and lead to long-term sustained release of vancomycin, and cefazolin to a lesser extent. Both antibiotics released from the ELP depot were shown to retain bioactivity very nearly equivalent to that of the free drug. Rehydration of a lyophilized ELP construct in the drug solution was performed to insure permeation of the solution and solute (drug) throughout the network prior to thermally-induced hydrophobic collapse. The hydrophobic collapse of ELP has been shown to “shed water” or reduce gel volume which has been a very effective means to entrap cells28 and presumably reduce pore size. Crosslinking of the ELP was pursued here, however, as ELP entrapment of highly hydrophilic drugs will lead to phase partitioning of the drug into the solution volume and not into the retained drug carrier. Crosslinking the ELP as performed here led to a molecular network that likely reduced pore size during thermally triggered hydrophobic collapse and minimized the substantial volumetric changes observed for uncrosslinked ELP. Drugs have been successfully loaded into crosslinked ELP membranes in previous work12,33, although the extent of crosslinking led to formation of a solid material that does not undergo thermally-triggered physical changes. In this study, crosslinking was used only to generate a molecular network that retained the properties of a thermosensitive gel capable of being injected. Such an ELP carrier able to entrap or be loaded with cefazolin and vancomycin, very hydrophilic and low MW antibiotics, could provide for sustained antibiotic release in the treatment of and prophylaxis against orthopaedic infections.
Cefazolin had a faster rate of drug release from ELP constructs, but no burst release effect, as compared to vancomycin under all testing conditions, suggesting that size or physical chemistry of the entrapped molecule is a key factor in determining entrapment efficiency and release rate from ELP. Furthermore, the time constant for release of cefazolin exhibited an unusual trend with amount of entrapped drug, with the lowest drug loading dose (i.e., 5 mg cefazolin) associated with the longest sustained-release. It appears that the cefazolin-loaded constructs become “saturated” above 5 mg of loaded drug, with little ability for the ELP to slow release of the low MW cefazolin at higher doses. There was no burst release effects for cefazolin at higher drug loading doses, however, indicating that the carrier retains an ability to initially interact with and entrap drug at these doses. Nonetheless, the time constant of release was so rapid as to confer only a minor benefit for the carrier in providing for sustained release of cefazolin. Vancomycin-loaded ELP constructs exhibited markedly different behaviors, with a trend of higher burst release fractions at higher drug loading doses. This supports the mechanism that ELP constructs become saturated by drug above a critical value, which fell below 5 mg for vancomycin. Once the critical drug dose is entrapped within the ELP construct, the kinetics of drug release were very similar with dramatically long time constants of ~ 520 hours. Thus, ELP demonstrated the desired behaviors of high drug entrapment and sustained release for the 1.5 kDa MW vancomycin. It is not known if the differences between cefazolin and vancomycin entrapment and release behaviors relate to modest differences in their hydrophilicity, charge or molecular weight; additional studies would be required to determine if ELP is most suitable for molecules above a molecular weight “cut-off”.
An interesting finding of this study was the significantly higher time constant of drug release for vancomycin when the concentration of ELP was increased from 150mg/ml to 225mg/ml, while maintaining drug loading dose. The prolonged release of vancomycin is likely attributed to greater collapse of the ELP network, and hence pore space available for diffusion, thereby limiting release of vancomycin into solution. Increasing the concentration of ELP had no effect on the release rate of cefazolin, suggesting that the crosslinking used may be associated with an apparent porosity after hydrophobic collapse that does not limit the smaller cefazolin molecule. It is possible that further increasing the concentration of ELP may slow the release rate of smaller molecules. Likewise, for molecules larger than vancomycin, it is possible that increasing ELP concentration could decrease release rates beyond what was observed here.
For all constructs, the antibiotic released was shown to be bioactive against relevant strains of bacteria. When drugs are conjugated or fused to ELPs through genetic design17,18,34,35, the ELP domain may interfere with accessibility to the drug that gives rise to reduced bioactivity. When ELP molecules are released into solvent, they may also non-specifically interact with drugs and reduce drug bioactivity. The more simple entrapment of a drug as performed here gave no evidence of ELP interfering with antibiotic bioactivity, either for drug released into the supernatant or for drug remaining in the ELP construct at the end of the experiment. This observation suggests that ELP entrapment of drugs in this system could be applied to other drugs that would not be appropriate for covalent coupling or genetically-designed fusion (e.g., antibodies, DNA, RNA).
An important design requirement for the ELP drug carrier system was a viscosity that was suitable for delivery via syringe and needle. The viscosity was comparable to values reported for synovial fluid, with values that were much lower than that for most injectable hyaluronan preparations (0.2-200 Pa-s36). This property will enable minimally invasive delivery of drug and carrier to intramedullary spaces, fracture sites, and other local sites for orthopaedic applications, and may find broader use for local drug delivery in other applications. Additional studies would be required to confirm localization of the injected ELP into the targeted compartment.
These experiments have demonstrated that an injectable, in situ forming ELP depot can provide for sustained release of antibiotics in vitro. ELPs have some important advantages for drug delivery as they are known to be biocompatible with tissue, tissue fluids, and blood (ASTM recommendations for materials and devices)21, they are biodegradable37, and elicit no immune response21. These are distinct and obvious advantages over the currently used drug carrier for orthopaedic applications, polymethylmethacrylate (PMMA). Although effective in drug release, they are not without limitations for infection treatment or prophylaxis.3,38,39. ELPs may also provide some advantages over recently developed synthetic polymeric systems that make use of harsh solvents during the entrapment process. The approach developed here has the potential to provide an alternative to the current standard-of-care for musculoskeletal system infections, and to additionally contribute to minimally-invasive prophylaxis strategies that are not currently explored. Further in vivo studies are needed, however, to characterize in vivo bio-localization, degradation, systemic exposure and drug release profiles, as well as the potential of this drug delivery vehicle to modify clinical outcomes.
Figure 2.
Release of vancomycin from ELP constructs prepared at 150mg/ml ELP (Group I samples). Data presented as averages and standard deviations for n=5 per group.
Acknowledgments
This work was supported with funds from the NIH (R01EB002263, NIH R21AR052745), and fellowships from Pratt-Gardner Fellows and Pratt Fellows program.
References
- 1.Darouiche RO. Current concepts - Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 2.Buchholz HW, Elson RA, Engelbrecht E, Lodenkamper H, Rottger J, Siegel A. Management of Deep Infection of Total Hip-Replacement. J Bone Joint Surg (Br) Volume. 1981;63(3):342–353. doi: 10.1302/0301-620X.63B3.7021561. [DOI] [PubMed] [Google Scholar]
- 3.Heijink A, Yaszemski MJ, Patel R, Rouse MS, Lewallen DG, Hanssen AD. Local antibiotic delivery with OsteoSet (R), DBX (R), and Collagraft (R) Clin Orthop. 2006;451:29–33. doi: 10.1097/01.blo.0000229319.45416.81. [DOI] [PubMed] [Google Scholar]
- 4.Garvin KL, Miyano JA, Robinson D, Giger D, Novak J, Radio S. Polylactide/polyglycolide antibiotic implants in the treatment of osteomyelitis. A canine model. J Bone Joint Surg (Am) 1994;76(10):1500–6. doi: 10.2106/00004623-199410000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Garvin K, Feschuk C. Polylactide-polyglycolide antibiotic implants. Clin Orthop. 2005;437:105–10. doi: 10.1097/01.blo.0000175720.99118.fe. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun JH, Mader JT. Treatment of osteomyelitis with a biodegradable antibiotic implant. Clin Orthop. 1997;341:206–14. [PubMed] [Google Scholar]
- 7.Gursel I, Korkusuz F, Turesin F, Alaeddinoglu NG, Hasirci V. In vivo application of biodegradable controlled antibiotic release systems for the treatment of implant-related osteomyelitis. Biomaterials. 2001;22(1):73–80. doi: 10.1016/s0142-9612(00)00170-8. [DOI] [PubMed] [Google Scholar]
- 8.Krasko MY, Golenser J, Nyska A, Nyska M, Brin YS, Domb AJ. Gentamicin extended release from an injectable polymeric implant. J Control Release. 2007;117(1):90–96. doi: 10.1016/j.jconrel.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, Setton LA. A thermally responsive biopolymer for intra-articular drug delivery. J Control Release. 2006;115(2):175–182. doi: 10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Shamji MF, Whitlatch LW, Friedman AH, Richardson WJ, Chilkoti A, Setton LA. An Injectable and In Situ-Gelling Biopolymer for Sustained Drug Release Delivery Following Perineural Administration. Spine. 2008;33(7):748–754. doi: 10.1097/BRS.0b013e3181695773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu WE, Dreher MR, Furgeson DY, Peixoto KV, Yuan H, Zalutsky MR, Chilkoti A. Tumor accumulation, degradation and pharmacokinetics of elastin-like polypeptides in nude mice. J Control Release. 2006;116(2):170–178. doi: 10.1016/j.jconrel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Kemppainen BW, Urry DW, Luan CX, Xu J, Swaim SF, Goel S. In vitro skin penetration of dazmegrel delivered with a bioelastic matrix. Int J Pharm. 2004;271(1-2):301–3. doi: 10.1016/j.ijpharm.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Urry DW. Free-energy transduction in polypeptides and proteins based on inverse temperature transitions. Prog Biophys Mol Biol. 1992;57(1):23–57. doi: 10.1016/0079-6107(92)90003-o. [DOI] [PubMed] [Google Scholar]
- 14.Urry DW, Pattanaik A, Xu J, Woods TC, McPherson DT, Parker TM. Elastic protein-based polymers in soft tissue augmentation and generation. J Biomater Sci Polym Ed. 1998;9(10):1015–48. doi: 10.1163/156856298x00316. [DOI] [PubMed] [Google Scholar]
- 15.Chilkoti A, Christensen T, MacKay JA. Stimulus responsive elastin biopolymers: Applications in medicine and biotechnology. Curr Opin Chem Biol. 2006;10(6):652–7. doi: 10.1016/j.cbpa.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer DE, Chilkoti A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: Examples from the elastin-like polypeptide system. Biomacromolecules. 2002;3(2):357–367. doi: 10.1021/bm015630n. [DOI] [PubMed] [Google Scholar]
- 17.Bidwell GL, 3rd, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther. 2005;4(7):1076–85. doi: 10.1158/1535-7163.MCT-04-0253. [DOI] [PubMed] [Google Scholar]
- 18.Shamji MF, Betre H, Kraus VB, Chen J, Chilkoti A, Pichika R, Masuda K, Setton LA. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: Sustained release of a local antiinflammatory therapeutic. Arthritis Rheum. 2007;56(11):3650–3661. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- 19.Liu JC, Heilshorn SC, Tirrell DA. Comparative cell response to artificial extracellular matrix proteins containing the RGD and CS5 cell-binding domains. Biomacromolecules. 2004;5(2):497–504. doi: 10.1021/bm034340z. [DOI] [PubMed] [Google Scholar]
- 20.Megeed Z, Winters RM, Yarmush ML. Modulation of single-chain antibody affinity with temperature-responsive elastin-like polypeptide linkers. Biomacromolecules. 2006;7(4):999–1004. doi: 10.1021/bm0507002. [DOI] [PubMed] [Google Scholar]
- 21.Urry DW, Parker TM, Reid MC, Gowda DC. Biocompatibility of the Bioelastic Materials, Poly(Gvgvp) and Its Gamma-Irradiation Cross-Linked Matrix - Summary of Generic Biological Test-Results. J Bioact Compat Poly. 1991;6(3):263–282. [Google Scholar]
- 22.Wang NZ, Urry DW, Swaim SF, Gillette RL, Hoffman CE, Hinkle SH, Coolman SL, Luan CX, Xu J, Kemppainen BW. Skin concentrations of thromboxane synthetase inhibitor after topical application with bioelastic membrane. J Vet Pharmacol Ther. 2004;27(1):37–43. doi: 10.1046/j.0140-7783.2003.00544.x. [DOI] [PubMed] [Google Scholar]
- 23.Trabbic-Carlson K, Setton LA, Chilkoti A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules. 2003;4(3):572–80. doi: 10.1021/bm025671z. [DOI] [PubMed] [Google Scholar]
- 24.Lim DW, Nettles DL, Setton LA, Chilkoti A. Rapid Cross-Linking of Elastin-like Polypeptides with (Hydroxymethyl)phosphines in Aqueous Solution. Biomacromolecules. 2007;8(5):1463–1470. doi: 10.1021/bm061059m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim DW, Nettles DL, Setton LA, Chilkoti A. In situ cross-linking of elastin-like polypeptide block copolymers for tissue repair. Biomacromolecules. 2008 Jan;9(1):222–30. doi: 10.1021/bm7007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truskey GA, Yuan F, Katz DF. Upper Saddle River. New Jersey: Prentice Hall; 2003. Transport Phenomena in Biological Systems. [Google Scholar]
- 27.Barry AL, Coyle MB, Thornsberry C, Gerlach EH, Hawkinson RW. Methods of Measuring Zones of Inhibition with the Bauer-Kirby Disk Susceptibility Test. Journal Clin Microbiol. 1979;10(6):885–889. doi: 10.1128/jcm.10.6.885-889.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Betre H, Setton LA, Meyer DE, Chilkoti A. Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules. 2002;3(5):910–916. doi: 10.1021/bm0255037. [DOI] [PubMed] [Google Scholar]
- 29.Hasselmann C. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) - European Committee for Antimicrobial Susceptibility Testing (EUCAST) -Determination of minimum inhibitory concentration (MIC) by agar dilution. Clin Microbiol Infect. 2000;6(5):1–7. [Google Scholar]
- 30.Geraci JE, Hermans PE. Vancomycin. Mayo Clin Proc. 1983;58(2):88–91. [PubMed] [Google Scholar]
- 31.Washington JA, Hermans PE, Martin WJ. In-Vitro Susceptibility of Staphylococci and Streptococci and Influence of Agar Media on Minimum Inhibitory Concentration. Mayo Clin Proc. 1970;45(7):527–535. [PubMed] [Google Scholar]
- 32.Safari M, Bjelle A, Gudmundsson M, Hogfors C, Granhed H. Clinical assessment of rheumatic diseases using viscoelastic parameters for synovial fluid. Biorheology. 1990;27:659–674. doi: 10.3233/bir-1990-27504. [DOI] [PubMed] [Google Scholar]
- 33.Wang NZ, Urry DW, Swaim SF, Gillette RL, Hoffman CE, Hinkle SH, Coolman SL, Luan CX, Xu J, Kemppainen BW. Skin concentrations of thromboxane synthetase inhibitor after topical application with bioelastic membrane. J Vet Pharmacol Ther. 2004;27(1):37–43. doi: 10.1046/j.0140-7783.2003.00544.x. [DOI] [PubMed] [Google Scholar]
- 34.Meyer DE, Trabbic-Carlson K, Chilkoti A. Protein purification by fusion with an environmentally responsive elastin-like polypeptide: Effect of polypeptide length on the purification of thioredoxin. Biotechnol Prog. 2001;17(4):720–728. doi: 10.1021/bp010049o. [DOI] [PubMed] [Google Scholar]
- 35.Trabbic-Carlson K, Liu L, Kim B, Chilkoti A. Expression and purification of recombinant proteins from Escherichia coli: Comparison of an elastin-like polypeptide fusion with an oligohistidine fusion. Protein Sci. 2004;13(12):3274–3284. doi: 10.1110/ps.04931604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borzacchiello A, Mayol L, Ambrosio L, Garskog O, Dahlqvist A. Rheological Characterization of Vocal Folds after Injection Augmentation in a Preliminary Animal Study. J Bioact Compat Polym. 2004;19:331–341. [Google Scholar]
- 37.Ong SR, Trabbic-Carlson KA, Nettles DL, Lim DW, Chilkoti A, Setton LA. Epitope tagging for tracking elastin-like polypeptides. Biomaterials. 2006;27(9):1930–1935. doi: 10.1016/j.biomaterials.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 38.DiCicco M, Duong T, Chu A, Jansen SA. Tobramycin and gentamicin elution analysis between two in situ polymerizable orthopedic composites. J Biomed Mat Res B Appl Biomater. 2003;65B(1):137–149. doi: 10.1002/jbm.b.10528. [DOI] [PubMed] [Google Scholar]
- 39.Mader JT, Stevens CM, Stevens JH, Ruble R, Lathrop JT, Calhoun JH. Treatment of experimental osteomyelitis with a fibrin sealant antibiotic implant. Clin Orthop. 2002;403:58–72. doi: 10.1097/00003086-200210000-00011. [DOI] [PubMed] [Google Scholar]