Abstract
Chemokines and their receptors play essential roles in the development and function of multiple tissues. Chemokine expression, particularly CXCL12 and its receptor CXCR4, has prognostic significance in several cancers apparently due to chemokine mediated growth and metastatic spread. These observations provide the rationale for pursuing CXCR4 inhibition for cancer chemotherapy. However, the multiple homeostatic functions of CXCR4 may preclude global inhibition as a therapeutic strategy. Here I review CXCR4 signaling and how it might differ in normal and transformed cells with special emphasis on the role that altered CXCR4 counter-regulation might play in tumor biology. I propose that CXCR4 mediates unique signals in cancer cells as a consequence of abnormal counter-regulation and that this results in novel biological responses. The importance of testing this hypothesis lies in the possibility that targeting abnormal CXCR4 signaling might provide an anti-tumor effect without disturbing normal CXCR4 functions.
Keywords: Chemokine, CXCR4, CXCL12, cancer, desensitization
Normal development depends upon chemokines and their receptors [1–4]. In many instances, tumor progression also appears to require chemokines, particularly CXCL12 and its receptor CXCR4 [5]. This creates a therapeutic challenge. CXCR4 inhibition has significant anti-tumor effects in vivo [6, 7] but prolonged CXCR4 antagonist therapy, as would be required in cancer treatment, has significant toxicity that is attributable to disturbed CXCR4 homeostatic functions [8, 9]. The effects of CXCL12 on cancer cells may be distinguishable from those on normal cells. Herein, I will review the early events in normal CXCR4 signaling as well as the mechanisms of CXCR4 counter-regulation. I will examine data that indicates CXCR4 signaling in cancer is dysregulated, resulting in novel biological responses in cancer cells. Understanding these differences in signaling may provide the leverage to develop therapies that correct abnormal CXCR4 signaling while leaving homeostatic CXCR4 functions intact.
CXCR4 signaling in normal cells
CXCR4, like all chemokine receptors belongs to the superfamily of seven transmembrane domain heterotrimeric G protein-coupled receptors (GPCR). There are a plethora of potential intracellular events that can occur downstream of CXCR4 activation. Here I will summarize only the early events; those that involve direct interactions with the ligand bound receptor (Figure 1).
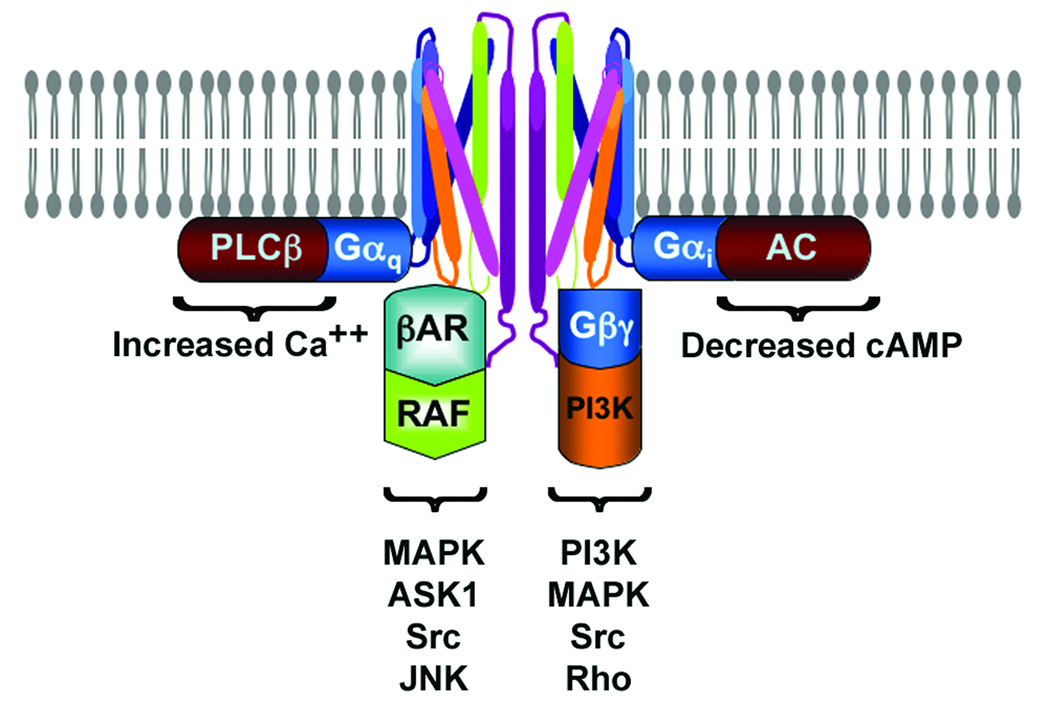
Figure 1. Ligand-Bound CXCR4 Dimers Signal Through Diverse Mediators.
A hypothetical dimer of CXCR4 is shown engaging Gαi and Gαq as well as Gβγ and β-arrestin (βAR). Each of these proteins transduce major components of CXCR4 signaling including: GαI inhibition of adenylyl cyclase (AC) and suppression of cAMP levels, Gαq - activation of phospholipase-β (PLC) and calcium flux, Gβγ - activation of PI3 kinase, MAP kinase (MAPK) as well as Src kinase and the monomeric G protein Rho. βAR – activation of MAPK, ASK1, Src and JNK.
CXCR4 signaling: The early events
Reminiscent of receptor tyrosine kinase signaling, CXCR4, and other GPCRs signal as receptor dimers [10]. Studies utilizing tagged CXCR4 constructs and bioluminescent and fluorescent resonance energy transfer techniques have demonstrated that CXCR4 exists as a homodimer under basal conditions and in the absence of CXCL12 [11–13]. Addition of CXCL12 promotes modest increases in bioluminescence resonance energy transfer (BRET) or fluorescence resonance energy transfer (FRET). This has alternately been interpreted to indicate either increased numbers of CXCR4 dimers or ligand-induced conformational changes, productive of more efficient energy transfer. The latter interpretation is supported in elegant studies performed by Percherancier et al. on the effects of CXCL12 and MCP-1 on heterodimers of their receptors CXCR4 and CCR2 [13]. In these experiments, BRET revealed CXCR4 and CCR2 heterodimers under basal conditions. When heterodimers composed of CXCR4-Rluc and CCR2-YFP receptor protomers were treated with MCP-1, BRET signal increased. When the opposite orientation heterodimer (CXCR4-YFP/CCR2-Rluc) was assayed, MCP-1 caused a significant decrease in BRET signal. As it is unlikely that these signal changes result from a difference in the number of heterodimers, these data suggest that the physical relationship between the luciferase and YFP moieties is affected by ligand and that the requisite directionality of energy transfer from Rluc to YFP results in similar ligand-induced conformational changes producing opposite effects on energy transfer. These studies support a model of GPCR signaling in which ligand-induced conformational changes in preformed receptor dimers is an initial step in receptor signaling. In addition they suggest caution when interpreting changes in resonance energy transfer signals.
CXCL12 binding to CXCR4 results in rapid tyrosine phosphorylation of the receptor in a JAK kinase dependent fashion [10]. In a series of experiments using pharmacological inhibitors of JAK kinases it was shown that the rapid recruitment of JAKs (JAK1, JAK2, JAK3 or TYK2) was required for CXCL12-induced calcium mobilization [10], as well as PI3 Kinase activation and tyrosine phosphorylation of focal adhesion proteins [14]. Inhibition of JAK kinases with AG490 or WHI-P131 abolished CXCL12 induced chemotaxis of CTS cells, thymocytes and primary hematopoietic progenitors [10, 14, 15]. Additionally, JAK3-deficient thymocytes and bone marrow progenitors exhibited impaired directed migration along a CXCL12 gradient [15]. Finally, JAK2-deficient γ2A cells have impaired CXCL12-induced Goci recruitment and calcium flux [16].
The studies described above suggest that JAK activity is essential to CXCR4 function. However, the importance of JAK kinases to CXCR4 signaling is called into question by additional data acquired in JAK2 and JAK3 deficient cells as well as HEK293 cells engineered to overexpress wildtype and dominant negative forms of JAK2 and JAK3 in which there are no effects of altered JAK expression on CXCL12-induced calcium flux or chemotaxis [17]. The basis for these discrepancies is unclear but a number of relevant factors can be identified. The use of pharmacological agents may be associated with non-specific effects resulting in the inhibition of non-target kinases. Differences in the level of expression of CXCR4 and downstream mediators of its signals could produce different results. In this regard, the JAK3 deficient thymocytes used by Soldevila et al. express lower than normal levels of CXCR4 protein and may exhibit deficient responses on this basis [17]. However, in the studies by Soriano et al., cells with similar levels of surface CXCR4 expression exhibited differing responses to CXCL12 that were correlated with differences in JAK expression or activity. Thus this potentially important pathway that could couple CXCR4 to cytokine signaling, tyrosine kinase pathways and immediate transcriptional responses through the STAT family of transcription factors needs to be better defined.
CXCR4 and G proteins
The family of chemokine receptors can couple to multiple Gα proteins [18, 19]. In the process of Gα activation, ligand-bound CXCR4 functions as a guanine nucleotide exchange factor or GEF, promoting the exchange of GDP for GTP within the Gα subunit [20]. This enables the association of Gα and the Gβγ subunits with their respective effectors.
There are over 20 known Gα subunit isoforms produced from 16 different genes in addition to 5 Gβ and 14 Gγ genes and subunits [21, 22]. The alpha subunits can be classified based on sequence homology into 4 groups: Gα(s/olf), Gα(i/o/ro/cone/gust/z), Gα(q/11/14/16) and Gα(12/13) (see [23] for review). The number of possible heterotrimers is large and several observations suggest that G proteins with specific subunit compositions have non-redundant functions in mediating GPCR signals. For example, somatostatin and muscarinic acetylcholine receptor regulation of L-type calcium channels requires heterotrimeric G proteins comprised with Gγ3 and Gγ4, respectively [24]. With regards to CXCR4 signaling, Holland et al. recently observed that CXCR4 mediated signals and invasive behaviors are differentially activated in breast cancer cells as a consequence of the stability of CXCR4-Gα-Gβγ complexes [25]. They suggest that varying biological responses to CXCL12 could result from changes in expression of G protein subunits with differing affinities for CXCR4.
Maghazachi was the first to show that CXCR4 could couple to multiple Gα isoforms [26]. By treating permeabilized NK cells with blocking antibodies directed against specific Gα subunits, he was able to demonstrate that SDF-induced chemotaxis and calcium flux involved coupling to Go or Gq. Interestingly, these studies also showed that CXCR4 could induce calcium flux through interaction with Gs. In HEK293 cells engineered to co-express CXCR4 and specific Gα proteins, Ling et al. found that CXCR4 could stimulate GTPγ-S loading of Gαi2 as well as Gαo1 [20]. Finally, Tan et al. demonstrated that CXCR4 must couple with Gi to activate Rac but with Gα13 in order to activate Rho in Jurkat T cells [27]. Clearly there is still much to know about how specific heterotrimeric G protein composition contributes to cell-specific responses to CXCL12 and what role altered patterns of G protein subunit expression plays in disease.
G protein signaling
The exchange of GTP for GDP on the Gα subunit of heterotrimeric G proteins results in conformational changes that expose effector interacting domains on the Gα subunit and the obligate Gβγ dimer which initiate Gα and Gβγ signaling [28]. The duration of these signals is determined by the rate of GTP hydrolysis to GDP, and re-establishment of the non-signaling conformation of the αβγ heterotrimer. As described below the rate of hydrolysis is determined by the intrinsic GTPase activity of Got proteins and the actions of a family of GTPase activating proteins known as the Regulators of G protein Signaling or RGS proteins.
Gα-dependent signals
While specific βγ isoforms influence heterotrimeric G protein signaling, the core properties of G protein action are determined by the identity of the involved Gα subunit. Some alpha subunits are expressed in a tissue-specific fashion, such as the restriction of Gαolf to the olfactory epithelium. While others, such as Gαi/o are more ubiquitous in their activity (see [23] for review).
Each class of Gα protein operates through specific effectors. Many commonly assayed functions of CXCR4 like chemotaxis and adenylyl cyclase (AC) inhibition are sensitive to treatment with Pertussis Toxin indicating they involve Gα proteins belonging to the i/o group. It is not clear what effectors are directly regulated through interaction with Gαo, but Gαi is a direct inhibitor of ACs 1, 3, 5, 6, 8 and 9 [21]. The inhibition of AC and suppression of cAMP alters PKA and EPAC activation [29] and is critical to CXCL12-induced chemotaxis, proliferation and survival signaling [7, 30, 31]
CXCL12-induced calcium flux, requires activation of phospholipase Cβ (PLCβ) and is also inhibited by Pertussis Toxin [32, 33]. Gαq is the only alpha subunit capable of activating PLCβ and it is insensitive to Pertussis Toxin [34, 35]. Thus, PLCβ activation must occur either via Gi/o βγ subunit function (see below), or as a consequence of sequential Gαi − Gαq effects. This kind of sequential G protein signaling occurs upon activation of the β-adrenergic receptor where initial Gαs activity results in increased cAMP levels and protein kinase A mediated phosphorylation of the receptor. This phosphorylation event allows the β-adrenergic receptor to switch to a Gi-coupled phase of signaling with enhanced ability to activate the MAP kinase pathway [36]. Calcium flux is an important regulator of calmodulin, CAM kinase and calcineurin. Alterations in the amplitude and time-course of calcium transients can promote differentiation or proliferation through the activation of NFAT or CREB, respectively [31, 37, 38].
Finally, the Gα12/13 family can also be engaged by CXCR4 and couple it with a great breadth of effectors. Several of these interactions have significant relevance to tumor biology. Gα12/13 proteins can directly stimulate Bruton’s tyrosine kinase [39]. Bruton’s Tyrosine Kinase is an inhibitor of Fas-mediated apoptosis and thus may contribute to genesis of leukemia and has recently gained attention as a therapeutic target [40]. Both Gα12 and Gα13 have also been shown to promote the release of β-catenin through interaction with cadherins [41]. This may be especially relevant to neuroectodermal tumors as β-catenin activation is increased in medulloblastoma and melanoma [42, 43]. Finally, CXCR4 mediated chemotaxis requires activation of Rho and Rac. While Gαi is necessary for Rac activation (most likely through Gβγ, see below) G12/13 is required as a direct activator of Rho through modulation of Rho-guanine nucleotide exchange factors [44, 45]. This pathway may be especially important to CXCR4’s function in cancer as constitutive activation of Rho-GTPase activity endows the PAR-1 GPCR with transforming ability [46].
Gβγ-dependent signals
While initial views of Gβγ function focused on its role in the juxta-membrane localization of Gα and its negative regulation of Gα activity through guanine-nucleotide dissociation inhibitor (GDI) functions, it is now clear that Gβγ plays a more critical and active role in the mediation of GPCR signaling (see [22] for review). Through direct interactions Gβγ can regulate multiple AC isoforms, ion channels, PLCβ and several critical kinases. The impact Gβγ has on cell biology may be influenced by the relative abundance of specific GPCRs and their coupled heterotrimeric G proteins. In this regard, the greater amount of Gi/o in neural tissues as compared to other heterotrimeric G proteins might imply that Gβγ signaling is particularly important to CXCR4’s role in development and oncogenesis within the neuroectoderm. Central to Gβγ function is the direct activation of PI3 kinase. This occurs through the interaction of Gβγ with p101, a protein associated with the p110 catalytic subunit of class I PI3 Kinase [47]. PI3 kinase is a critical regulator of survival signaling through direct modulation of effectors of apoptosis and by regulating transcription of pro-apoptotic and pro-survival genes [48]. The importance of PI3 kinase activation to oncogenesis is underscored by the frequency with which the primary negative regulator of its function, PTEN, is mutated or deleted in cancer [49, 50].
GPCRs can also activate a second primary regulator of proliferation and survival, the MAP kinase pathway [51, 52]. The mechanism of MAP kinase pathway activation appears to be secondary to PI3 kinase activation [53]. The increase in inositol triphosphate that follows PI3 kinase activation enhances the activation of non-receptor tyrosine kinases such as Src [54, 55]. Src in turn phosphorylates Shc resulting in increased Shc-Grb2-Sos formation and activation of Ras and the MAP kinase pathway [56, 57]. These pathways are essential for CXCR4-mediated proliferation; survival and chemotaxis, identifying Gβγ function as central to CXCR4’s role in cancer [31].
Heterotrimeric G protein independent signals
Ligand-bound CXCR4, like all GPCRs, is a substrate for a family of serine/threonines kinases known as G protein Receptor Kinases or GRKs [58, 59]. GRK-mediated phosphorylation of CXCR4 creates a binding site for β-arrestins. The binding of β-arrestin to CXCR4 promotes the uncoupling of ligand-bound receptor from heterotrimeric G proteins (see discussion of desensitization below) and CXCR4 internalization. The consequence of this sequence of events is inhibition of CXCL12-induced GTP-γS loading and termination of AC inhibition and cAMP suppression [60]. The CXCR4- β-arrestin complex binds the monomeric GTPase Raf [61] potently activating the Erk1/2 MAP kinase pathway. In addition β-arrestin recruits and activates Ask1 and the p38 MAP kinase pathway [62]. Activation of both the Erk1/2 and p38 pathways is especially important for CXCL12-induced chemotaxis and its role in metastasis [63]. In other receptor systems arrestins have also been shown to bind to and activate Src [64] and c-Jun NH2-terminal kinase 3 [65].
In addition to modulating chemotactic responses, arrestins appear to block GPCR-induced apoptosis. In mouse embryonic fibroblasts lacking arrestins 2 and 3, activation of multiple GPCRs with their cognate ligands, including FPR, IL-8, angiotensin II and vasopressin receptors resulted in caspase 9 and 3 dependent cytochrome c release and apoptosis [66]. Apoptosis induced in the absence of arrestins required receptor phosphorylation and was not the simple result of sustained receptor activation (lack of desensitization). Interestingly, activation of CXCR4 in MEFs null for arrestin 2 and 3 did not induce apoptosis.
Counter-regulation of CXCR4 signaling
CXCR4 signaling is counter-regulated at multiple levels. These counter-regulatory mechanisms can shape CXCR4 effects by diminishing one aspect of signaling, such as AC inhibition while enhancing another such as Erk1/2 activation. In addition, counter-regulation constrains CXCR4 signals, preventing excessive or inappropriate activation of downstream pathways. In this section I will review the primary modes of CXCR4 counter-regulation in anticipation of discussing abnormal counter-regulation as the basis for CXCR4 function in cancer.
GTPase activity is enhanced by RGS proteins
The activated, GTP-bound state of Gαi is terminated by the intrinsic GTPase activity of the alpha subunit, which is stimulated by RGS proteins [67]. These proteins serve as the frontline against excessive heterotrimeric G protein signaling and thus are critical to the maintenance of normal GPCR function. There are more than 30 members of the mammalian RGS family of proteins [68]. While initial assessments of their role focused on the relatively simple acceleration of GTP hydrolysis by Gα GTPases, more recently their interactions with additional signaling intermediaries has been characterized and yielded a more comprehension picture of how RGS proteins modulate GPCR and other protein signaling.
The best-described roles for RGS regulation of CXCR4 function are in the development of lymphocytes and megakaryocytes. RGS1 [69], RGS 13 [70, 71] and RGS 16 [71, 72] exhibit developmental regulation that inhibits CXCR4 signaling and CXCL12-induced chemotaxis at critical stages of T cell maturation. Similarly RGS 16 expression inhibits CXCL12-induced chemotaxis in mature megakaryocytes [73]. With regard to the neuroectoderm, RGS2 and RGS4 are highly expressed in the brain and have been shown to modulate pain perception and neurotransmitter signaling [68, 74].
Desensitization serves as a switch between heterotrimeric G protein dependent and independent pathways
GRK-mediated CXCR4 phosphorylation promotes association of the receptor with β-arrestin and clathrin, resulting in receptor internalization in a dynamin-dependent fashion and a shift in downstream signals [75–77]. This aggregate process is referred to as receptor desensitization [58, 59] and the net effect is an inhibition of G-protein dependent signaling reflected in decreased GTP-γS loading, decreased calcium flux and loss of AC inhibition [60, 78]. In addition, desensitization and β-arrestin binding activates Raf and Ask1 resulting in the further stimulation of the Erk 1/2 and p38 MAP kinase pathways. Desensitization initiated by CXCL12 binding is referred to as homologous desensitization while desensitization initiated by activation of other receptors is referred to as heterologous desensitization.
Multiple serine and threonines in the carboxy-tail and third cytoplasmic loop of CXCR4 can be phosphorylated. Orsini et al. found that phosphorylation of serines 338/339 were essential for homologous desensitization [75]. Woerner et al. found that only serine 339 and not serine 338 was phosphorylated in a CXCL12-dependent manner [79]. The importance of the carboxy tail to arrestin-mediated internalization is also indicated by studies of C-terminal truncations of CXCR4 [80].
GRKs involved in CXCR4 desensitization appear to vary in a cell type specific fashion. Targeted deletion of GRK6 results in failed CXCL12-induced neutrophil and lymphocyte chemotaxis [81, 82]. GRK2 activity correlates with CXCR4 phosphorylation and cAMP regulation in astrocytes [30], and Balabanian et al. demonstrated that GRK3 also regulates CXCR4 desensitization [83].
The effects of arrestin on GTP-γS loading and cAMP accumulation require arrestin interaction with the carboxy-tail of CXCR4 and are abrogated by truncation of the receptor [60]. The effects of arrestin on CXCR4 internalization and Erk activation do not require the carboxy tail and instead appear to result from arrestin interaction with the 3rd cytoplasmic loop [60, 80]. This has particular significance to the pathological increase in CXCL12-induced chemotaxis observed in the immunodeficiency disorder WHIM syndrome [83–85].
CXCR4 signaling in cancer
CXCR4 function in cancer and normal cells differs. For example, CXCL12 induces apoptosis in primary cultures of neonatal astrocytes but promotes growth through enhanced survival in cultures of astrocytoma cells [7, 30]. Similarly, neuroblastoma [86] and melanoma [87] cells exhibit proliferation in response CXCL12 while growth of their normal counterparts is not known to do so. In each of these neuroectodermal cancers the level of CXCR4 expression has prognostic significance suggesting that the acquisition of this novel cancer-promoting effect is related, in part, to increased receptor numbers and/or an increase in the strength of receptor signals [87–90]. However, under normal circumstances excessive activation of CXCR4 is stringently counter-regulated and increased receptor activation alone may not be enough to drive abnormal CXCL12-induced cancer growth. In this final section I will consider whether dysregulation of CXCR4 signaling results in the genesis of novel biological effects. The importance of recognizing and understanding dysregulated CXCR4 signals lies in the therapeutic opportunity it affords. As extended inhibition of CXCR4 signaling has thus far proved toxic [8, 9], it may be that normalization, rather than inhibition of CXCR4 would provide therapeutic benefit with fewer side effects.
The work of multiple investigators has led to the hypothesis that the CXCL12-CXCR4 pathway plays a significant role in astrocytoma biology [7, 79, 89, 91–93]. An important validation of this hypothesis is the prognostic significance of CXCL12 and CXCR4 expression. Bian at al. found that patients with CXCR4-positive high-grade gliomas had significantly reduced survival compared to patients with gliomas that did not express CXCR4 [89]. Similarly, Calatozzolo, et al, found that CXCL12 expression was a more powerful predictor of time-to-progression than histological subtype in oligodendroglioma or oligoastrocytoma [91].
Among the potential mechanisms whereby increased CXCR4 expression could alter the nature of cellular responses is by modifying the relative abundance of CXCR4 homo- and hetero-dimers. The presence of homo- and hetero-dimers of chemokine receptors at baseline suggests that “normal” chemokine effects could result from the balance of signals initiated from both dimer species. Alterations in the numbers of CXCR4 molecules could therefore shift the balance towards homodimers or result in the formation of rare, low-affinity heterodimers with receptor types that do not normally pair with CXCR4 when receptor numbers are lower.
Changes in the balance of homo- and hetero-dimers as well as the formation of novel heterodimers could have significant effects on signaling and biology. Heterodimers formed between CXCR4 and CCR2 and between CXCR4 and δ-opioid receptor are present under basal conditions in primary isolates of T lymphocytes and blood mononuclear cells, respectively [94, 95]. In both instances the presence of heterodimers has a significant effect on response to each ligand. While the presence of CXCR4 - δ-opioid receptor heterodimers had no effect on response to CXCL12 alone, co-stimulation with the enkephalin δ-opioid agonist DPDPE blocked all CXCL12-induced signaling and inhibited chemotaxis in vivo as measured by the accumulation of mononuclear cells in peritoneal fluid after intraperitoneal injection of the combined receptor agonists [95]. This was not due to heterologous desensitization as no increase in CXCR4 phosphorylation was detected in MonoMac-1 cells after DRDPE treatment. Rather, it appeared that co-stimulation of the heterodimer resulted in a silent receptor. Similarly, Mellado et al. showed that co-stimulation of CCR2-CCR5 heterodimers with CCL5 and CCL2 led to novel Gαq/11 activation that was relatively resistant to regulation by desensitization [96]. This was in contrast to Gαi mediated signals which originated from homodimers of CCR2 and CCR5, which were readily desensitized. Thus changes in the level of CXCR4 expression alone, or in combination with changes in other chemokine receptors could alter the profile of receptor homo- and hetero-dimers. This in turn could alter signaling in more than one chemokine dependent pathway and might even result in the acquisition of novel signals not possessed by any of the component receptors when functioning as homodimers.
Even in the absence of an altered receptor dimer profile, the opportunity to distort CXCR4 functions exists through modulation of the counter-regulatory mechanisms. Consistent with the known role of Gi-dependent signals in chemotaxis, loss of RGS expression or function generally results in increased chemotactic responses. This was demonstrated in lymphocytes and could have implications in leukemia and lymphoma (see above). RGS4 is expressed in neuroblastoma cells where it inhibits signaling through Gαi and Gαq [97]. RGS2 is expressed in pheochromocytoma derived PC12 cells where it interacts with microtubules and promotes neurite outgrowth in response to nerve growth factor [98]. Loss of RGS attenuates the differentiating effects of NGF, a function that could be relevant to transformation within the neuroectoderm. Unexpectedly, increased RGS 3 and 4 expression enhanced U373 glioma cell adhesion and migration [99] suggesting that the role of CXCR4 in cancer may not be a simple matter of more or less CXCR4 signaling.
Astrocytes, pre-cancerous astrocytes and astrocytoma cells
The activation of CXCR4 in the setting of RGS deficiency can still be counter-regulated through the process of desensitization. To determine whether such dysregulation of CXCR4 signaling was important to tumorigenesis, Warrington et al. examined a spectrum of cells, from normal to malignant, derived from the astrocytoma lineage. As an intermediate between normal and malignant they utilized astrocytes with complete loss of the tumor suppressor neurofibromin. Neurofibromin is mutated in the autosomal dominant disorder neurofibromatosis Type 1, which predisposes affected patients to multiple cancers including benign and malignant astrocytomas [100].
Significant differences in wildtype and Nf1−/− astrocyte responses to CXCL12 were evident in in vitro studies. Primary cultures of wildtype neonatal astrocytes exhibited an increase in apoptosis while Nf1−/− astrocytes displayed a decrease in apoptosis when treated with CXCL12 [30]. These opposed effects were dependent upon differences in CXCL12-induced cAMP suppression. Loss of neurofibromin resulted in diminished CXCR4 desensitization and prolonged AC inhibition. The molecular basis for altered desensitization was increased Erk-mediated phosphorylation and inhibition of GRK2 in Nf1−/− astrocytes. This resulted in a decrease in CXCL12-induced CXCR4 phosphorylation. Inhibition of Erk activation with the MEK inhibitor PD98059 led to re-activation of GRK2 and normalization of CXCL12-induced CXCR4 phosphorylation and cAMP responses. These data were consistent with studies that identified abnormal suppression of cAMP as the basis for CXCL12-induced medulloblastoma and glioblastoma growth [7, 79]. Together these results suggest that altered CXCR4 counter-regulation gives rise to novel CXCR4 signals and functions, productive of abnormal growth promoting effects.
Alterations in GPCR desensitization play a pathophysiological role in other diseases and cancers. Beautiful work done by Balabanian et al. demonstrated that mutation of the carboxy-tail of CXCR4 alters its internalization without abrogating its interaction with arrestins. This leads to aberrant activation of arrestin from the cell surface and enhanced chemotaxis, which contributes to the disease [83–85].
Model for CXCR4 function in cancer
Available data suggest a model for CXCR4 function in cancer in which the kinetics and magnitude of heterotrimeric G protein dependent and independent phases of signaling are altered by increased levels of receptor expression, coupled with altered counter-regulation. Normally, these phases of CXCR4 signaling have limited overlap as rapid desensitization and internalization terminate the heterotrimeric G protein phase of signaling and initiate the β-arrestin phase of signaling (Figure 2). I propose a model in which altered desensitization results in a greater overlap between these phases of signaling as the heterotrimeric G protein component is prolonged while the β-arrestin dependent component initiates. This overlap in pathway activation generates a novel signal and novel cellular response such as the survival response exhibited by Nf1−/− astrocytes and malignant astrocytoma cells. This model makes several predictions. First, CXCR4 function in cancer may not be a simple recapitulation of its role in development. Second, over-expression of CXCR4 in otherwise intact cells may not have profound effects on growth. Further, knockout of CXCR4 in genetically engineered mouse models of cancer may not affect tumorigenesis as normal CXCR4 signals do not contribute to transformation. One test of this hypothesis will be altering CXCR4 counter-regulation in tumor-prone mice and determining whether this will increase tumor frequency.
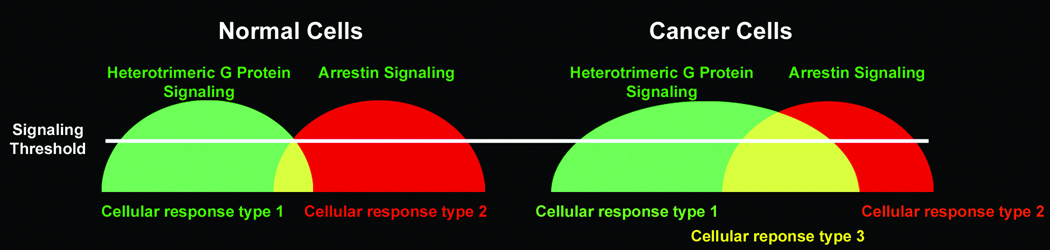
Figure 2. Altered CXCR4 Signaling Results in Novel cellular Response.
In normal cells CXCR4 signaling can be considered as occurring in two phases or humps; the heterotrimeric G protein dependent and β-arrestin dependent phases. Cellular responses are determined by whether signal strength exceeds a threshold (white line). Under normal circumstances the two phases of CXCR4 signaling are temporarily distinguishable as the rapid desensitization of AC inhibition or calcium flux is coincident with the onset of β-arrestin dependent signals. I propose that altered desensitization results in greater overlap between these phases of signaling as the heterotrimeric G protein dependent component is extended into the β-arrestin dependent component. The resultant merge of the two phases into one phase or hump results in an entirely novel signal and cellular response such as the abnormal survival response in astrocytoma cells.
It will be essential to explore these possibilities as differences between normal and tumor CXCR4 signaling and function may provide a critical therapeutic opportunity. Currently, the application of CXCR4 antagonist therapy for cancer is limited by excessive toxicity of global CXCR4 inhibition. If correction of abnormal CXCR4 signaling were possible without inhibition of global CXCR4 signaling, there may be therapeutic benefit without excessive toxicity.
Acknowledgements
I would like to thank Dr. David Wilson for helpful comments on the manuscript. This work was supported by the National Cancer Institute (RO1CA118389) and the Brain Tumor Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 2.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 3.Ma Q, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommer L, Civenni G. Chemokines and Cancer Stem Cells: Potential Implications in Metastasis. Seminars in Cancer Biology. 2008 doi: 10.1016/j.semcancer.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Rubin JB, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, et al. Blocking CXCR4-Mediated Cyclic AMP Suppression Inhibits Brain Tumor Growth In vivo. Cancer Res. 2007;67:651–658. doi: 10.1158/0008-5472.CAN-06-2762. [DOI] [PubMed] [Google Scholar]
- 8.Hendrix CW, et al. Safety, Pharmacokinetics, and Antiviral Activity of AMD3100, a Selective CXCR4 Receptor Inhibitor, in HIV-1 Infection. J Acquir Immune Defic Syndr. 2004;37:1253–1262. doi: 10.1097/01.qai.0000137371.80695.ef. [DOI] [PubMed] [Google Scholar]
- 9.Hendrix CW, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR- 4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vila-Coro AJ, et al. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. Faseb J. 1999;13:1699–1710. [PubMed] [Google Scholar]
- 11.Babcock GJ, Farzan M, Sodroski J. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem. 2003;278:3378–3385. doi: 10.1074/jbc.M210140200. [DOI] [PubMed] [Google Scholar]
- 12.Toth PT, Ren D, Miller RJ. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1alpha and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J Pharmacol Exp Ther. 2004;310:8–17. doi: 10.1124/jpet.103.064956. [DOI] [PubMed] [Google Scholar]
- 13.Percherancier Y, et al. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem. 2005;280:9895–9903. doi: 10.1074/jbc.M411151200. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE. Janus kinase 2 is involved in stromal cell-derived factor-1alpha-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood. 2001;97:3342–3348. doi: 10.1182/blood.v97.11.3342. [DOI] [PubMed] [Google Scholar]
- 15.Soldevila G, et al. Impaired chemokine-induced migration during T-cell development in the absence of Jak 3. Immunology. 2004;112:191–200. doi: 10.1111/j.1365-2567.2004.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soriano SF, et al. Chemokines integrate JAK/STAT and G-protein pathways during chemotaxis and calcium flux responses. Eur J Immunol. 2003;33:1328–1333. doi: 10.1002/eji.200323897. [DOI] [PubMed] [Google Scholar]
- 17.Moriguchi M, et al. CXCL12 signaling is independent of Jak2 and Jak3. J Biol Chem. 2005;280:17408–17414. doi: 10.1074/jbc.M414219200. [DOI] [PubMed] [Google Scholar]
- 18.Arai H, Charo IF. Differential regulation of G-protein-mediated signaling by chemokine receptors. J Biol Chem. 1996;271:21814–21819. doi: 10.1074/jbc.271.36.21814. [DOI] [PubMed] [Google Scholar]
- 19.Kuang Y, Wu Y, Jiang H, Wu D. Selective G protein coupling by C-C chemokine receptors. J Biol Chem. 1996;271:3975–3978. doi: 10.1074/jbc.271.8.3975. [DOI] [PubMed] [Google Scholar]
- 20.Ling K, et al. Five-transmembrane domains appear sufficient for a G protein-coupled receptor: functional five-transmembrane domain chemokine receptors. Proc Natl Acad Sci U S A. 1999;96:7922–7927. doi: 10.1073/pnas.96.14.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Offermanns S. In vivo functions of heterotrimeric G-proteins: studies in Galpha-deficient mice. Oncogene. 2001;20:1635–1642. doi: 10.1038/sj.onc.1204189. [DOI] [PubMed] [Google Scholar]
- 22.Schwindinger WF, Robishaw JD. Heterotrimeric G-protein betagamma-dimers in growth and differentiation. Oncogene. 2001;20:1653–1660. doi: 10.1038/sj.onc.1204181. [DOI] [PubMed] [Google Scholar]
- 23.McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. G-protein signaling: back to the future. Cell Mol Life Sci. 2005;62:551–577. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993;259:832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- 25.Holland JD, et al. Differential functional activation of chemokine receptor CXCR4 is mediated by G proteins in breast cancer cells. Cancer Res. 2006;66:4117–4124. doi: 10.1158/0008-5472.CAN-05-1631. [DOI] [PubMed] [Google Scholar]
- 26.Maghazachi AA. Role of the heterotrimeric G proteins in stromal-derived factor-1alpha-induced natural killer cell chemotaxis and calcium mobilization. Biochem Biophys Res Commun. 1997;236:270–274. doi: 10.1006/bbrc.1997.6937. [DOI] [PubMed] [Google Scholar]
- 27.Tan W, Martin D, Gutkind JS. The Galpha13-Rho signaling axis is required for SDF-1-induced migration through CXCR4. J Biol Chem. 2006;281:39542–39549. doi: 10.1074/jbc.M609062200. [DOI] [PubMed] [Google Scholar]
- 28.Rebois RV, Warner DR, Basi NS. Does subunit dissociation necessarily accompany the activation of all heterotrimeric G proteins? Cell Signal. 1997;9:141–151. doi: 10.1016/s0898-6568(96)00133-7. [DOI] [PubMed] [Google Scholar]
- 29.Kopperud R, Krakstad C, Selheim F, Doskeland SO. cAMP effector mechanisms. Novel twists for an 'old' signaling system. FEBS Lett. 2003;546:121–126. doi: 10.1016/s0014-5793(03)00563-5. [DOI] [PubMed] [Google Scholar]
- 30.Warrington NM, et al. Spatiotemporal differences in CXCL12 expression and cyclic AMP underlie the unique pattern of optic glioma growth in neurofibromatosis type 1. Cancer Res. 2007;67:8588–8595. doi: 10.1158/0008-5472.CAN-06-2220. [DOI] [PubMed] [Google Scholar]
- 31.Gross N, Meier R. Chemokines in Neuroectodermal Cancers: The Crucial Growth Signal from the Soil. Seminars in Cancer Biology. 2008 doi: 10.1016/j.semcancer.2008.10.009. This issue. [DOI] [PubMed] [Google Scholar]
- 32.Smrcka AV, Hepler JR, Brown KO, Sternweis PC. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991;251:804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- 33.Klein RS, et al. SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–1981. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- 34.Exton JH. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu Rev Pharmacol Toxicol. 1996;36:481–509. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- 35.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 37.Jones EA, Sun D, Kobierski L, Symes AJ. NFAT4 is expressed in primary astrocytes and activated by glutamate. J Neurosci Res. 2003;72:191–197. doi: 10.1002/jnr.10584. [DOI] [PubMed] [Google Scholar]
- 38.Lipskaia L, Lompre AM. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol Cell. 2004;96:55–68. doi: 10.1016/j.biolcel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Y, et al. The G protein G alpha12 stimulates Bruton's tyrosine kinase and a rasGAP through a conserved PH/BM domain. Nature. 1998;395:808–813. doi: 10.1038/27454. [DOI] [PubMed] [Google Scholar]
- 40.Uckun FM, Tibbles HE, Vassilev AO. Bruton's tyrosine kinase as a new therapeutic target. Anticancer Agents Med Chem. 2007;7:624–632. doi: 10.2174/187152007784111331. [DOI] [PubMed] [Google Scholar]
- 41.Meigs TE, Fields TA, McKee DD, Casey PJ. Interaction of Galpha 12 and Galpha 13 with the cytoplasmic domain of cadherin provides a mechanism for beta-catenin release. Proc Natl Acad Sci U S A. 2001;98:519–524. doi: 10.1073/pnas.021350998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raffel C. Molecular biology of the primitive neuroectodermal tumor: a review. Neurosurg Focus. 1999;7:e2. doi: 10.3171/foc.1999.7.2.4. [DOI] [PubMed] [Google Scholar]
- 43.Bonitsis N, Batistatou A, Karantima S, Charalabopoulos K. The role of cadherin/catenin complex in malignant melanoma. Exp Oncol. 2006;28:187–193. [PubMed] [Google Scholar]
- 44.Hart MJ, et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 45.Fukuhara S, Chikumi H, Gutkind JS. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett. 2000;485:183–188. doi: 10.1016/s0014-5793(00)02224-9. [DOI] [PubMed] [Google Scholar]
- 46.Whitehead IP, Zohn IE, Der CJ. Rho GTPase-dependent transformation by G proteincoupled receptors. Oncogene. 2001;20:1547–1555. doi: 10.1038/sj.onc.1204188. [DOI] [PubMed] [Google Scholar]
- 47.Stephens L, et al. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994;77:83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 48.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 49.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Rasheed BK, Wiltshire RN, Bigner SH, Bigner DD. Molecular pathogenesis of malignant gliomas. Curr Opin Oncol. 1999;11:162–167. doi: 10.1097/00001622-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Crespo P, Xu N, Simonds WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 52.Della Rocca GJ, et al. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Ilasaca M, et al. Requirement of phosphatidylinositol-3 kinase for activation of JNK/SAPKs by PDGF. Biochem Biophys Res Commun. 1997;232:273–277. doi: 10.1006/bbrc.1997.6289. [DOI] [PubMed] [Google Scholar]
- 54.Pleiman CM, Hertz WM, Cambier JC. Activation of phosphatidylinositol-3' kinase by Src-family kinase SH3 binding to the p85 subunit. Science. 1994;263:1609–1612. doi: 10.1126/science.8128248. [DOI] [PubMed] [Google Scholar]
- 55.Touhara K, Hawes BE, van Biesen T, Lefkowitz RJ. G protein beta gamma subunits stimulate phosphorylation of Shc adapter protein. Proc Natl Acad Sci U S A. 1995;92:9284–9287. doi: 10.1073/pnas.92.20.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Biesen T, et al. Receptor-tyrosine-kinase- and G beta gamma-mediated MAP kinase activation by a common signalling pathway. Nature. 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- 57.Cazaubon SM, et al. Endothelin induces tyrosine phosphorylation and GRB2 association of Shc in astrocytes. J Biol Chem. 1994;269:24805–24809. [PubMed] [Google Scholar]
- 58.Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Recent Prog Horm Res. 1996;51:319–351. discussion 352–3. [PubMed] [Google Scholar]
- 59.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 60.Cheng ZJ, et al. beta-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4. J Biol Chem. 2000;275:2479–2485. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- 61.Luttrell LM, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 63.Pistoia V, Raffaghello L, Cocco C, Corrias MV, Airoldi I. Chemokines in Neuroectodermal Tumour Progression and Metastasis. Semin Cancer Biol. 2008 doi: 10.1016/j.semcancer.2008.10.003. This issue. [DOI] [PubMed] [Google Scholar]
- 64.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 65.McDonald PH, et al. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 66.Revankar CM, Vines CM, Cimino DF, Prossnitz ER. Arrestins block G protein-coupled receptor-mediated apoptosis. J Biol Chem. 2004;279:24578–24584. doi: 10.1074/jbc.M402121200. [DOI] [PubMed] [Google Scholar]
- 67.Druey KM, Blumer KJ, Kang VH, Kehrl JH. Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family. Nature. 1996;379:742–746. doi: 10.1038/379742a0. [DOI] [PubMed] [Google Scholar]
- 68.Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther. 2007;116:473–495. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piovan E, et al. Differential regulation of hypoxia-induced CXCR4 triggering during B-cell development and lymphomagenesis. Cancer Res. 2007;67:8605–8614. doi: 10.1158/0008-5472.CAN-06-4722. [DOI] [PubMed] [Google Scholar]
- 70.Shi GX, Harrison K, Wilson GL, Moratz C, Kehrl JH. RGS13 regulates germinal center B lymphocytes responsiveness to CXC chemokine ligand (CXCL)12 and CXCL13. J Immunol. 2002;169:2507–2515. doi: 10.4049/jimmunol.169.5.2507. [DOI] [PubMed] [Google Scholar]
- 71.Estes JD, et al. Follicular dendritic cell regulation of CXCR4-mediated germinal center CD4 T cell migration. J Immunol. 2004;173:6169–6178. doi: 10.4049/jimmunol.173.10.6169. [DOI] [PubMed] [Google Scholar]
- 72.Lippert E, et al. Role of regulator of G protein signaling 16 in inflammation-induced T lymphocyte migration and activation. J Immunol. 2003;171:1542–1555. doi: 10.4049/jimmunol.171.3.1542. [DOI] [PubMed] [Google Scholar]
- 73.Berthebaud M, et al. RGS16 is a negative regulator of SDF-1-CXCR4 signaling in megakaryocytes. Blood. 2005;106:2962–2968. doi: 10.1182/blood-2005-02-0526. [DOI] [PubMed] [Google Scholar]
- 74.Saugstad JA, Marino MJ, Folk JA, Hepler JR, Conn PJ. RGS4 inhibits signaling by group I metabotropic glutamate receptors. J Neurosci. 1998;18:905–913. doi: 10.1523/JNEUROSCI.18-03-00905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Orsini MJ, Parent JL, Mundell SJ, Marchese A, Benovic JL. Trafficking of the HIV coreceptor CXCR4: role of arrestins and identification of residues in the C-terminal tail that mediate receptor internalization. J Biol Chem. 2000;275:25876. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- 76.Signoret N, et al. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139:651–664. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 78.Haribabu B, et al. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J Biol Chem. 1997;272:28726–28731. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- 79.Woerner BM, Warrington NM, Kung AL, Perry A, Rubin JB. Widespread CXCR4 activation in astrocytomas revealed by phospho-CXCR4-specific antibodies. Cancer Res. 2005;65:11392–11399. doi: 10.1158/0008-5472.CAN-05-0847. [DOI] [PubMed] [Google Scholar]
- 80.Roland J, et al. Role of the intracellular domains of CXCR4 in SDF-1-mediated signaling. Blood. 2003;101:399–406. doi: 10.1182/blood-2002-03-0978. [DOI] [PubMed] [Google Scholar]
- 81.Fong AM, et al. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci U S A. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vroon A, et al. GRK6 deficiency is associated with enhanced CXCR4-mediated neutrophil chemotaxis in vitro and impaired responsiveness to G-CSF in vivo. J Leukoc Biol. 2004;75:698–704. doi: 10.1189/jlb.0703320. [DOI] [PubMed] [Google Scholar]
- 83.Balabanian K, et al. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J Clin Invest. 2008;118:1074–1084. doi: 10.1172/JCI33187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lagane B, et al. CXCR4 dimerization and beta-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood. 2008;112:34–44. doi: 10.1182/blood-2007-07-102103. [DOI] [PubMed] [Google Scholar]
- 85.Balabanian K, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449–2457. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 86.Meier R, et al. The chemokine receptor CXCR4 strongly promotes neuroblastoma primary tumour and metastatic growth, but not invasion. PLoS ONE. 2007;2:e1016. doi: 10.1371/journal.pone.0001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scala S, et al. Human melanoma metastases express functional CXCR4. Clin Cancer Res. 2006;12:2427–2433. doi: 10.1158/1078-0432.CCR-05-1940. [DOI] [PubMed] [Google Scholar]
- 88.Tucci MG, et al. Involvement of E-cadherin, beta-catenin, Cdc42 and CXCR4 in the progression and prognosis of cutaneous melanoma. Br J Dermatol. 2007;157:1212–1216. doi: 10.1111/j.1365-2133.2007.08246.x. [DOI] [PubMed] [Google Scholar]
- 89.Bian XW, et al. Preferential expression of chemokine receptor CXCR4 by highly malignant human gliomas and its association with poor patient survival. Neurosurgery. 2007;61:570–578. doi: 10.1227/01.NEU.0000290905.53685.A2. discussion 578–9. [DOI] [PubMed] [Google Scholar]
- 90.Russell HV, Hicks J, Okcu MF, Nuchtern JG. CXCR4 expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. J Pediatr Surg. 2004;39:1506–1511. doi: 10.1016/j.jpedsurg.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 91.Calatozzolo C, et al. Prognostic value of CXCL12 expression in 40 low-grade oligodendrogliomas and oligoastrocytomas. Cancer Biol Ther. 2006;5:827–832. doi: 10.4161/cbt.5.7.2838. [DOI] [PubMed] [Google Scholar]
- 92.Rempel SA, Dudas S, Ge S, Gutierrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res. 2000;6:102–111. [PubMed] [Google Scholar]
- 93.Salmaggi A, et al. CXCL12 expression is predictive of a shorter time to tumor progression in low-grade glioma: a single-institution study in 50 patients. J Neurooncol. 2005;74:287–293. doi: 10.1007/s11060-004-7327-y. [DOI] [PubMed] [Google Scholar]
- 94.Sohy D, Parmentier M, Springael JY. Identification and localization of Allosteric transinhibition by specific antagonists in CCR2/CXCR4 heterodimers. J Biol Chem. 2007;282:30062–30069. doi: 10.1074/jbc.M705302200. [DOI] [PubMed] [Google Scholar]
- 95.Pello OM, et al. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur J Immunol. 2008;38:537–549. doi: 10.1002/eji.200737630. [DOI] [PubMed] [Google Scholar]
- 96.Mellado M, et al. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. Embo J. 2001;20:2497–2507. doi: 10.1093/emboj/20.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leone AM, Errico M, Lin SL, Cowen DS. Activation of extracellular signal-regulated kinase (ERK) and Akt by human serotonin 5-HT(1B) receptors in transfected BE(2)-C neuroblastoma cells is inhibited by RGS4. J Neurochem. 2000;75:934–938. doi: 10.1046/j.1471-4159.2000.0750934.x. [DOI] [PubMed] [Google Scholar]
- 98.Heo K, et al. RGS2 promotes formation of neurites by stimulating microtubule polymerization. Cell Signal. 2006;18:2182–2192. doi: 10.1016/j.cellsig.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 99.Tatenhorst L, Senner V, Puttmann S, Paulus W. Regulators of G-protein signaling 3 and 4 (RGS3, RGS4) are associated with glioma cell motility. J Neuropathol Exp Neurol. 2004;63:210–222. doi: 10.1093/jnen/63.3.210. [DOI] [PubMed] [Google Scholar]
- 100.Rubin JB, Gutmann DH. Neurofibromatosis type I - a model for nervous system tumour formation? Nature Cancer Reviews. 2005;5:557–564. doi: 10.1038/nrc1653. [DOI] [PubMed] [Google Scholar]