Abstract
The regulation mechanism of interferon (IFN) and IFN-stimulated genes is a very complex procedure and is dependent on cell types and virus species. We observed molecular changes related to anti-viral responses in endothelial cells during Hantaan virus (HTNV) infection. We found that there are two patterns of gene expression, the first pattern of gene expression being characterized by early induction and short action, as in that of type I IFNs,' and the other being characterized by delayed induction and long duration, as those of IRF-7, MxA, and TAP-1/2. Even though there are significant differences in their induction folds, we found that all of IFN-α/β, IRF-3/7, MxA, and TAP-1/2 mRNA expressions reached the peak when the viral replication was most active, which took place 3 days of post infection (d.p.i.). In addition, an interesting phenomenon was observed; only one gene was highly expressed in paired genes such as IFN-α/β (3/277-folds), IRF-3/7 (2.2/29.4-folds), and TAP-1/2 (26.2/6.1-folds). Therefore, IFN-β, IRF-7, and TAP-1 seem to be more important for the anti-viral response in HTNV infection. MxA was increased to 296-folds at 3 d.p.i. and kept continuing 207-folds until 7 d.p.i.. The above results indicate that IFN-β works for an early anti-viral response, while IRF7, MxA, and TAP-1 work for prolonged anti-viral response in HTNV infection.
Keywords: Hantaan Virus, IFN-α/β, IRF-3/7, MxA, Endothelial Cells
INTRODUCTION
Interferons (IFNs) are a family of cytokines with various biological activities and play a critical role in mediating antiviral responses (1). It is believed that IFN-α/β are regulated by two phases that are associated with interferon regulatory factor-3 (IRF-3) and IRF-7 (2, 3). IRF-3 is constitutively expressed in the cytoplasm of most types of cells. The activated IRF-3 (by phosphorylation) translocates to the nucleus following viral infection and induces type I IFN. IRF-7 is constitutively expressed in lymphoid cells, especially in plasmocytoid dendritic cells, and is inducible in other cell types through viral infection (4, 5). IRF-7 has been characterized as a master regulator of the production of type I IFN (6). It has recently been reported that the regulation of type I IFN is dependent on cell type, and is mainly under the control of IRF-7 in plasmocytoid dendritic cells (5, 7).
Hantaviruses, enveloped viruses with three segmented single-stranded RNAs, belong to the family Bunyaviridae (8). These viruses cause two diseases in human: hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) (9, 10). HFRS is mainly caused by Hantaan virus (HTNV), Seoul viruses, Puumala virus, and Dobrava virus, while HPS is caused by Sin Nombre virus, Andes virus, and New York virus. In both diseases, the vascular endothelium is a prime target for virus infection even though the main target organs differ in HFRS and HPS. Antiviral responses to Hantaviruses are mediated by MxA, which is induced by interferon and interferon-stimulate genes (ISGs) (11, 12). MxA protein, a small GTPase, interferes with the production of some RNA viruses (13, 14). MxA normally accumulates in the cytoplasm of IFN-treated cells (13). However, the association of the MxA and IRFs in endothelial cells remains pooly understood.
Antigen presentation is a very important mechanism for the clearance of virus-infected cells. The molecules involved in antigen presentation, including major histocompatibility complex (MHC) and transporter associated with antigen processing (TAPs), are known to be regulated by IFNs. MHC class I molecules, which serve as target structures for cytotoxic T cells, could be relevant for hantavirus-associated pathogenesis in terms of the severity of the clinical course in humans (15, 16). TAP protein transports epitope peptide to MHC in the endoplasmic reticulum (17). MHC class I molecules have been found to be induced on HTNV-infected endothelial cells (18). However, the mechanisms by which HTNV enhances MHC class I surface expression remain unclear.
In this study, we examined the comparative antiviral responses of IFNs and IFN-stimulated genes during HTNV infection in endothelial cells.
MATERIALS AND METHODS
Cells and virus
Human umbilical vein endothelial cells were purchased from Modern Tissue Technologies (MTT, Seoul, Korea) and grew in EGM-2 (Cambrex, Walkersville, U.S.A.) supplemented with 10% FBS, hFGT, R3-igf-1, ascorbic acid, VEGF, hEGF, heparin, and hydrocortisone. The cells were incubated in a humidified atmosphere with 5% CO2 at 37℃. The Hantaan virus ROK 84-105 strain, which was provided by Dr. Ho Wang Lee, was propagated in Vero E6 cells (ATCC CRL-1586). After 7 days of viral infection, culture supernatant of Vero E6 cells was harvested and centrifuged at 1,500 rpm for 15 min. Aliquots of the centrifuged supernatant were stored at -70℃ until use. Endothelial cells were infected with HTNV at a multiplicity of infection (M.O.I) of 1 in culture dishes (100 mm) for reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blot analysis.
RT-PCR and Real-time PCR
RNA was isolated from endothelial monolayers using RNA Bee (TEL-TEST, Friendswood, Texas, U.S.A.). Total RNA (1 µg) was reverse transcribed in a 20 µg volume containing 15 unites AMV reverse transcriptase (Promega, Madison, U.S.A.), 10 mM dNTP mix, and 1 µg/mL of oligo (dT)15 primer. Each reverse transcription reaction was carried out for 15 min at 42℃ and then stopped by denaturing for 5 min at 95℃. Subsequently, sequences were amplified from cDNA by PCR in a total volume of 50 µL containing 5 µL of RT reaction buffer included 25 mM MgCl2, 2.5 mM dNTP mixed, 25 pmole of each primer, and 2.5 units of DNA polymerase (Bio Tools, Madrid, Spain) in RNase-free distilled water. After a hot start, the amplification profile for HTNV S segment and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was 35 cycles of 1 min denaturation at 95℃ and 1 min extension at 72℃. After amplification, aliquots of the PCR reactions were separated on a 1.5% agarose gel containing ethidium bromide and photographed.
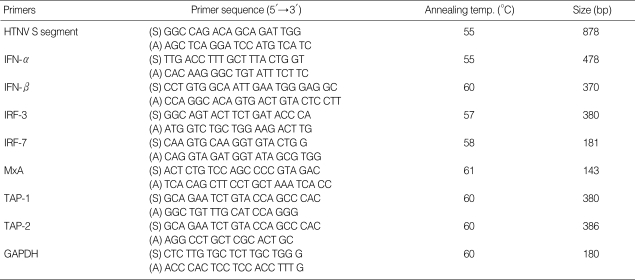
Real-time PCR for IFN-α/β, IRF-3/7, MxA, and TAP1/2 was performed using the LightCycler (Roche, Mannheim, Germany) according to the manufacturer's instructions. Real-time PCR was performed in a total volume of 20 µL of LightCycler FastStart DNA SYBR Green I mix (Roche) containing primer and 2 µL of cDNA. PCR amplification was preceded by incubation of the mixture for 10 min at 95℃, and the amplification step consisted of 45 cycles of denaturation, annealing, and extension. Denaturation was performed for 10 sec at 95℃, annealing was performed for 5 sec at an appropriate temperature depending on each primer, and extension was performed at 72℃ for 10 sec, with fluorescence detection at 72℃ after each cycle. After the final cycle, melting point analyses of all samples were performed within the range of 65 to 95℃ with continuous fluorescence detection. Expression levels of GAPDH were used for sample normalization. Results for each gene were expressed as the relative expression level compared with control HuVEC. The oligonucleotides used in the PCR experiments were described in Table 1.
Table 1.
PCR condition: primers sequences, annealing temperature, and gene fragment size
HTNV, Hantaan virus; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blot
For immunoblots, confluent monolayers of HuVECs were lysed with lysis buffer. Electrophoration was performed on 10-12% SDS-polyacrylamide gels for 2 hr, and the gels were then transferred onto nitrocellulose transfer membranes (Protran, Keene, U.S.A.). Membranes were washed and incubated for 1 hr at room temperature with primary antibodies in a 1:200-500 dilution buffer (5% skim milk in 1×Tris-Buffered saline with Tween 20 [TBST]). Membranes were washed three times with TBST and incubated with secondary antibodies for 1 hr at room temperature. Immunoblots were developed with enhanced chemiluminescence agents according to the instructions of manufacturer (SuperSignal West Pico Chemiluminescent Substrate: PIERCE, Rockford, U.S.A.) and exposed to imaging film.
Fluorescence-activated cell sorting (FACS)
Cells were trypsinized and washed once with phosphate-buffered saline (PBS). Cells were incubated with FITC-conjugated anti-major histocompatibility complex (MHC) class I molecules for 45 min at 4℃. Cells were washed with PBS, and were analyzed using a FACScan cytometer (Becton-Dickinson, Mountain View, CA, U.S.A.). A total of 10,000 cells were counted, and the fluorescence intensity (FI) of the product was calculated as follows: FI=[Percentage of positive cells (M2 gate)×Mean fluorescence of M2]-Background fluorescence (Isotype fluorescence)
Antibodies
Polyclonal rabbit anti-IRF3 (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) and polyclonal rabbit anti-IRF7 (Santa Cruz, CA, U.S.A.), monoclonal mouse anti-MxA (provided from Dr. Otto Haller, Germany), monoclonal mouse anti-human HLA A/B/C (BD Bioscience, CA, San Jose, U.S.A.), and monoclonal mouse anti-γ-tubulin (Sigma, St. Louise, U.S.A.) antibodies were used as primary antibodies. Goat anti-mouse IgG-HRP and goat anti-rabbit IgG (H+L) HRP conjugate (Zymed, San Francisco, CA, U.S.A.) were used as secondary antibodies for Western blots.
Quantitative analysis of RT-PCR and Western blot
The band densities of RT-PCR and Western blot were measured with the Scion Image Beta 4.0.2 (Scion Co., Frederick, MD, U.S.A.) program. For the quantitative analysis of RT-PCR and Western blot, each of the bands was compared with GAPDH and γ-tubulin.
RESULTS
Messenger RNA Expression Profiles of HTNV, IFN, and IFN-stimulated genes
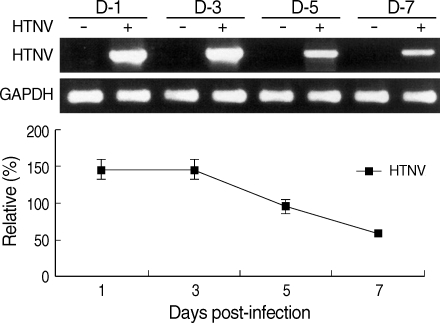
The mRNA expressions of HTNV, IFN, and IFN-stimulated genes such as IFN-α/β, IRF-3/7, MxA, and TAP-1/2 were examined by RT-PCR or real-time-PCR. Hantaan virus gene expression was detected from 1 day of post-infection (d.p.i.) until 7 d.p.i., but the amounts of mRNA decreased after 5 d.p.i. (Fig. 1).
Fig. 1.
Hantaan virus gene expression. HTNV S segment mRNA expression was assessed via RT-PCR. Quantitative analysis was performed using the Scion Image Beta 4.0.2 program. Density of the HTNV S segment was measured by comparison with GAPDH bands.
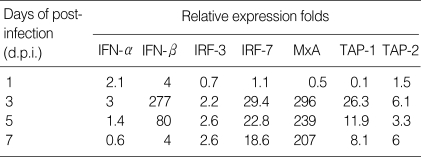
The mRNA expression of IFN-α and IFN-β showed a similar pattern, but the amounts of gene expression were significantly different. IFN-α/β slightly induced as early as first day post-HTNV infection and reached their peaks at 3 d.p.i. before decreasing. The gene expression of IFN-β increased to 277-folds at 3 d.p.i., and then reduced to 4-folds at 7 d.p.i. However, the gene expression of IFN-α increased to only 3-folds at 3 d.p.i. (Table 2).
Table 2.
Results of real-time PCR
The mRNA expression of IRF-3 was induced only 2.2-2.6 folds from 3 to 7 d.p.i.. Whereas, IRF-7 mRNA increased to 29-folds at 3 d.p.i. and gradually decreased to 18-folds at 7 d.p.i. in HTNV-infected HuVECs (Table 2). MxA mRNA was highly expressed in HTNV-infected HuVECs from 3 d.p.i. (296-folds) and maintained high levels until 7 d.p.i. (207-folds) (Table 2). The mRNA expression of TAP-1 peaked at 3 d.p.i. (26-folds) and then gradually decreased during HTNV infection. However, TAP-2 mRNA maintained low levels (6 folds) from 3 d.p.i. to 7 d.p.i. (Table 2).
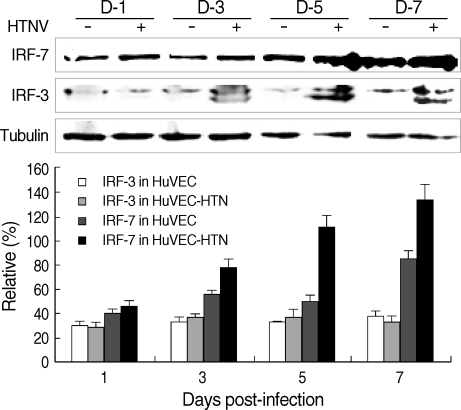
Western blot of MxA and IFR-3/7 proteins
To confirm the expression of MxA and IRF-3/7 proteins by HTNV infection, control and HTNV-infected cells were subjected to Western blotting analysis.
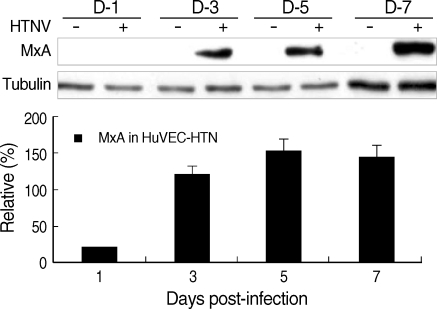
Similar to MxA mRNA expression, MxA proteins were highly expressed in HTNV-infected cells, but not in control cells (Fig. 2).
Fig. 2.
MxA protein expression in HuVECs. Western blot was performed in HuVECs from 1 to 7 d.p.i.. Quantitative analysis was performed using the Scion Image Beta 4.0.2 program. The densities of MxA bands were measured by comparison with tubulin bands.
IRF-7 protein (75 kDa) was expressed at 20-60% higher levels in HTNV-infected HuVECs compared to controls from 3 to 7 d.p.i. (Fig. 3). The protein expression of IRF-3 was detected in both HTNV-infected and control cells, and there were no differences between the two groups (Fig. 3).
Fig. 3.
IRF-3/7 protein expression in HuVECs. Western blot was performed in HuVECs from 1 to 7 d.p.i.. Quantitative analysis was performed using the Scion Image Beta 4.0.2 program. The densities of IRF-3/7 bands were measured by comparison with tubulin bands.
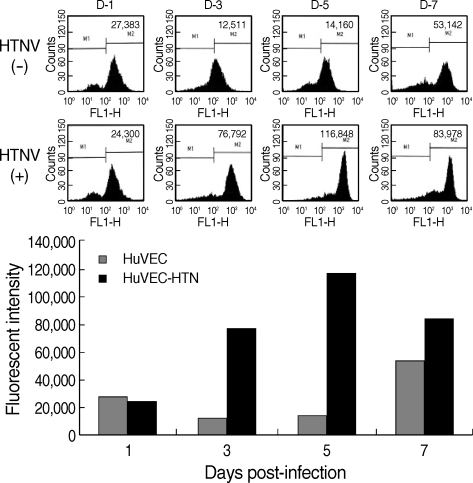
Surface expression of MHC class I molecules
Surface expression of MHC class I was examined in control and HTNV-infected HuVECs by FACS analysis. MHC class I increased from 3 to 7 d.p.i. (Fig. 4). The surface expressions increased 6.1-folds and 8.2-folds in HTNV-infected HuVECs at 3 and 5 d.p.i., respectively (Fig. 4).
Fig. 4.
Surface expression of MHC class I antigen in HuVECs was analyzed by FACS.
DISCUSSION
Understanding of antiviral responses is important for the study of host defense mechanism against viruses. In this study, molecules related to antiviral response and viral clearance such as type I IFNs, IRF-3/7, MxA, TAP-1/2, and MHC were investigated in HuVECs during HTNV infection.
IFN-α/β are synthesized in response to virus infection, and is produced at low levels in the absence of virus infection (19). It is known that constitutively expressed IRF-3 is activated by viral infection, and the activated IRF-3 is capable of inducing a limited subset of type I IFN genes, while IRF-7 activates a broad spectrum of IFN isotypes. IFN-α genes are differentially regulated by positive feedback through interferon regulatory factor-7 (3). In this study, we found that IFN-β, IRF-7, MxA and TAP-1 gene expressions were greatly induced by HTNV infection. IFN-α/β mRNA expressions were slightly induced as early as 1 day after HTNV infection, while other IFN-related genes did not induced yet in this period. The mRNA expression of IFN-α increased to 2.1, 3, 1.4, and 0.6-folds, and IFN-β increased to 4, 277, 80, and 4-folds, while that of IRF-7 increased to 1.1, 29.4, 22.8, and 18.6-folds at 1, 3, 5, and 7 d.p.i., respectively (Table 2). Thus, type I IFNs induced the IRF7 and IRF7 seemed to be a negative regulator for type I IFNs, not a positive regulator in HTNV-infected HuVECs. Therefore, the IFN-β seems to be a main interferon response in HTNV infection and works as a shortacting molecule for the induction of interferon-stimulated genes in an early HTNV replication phase.
MxA, one of the IFN-induced molecules, inhibits viral multiplication of some RNA viruses such as Orthomyxoviridae (Influenza A/C virus and Thogoto virus), Rhabdoviridae (Vesicular stomatitis virus), Paramyxoviridae (Measles virus and Parainfluenza 3), Togaviridae (Semliki Forest virus), and Bunyaviridae (LaCrosse virus and Hantavirus) (14, 20). In this study, MxA mRNA increased to very high levels such as 296, 239, and 207-folds at 3, 5, and 7 d.p.i., respectively (Table 2). It is known that the human MxA protein is induced by IFN-α/β (13, 21). However, our results showed that the mRNA expression pattern of MxA was similar with that of IRF7. These results suggest that the early phase of MxA gene expression is induced by type I IFN but the late phase might be regulated by either IRF7 or other factors rather than type I IFN. IRF-7 and MxA expressed continuously from 3 to 7 d.p.i. and this represented that they work as long-lasting anti-viral response molecules during HTNV-infection (Table 2). When compared to mRNA changes, 75 kDa IRF7 protein expression was not significantly changed (Fig. 3). However, highly expressed 40-42 kDa IRF-7 was detected only in the cytoplasm of HTNV-infected cells (data not shown). This small IRF-7 might be one of the isoform of IRF-7 or the delayed-degradation form of IRF-7 (5). Until now, we do not know how the small-sized IRF-7 were produced by HTNV. Therefore, further studies are necessary to confirm these findings.
The IRF-3 mRNA expression pattern was different from other genes. The IRF3 mRNA expression was lower than 3-folds in all time courses. Therefore, there was no significant changes in the gene expression of IRF3. The IRF3 protein levels were unchanged by HTNV-infection during all of the time courses. These results are consistent with other reports that IRF-3 transcription is constitutive and is not induced by viral infection, but activation is dependent on phosphorylation upon viral infection (19, 22).
Viral antigen is presented to the cell surface through the TAP-mediated peptide transport to the MHC class I molecules in the endoplasmic reticulum and surface expression of MHC I. According to the virus species and cell type, two opposite results occur. The first one is to increase viral clearance and the other is to decrease it through the regulation of TAPs and MHC class I molecules (23). In this study, TAP-1 mRNA levels increased 26.3 folds at 3 d.p.i. and then decreased gradually until 8.1-folds at 7 d.p.i.. TAP-2 mRNA expression levels were lower than 6-folds (Table 2). IFN-γ treatments lead to the induction of TAP-1 expression in macrophages (24). We used endothelial cells, and there was no IFN-γ production. Therefore, TAP-1/2 gene expression might be associated with type I IFN or IFN-stimulated genes other than IFN-γ in HTNV-infected HuVECs. The expression of MHC class I molecules increased from 3 to 7 d.p.i., and it was 8.2-fold higher in HTNV-infected HuVECs than in controls at 5 d.p.i. (Fig. 4).
We attempted to find the gene expression kinetics of interferon and interferon-stimulated genes in endothelial cells following infection with Hantaan virus. The type I IFN responded at an early period of viral infection, whereas the induction of IRF-7 and MxA, TAP-1 was delayed and maintained at a high level until 7 d.p.i.. The MHC class I surface expression reached its peak at 5 d.p.i.. These results suggest that anti-viral responses in endothelial cells following HTNV-infection occur in two steps, the early response by type I IFNs and the late response through the interferon-stimulated genes and antigen presentation.
Footnotes
This research was supported by the Yeungnam University Research Grants in 2004.
References
- 1.Sen GC. Viruses and interferons. Annu Rev Microbiol. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 3.Marie I, Durbin JE, Levy DE. Differential viral induction of distinct Interferon-α gene by positive feedback through Interferon Regulatory Factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izaguirre A, Barnes BJ, Amrute S, Yeow WS, Megjugorac N, Dai J, Feng D, Chung E, Pitha PM, Fitzgerald-Bocarsly P. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J Leukoc Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- 5.Prakash A, Levy DE. Regulation of IRF7 through cell type-specific protein stability. Biochem Biophys Res Commun. 2006;342:50–56. doi: 10.1016/j.bbrc.2006.01.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 7.Prakash A, Smith E, Lee CK, Levy DE. Tissue-specific positive feedback requirements for production of type I interferon following virus infection. J Biol Chem. 2005;280:18651–18657. doi: 10.1074/jbc.M501289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmaljohn CS, Hasty SE, Harrison SA, Dalrymple JM. Characterization of Hantaan virus, the prototype virus of hemorrhagic fever with renal syndrome. J Infect Dis. 1983;148:1005–1012. doi: 10.1093/infdis/148.6.1005. [DOI] [PubMed] [Google Scholar]
- 9.Linderholm M, Elgh F. Clinical characteristics of hantavirus infections on the Eurasian continent. Curr Top Microbiol Immunol. 2001;256:135–151. doi: 10.1007/978-3-642-56753-7_8. [DOI] [PubMed] [Google Scholar]
- 10.Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, Sanchez A, Childs J, Zaki S, Peters CJ. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- 11.Kanerva M, Melen K, Vaheri A, Julkunen I. Inhibition of Puumala and Tula hantaviruses in Vero cells by MxA protein. Virology. 1996;224:55–62. doi: 10.1006/viro.1996.0506. [DOI] [PubMed] [Google Scholar]
- 12.Alff PJ, Gavrilovskaya IN, Gorbunova E, Endriss K, Chong Y, Geimonen E, Sen N, Reich NC, Mackow ER. The pathogenic NY-1 hantavirus G1 cytoplasmic tail inhibits RIG-I- and TBK-1-directed interferon responses. J Virol. 2006;80:9676–9686. doi: 10.1128/JVI.00508-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haller O, Frese M, Kochs G. Mx proteins: mediators of innate resistance to RNA viruses. Rev Sci Tech. 1998;17:220–230. doi: 10.20506/rst.17.1.1084. [DOI] [PubMed] [Google Scholar]
- 14.Staeheli P, Pitossi F, Pavlovic J. Mx proteins: GTPases with antiviral activity. Trends Cell Biol. 1993;3:268–272. doi: 10.1016/0962-8924(93)90055-6. [DOI] [PubMed] [Google Scholar]
- 15.Makela S, Mustonen J, Ala-Houhala I, Hurme M, Partanen J, Vapalahti O, Vaheri A, Pasternack A. Human leukocyte antigen-B8-DR3 is a more important risk factor for severe Puumala hantavirus infection than the tumor necrosis factor-alpha(-308) G/A polymorphism. J Infect Dis. 2002;186:843–846. doi: 10.1086/342413. [DOI] [PubMed] [Google Scholar]
- 16.Mustonen J, Partanen J, Kanerva M, Pietila K, Vapalahti O, Pasternack A, Vaheri A. Genetic susceptibility to severe course of nephropathia epidemica caused by Puumala hantavirus. Kidney Int. 1996;49:217–221. doi: 10.1038/ki.1996.29. [DOI] [PubMed] [Google Scholar]
- 17.Lankat-Buttgereit B, Tampe R. The transporter associated with antigen processing: function and implications in human diseases. Physiol Rev. 2002;82:187–204. doi: 10.1152/physrev.00025.2001. [DOI] [PubMed] [Google Scholar]
- 18.Raftery MJ, Kraus AA, Ulrich R, Kruger DH, Schonrich G. Hantavirus infection of Dendritic cells. J Virol. 2002;76:10724–10733. doi: 10.1128/JVI.76.21.10724-10733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct triggering of the type I interferon system by virus infection: activationof a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frese M, Kochs G, Feldmann H, Hertkorn C, Haller O. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J Virol. 1996;70:915–923. doi: 10.1128/jvi.70.2.915-923.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haller O, Kochs G. Interferon-induced Mx proteins: dynamin-like GTPases with antiviral activity. Traffic. 2002;3:710–717. doi: 10.1034/j.1600-0854.2002.31003.x. [DOI] [PubMed] [Google Scholar]
- 22.Lin R, Heylbroeck C, Pitha PM, Hiscott J. Virus-dependent phosphorylation of the IRF-3 transactivation potential, and proteasome-mediated degradation. Mol Cell biol. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momburg F, Mullbacher A, Lobigs M. Modulation of transporter associated with antigen processing (TAP)-mediated peptide import into the endoplasmic reticulum by flavivirus infection. J Virol. 2001;75:5663–5671. doi: 10.1128/JVI.75.12.5663-5671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cecil AA, Klemsz MJ. p38 activation through Toll-like receptors modulates IFN-gamma-induced expression of the Tap-1 gene only in macrophages. J Leukoc Biol. 2004;75:560–568. doi: 10.1189/jlb.0803375. [DOI] [PubMed] [Google Scholar]