Abstract
Prostaglandins have been implicated in several neovascular diseases. In the present study, we found elevated FP receptor and vascular endothelial growth factor (VEGF) expression colocalized in glandular epithelial and vascular cells lining the blood vessels in endometrial adenocarcinomas. We investigated the signaling pathways activated by the FP receptor and their role in modulating VEGF expression in endometrial adenocarcinoma (Ishikawa) cells. Ishikawa cells were stably transfected with FP receptor cDNA in the sense or antisense orientations. Treatment of Ishikawa cells with prostaglandin F2α (PGF2α) rapidly induced transphosphorylation of the epidermal growth factor receptor (EGFR) and phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 via the FP receptor. Activation of EGFR-Ras-mitogen-activated protein kinase/ERK kinase (MEK) signaling via the FP receptor resulted in an increase in VEGF promoter activity, expression of VEGF mRNA, and secretion of VEGF protein. These effects of PGF2α on the FP receptor could be abolished by treatment of cells with a specific FP receptor antagonist, chemical inhibitors of c-Src, matrix metalloproteinase, and EGFR kinase or by inactivation of signaling with dominant-negative mutant isoforms of EGFR, Ras, or MEK or with small inhibitory RNA oligonucleotides targeted against the EGFR. Finally, we confirmed that PGF2α could potentiate angiogenesis in endometrial adenocarcinoma explants by transactivation of the EGFR and induction of VEGF mRNA expression.
Introduction
Over the past decade, a role has been established for cyclooxygenase (COX) enzymes and prostaglandins in the development and progression of inflammation and cancer (1, 2). Of particular significance to female reproductive health are the observations that reproductive tract cancers, such as endometrial adenocarcinoma and cervical carcinoma, which highly express COX enzymes, also contain elevated levels of prostaglandins and expression and signaling of prostanoid G protein–coupled receptors (GPCR; refs. 3-6). These findings suggest that aberrant prostanoid receptor signaling may be key toward the regulation or progression of female reproductive tract pathologies, such as endometrial adenocarcinoma.
In the reproductive tract, the E and F series of prostaglandins are the most abundantly biosynthesized prostanoids, and prostaglandin F2α (PGF2α) is a major metabolite of COX enzymes in human endometrium (7, 8). Currently, there are three known isoforms of COX enzyme, COX-1, COX-2, and COX-3 (a splice variant of COX-1; refs. 3, 9). PGF2α is biosynthesized from arachidonic acid by a series of oxidation steps by COX enzymes and PGF synthase, respectively (10). Following biosynthesis, PGF2α is transported out of the cell by a prostaglandin transporter (11) where it exerts an autocrine/paracrine function through GPCR-mediated interaction. The GPCR for the human PGF2α (FP receptor) has been cloned, and its activation leads to coupling to the G protein Gq and release of inositol-1,4,5-trisphosphate and diacylglycerol (12).
Recently, we have reported elevated expression of the FP receptor in human endometrial adenocarcinomas and ascertained a role for PGF2α in enhancing the proliferation of endometrial adenocarcinoma cells (6), confirming an autocrine/paracrine regulation of neoplastic cell function by PGF2α-FP receptor interaction. Prostanoid biosynthesis and signaling, including PGF2α, have been implicated in numerous endometrial pathologies, including excessive menstrual bleeding (menorrhagia), endometriosis, and dysmenorrhea (7, 13, 14).
Like many other solid tumors, the growth and proliferation of endometrial adenocarcinoma is dependent on angiogenesis, the formation of new blood vessels from the preexisting vascular bed (15). Of the numerous proangiogenic factors reported to date, vascular endothelial growth factor (VEGF) is the progenitor in the mediation of tumor angiogenesis (16). Elevated expression of VEGF in human tumor biopsies has been reported in various carcinomas, including endometrial adenocarcinoma (17). Recently, a role for the E-series prostanoid receptors in angiogenesis has been established ( for review, see ref. 3). However, little is known about FP receptor signaling to angiogenic factors, such as VEGF.
In this study, we investigated the potential role of elevated FP receptor expression and signaling in modulating vascular function in endometrial adenocarcinomas. We found elevated FP receptor and VEGF expression in endometrial adenocarcinomas, colocalized in glandular epithelial and endothelial cells. We investigated the signal transduction pathways associated with VEGF expression via the FP receptor using an endometrial adenocarcinoma (Ishikawa) cell model system. We found that PGF2α rapidly augments the activation of the extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathway in a c-Src-, matrix metalloproteinase (MMP)-, epidermal growth factor receptor (EGFR)-, and Ras-dependent manner via the FP receptor. Activation of the EGFR-Ras-mitogen-activated protein kinase (MAPK)/ERK kinase (MEK)-ERK1/2 pathway resulted in an increase in VEGF promoter activity, VEGF mRNA expression, and secretion of VEGF protein. Finally, using human endometrial adenocarcinoma explants, we confirmed that PGF2α could potentiate tumorigenesis in situ by transactivating the EGFR and inducing the expression of VEGF to promote angiogenesis.
Materials and Methods
Reagents
Culture medium was purchased from Life Technologies (Paisley, United Kingdom). Penicillin-streptomycin and FCS were purchased from PAA Laboratories (Middlesex, United Kingdom). The c-Myc (sc-40), EGFR (sc-03), phospho-EGFR (sc-12351), and ERK (sc-93) antibodies were purchased from Santa Cruz Biotechnology/Autogen-Bioclear (Wiltshire, United Kingdom). The FP receptor antibody (101802) was purchased from Cayman Chemical Co./Alexis Corp. (Nottingham, United Kingdom). Anti-phospho-p42/p44 ERK (9101), phospho-p38 (9211), total p38 (9212), phospho–c-Jun NH2-terminal kinase (JNK; 9251), and total JNK (9252) antibodies were purchased from Cell Signaling Technologies/New England Biolabs (Herts, United Kingdom). The CD31 antibody was purchased from DAKO Corp. (High Wycombe, United Kingdom). Alkaline phosphatase secondary antibodies, indomethacin, PBS, bovine serum albumin, and PGF2α were purchased from Sigma Chemical Co. (Dorset, United Kingdom). ECF chemiluminescence system was purchased from Amersham Biosciences (Buckinghamshire, United Kingdom). PD98059 (18.7 mmol/L stock in DMSO), PP2 (10 mmol/L stock in DMSO), GM6001 (10 mmol/L stock in DMSO), GF109203X (10 mmol/L stock in DMSO), and AG1478 (10 mmol/L stock in DMSO) were purchased from Calbiochem (Nottingham, United Kingdom) and stored at -20°C.
Patients and Tissue Collection
Endometrial adenocarcinoma tissue (n = 25) was collected from women undergoing hysterectomy who had been prediagnosed to have adenocarcinoma of the uterus. All women were postmenopausal and had received no treatment before surgery. The ages of the patients ranged from 50 to 71 years with a median age of 60.5 years. Hysterectomy specimens for adenocarcinoma were collected from the operating theatre and placed on ice. With minimal delay, the specimens were opened by a gynecologic pathologist. Small samples (~5 mm-3 cm) of polypoidal adenocarcinoma tissue were collected from the endometrial lumen, transferred into neutral-buffered formalin (and wax embedded for immunofluorescence studies), snap frozen in dry ice, and stored at -70°C ( for RNA extraction), and placed in RPMI 1640 containing 2 mmol/L L-glutamine, 100 units penicillin, 100 μg/mL streptomycin, and 3 μg/mL indomethacin (to inhibit endogenous COX activity) for in vitro culture. The diagnosis of adenocarcinoma was confirmed histologically in all cases and the percentage of tumor cells to stroma was estimated to be ~75%:25%. Normal endometrial tissue (n = 10) at different stages of the menstrual cycle was collected from women undergoing surgery for minor gynecologic procedures with no underlying endometrial pathology with an endometrial suction curette (Pipelle, Laboratoire CCD, Paris, France) from women with regular menstrual cycles (25-35 days) and processed exactly as described above. The ages of the control women ranged from 21 to 39 years with a median age of 30.5 years. None of the control women had received a hormonal preparation in the 3 months preceding biopsy collection. Biopsies were dated according to the stated last menstrual period and confirmed by histologic assessment according to the criteria of Noyes et al. (18). Ethical approval was obtained from Lothian Research Ethics Committee and written informed consent was obtained from all subjects before tissue collection.
Cell Culture
Ishikawa endometrial adenocarcinoma cells (European Collection of Cell Culture, Wiltshire, United Kingdom) were maintained as described previously (6). Stable FP transfectant cells were maintained under the same conditions with the addition of a maintenance dose of 200 μg/mL G418.
FP Receptor Amplification and Cell Transfections
The FP receptor cDNA clone and stable cell lines were constructed by Cytomix Ltd. (Cambridge, United Kingdom). The FP receptor cDNA was amplified from a human lung cDNA library from the Genbank accession no. NM_000959 by PCR using the proofreading polymerase Pfu (Stratagene, Amsterdam, The Netherlands). The product was directionally cloned into the mammalian expression vector gWIZ3.0 (Gene Therapy Systems, Cambridge, United Kingdom), in both sense and antisense directions, and the orientation of the construct was confirmed by automated DNA sequencing. The FP receptor cDNA was transfected into Ishikawa cells at passage 10 in the sense and antisense directions. Control cells transfected with pcDNA6/V5/His/LacZ (Invitrogen, De Schelp, The Netherlands) cDNA and assayed with β-galactosidase were used to determine a transfection efficiency of 45 ± 5%. Individual cell populations were selected for with addition of 800 μg/mL G418. Full selection was confirmed by the 100% death of nontransfected control cells, and 11 single FP receptor sense and antisense clones were selected and expanded for Western blot analysis. The clones with the highest level of FP receptor expression [FP sense (FPS)] and lowest receptor expression [FP antisense (FPAS)] were expanded and stored in liquid nitrogen. Based on the relative quantification of FP receptor expression, two sense clones (S32 and S22) and two antisense clones (A14 and A19) exhibiting similar phenotypic and biochemical alterations were supplied. The results of our studies using the S32 and A19 clones are presented here.
Immunofluorescent Microscopy
Cells
Approximately 10,000 wild-type (WT), FPS, and FPAS cells were seeded in chamber slides, fixed in methanol, washed in PBS, and blocked using 5% normal swine serum. Subsequently, the cells were incubated with polyclonal rabbit anti–FP receptor antibody at a dilution of 1:50 at 4°C for 18 hours followed by swine anti-rabbit tetramethylrhodamine isothiocyanate (DAKO) at 25°C for 20 minutes and counterstained with To-Pro-3 (Molecular Probes, Inc., Eugene, OR) at a dilution of 1:2,000 for 2 minutes. Cells were then mounted in Permafluor (Immunotech-Coulter, Buckinghamshire, United Kingdom) and coverslipped. Control cells were incubated with rabbit IgG.
Tissues
FP receptor and VEGF or CD31 protein expression were colocalized in endometrial adenocarcinomas (n = 12) by dual immunofluorescence immunohistochemistry. Tissue sections were prepared as described previously (6) and blocked using 5% normal horse ( for FP/VEGF) or normal goat ( for FP/CD31) serum diluted in PBS. Subsequently, sections were incubated with either goat anti-VEGF antibody at a dilution of 1:50 or mouse anti-CD31 antibody (1:30) for 18 hours at 4°C. Control sections were incubated with goat or mouse IgG, respectively. Thereafter, sections were washed with PBS and incubated with biotinylated horse antigoat for FP/VEGF or goat anti-mouse IgG (DAKO) followed by incubation with the fluorochrome streptavidin Alexa Fluor 488 (Molecular Probes) diluted 1:200 in PBS. Sections were reblocked with 5% normal goat serum diluted in PBS and incubated with rabbit anti–FP receptor antibody at a dilution of 1:100 at 4°C for 18 hours. Control sections were incubated with rabbit IgG. Thereafter, the cells were washed in PBS and incubated with the fluorochrome streptavidin Alexa Fluor 546 diluted 1:200 in PBS at 25°C for 20 minutes. Cells were mounted in Permafluor and coverslipped. Fluorescent images were visualized and photographed using a Carl Zeiss (Jena, Germany) laser scanning microscope LM510.
Protein Extraction
Cells
For EGFR transactivation studies, 3 × 106 cells were seeded in 10 cm dishes, and for MAPK studies, 1 × 106 cells were seeded in 5 cm dishes. The following day cells were incubated in serum-free culture medium and 8.4 μmol/L indomethacin (a dual COX enzyme inhibitor used to inhibit endogenous prostanoid biosynthesis) for at least 16 hours. The next day, cells were pretreated with specific inhibitors for c-Src (PP2, 10 μmol/L), MMP (GM6001, 10 μmol/L), EGFR kinase (AG1478, 100 nmol/L), or MEK (PD98059, 50 μmol/L) or FP receptor antagonist AL8810 (Calbiochem) or vehicle for 1 hour before stimulation with 100 nmol/L PGF2α ( for the period specified in the figure legends) or vehicle. Following stimulation with PGF2α, proteins were extracted and quantified as described previously (6). The protein content in the supernatant fraction was determined using protein assay kits (Bio-Rad, Hemel Hempstead, United Kingdom).
Tissue
For EGFR transactivation studies, carcinoma tissue (n = 4) explants were finely chopped using a sterile scalpel blade and incubated in serum-free medium for at least 12 hours containing penicillin/streptomycin (as described previously) and 8.4 μmol/L indomethacin. The next day, tissue was pretreated with 50 μmol/L AL8810 or 100 nmol/L AG1478 for 1 hour before stimulation with 100 nmol/L PGF2α for 10 minutes. Following stimulation with PGF2α, tissue was washed with PBS and protein was harvested by homogenization in protein lysis buffer, clarified by centrifugation, and assayed as described above before immunoprecipitation and/or Western blot analysis.
Immunoprecipitation and Western Blot Analysis
For immunoprecipitation studies, equal amounts of protein were incubated with specific EGFR or c-Myc antibody preconjugated to protein A-Sepharose overnight at 4°C with gentle rotation. Beads were washed extensively with lysis buffer and immune complexes were solubilized in Laemmli buffer [125 mmol/L Tris-HCl (pH 6.8), 4% SDS, 5% 2-mercaptoethanol, 20% glycerol, 0.05% bromophenol blue] and then boiled for 5 minutes. For FP receptor expression in cells and MAPK studies, a total of 50 μg of protein was resuspended in 20 μL Laemmli buffer. Proteins were resolved and immunoblotted as described previously (6) and incubated with specific primary and alkaline phosphatase–conjugated secondary antibodies. Immunoreactive proteins were visualized by the ECF chemiluminescence system according to the manufacturer’s instructions. Proteins were revealed and quantified by PhosphorImager analysis using the Typhoon 9400 system (Molecular Dynamics/Amersham Biosciences). Relative density in immunoblots was calculated by dividing the value obtained from the phosphorylated blots by the value obtained from total protein blots and expressed as fold above vehicle controls. All data are presented as mean ± SE.
Total Inositol Phosphate Assays
Total inositol phosphate production was measured in Ishikawa WT, FPS, and FPAS cells treated either with vehicle, 100 nmol/L PGF2α, or PGF2α and increasing doses of AL8810 and assayed as described previously (19). Data are presented as mean ± SE.
Taqman Quantitative Reverse Transcription-PCR
FP receptor and VEGF (n = 25 carcinoma and n = 10 normal tissues) expression in endometrial tissues and VEGF expression in FPS cells was measured by quantitative reverse transcription-PCR (RT-PCR) analysis as described previously (20). Cells were synchronized by serum withdrawal for at least 12 hours in serum-free medium containing 8.4 μmol/L indomethacin. Carcinoma explants were finely chopped using a sterile scalpel blade and incubated in serum-free medium for at least 12 hours. Thereafter, medium was removed and replaced with fresh medium containing indomethacin with either 100 nmol/L PGF2α, vehicle, or 100 nmol/L PGF2α and chemical inhibitor for the time indicated in the figure legends. RNA was extracted using Tri-reagent (Sigma Chemical) following the manufacturer’s guidelines. Once extracted and quantified, RNA samples were reverse transcribed and subjected to RT-PCR analysis using an ABI Prism 7700 as described previously (6, 20). VEGF and FP primers and probe for quantitative PCR were designed using the PRIMER express program (PE Applied Biosystems, Warrington, United Kingdom) as described previously (6, 20). Data were analyzed and processed using Sequence Detector version 1.6.3 (PE Applied Biosystems). Expression of VEGF/FP was normalized to RNA loading for each sample using the 18S rRNA as an internal standard. Results are expressed as fold increase above vehicle treated cells.
Transient Transfections
Cell signaling to ERK1/2 was determined using dominant-negative (DN) isoforms targeted against Ras GTPase, EGFR, and MEK. The transfection efficiency of Ishikawa cells had been determined as described earlier and the plasmids for the various ERK cascade members were described recently (21-24). In addition, small interfering RNAs (siRNA) were used to abolish EGFR expression and function using a siRNA/siAB assay kit (Upstate, Milton Keynes, United Kingdom). Transfection efficiency of siRNA experiments was determined by immunofluorescence microscopy as 48 ± 5% using a fluorescein-labeled siRNA. Briefly, Ishikawa sense cells were seeded to a density of 5 × 105 cells per well in 6 cm dishes and exposed to either 300 nmol/L EGFR siRNA or 100 nmol/L control random siRNA or 5 μg c-Myc-ERK1/2 and DN-Ras, DN-EGFR, DN-MEK, or empty vector plasmid (pcDNA3) in the presence of Superfect (Qiagen, Crawley, United Kingdom) for 4 hours and then cultured for 24 hours in complete medium. Thereafter, cells were serum starved for 24 hours in medium containing indomethacin and then exposed to 100 nmol/L PGF2α or vehicle for 10 minutes. Thereafter, cells were lysed and protein was quantified and subjected to Western blot analysis as described above.
VEGF ELISA
Secreted VEGF was measured by ELISA. Cells were first synchronized by serum withdrawal for 12 hours in serum-free medium containing 8.4 μmol/L indomethacin. Thereafter, medium was removed and replaced with fresh serum-free medium containing indomethacin and vehicle, 100 nmol/L PGF2α, or 100 nmol/L PGF2α and AL8810 for 24 hours. Culture medium was removed and VEGF protein was measured using a Human VEGF ELISA kit according to the manufacturer’s instruction (Oncogene, Nottingham, United Kingdom). Data are expressed as fold increase where the amount of VEGF secreted in treated cells is divided by the amount secreted in cells treated with the vehicle. The data are presented as mean ± SE from three independent experiments.
VEGF Luciferase Reporter Assays
The VEGF reporter plasmid (kindly supplied by Prof. Keping Xie, Department of Gastrointestinal Medical Oncology and Cancer Biology, The University of Texas M.D. Anderson Cancer Center, Houston, TX; ref. 25) containing the firefly luciferase reporter was cotransfected into Ishikawa cells in triplicate with an internal control pRL-TK (containing the Renilla luciferase coding sequence; Promega, Southampton, United Kingdom) and either control vector (pcDNA3.0) or vector encoding a DN-Ras, DN-EGFR, and DN-MEK. Cells were transfected using Superfect for 6 hours. The following day, the cells were serum starved for at least 18 hours with indomethacin before stimulation for 8 hours with vehicle, PGF2α, or PGF2α and AL8810. The activity of both firefly and Renilla luciferase was determined using the dual luciferase assay kit. Fold increase in luciferase activity was calculated by dividing the relative luciferase activity in cells treated with PGF2α by the relative luciferase activity in cells treated with vehicle.
Statistics
Where appropriate, data were subjected to statistical analysis with ANOVA and Fisher’s protected least significant difference tests (Statview 5.0, Abacus Concepts, Inc., Carpenteria, CA).
Results
FP receptor and vascular endothelial growth factor expression in endometrial adenocarcinoma and normal endometrium
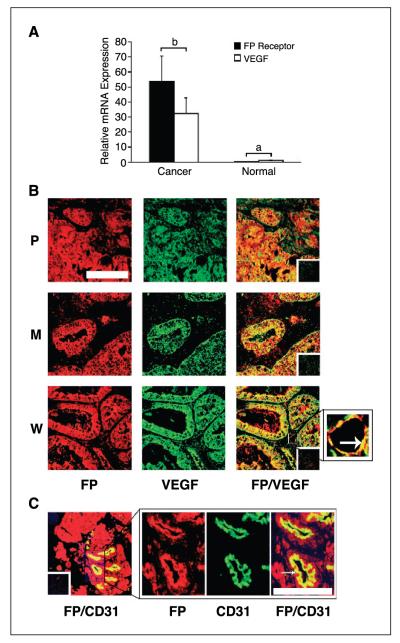
The expression of FP receptor and VEGF mRNA was significantly up-regulated in all cases of endometrial adenocarcinoma investigated compared with normal endometrium (P < 0.05) as determined by Taqman quantitative RT-PCR analysis (Fig. 1A). No correlation was observed between levels of expression of FP receptor and VEGF mRNA and grade or stage of carcinoma. The relative expression of FP receptor and VEGF in endometrial adenocarcinomas was determined to be 53.7 ± 16.9 and 32.5 ± 10.2, respectively, compared with expression in normal endometrium (0.4 ± 0.12 and 1.12 ± 0.25 for FP receptor and VEGF, respectively).
Figure 1.
A, relative mRNA expression of FP receptor and VEGF in endometrial adenocarcinoma (n = 25) and normal endometrium (n = 10) as determined by real-time quantitative RT-PCR analysis. B, localization of the site of expression of FP receptor (red) and VEGF (green) and colocalization of FP receptor with VEGF (merged; yellow) in poorly (P), moderately (M), and well-differentiated (W) endometrial adenocarcinomas, respectively. Arrow, blood vessel under high magnification. Bar, 100 μm. C, localization of the site of expression of FP receptor (red) and CD31 (green) and colocalization of FP receptor with CD31 (merged; yellow) in the endothelial cells of the blood vessels. Representative sample of a moderately differentiated adenocarcinoma. Insets, negative controls. Bar, 10 μm. P < 0.05, b is significantly different from a.
Colocalization of FP receptor with vascular endothelial growth factor or CD31 in endometrial adenocarcinomas
FP receptor and VEGF expression was colocalized in endometrial adenocarcinomas by dual immunofluorescence immunohistochemistry (Fig. 1B). FP receptor expression and VEGF expression were localized together (FP/VEGF) in the glandular epithelial and endothelial compartment in all poorly, moderately, and well-differentiated endometrial adenocarcinomas. To confirm that FP receptor was localized to the endothelial cells of blood vessels, we did dual immunofluorescence immunohistochemistry on tissue sections using antibodies raised against the FP receptor and CD31 endothelial cell marker (Fig. 1C). FP receptor (FP) colocalized (FP/CD31) in the blood vessels with CD31 in all endometrial adenocarcinomas. Incubating sections with nonimmune IgG from the host species abolished the immunoreactivity.
FP receptor expression in Ishikawa cells
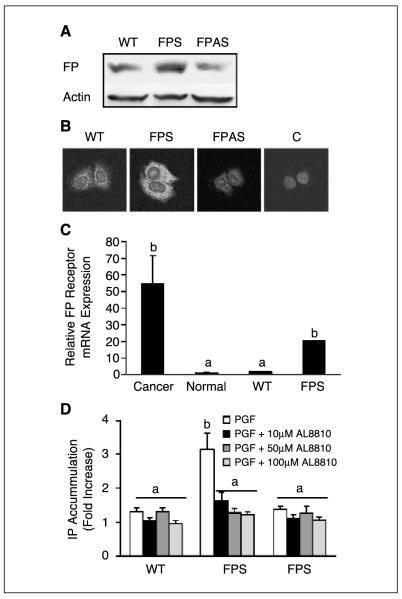
FP receptor expression was assessed in stably transfected Ishikawa cells overexpressing the FP receptor in the sense (FPS) and antisense (FPAS) orientations. Western blot analysis (Fig. 2A) and immunofluorescence microscopy (Fig. 2B) showed elevated FP receptor expression in FPS cells and reduced FP expression in FPAS cells compared with WT cells. FP receptor expression was localized to the plasma membrane (Fig. 2B) and was abolished by incubating cells with rabbit IgG in place of antibody (Fig. 2B, control representative of FPS cells, and Fig. 2C). Quantitative RT-PCR analysis was then used to compare FP receptor expression in WT and FPS Ishikawa cells with FP receptor expression detected in normal endometrium and endometrial adenocarcinomas in vivo. As shown in Fig. 2C, the relative FP receptor mRNA expression in FPS cells and adenocarcinoma tissues was significantly greater than the FP receptor expression in WT Ishikawa cells or normal endometrial tissue (P < 0.05). FP receptor couples to Gq resulting in increased formation of inositol phosphate. We measured total inositol phosphate accumulation in WT, FPS, and FPAS cells in response to administration of 100 nmol/L PGF2α. A significant increase in inositol phosphate accumulation was observed in FPS cells compared with WT and FPAS cells following treatment with PGF2α (P < 0.05; Fig. 2D). Treatment of FPS cells with the selective FP receptor antagonist AL8810 (26) dose-dependently reduced the agonist-induced inositol phosphate accumulation (P < 0.05; Fig. 2D).
Figure 2.
A, representative Western blot of FP receptor expression in WT, FPS, and FPAS cells. Cells were immunoblotted with specific FP receptor antibody and normalized for loading against β-actin on the same blot. B, immunofluorescence microscopy done on WT, FPS, and FPAS cells using a specific FP receptor primary antibody. Control cells (C) were incubated with normal IgG in place of primary antibody (representative FPS cells incubated with IgG). C, relative expression of FP receptor mRNA in endometrial adenocarcinoma (n = 25) and normal endometrial tissues (n = 10) compared with FP receptor mRNA expression in Ishikawa WT and FPS cell lines. D, inositol phosphate (IP) accumulation was assessed in WT, FPS, and FPAS cells in response to administration of vehicle, 100 nmol/L PGF2α, or 100 nmol/L PGF2α and increasing doses of specific FP receptor antagonist AL8810. Columns, mean of three independent experiments; bars, SE. P < 0.05, b is significantly different from a.
Prostaglandin F2α-FP receptor activation of mitogen-activated protein kinase signaling
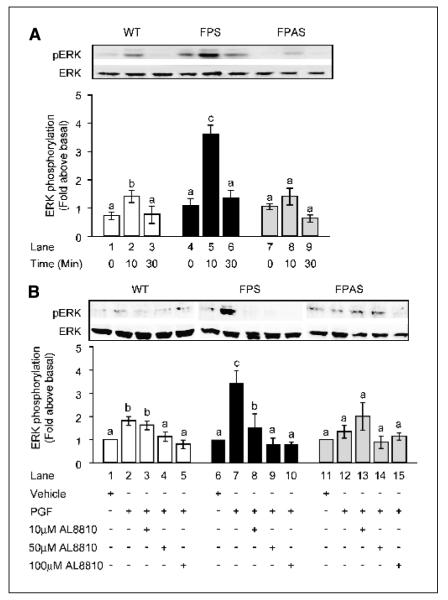
The effect of PGF2α on the activation of the downstream MAPK signaling pathways (ERK1/2, p38, and JNK) was determined after treatment of WT, FPS, and FPAS Ishikawa cells with 100 nmol/L PGF2α. Stimulation of Ishikawa cells with PGF2α caused a rapid and augmented activation of ERK1/2 in FPS compared with WT and FPAS cells (Fig. 3A). The peak of ERK1/2 activation was observed after 10 minutes in Ishikawa FPS treated with 100 nmol/L PGF2α (Fig. 3A, lane 5) compared with cells treated with vehicle alone (Fig. 3A, lane 4). No significant phosphorylation of p38 or JNK MAPK was observed in WT, FPS, or FPAS cells following treatment of cells with 100 nmol/L PGF2α within the period of activation of ERK1/2 (data not shown). Cotreatment of Ishikawa WT, FPS, and FPAS cells with the selective FP receptor antagonist AL8810 dose-dependently inhibited the activation of ERK1/2, indicating that the ERK1/2 activation was mediated via direct PGF2α-FP receptor interaction (Fig. 3B).
Figure 3.
Effect of PGF2α on ERK1/2 signaling in WT, FPS, and FPAS Ishikawa cells. A, Ishikawa cells were stimulated with 100 nmol/L PGF2α for 0, 10, and 30 minutes and subjected to immunoblot analysis using antibody against phosphorylated ERK1/2 (top). The total amount of ERK in cell lysates was determined by reprobing the same blot with antibody recognizing total protein (bottom). B, Ishikawa WT, FPS, and FPAS cells were pretreated for 1 hour with increasing doses of specific FP receptor antagonist (AL8810) or vehicle followed by stimulation with vehicle, 100 nmol/L PGF2α, or PGF2α and antagonist for 10 minutes. For each, a representative Western blot is shown, with semiquantitative analysis of ERK phosphorylation determined as described in Materials and Methods. Columns, mean of four independent experiments; bars, SE. P < 0.05, b is significantly different from a; P < 0.01, c is significantly different from a and b. -, absence of agent; +, presence of agent.
Prostaglandin F2α-FP receptor activation of extracellular signal-regulated kinase 1/2 signaling requires epidermal growth factor receptor kinase activity
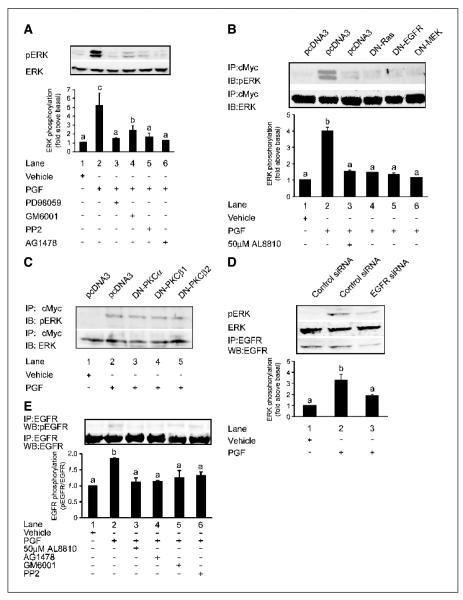
We next evaluated the effect of the selective inhibitors of MEK (PD98059), MMP (GM6001), c-Src (PP2), and EGFR kinase (AG1478) on the PGF2α-induced activation of ERK1/2 signaling in FPS cells. Treatment of FPS cells with 100 nmol/L PGF2α induced a rapid phosphorylation of ERK1/2 (Fig. 4A, lane 2) compared with FPS cells treated for the same period with vehicle alone (Fig. 4A, lane 1). This elevation in ERK1/2 phosphorylation was abolished by treatment of FPS cells with PD98059 (Fig. 4A, lane 3), GM6001 (Fig. 4A, lane 4), PP2 (Fig. 4A, lane 5), or AG1478 (Fig. 4A, lane 6). These results implicate the involvement of c-Src, MMP, and EGFR in PGF2α-FP receptor signaling to ERK1/2. To further explore a role for the EGFR and the small GTPase Ras in the activation of ERK1/2 by PGF2α, we cotransfected FPS cells with the cDNA for c-Myc-tagged ERK with either DN-Ras, DN-EGFR, and DN-MEK or an empty vector (pcDNA3.0). FPS cells were then treated with either vehicle, 100 nmol/L PGF2α, or 100 nmol/L PGF2α and AL8810 for 10 minutes. The tagged ERK was immunoprecipitated with anti-c-Myc antibodies and ERK activity was determined by Western blotting as described in Materials and Methods. PGF2α rapidly phosphorylated ERK1/2 in FPS cells (Fig. 4B, lane 2). This activation of ERK1/2 was abolished by cotreatment with the FP receptor antagonist (AL8810, Fig. 4B, lane 3) or by cotransfection with the DN-Ras (Fig. 4B, lane 4), DN-EGFR (Fig. 4B, lane 5), and DN-MEK (Fig. 4B, lane 6). Preliminary proteomic phosphosite array studies (Kinexus Bioinformatics Corp., Vancouver, British Columbia, Canada) done by our laboratory showed that the predominant isoforms of protein kinase C (PKC) activated by prostanoids in our Ishikawa cells were PKCα, PKCβ1, and PKCβ2.4 We investigated whether PGF2α-FP signaling to ERK1/2 occurred via activation of these PKC isoforms by cotransfecting FPS cells with the cDNA for c-Myc-tagged ERK with either DN-PKCα, DN-PKCβ1, or DN-PKCβ2 or an empty vector (pcDNA3.0). FPS cells were then treated with either vehicle or 100 nmol/L PGF2α for 10 minutes and the cells were immunoprecipitated and immunoblotted as described above. No reduction in ERK phosphorylation was observed in cells transfected with the DN-PKCα (Fig. 4C, lane 3), DN-PKCβ1 (Fig. 4C, lane 4), or DN-PKCβ2 (Fig. 4C, lane 5), implicating that PGF2α-FP receptor signaling to ERK1/2 did not occur via these PKC isoforms (Fig. 4C). We further coincubated FPS cells with the specific PKC inhibitor GF109203X (data not shown), which also did not abolish the PGF2α-induced activation of ERK1/2, confirming that PKC was not involved in the PGF2α-induced activation of ERK1/2.
Figure 4.
Representative Western blots showing activation of ERK1/2 and EGFR signaling in FPS Ishikawa cells. A, FPS cells were pretreated for 1 hour with inhibitors or vehicle followed by stimulation with vehicle (control; lane 1), 100 nmol/L PGF2α (lane 2), 100 nmol/L PGF2α and PD98059 (lane 3), 100 nmol/L PGF2α and GM6001 (lane 4), 100 nmol/L PGF2α and PP2 (lane 5), and 100 nmol/L PGF2α and AG1478 (lane 6) for 10 minutes. B, Ishikawa FPS cells were transfected with c-Myc-tagged ERK cDNA together with pcDNA3 (control empty vector) cDNA or cDNA encoding DN-Ras, DN-EGFR, or DN-MEK. Cells were pretreated for 1 hour with 50 μmol/L AL8810 or vehicle followed by stimulation with vehicle (control), 100 nmol/L PGF2α, or 100 nmol/L PGF2α and 50 μmol/L AL8810 for 10 minutes. C, Ishikawa FPS cells were transfected with c-Myc-tagged ERK cDNA together with pcDNA3 cDNA or cDNA encoding DN-PKCα, DN-PKCβ1, and DN-PKCβ2. Cells were stimulated with vehicle (control) or 100 nmol/L PGF2α for 10 minutes. D, Ishikawa FPS cells were transfected with control random siRNA or siRNA targeted against the EGFR. Cells were stimulated with vehicle or 100 nmol/L PGF2α for 10 minutes and subjected to immunoblot analysis for phosphorylated ERK1/2, total ERK, or immunoprecipitated (IP) and immunoblotted (WB) with antisera against EGFR (bottom). E, representative Western blot showing PGF2α transactivation of EGFR signaling in FPS cells. FPS cells were pretreated for 1 hour with inhibitors, antagonist, or vehicle followed by stimulation with vehicle (control; lane 1), 100 nmol/L PGF2α (lane 2), 100 nmol/L PGF2α and AL8810 (lane 3), 100 nmol/L PGF2α and AG1478 (lane 4), 100 nmol/L PGF2α and GM6001 (lane 5), and 100 nmol/L PGF2α and PP2 (lane 6) for 10 minutes. After lysis, EGFR was immunoprecipitated with anti-EGFR antibody and tyrosine-phosphorylated EGFR was detected by immunoblotting with anti-phospho-EGFR antibody (top). The total amount of EGFR in immunoprecipitates was determined by reprobing the same blot with anti-EGFR antibody (bottom). Immunoblots were quantified as described in Materials and Methods. Columns, mean of four independent experiments; bars, SE. P < 0.05, b is significantly different from a; P < 0.01, c is significantly different from a and b. -, ab- sence of agent; +, presence of agent.
The involvement of the EGFR in transducing ERK1/2 signaling in FPS cells was confirmed using RNA interference. FPS cells were transfected with either control random siRNA or siRNA duplexes targeted against the EGFR. FPS cells were treated with either vehicle or 100 nmol/L PGF2α for 10 minutes. ERK1/2 phosphorylation was significantly reduced in cells transfected with EGFR siRNA (Fig. 4D, lane 3) compared with FPS cells transfected with control random siRNA (Fig. 4D, lane 2). EGFR levels in cells transfected with EGFR siRNA (Fig. 4D, lane 3) was significantly reduced by 68.2 ± 1.3% compared with control transfected cells (Fig. 4D, lane 2; P < 0.05).
Prostaglandin F2α-FP receptor stimulation transactivates the epidermal growth factor receptor
Because ERK1/2 signaling in FPS cells could be inhibited by the EGFR protein tyrosine kinase inhibitor AG1478 as well as by transfecting FPS cells with DN-EGFR or by targeted down-regulation of EGFR with RNA interference, we investigated PGF2α-induced transphosphorylation of the EGFR in FPS cells. Ishikawa FPS cells were treated with 100 nmol/L PGF2α or vehicle and EGFR tyrosine phosphorylation was assessed by immunoprecipitation and Western blotting. A significant increase in EGFR tyrosine phosphorylation compared with vehicle stimulation was observed in Ishikawa FPS cells stimulated with PGF2α (Fig. 4E, lane 2). Treatment of cells with AL8810 (Fig. 4E, lane 3), AG1478 (Fig. 4E, lane 4), GM6001 (Fig. 4E, lane 5), or PP2 (Fig. 4E, lane 6) significantly reduced the PGF2α-induced EGFR tyrosine phosphorylation (P < 0.05).
Prostaglandin F2α-FP receptor activation induces vascular endothelial growth factor expression and secretion in Ishikawa cells
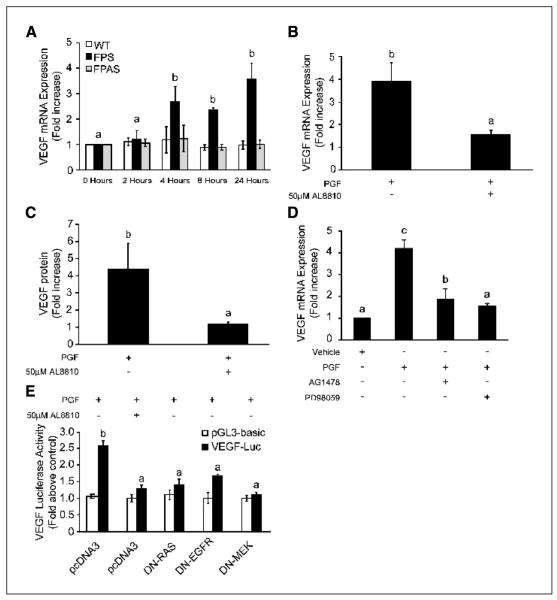
The role of PGF2α-FP receptor signaling on the expression of VEGF was investigated by quantitative RT-PCR analysis after stimulation of WT, FPS, and FPAS cells with 100 nmol/L PGF2α or vehicle for 0, 2, 4, 8, and 24 hours. As shown in Fig. 5A, PGF2α stimulation resulted in a significant fold increase in the expression of VEGF in Ishikawa FPS cells at 4, 8, and 24 hours (P < 0.01). However, no such increase in the expression of VEGF was observed in WT or FPAS cells. Treatment of cells with PGF2α and AL8810 significantly reduced the PGF2α-induced expression of VEGF mRNA at 8 hours (Fig. 5B; P < 0.05), indicating that the effect of PGF2α on VEGF is exerted via the FP receptor. We next investigated whether VEGF protein was secreted in the culture medium of FPS cells treated with 100 nmol/L PGF2α. As shown in Fig. 5C, PGF2α treatment of FPS cells induced a significant elevation in VEGF protein in the culture medium at 24 hours (P < 0.01). Cotreatment of cells with PGF2α and AL8810 abolished the secretion of VEGF into the culture medium (P < 0.05). No significant elevation in secreted VEGF protein was observed in FPS cells at earlier time points and no elevation in VEGF protein secretion was observed in WT and FPAS cells following treatment with PGF2α (data not shown).
Figure 5.
VEGF expression and secretion in Ishikawa cells. A, VEGF expression in Ishikawa WT, FPS, and FPAS cells was measured by real-time quantitative RT-PCR analysis following treatment of cells for 0, 2, 4, 8, and 24 hours with vehicle or 100 nmol/L PGF2α. B, VEGF expression in FPS cells at 8 hours. Cells were treated with 100 nmol/L PGF2α in the absence or presence of FP receptor antagonist (AL8810). C, secretion of VEGF protein into the culture medium of FPS cells was measured by ELISA. FPS cells were treated for 24 hours with vehicle, 100 nmol/L PGF2α, or 100 nmol/L PGF2α and AL8810. D, VEGF expression in Ishikawa FPS cells in response to treatment with chemical inhibitors of EGFR and ERK signaling as measured by real-time quantitative RT-PCR analysis. Cells were treated for 8 hours with vehicle or 100 nmol/L PGF2α. In parallel, cells were treated with 100 nmol/L PGF2α and AG1478 or 100 nmol/L PGF2α and PD98059. E, VEGF promoter activity in FPS cells transfected with the full-length VEGF firefly luciferase promoter (pGL3-VEGF-luc; filled columns) or empty firefly luciferase control vector (pGL3-basic; open columns). FPS cells were transfected with pGL3-basic or pGL3-VEGF-luc and pRL-TK (Renilla luciferase) and cotransfected with either pcDNA3 (control empty vector cDNA) or cDNA encoding DN-Ras, DN-EGFR, and DN-MEK. Following transfection, the cells were incubated for 8 hours with either vehicle, 100 nmol/L PGF2α, or 100 nmol/L PGF2α and AL8810, and firefly and Renilla luciferase activity was measured for the calculation of specific VEGF promoter activity as described in Materials and Methods. Columns, mean of four independent experiments; bars, SE. P < 0.05, b is significantly different from a; P < 0.01, c is significantly different from a and b. -, absence of agent; +, presence of agent.
The role of the EGFR and ERK1/2 signaling pathways in mediating the effects of PGF2α-FP interaction on the expression of VEGF in FPS cells was investigated by quantitative RT-PCR analysis. As shown in Fig. 5D, treatment of FPS cells with 100 nmol/L PGF2α produced a 4.2 ± 0.4–fold increase in VEGF mRNA expression, which was significantly reduced by the EGFR tyrosine kinase inhibitor AG1478 (P < 0.05) and abolished by the selective MEK inhibitor PD98059 (P < 0.05). To further confirm that VEGF expression was mediated via the EGFR and Ras-MEK-ERK1/2 pathways, we used a luciferase reporter plasmid in which the full-length VEGF 5′ flanking sequence was fused to the firely luciferase coding sequence in the pGL3-basic vector (25). Additionally, a luciferase reporter without any VEGF promoter sequences (pGL3-basic) was used as a control. Cells were cotransfected with the pRL-TK (Renilla luciferase vector as an internal control) with either DN-Ras, DN-EGFR, and DN-MEK or an empty vector (pcDNA3.0) as a control. As shown in Fig. 5E, treatment of control vector-transfected cells with 100 nmol/L PGF2α significantly elevated VEGF promoter activity by 2.5 ± 0.1–fold (P < 0.01). This elevation in VEGF promoter activity was abolished by cotreatment of the cells with PGF2α and AL8810 and significantly reduced by cotransfection of cells with DN-EGFR, DN-Ras, or DN-MEK (P < 0.01). Cells transfected with the control luciferase reporter, without any VEGF sequence (pGL3-basic), showed no significant alteration in luciferase activity in response to treatment (Fig. 5E).
Prostaglandin F2α-FP receptor activation induces the expression of vascular endothelial growth factor in endometrial adenocarcinomas via transactivation of the epidermal growth factor receptor
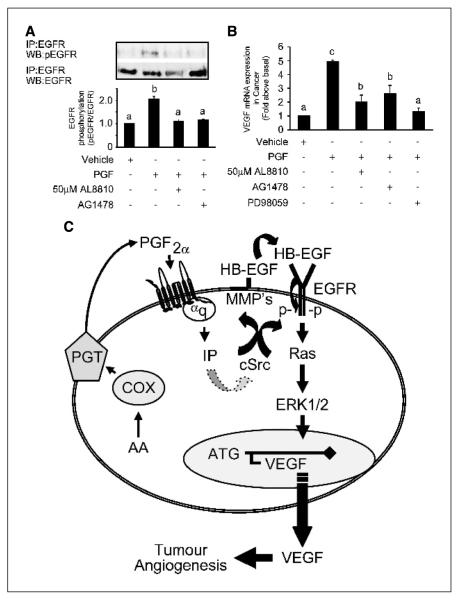
To correlate our findings obtained using the Ishikawa FP receptor model system with PGF2α signaling to VEGF in endometrial adenocarcinomas in vivo, we also used endometrial adenocarcinoma tissue explants. Endometrial adenocarcinoma tissues were treated with 100 nmol/L PGF2α or vehicle alone for 10 minutes and EGFR phosphorylation was assessed by Western blotting. A significant increase in tyrosine phosphorylation of EGFR was observed in endometrial adenocarcinoma explants (Fig. 6A, lane 2; P < 0.05) in response to stimulation with PGF2α compared with control tissue (Fig. 6A, lane 1; P < 0.05). Treatment of endometrial adenocarcinoma explants with AL8810 or AG1478 abolished this action of PGF2α (Fig. 6A, lanes 3 and 4, respectively; P < 0.05). We next incubated endometrial adenocarcinoma explants with either vehicle or PGF2α in the absence or presence of AL8810, AG1478, or PD98059 for 24 hours and assessed VEGF mRNA expression by quantitative RT-PCR analysis (Fig. 6B). PGF2α significantly elevated the expression of VEGF in endometrial adenocarcinoma explants by 4.9 ± 0.4–fold compared with vehicle-treated tissue (P < 0.01). This elevation in expression of VEGF was significantly inhibited by cotreatment of tissue explants with AL8810, AG1478, and PD98059 (P < 0.05). These data obtained from the endometrial adenocarcinoma explants agree with the data obtained in the Ishikawa cell FP receptor model system and imply that PGF2α-FP receptor interaction can potentiate tumor angiogenesis in endometrial adenocarcinomas by enhancing the expression of proangiogenic factors, such as VEGF, following the activation of the EGFR and ERK1/2 pathways mediated by the actions of c-Src and MMPs (Fig. 6C).
Figure 6.
PGF2α transactivation of the EGFR and activation of VEGF in endometrial adenocarcinomas. A, endometrial adenocarcinoma biopsy explants were pretreated for 1 hour with inhibitors or vehicle followed by stimulation with vehicle (control, lane 1), 100 nmol/L PGF2α (lane 2), 100 nmol/L PGF2α and AL8810 (lane 3), or 100 nmol/L PGF2α and AG1478 (lane 4) for 10 minutes. After lysis, EGFR was immunoprecipitated with anti-EGFR antisera and tyrosine-phosphorylated EGFR was detected by immunoblotting with anti-phospho-EGFR antibody. The total amount of EGFR in immunoprecipitates was determined by reprobing the same blot with total EGFR antibody. Semiquantitative analysis of EGFR phosphorylation was determined as described in Materials and Methods. B, VEGF mRNA expression in endometrial adenocarcinoma in response to 100 nmol/L PGF2α as determined by quantitative RT-PCR analysis. Endometrial adenocarcinoma explants were treated with vehicle, 100 nmol/L PGF2α, 100 nmol/L PGF2α and AL8810, 100 nmol/L PGF2α and AG1478, and 100 nmol/L PGF2α and PD98059 for 24 hours. Columns, mean of four independent experiments; bars, SE. P < 0.05, b is significantly different from a; P < 0.01, c is significantly different from a and b. -, absence of agent; +, presence of agent. C, schematic representation of PGF2α-FP receptor signaling to ERK in endometrial adenocarcinoma cells. Intracellular PGF2α produced via the actions of COX enzymes is actively transported out of the cell (PGT) and interacts with FP receptors in an autocrine/paracrine manner to activate inositol-1,4,5-trisphosphate. Activation of inositol phosphate can potentially initiate ERK signaling via the activation of c-Src and MMPs and transphosphorylation of the EGFR, culminating in the transcription and release of VEGF to promote tumor angiogenesis by acting on adjacent endothelial cells.
Discussion
A relationship between PGF2α and vascular dysfunction of the endometrium has been described (13, 27, 28). Elevated PGF2α concentrations of up to 2 μmol/L have been reported in the menstrual fluid of women with dysmenorrhea (7). This concentration is 10-fold greater than that observed for normal women with painless periods (7). In addition, a correlation between PGF2α concentration and excessive menstrual blood loss has also been reported in patients diagnosed with menorrhagia (13, 28, 29). Recent findings in our laboratory have shown elevated COX-2 expression and synthesis and signaling of prostanoids, including PGF2α, in human endometrial adenocarcinomas (4). Moreover, we have recently reported elevated expression of the FP receptor in endometrial adenocarcinomas compared with normal endometrium and ascertained a role for PGF2α-FP receptor signaling in tumorigenesis by enhancing proliferation of endometrial adenocarcinoma cells (6). In the present study, we show colocalized and elevated expression of FP receptor and VEGF within the glandular epithelial cells and endothelial cells lining the blood vessels in endometrial adenocarcinomas. These findings suggest a possible paracrine regulation of tumor vascular function by PGF2α via the FP receptor.
To elucidate the molecular mechanisms whereby PGF2α, via the FP receptor, could modulate the expression of proangiogenic factors, such as VEGF, and thereby promote tumor angiogenesis, we overexpressed FP receptor in Ishikawa cells by introducing the FP receptor cDNA in the sense and antisense orientations. This was done to raise the levels of FP receptor in the Ishikawa cell line to the levels observed in endometrial adenocarcinomas to create a model system that best represents FP receptor signaling in endometrial adenocarcinoma in vivo. Furthermore, because at least nine prostanoid receptors have been detected to date, any attempt to dissect out the signaling of a given receptor is made difficult due to the multiplicity of different signaling pathways and complex networks of cross-talk between them. Hence, our model presented here provides a unique opportunity to dissect out the signaling of a single receptor (i.e., the FP receptor). Elevated FP receptor expression in Ishikawa FPS cells compared with WT and FPAS cells was confirmed by Western blot analysis, immunofluorescence microscopy, and accumulation of inositol phosphate and localized to the plasma membrane, indicating that the overexpressed receptors were trafficked to the correct intracellular compartment. The expression of FP receptor in FPS cells also correlated closely with levels of expression of FP receptor in endometrial adenocarcinomas, and the increase in inositol phosphate accumulation in FPS cells compared with WT and FPAS cells was similar to that observed in endometrial adenocarcinoma explants stimulated with PGF2α, which we reported previously (6). We therefore propose that the Ishikawa FP receptor cell line is a good model system for investigating the molecular signal transduction pathways mediating the role of PGF2α in endometrial adenocarcinoma cells via the FP receptor, which may be involved in the development and/or progression of endometrial tumors in vivo.
The integrated response to GPCR coupling results in activation of numerous effector signaling pathways, including the MAPK pathway (30). The MAPK pathway is a key signaling mechanism that regulates many cellular functions, such as growth, differentiation, and transformation (30, 31). The upstream component of the ERK-MAPK pathway is the GTPase Ras, which activates the serine/threonine kinase Raf that in turn phosphorylates and activates ERK1/2 (32, 33). We examined PGF2α activation of the downstream MAPK cascades (ERK, p38, and JNK). We found that, within our experimental paradigms, PGF2α induced a rapid increase in ERK (but not p38 or JNK) phosphorylation. This PGF2α-induced effect was significantly elevated in FPS cells compared with WT and FPAS cells and was mediated via the FP receptor, because ERK1/2 phosphorylation in FPS cells could be abolished by cotreatment of the cells with the specific FP receptor antagonist (AL8810). Furthermore, we have shown that PGF2α-induced phosphorylation of ERK1/2 in FPS cells occurs in a c-Src-, MMP-, EGFR-, and Ras-dependent manner, because ERK1/2 phosphorylation could be inhibited with specific chemical inhibitors of c-Src (PP2), MMP (GM6001), and EGFR kinase (AG1478) or by cotransfection of FPS cells with DN-EGFR, DN-Ras, and DN-MEK or by siRNA targeted against the EGFR. Interestingly, we observed that PGF2α-induced phosphorylation of ERK1/2 occurred independently of PKC activation.
Recent data in our laboratory have indicated that transphosphorylation of the EGFR by PGF2α is crucial for transducing mitogenic signaling and cell growth (6). Several mechanisms are proposed for the transactivation of EGFR by GPCRs (34-36). One of these mechanisms involves the activation of transmembrane MMP and extracellular release of heparin-binding EGF (HB-EGF) from its latent membrane-spanning precursor in the plasma membrane. Once cleaved, the HB-EGF ligand can associate with and activate the EGFR and induce ERK1/2 activation. Alternatively, activation the EGFR can also occur via the activation of the c-Src family of nonreceptor tyrosine kinases (34-36). We found that the effect of PGF2α on the transphosphorylation of EGFR and ERK activation in FP receptor–expressing Ishikawa cells seems to be mediated by c-Src as well as MMPs. Recently, Guerrero et al. (37) have also shown transactivation of the EGFR by c-Src as well as MMPs, suggesting that EGFR signaling can be mediated via an intracellular c-Src-dependent as well as extracellular MMP-dependent mechanism coactivated in the same cell in a cell context–dependent manner.
Tumor growth is dependent on angiogenesis. To sustain and facilitate growth in an environment where oxygen and nutrients are limiting, cancer cells produce a variety of factors, including VEGF, to create a proangiogenic environment and promote neovascularization (38, 39). VEGF is a heparin-binding glycoprotein having potent angiogenic, mitogenic, and vascular permeability-enhancing properties that are specific for endothelial cells (40). Our data show that PGF2α-FP receptor activation results in elevated expression and secretion of VEGF in Ishikawa FP cells and endometrial adenocarcinoma explants. Our approach using chemical inhibitors of signal transduction or DN mutant isoforms of signaling effectors confirm further the involvement of the EGFR to ERK1/2 signaling in the activation of VEGF expression and secretion following PGF2α-FP receptor interaction. Elevated VEGF expression and secretion would in turn exert a paracrine function on endothelial cells to promote angiogenesis, thus enhancing blood flow to the tumor and creating an environment to sustain tumor growth. Such a mechanism has been proposed for the action of another prostanoid (i.e., prostaglandin E2), via its interaction with the EP2 receptor (20, 38, 41, 42).
Taken together, our findings using the Ishikawa FP receptor model system and which have been confirmed using endometrial adenocarcinoma explants show for the first time that PGF2α-FP receptor interaction can promote the transcription and release of a potent proangiogenic factor (i.e., VEGF) via the transactivation of the EGFR leading to ERK1/2 signaling as depicted schematically in Fig. 6C. Moreover, we propose that targeted inhibition of FP receptor, EGFR, and ERK function in endometrial carcinomas could effectively block the signaling and transcription of target genes, such as VEGF, associated with angiogenesis. High EGFR levels in endometrial cancer have been correlated with poor histopathologic grading, greater invasiveness, and reduced patient survival (43, 44). Blockade of EGFR signaling with an orally active EGFR tyrosine kinase inhibitor has been used successfully in carcinomas of nude mice to inhibit tumor angiogenesis by reducing VEGF expression (45). Similarly, the use of an orally active inhibitor of the Ras-ERK cascade has been proven efficacious in treatment of metastatic melanoma (46). Recently, Torrance et al. (47) have shown that a combinatorial approach using a nonselective COX enzyme inhibitor in combination with an inhibitor of EGFR kinase can reduce polyp formation in APCΔ716 mice more effectively than either compound on their own (47). In light of this latter finding and our observations presented herein, an ERK inhibitor or EGFR tyrosine kinase inhibitor in combination with a FP receptor antagonist may be of clinical relevance as an efficacious therapy for women with endometrial adenocarcinoma. Our data may also have relevance for other endometrial pathologies that are associated with disturbed vascular function and elevated prostanoid biosynthesis, such as menorrhagia, dysmenorrhea, and endometriosis.
Acknowledgments
We thank J. Creiger for assistance with sample collection.
Footnotes
K.J. Sales and H.N. Jabbour, unpublished observation.
References
- 1.Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73. [PubMed] [Google Scholar]
- 2.Lipsky PE. Role of cyclooxygenase-1 and -2 in health and disease. Am J Orthop. 1999;28:8–12. [PubMed] [Google Scholar]
- 3.Sales KJ, Jabbour HN. Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction. 2003;126:559–67. doi: 10.1530/rep.0.1260559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabbour HN, Milne SA, Williams ARW, Anderson RA, Boddy SC. Expression of COX-2 and PGE synthase and synthesis of PGE2 in endometrial adenocarcinoma: a possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. Br J Cancer. 2001;85:1023–31. doi: 10.1054/bjoc.2001.2033. [DOI] [PubMed] [Google Scholar]
- 5.Sales KJ, Katz AA, Davis M, et al. Cyclooxygenase-2 expression and prostaglandin E2 synthesis are up-regulated in carcinomas of the cervix: a possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. J Clin Endocrinol Metab. 2001;86:2243–9. doi: 10.1210/jcem.86.5.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sales KJ, Milne SA, Williams AR, Anderson RA, Jabbour HN. Expression, localization, and signaling of prostaglandin F2α receptor in human endometrial adenocarcinoma: regulation of proliferation by activation of the epidermal growth factor receptor and mitogen-activated protein kinase signaling pathways. J Clin Endocrinol Metab. 2004;89:986–93. doi: 10.1210/jc.2003-031434. [DOI] [PubMed] [Google Scholar]
- 7.Lumsden MA, Kelly RW, Baird DT. Primary dysmenorrhoea: the importance of both prostaglandins E2 and F2α. Br J Obstet Gynaecol. 1983;90:1135–40. doi: 10.1111/j.1471-0528.1983.tb06460.x. [DOI] [PubMed] [Google Scholar]
- 8.Hofer G, Bieglmayer C, Kopp B, Janisch H. Measurement of eicosanoids in menstrual fluid by the combined use of high pressure chromatography and radioimmunoassay. Prostaglandins. 1993;45:413–26. doi: 10.1016/0090-6980(93)90118-q. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekharan NV, Dai H, Roos KL, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99:13926–31. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan BS, Satriano JA, Pucci M, Schuster VL. Mechanism of prostaglandin E2 transport across the plasma membrane of HeLa cells and Xenopus oocytes expressing the prostaglandin transporter “PGT’’. J Biol Chem. 1998;273:6689–97. doi: 10.1074/jbc.273.12.6689. [DOI] [PubMed] [Google Scholar]
- 12.Abramovitz M, Boie Y, Nguyen T, et al. Cloning and expression of a cDNA for the human prostanoid FP receptor. J Biol Chem. 1994;269:2632–6. [PubMed] [Google Scholar]
- 13.Smith SK, Abel MH, Kelly RW, Baird DT. Prostaglandin synthesis in the endometrium of women with ovular dysfunctional uterine bleeding. Br J Obstet Gynaecol. 1981;88:434–42. doi: 10.1111/j.1471-0528.1981.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 14.Rees MC, Anderson AB, Demers LM, Turnbull AC. Prostaglandins in menstrual fluid in menorrhagia and dysmenorrhoea. Br J Obstet Gynaecol. 1984;91:673–80. doi: 10.1111/j.1471-0528.1984.tb04829.x. [DOI] [PubMed] [Google Scholar]
- 15.Folkman J. Angiogenesis and angiogenesis inhibition: an overview. EXS. 1997;79:1–8. doi: 10.1007/978-3-0348-9006-9_1. [DOI] [PubMed] [Google Scholar]
- 16.Jain RK, Booth MF. What brings pericytes to tumor vessels? J Clin Invest. 2003;112:1134–6. doi: 10.1172/JCI20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidi AJ, Abu-Jawdeh G, Tognazzi K, Dvorak HF, Brown LF. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in endometrial carcinoma. Cancer. 1996;78:454–60. doi: 10.1002/(SICI)1097-0142(19960801)78:3<454::AID-CNCR12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 19.Berg KA, Clarke WP, Sailstad C, Saltzman A, Maayani S. Signal transduction differences between 5-hydroxytryptamine type 2a and type 2c receptor systems. Mol Pharmacol. 1994;46:477–84. [PubMed] [Google Scholar]
- 20.Sales KJ, Maudsley S, Jabbour HN. Elevated prostaglandin EP2 receptor in endometrial adenocarcinoma cells promotes vascular endothelial growth factor expression via cyclic 3′,5′-adenosine monophosphate-mediated transactivation of the epidermal growth factor receptor and extracellular signal-regulated kinase 1/2 signaling pathways. Mol Endocrinol. 2004;18:1533–45. doi: 10.1210/me.2004-0022. [DOI] [PubMed] [Google Scholar]
- 21.Levi NL, Hanoch T, Benard O, et al. Stimulation of Jun N-terminal kinase (JNK) by gonadotropin-releasing hormone in pituitary σ t3-1 cell line is mediated by protein kinase C, c-Src, and cdc42. Mol Endocrinol. 1998;12:815–24. doi: 10.1210/mend.12.6.0120. [DOI] [PubMed] [Google Scholar]
- 22.Benard O, Naor Z, Seger R. Role of dynamin, src, and ras in the protein kinase C-mediated activation of ERK by gonadotropin-releasing hormone. J Biol Chem. 2001;276:4554–63. doi: 10.1074/jbc.M006995200. [DOI] [PubMed] [Google Scholar]
- 23.Bonfil D, Chuderland D, Kraus S, et al. Extracellular signal-regulated kinase, Jun N-terminal kinase, p38, and c-Src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone β-subunit promoter. Endocrinology. 2004;145:2228–44. doi: 10.1210/en.2003-1418. [DOI] [PubMed] [Google Scholar]
- 24.Harris D, Bonfil D, Chuderland D, et al. Activation of MAPK cascades by GnRH: ERK and Jun N-terminal kinase are involved in basal and GnRH-stimulated activity of the glycoprotein hormone LHβ-subunit promoter. Endocrinology. 2002;143:1018–25. doi: 10.1210/endo.143.3.8675. [DOI] [PubMed] [Google Scholar]
- 25.Shi Q, Le X, Abbruzzese JL, et al. Constitutive sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61:4143–54. [PubMed] [Google Scholar]
- 26.Griffin BW, Klimko P, Crider JY, Sharif NA. Al-8810: a novel prostaglandin F2α analog with selective antagonist effects at the prostaglandin F2α (FP) receptor. J Pharmacol Exp Ther. 1999;290:1278–84. [PubMed] [Google Scholar]
- 27.Lundstrom V, Green K. Endogenous levels of prostaglandin F2α and its main metabolites in plasma and endometrium of normal and dysmenorrheic women. Am J Obstet Gynecol. 1978;130:640–6. doi: 10.1016/0002-9378(78)90320-4. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann GE, Rao CV, Barrows GH, Sanfilippo JS. Topography of human uterine prostaglandin E and F2α receptors and their profiles during pathological states. J Clin Endocrinol Metab. 1983;57:360–6. doi: 10.1210/jcem-57-2-360. [DOI] [PubMed] [Google Scholar]
- 29.Adelantado JM, Rees MC, Bernal A Lopez, Turnbull AC. Increased uterine prostaglandin E receptors in menorrhagic women. Br J Obstet Gynaecol. 1988;95:162–5. doi: 10.1111/j.1471-0528.1988.tb06846.x. [DOI] [PubMed] [Google Scholar]
- 30.Naor Z, Benard O, Seger R. Activation of MAPK cascades by G-protein-coupled receptors: the case of gonadotropin-releasing hormone receptor. Trends Endocrinol Metab. 2000;11:91–9. doi: 10.1016/s1043-2760(99)00232-5. [DOI] [PubMed] [Google Scholar]
- 31.Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 32.Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637–43. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 33.Kranenburg O, Moolenaar WH. Ras-MAP kinase signaling by lysophosphatidic acid and other G-protein-coupled receptor agonists. Oncogene. 2001;20:1540–6. doi: 10.1038/sj.onc.1204187. [DOI] [PubMed] [Google Scholar]
- 34.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–60. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 35.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–83. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 36.Maudsley S, Pierce KL, Zamah AM, et al. The β2-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem. 2000;275:9572–80. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- 37.Guerrero J, Santibanez JF, Gonzalez A, Martinez J. EGF receptor transactivation by urokinase receptor stimulus through a mechanism involving src and matrix metalloproteinases. Exp Cell Res. 2004;292:201–8. doi: 10.1016/j.yexcr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Tsujii M, Kawano S, Tsuji S, et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–16. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 39.Sales KJ, Katz AA, Howard B, et al. Cyclooxygenase-1 is up-regulated in cervical carcinomas: autocrine/paracrine regulation of cyclooxygenase-2, PGE receptors and angiogenic factors by cyclooxygenase-1. Cancer Res. 2002;62:424–32. [PMC free article] [PubMed] [Google Scholar]
- 40.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 41.Seno H, Oshima M, Ishikawa T, et al. Cyclooxygenase-2 and prostaglandin E2 receptor EP2-dependent angiogenesis in APCΔ716 mouse intestinal polyps. Cancer Res. 2002;62:506–11. [PubMed] [Google Scholar]
- 42.Sonoshita M, Takaku K, Sasaki N, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in APCΔ714 knockout mice. Nat Med. 2001;7:1048–51. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 43.Scambia G, Panici P Benedetti, Ferrandina G, et al. Significance of epidermal growth factor receptor expression in primary human endometrial cancer. Int J Cancer. 1994;56:26–30. doi: 10.1002/ijc.2910560106. [DOI] [PubMed] [Google Scholar]
- 44.Niikura H, Sasano H, Matsunaga G, et al. Prognostic value of epidermal growth factor receptor expression in endometrioid endometrial carcinoma. Hum Pathol. 1995;26:892–6. [Google Scholar]
- 45.Kedar D, Baker CH, Killion JJ, Dinney CP, Fidler IJ. Blockade of the epidermal growth factor receptor signaling inhibits angiogenesis leading to regression of human renal cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 2002;8:3592–600. [PubMed] [Google Scholar]
- 46.Collisson EA, De A, Suzuki H, Gambhir SS, Kolodney MS. Treatment of metastatic melanoma with an orally available inhibitor of the Ras-Raf-MAPK cascade. Cancer Res. 2003;63:5669–73. [PubMed] [Google Scholar]
- 47.Torrance CJ, Jackson PE, Montgomery E, et al. Combinatorial chemoprevention of intestinal neoplasia. Nat Med. 2000;6:1024–8. doi: 10.1038/79534. [DOI] [PubMed] [Google Scholar]