Abstract
BACKGROUND
Although the mechanisms underlying the causes of heavy menstrual blood loss (MBL) remain to be elucidated, prostaglandins have been previously implicated. This study was initiated to elucidate a pattern of expression of the various components of the cyclooxygenase (COX)–prostaglandin signalling pathways present in the endometrium of women with normal and heavy MBLs.
METHODS
Endometrial biopsies were collected at different stages of the menstrual cycle from women who underwent measurement of MBL. Tissue was divided for either examination of gene expression by quantitative RT–PCR analysis or in vitro culture experimentation.
RESULTS
Analysis of gene expression demonstrated a significant elevation in expression of COX-1 and COX-2 mRNA in endometrium obtained from women with heavy MBL when compared with endometrium obtained from women with normal MBL. Tissue culture with PGE2 stimulation caused a significantly elevated production of cyclic AMP (cAMP) by endometrium of women with heavy MBL when compared with normal MBL. Expression of phosphodiesterase 4B, an enzyme involved in cAMP breakdown, was reduced in these same endometrial samples obtained from women with heavy MBL.
CONCLUSIONS
These data identify the E series prostaglandin receptors and their signalling pathways as potential therapeutic targets in the treatment of heavy menstruation.
Keywords: cyclooxygenase, heavy menstruation, menorrhagia, prostaglandin receptors
Introduction
Initial studies of menstrual fluid identified vasoactive substances with the capabilities of inducing contractions in strips of ileal muscle (Pickles, 1957). These were subsequently identified as prostaglandins F2α (PGF2α) and E2 (PGE2), the two most abundant prostaglandins found in the endometrium and menstrual fluid (Lumsden et al., 1983). Evidence has since continued to mount, supporting a role for prostaglandins in menstruation (Baird et al., 1996).
In the prostaglandin synthesis pathway, the cyclooxygenase (COX) enzymes generate PGH2 from arachidonic acid. There are two main isoforms of the COX enzyme: COX-1 and COX-2. COX-1 is constitutively expressed in many tissues and generates prostaglandins for normal physiological function, whereas COX-2 is rapidly induced in cells in response to varied stimuli (Vane et al., 1998). Once synthesized, PGH2 acts as an intermediary for a specific terminal prostaglandin synthase enzyme. Synthesized prostaglandins mediate their actions via seven-transmembrane G-protein coupled receptors (GPCRs). PGE2 can couple to four subtypes of GPCRs, which have been pharmacologically classified as EP1, EP2, EP3 and EP4 (Coleman et al., 1994). These receptors are often co-expressed together in the same cell and, utilize alternate and, in some cases, opposing intracellular signalling pathways (Ashby, 1998). EP2 and EP4 receptors, previously demonstrated in human endometrium (Milne et al., 2001), are coupled to G proteins (Gαs) and adenylyl cyclase, resulting in the increased formation of cyclic AMP (cAMP). PDE4 is a member of the phosphodiesterase family of enzymes, which has been previously demonstrated in human endometrium (Bartsch et al., 2004), and is specific for the hydrolysis of cAMP (Sanz et al., 2005). The level of accumulation of the second messenger, cAMP, in response to prostaglandin signalling may therefore be dependent on the level of PDE4 activity. The PGE2 pathway has previously been implicated in the problem of heavy menstrual blood loss (MBL) (Willman et al., 1976; Smith et al., 1981; Adelantado et al., 1988), although specific signalling mechanisms have yet to be elucidated. Additional prostaglandins include prostacyclin and thromboxane, which act upon their respective receptors (IP and TBXR). Prostacyclin and thromboxane are best known for their effects on the vascular endothelium, where their synthesis is well noted (Ullrich et al., 2001). Menstruation is an active process whereby the upper two-thirds of the endometrium, the functional layer, are shed and regenerated on a cyclical basis. Menstrual problems account for much of the morbidity that occurs in women of reproductive age. Thirty percentage of women consider their menstruation to be excessive, rising to 50% in perimenopausal women (Prentice, 1999, 2000). Management typically involves invasive surgery. In 2002–3, over 13 000 surgical procedures (hysterectomy and endometrial ablation) were performed in UK for complaints of heavy bleeding (Reid and Mukri, 2005). An estimated 3.5 million workdays are lost annually (Weeks et al., 2000).
The objective definition of heavy MBL is often based on the measurement of menstrual haemoglobin content. This method of measurement was first described in Scandinavian studies, which demonstrated the mean MBL to be 40 ml. Regular MBL in excess of 63 ml was associated with iron deficiency anaemia (Hallberg, 1964; Hallberg et al., 1966). The 90th centile for measured blood loss was 80 ml and this has traditionally been accepted as the upper limit of normal in clinical evaluation of MBL.
Previous investigations into the aetiology of heavy MBL have not been able to discover any differences in circulating steroid hormone levels (Eldred and Thomas, 1994) or any specific histological differences within the endometrium (Rees et al., 1984) of women with heavy MBL when compared with women with normal MBL. Additionally, there is no difference in endometrial expression of estrogen and progesterone receptors (Critchley et al., 1994). However, evidence does exist implicating local mediators, in particular, prostaglandins (Sales and Jabbour, 2003). Increased levels of total prostaglandins have been found in endometrium taken from women with heavy MBL (Smith et al., 1981). Furthermore, treatment using inhibitors of COX enzymes has repeatedly been shown to reduce MBL (Cameron et al., 1990; Coulter et al., 1995; Bonnar and Sheppard, 1996), implicating disturbances of prostaglandin pathways in the aetiology of excessive menstrual bleeding, although the mechanisms underlying the cause of heavy blood loss remain to be elucidated.
This study was initiated to determine a pattern of expression of the various components of the COX–prostaglandin signalling pathways present in the endometrium of women with normal and heavy MBLs (>80 ml). This is based on the hypothesis that disturbances of local mediator signalling, including prostaglandin signalling, are implicated in the aetiology of heavy MBL.
Materials and methods
Tissue collection and measurement of MBL
Patients complaining of heavy menstruation were recruited from the gynaecological outpatient setting. Ethical approval was obtained from the Lothian Research Ethics Committee and written informed consent was obtained from all subjects before tissue collection. All subjects were aged 18–50 (range 22–49; mean 40 years of age). All subjects reported regular menstrual cycles (cycle length 21–35 days), with no unscheduled, non-menstrual bleeding. No woman had received hormonal preparation in the 3 months preceding biopsy collection. Patients were clinically examined and clinical pelvic abnormalities, such as an enlarged uterus, were further investigated by pelvic ultrasound imaging. Patients with known uterine pathology such as fibroid disease and endometriosis were excluded from the study.
Endometrial biopsies (n = 26) were collected for research purposes at different stages of the menstrual cycle with an endometrial suction curette (Pipelle, Laboratoire CCD, Paris, France). No biopsies were taken during the menstrual phase of the cycle because of technical difficulties in obtaining sufficient quantities of endometrium suitable for RNA analysis. Biopsies were dated according to stated last menstrual period (LMP) and dating was confirmed by histological assessment according to criteria of Noyes et al. (1950). Furthermore, circulating estradiol and progesterone serum levels were measured at the time of biopsy collection and were consistent for both LMP and histological assessment of the menstrual cycle stage. This is a robust method for characterizing endometrial samples. Detailed gene microarray studies support this method for characterizing endometrial samples with consistency across these three parameters (Critchley et al., 2006; Talbi et al., 2006).
Inconsistencies between measured circulating hormone levels, stated day of LMP and/or histological assessment, led to exclusion of three tissue biopsies from the study. Table I provides a summary of the biopsies (n = 23) used within this work.
Table I.
Summary of collected endometrial biopsies
| Stage of menstrual cycle | Number of biopsies |
|
|---|---|---|
| Menstrual blood loss (MBL) ≤80 ml |
MBL >80 ml | |
| Proliferative | 7 | 5 |
| Secretory | 6 | 5 |
Immediately after collection, tissue was divided, transferred into RNA Later (Ambion Inc., Huntingdon, UK) and stored at -70°C (for RNA extraction), fixed in neutral-buffered formalin for wax embedding (for histological dating) and placed in Rosewell Park Memorial Institute (RPMI) 1640 medium (containing 2 mM L-glutamine, 100 U penicillin and 100 μg ml-1 streptomycin) and transported to the laboratory for in vitro culture.
In addition to consenting to provide an endometrial biopsy, patients agreed to undergo measurement of MBL over one menstruation. Measurement was based on a modified alkaline–haematin method as previously described (Hallberg, 1964). Briefly, used sanitary products were added to a measured volume of 5% sodium hydroxide (between 2 and 4 l). The contents were then left for 48 h to allow conversion of haemoglobin to haematin. During this same time period, a 1 in 200 dilution of the patient’s venous blood in 5% sodium hydroxide was made and additionally stored. After 48 h, an aliquot of sodium hydroxide was removed from the volume soaking the sanitary products and filtered through hardened filter paper (Whartman No. 54, Maidstone, UK). The optical density (OD) of MBL solution and venous blood sample were then measured using spectrophotometry at 546 nm (A546).
MBL was then calculated as a quantity of patient’s own venous blood using the following equation (van Eijkeren et al., 1986):
Validation work for this method of measuring MBL has previously been carried out (data not shown).
The range of measured MBL was between 10 and 567 ml with a median MBL of 42 ml for the normal group (MBL >80 ml) and a median MBL of 183 ml for the heavy group (MBL >80 ml). All patients provided informed consent for collection of an endometrial biopsy during the month proceeding or immediately following collection of their menstrual loss.
Whole tissue cAMP assay
Endometrial biopsies from proliferative and secretory phases of the menstrual cycle were minced finely with scissors and incubated overnight in RPMI medium containing 3 μg ml-1 indomethacin (an inhibitor of COX-1 and COX-2 enzymes). Following overnight treatment, approximately one-third of the tissue was removed and stored at -20°C. The remaining tissue was incubated in the same medium containing 1 mM 1-methyl-3-isobutylxanthine (IBMX, Sigma, Poole, UK) for 30 min. (IBMX inhibits the action of phosphodiesterases and prevents the rapid degradation of cAMP.) It was then divided into two portions and treated with control medium or 100 nM PGE2 for 10 min. Tissue was then lysed in 0.1 M HCl and frozen until assayed. Cyclic AMP concentration was measured by enzyme-linked immunosorbent assay (Biomol, Affiniti, Exeter, UK) in accordance with the manufacturer’s instructions and normalized to protein concentration determined by protein assay according to the manufacturer’s instructions (Bio-Rad, Hemel Hempstead, UK).
Taqman quantitative reverse transcriptase–polymerase chain reaction
The expression of COX enzymes across the menstrual cycle and the expression of prostanoid receptors in endometrium characterized by MBL were investigated by quantitative RT–PCR. Total RNA was extracted from endometrial biopsies using the commercially available product RNeasy Midi Kit (Qiagen Ltd, Sussex, UK), according to the manufacturer’s instructions. Each tissue sample was able to provide sufficient quantities of RNA for the complete analysis of our target genes. Samples were treated for DNA contamination by DNA digestion during RNA purification. Following extraction, total RNA was eluted in 150 μl of nuclease-free water and stored at -80°C. Quality of RNA was assessed using the Agilent 2100 Bioanalyser system in combination with RNA6000nano chips (Agilent Technologies, Cheshire, UK). Only RNA that displayed intact 18S and 28S peaks was reverse transcribed to cDNA for real-time PCR analysis.
Once extracted and quantified, RNA samples were reverse transcribed as previously described (Milne et al., 2001; Sales et al., 2004). Thereafter, cDNA samples were stored at -20°C. A tube with no reverse transcriptase was included to control for any DNA contamination.
Real-time quantitative PCR was performed using an ABI 7700 Sequence Detection System (Applied Biosystems, Warrington, UK) as previously described (Milne et al., 2001; Sales et al., 2004) using duplicate samples. A no-template control (containing water) was included. The inclusion of ribosomal 18S primers and probes was used to act as a housekeeping gene for each reaction mix. All primers and probes were designed using the PRIMER express programme (Applied Biosystems) and their sequences can be found in Table II.
Table II.
Sequences of primers and probes used for Taqman RT-PCR analysis
| Cyclooxygenase-1 (COX-1) | Forward Reverse Probe |
5′-TGT TCG GTG TCC AGT TCC AAT A-3′ 5′-ACC TTG AAG GAG TCA GGC ATG AG-3′ 5′-CGC AAC CGC ATT GCC ATG GAG T-3′ |
| Cyclooxygenase-2 (COX-2) | Forward Reverse Probe |
5′-CCT TCC TCC TGT GCC TGA TG-3′ 5′-ACA ATC TCA TTT GAA TCA GGA AGC T-3′ 5′-TGC CCG ACT CCC TTG GGT GTC A-3′ |
| Prostaglandin E2 receptor type 1 (EP1) | Forward Reverse Probe |
5′-AGA TGG TGG GCC AGC TTG T-3′ 5′-GCC ACC AAC ACC AGC ATT G-3′ 5′-CAG CAG ATG CAC GAC ACC ACC ATG-3′ |
| Prostaglandin E2 receptor type 2 (EP2) | Forward Reverse Probe |
5′-GAC CGC TTA CCT GCA GCT GTA C-3′ 5′-TGA AGT TGC AGG CGA GCA-3′ 5′-CCA CCC TGC TGC TGC TTC TCA TTG TCT-3′ |
| Prostaglandin E2 receptor type 3 (EP3) | Forward Reverse Probe |
5′-GAC GGC CAT TCA GCT TAT GG-3′ 5′-TTG AAG ATC ATT TTC AAC ATC ATT ATC A-3′ 5′-CTG TCG GTC TGC TGG TCT CCG CTC-3′ |
| Prostaglandin E2 receptor type 4 (EP4) | Forward Reverse Probe |
5′-ACG CCG CCT ACT CCT ACA TG-3′ 5′-AGA GGA CGG TGG CGA GAA T-3′ 5′-ACG CGG GCT TCA GCT CCT TCC T-3′ |
| Prostaglandin F2α receptor (FP) | Forward Reverse Probe |
5′-GCA GCT GCG CTT CTT TCA A-3′ 5′-CAC TGT CAT GAA GAT TAC TGA AAA AAA TAC-3′ 5′-CAC AAC CTG CCA GAC GGA AAA CCG-3′ |
| Thromboxane receptor (TBXR) | Forward Reverse Probe |
5′-TGG TGG TGG CCA GCG T-3′ 5′-CGG GTT TCG CAG CAC TGT-3′ 5′-TGC CCC TTC TGG TCT TCA TCG CCC-3′ |
| Prostacyclin receptor (IP) | Forward Reverse Probe |
5′-GCC CTC CCC CTC TAC CAA-3′ 5′-TTT TCC AAT AAC TGT GGT TTT TGT G-3′ 5′-CCA AGA GCC AGC CCC CTT TCT GC-3′ |
| Phosphodiesterase 4B | Forward Reverse Probe |
5′-CCT TCA GTA GCA CCG GAA TCA-3′ 5′-CAA ACA AAC ACA CAG GCA TGT AGT T-3′ 5′-AGC CTG CAG CCG CTC CAG CC-3′ |
| 18S | Forward Reverse Probe |
5′-CGT CTA CCA CAT CCA AGG AA-3′ 5′-GCT GGA ATT ACG GGG GCT-3′ 5′-TCG TGG CAC CAG ACT TGC CCT C-3′ |
Data were analysed and processed using Sequence Detector version 1.6.3 (Applied Biosystems) according to manufacturer’s instructions. Expression of target genes was normalized to RNA loading for each sample using 18S ribosomal RNA as an internal standard. All results were expressed relative to a control standard (cDNA obtained from a single sample of endometrial tissue and included in all experiments).
Statistical analysis
The quantitative RT–PCR data did not consistently fulfil the assumptions necessary for using analysis of variance (ANOVA) and t-test; therefore, the non-parametric Mann–Whitney test was used (Graphpad InStat3) and statistical significance accepted when P < 0.05. Cyclic AMP assay data were subjected to statistical analysis with ANOVA and Fishers PLSD tests (Statview 4.0; Abacus Concepts Inc., Piscataway, NJ, USA) and statistical significance accepted when P < 0.05.
Results
Quantitative RT–PCR analysis of genes involved with prostaglandin signalling
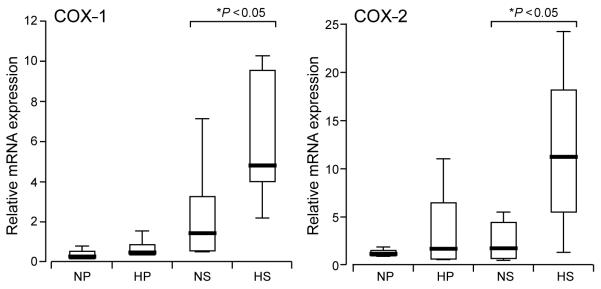
Analysis of mRNA expression for a variety of genes associated with prostanoid signalling within the endometrium by quantitative RT–PCR was performed. Analysis of COX-1 and COX-2 mRNA expressions demonstrated a significant increase in secretory endometrium of women with MBL in excess of 80 ml when compared with endometrium of women with MBL <80 ml (Figure 1).
Figure 1.
Box plot demonstrating relative cyclooxygenase-1 (COX-1) and COX-2 mRNA expression in proliferative and secretory endometrium of women with normal and heavy measured menstrual blood losses (MBLs) (normal MBL ≤80 ml and heavy MBL >80 ml). The data are presented as box-and-whisker plots: box represents the 25th and 75th percentiles and the heavy bar represents the median. The whiskers are the 10th and 90th percentiles. NP, normal proliferative (n = 7); HP, heavy proliferative (n = 5); NS, normal secretory (n = 6); HS, heavy secretory (n = 5) Asterisk indicates significant difference (P < 0.05).
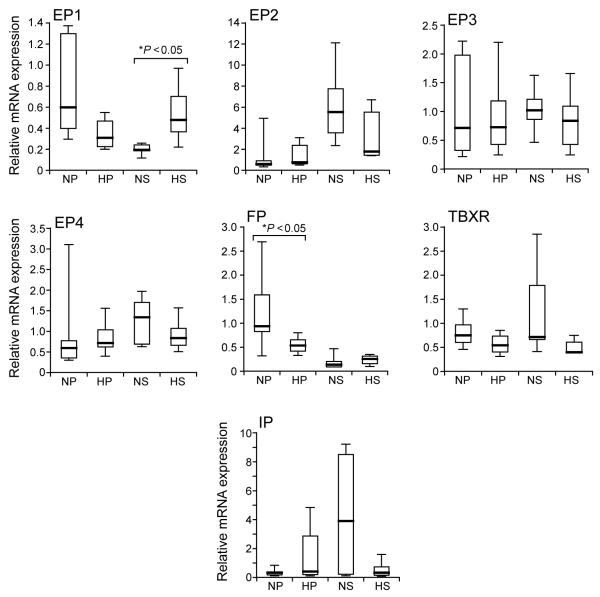
No significant differences in endometrial expression were detected for prostacyclin or thromboxane receptors. Regarding the receptors for PGE2, no significant differences in expression were detected between the two groups of endometrial samples for EP2, EP3 and EP4. The mRNA expression of EP1 was significantly increased in secretory endometrium of women with heavy MBL when compared with normal MBL. Finally, mRNA expression of the receptor for PGF2α (FP) was significantly reduced in proliferative endometrium of women with heavy MBL when compared with normal MBL (Figure 2).
Figure 2.
Composite figure of box plot graphs demonstrating relative mRNA expression for prostanoid receptors in proliferative and secretory endometrium of women with normal and heavy measured menstrual blood losses (MBLs) (normal MBL ≤80 ml and heavy MBL >80 ml). The data are presented as box-and-whisker plots: box represents the 25th and 75th percentiles and the heavy bar represents the median. The whiskers are the 10th and 90th percentiles. NP, normal proliferative (n = 7); HP, heavy proliferative (n = 5); NS, normal secretory (n = 6); HS, heavy secretory (n = 5). EP1 to EP4 are the receptors for prostaglandin E2; FP is the receptor for prostaglandin F2α; TBXR is the receptor for thromboxane and IP is the receptor for prostacyclin. Asterisk indicates significant difference (P < 0.05).
Functional PGE2 receptor signalling in endometrium of women with measured MBL
The PGE2 receptors, EP2 and EP4, are known to activate the cAMP/protein kinase A pathway within the human endometrium (Milne et al., 2001; Regan, 2003). To investigate the potential differences in PGE2 receptor function between endometrial samples of women with measured MBL, we therefore looked at cAMP production as an end-point for receptor function.
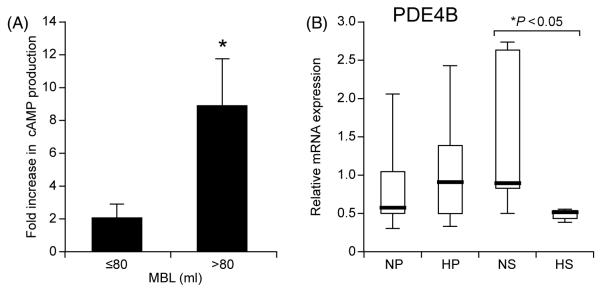
Cyclic AMP production in response to treatment with 100 nM PGE2 was higher in endometrial tissue explants collected from women with heavy MBL when compared with endometrium of women with normal MBL (Figure 3A).
Figure 3.
(A) Cyclic AMP (cAMP) production by endometrial tissue explants in response to a 10 min stimulation with 100 nM PGE2 or vehicle. Graph shows average fold increase in cAMP production by endometrium of women with normal and heavy menstrual blood losses (MBLs) (normal MBL ≤80 ml and heavy MBL >80 ml). Asterisk indicates significant elevated cAMP production (P < 0.05). (B) Phosphodiesterase 4B (PDE4B) mRNA expression in endometrium of women with normal and heavy MBLs (normal MBL ≤80 ml and heavy MBL >80 ml) as determined by real-time quantitative RT–PCR. The data are presented as box-and-whisker plots: box represents the 25th and 75th percentiles and the heavy bar represents the median. The whiskers are the 10th and 90th percentiles. NP, normal proliferative (n = 7); HP, heavy proliferative (n = 5); NS, normal secretory (n = 6); HS, heavy secretory (n = 5). Asterisk indicates significant difference (P < 0.05).
As described earlier, the analysis of prostanoid receptor mRNA expression did not detect any significant differences for EP2 or EP4 in endometrium of women with measured MBL. Therefore, in order to investigate whether effects at the post-receptor level could explain this enhanced functionality of the EP receptor, we investigated the expression of phosphodiesterase isotype 4B (PDE4B) in the same endometrial samples. Quantitative RT–PCR analysis demonstrated a significant reduction in the expression of PDE4B in secretory endometrium of women with heavy MBL, compared with normal MBL (Figure 3B).
Discussion
This study demonstrates significantly elevated levels in mRNA expression of both COX-1 and COX-2 enzymes in endometrium obtained from women with measured heavy MBL. These data suggest that it is important to use a general inhibitor of both COX-1 and COX-2 enzyme action in treating the complaint of heavy menstruation. Mefenamic acid, a COX inhibitor and a member of the Fenamate family of drugs, is routinely used as a first-line treatment for menorrhagia. As well as inhibiting prostaglandin synthesis, it has been shown to inhibit binding of PGE2 to its receptors (Rees et al., 1988). In keeping with this dual mode of action, the PGE2 pathway has previously been implicated in the problem of heavy MBL. Elevated levels of PGE2 have been found in the endometrium of women complaining of heavy periods (Willman et al., 1976) and further experiments confirmed an apparent shift in synthesis in favour of PGE2 over PGF2α in the endometrium of women with heavy MBL (Smith et al., 1981).
Additionally, our data demonstrate an enhanced PGE2–EP-induced cAMP production in the endometrium obtained from women with heavy MBL; however, the expression of EP2 and EP4 receptors, which are known to couple to cAMP production, does not display any significant correlation with measured MBL in our studies. Previous data have demonstrated an increase in PGE2 binding sites in uterine tissue associated with the complaint of heavy MBL (Adelantado et al., 1988), although specific receptor subtypes were not identified. Indeed, it is plausible that EP2 and EP4 receptor numbers could be regulated at the post-transcriptional level, resulting in increased PGE2 receptor binding sites. PGF2α and PGE2 are two prominent prostaglandins found in human endometrium (Lumsden et al., 1986). In the endometrium, PGF2α receptor (FP) and PGE2 receptors, EP2 and EP4, are responsible for their respective ligand action. Although there was a reduction in the expression of FP in the endometrium taken from women with heavy MBL, no changes in the expression of EP2 and EP4 were found. Therefore, a shift of endometrial prostaglandin signalling in favour of the PGE2 pathway over the PGF2α pathway may exist in the endometrium of women with heavy MBL. Previous work has shown a decrease in the PGF2α/PGE2 ratio in the endometrium of women with measured heavy MBL when compared with women with normal MBL (Smith et al., 1981).
The COX-2–PGE2–EP pathway has previously been shown to influence angiogenic factors such as vascular endothelial growth factor through a mechanism mediated by cAMP (Sonoshita et al., 2001). Therefore, the enhanced ability for cAMP production by endometrium taken from women with heavy MBL may have important effects on the expression of angiogenic factors. Altered endothelial function as a result of disturbances to angiogenic factors has previously been implicated in excessive MBL (Kooy et al., 1996).
Thromboxane and prostacyclin are implicated in platelet function and vascular haemostasis (Grosser et al., 2006) and there is a strong expression of their respective receptors in the endometrial vascular compartment (Milling Smith et al., 2006; Battersby, unpublished data). However, no differences in the expression of the genes involved in their signalling pathways downstream of COX enzymes were detected between the two groups of the endometrium of women with heavy and normal MBLs.
PDEs are a large family of enzymes that are responsible for the hydrolysis of cyclic nucleotides (Sanz et al., 2005). PDE4B is an isoenzyme that is found in the human endometrium (Bartsch et al. 2001, 2004) and shows specificity for hydrolysis of cAMP. We have shown that in addition to an enhanced ability to produce cAMP in response to PGE2, endometrium taken from women with heavy MBL expresses a significantly reduced level of PDE4B isoform mRNA.
In summary, increased expression of the rate-limiting COX enzymes in the endometrium of women with heavy MBL will lead to an increase in prostaglandin production and signalling. In addition, enhanced functionality of the EP receptors coupled to cAMP production could in part be explained by a reduction in PDE4B expression within endometrium of women with heavy MBL.
Our data, therefore, present a novel mechanism of endometrial prostaglandin signalling that may lead to the complaint of heavy menstruation. Enhanced COX–PGE2 signalling and reduced PDE4B expression in the endometrium of women with heavy MBL present us with new therapeutic opportunities in the treatment of heavy menstrual bleeding.
Acknowledgements
A Clinical Research Fellowship was awarded to O.P.M.S. from the University of Edinburgh College of Medicine and Veterinary Medicine. Further financial support was provided by the Medical Research Council (MRC grants G0000066 and G0500047 to H.O.D.C. and core MRC funding to H.N.J.). The authors would like to thank Professor Rodney Kelly and Dr Kurt Sales for their valuable input to this work.
References
- Adelantado JM, Rees MC, Bernal A Lopez, Turnbull AC. Increased uterine prostaglandin E receptors in menorrhagic women. Br J Obstet Gynaecol. 1988;95(2):162–165. doi: 10.1111/j.1471-0528.1988.tb06846.x. [DOI] [PubMed] [Google Scholar]
- Ashby B. Co-expression of prostaglandin receptors with opposite effects: a model for homeostatic control of autocrine and paracrine signaling. Biochem Pharmacol. 1998;55(3):239–246. doi: 10.1016/s0006-2952(97)00241-4. [DOI] [PubMed] [Google Scholar]
- Baird DT, Cameron ST, Critchley HO, Drudy TA, Howe A, Jones RL, Lea RG, Kelly RW. Prostaglandins and menstruation. Eur J Obstet Gynecol Reprod Biol. 1996;70(1):15–17. doi: 10.1016/s0301-2115(96)02568-7. [DOI] [PubMed] [Google Scholar]
- Bartsch O, Bartlick B, Ivell R. Relaxin signalling links tyrosine phosphorylation to phosphodiesterase and adenylyl cyclase activity. Mol Hum Reprod. 2001;7(9):799–809. doi: 10.1093/molehr/7.9.799. [DOI] [PubMed] [Google Scholar]
- Bartsch O, Bartlick B, Ivell R. Phosphodiesterase 4 inhibition synergizes with relaxin signaling to promote decidualization of human endometrial stromal cells. J Clin Endocrinol Metab. 2004;89(1):324–334. doi: 10.1210/jc.2003-030498. [DOI] [PubMed] [Google Scholar]
- Bonnar J, Sheppard BL. Treatment of menorrhagia during menstruation: randomised controlled trial of ethamsylate, mefenamic acid, and tranexamic acid. BMJ. 1996;313(7057):579–582. doi: 10.1136/bmj.313.7057.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron IT, Haining R, Lumsden MA, Thomas VR, Smith SK. The effects of mefenamic acid and norethisterone on measured menstrual blood loss. Obstet Gynecol. 1990;76(1):85–88. [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46(2):205–229. [PubMed] [Google Scholar]
- Coulter A, Kelland J, Peto V, Rees MC. Treating menorrhagia in primary care. An overview of drug trials and a survey of prescribing practice. Int J Technol Assess Health Care. 1995;11(3):456–471. doi: 10.1017/s0266462300008679. [DOI] [PubMed] [Google Scholar]
- Critchley HO, Abberton KM, Taylor NH, Healy DL, Rogers PA. Endometrial sex steroid receptor expression in women with menorrhagia. Br J Obstet Gynaecol. 1994;101(5):428–434. doi: 10.1111/j.1471-0528.1994.tb11917.x. [DOI] [PubMed] [Google Scholar]
- Critchley HO, Robertson KA, Forster T, Henderson TA, Williams AR, Ghazal P. Gene expression profiling of mid to late secretory phase endometrial biopsies from women with menstrual complaint. Am J Obstet Gynecol. 2006;195(2):406e1–406e16. doi: 10.1016/j.ajog.2006.05.002. [DOI] [PubMed] [Google Scholar]
- van Eijkeren MA, Scholten PC, Christiaens GC, Alsbach GP, Haspels AA. The alkaline hematin method for measuring menstrual blood loss—a modification and its clinical use in menorrhagia. Eur J Obstet Gynecol Reprod Biol. 1986;22(5-6):345–351. doi: 10.1016/0028-2243(86)90124-3. [DOI] [PubMed] [Google Scholar]
- Eldred JM, Thomas EJ. Pituitary and ovarian hormone levels in unexplained menorrhagia. Obstet Gynecol. 1994;84(5):775–778. [PubMed] [Google Scholar]
- Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116(1):4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg N. Determination of menstrual blood loss. Scand J Clin Lab Invest. 1964;16:244–248. [PubMed] [Google Scholar]
- Hallberg L, Hogdahl AM, Nilsson L, Rybo G. Menstrual blood loss—a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand. 1966;45(3):320–351. doi: 10.3109/00016346609158455. [DOI] [PubMed] [Google Scholar]
- Kooy J, Taylor NH, Healy DL, Rogers PA. Endothelial cell proliferation in the endometrium of women with menorrhagia and in women following endometrial ablation. Hum Reprod. 1996;11(5):1067–1072. doi: 10.1093/oxfordjournals.humrep.a019298. [DOI] [PubMed] [Google Scholar]
- Lumsden MA, Kelly RW, Abel MH, Baird DT. The concentrations of prostaglandins in endometrium during the menstrual cycle in women with measured menstrual blood loss. Prostaglandins Leukot Med. 1986;23(2-3):217–227. doi: 10.1016/0262-1746(86)90189-7. [DOI] [PubMed] [Google Scholar]
- Lumsden MA, Kelly RW, Baird DT. Primary dysmenorrhoea: the importance of both prostaglandins E2 and F2 alpha. Br J Obstet Gynaecol. 1983;90(12):1135–1140. doi: 10.1111/j.1471-0528.1983.tb06460.x. [DOI] [PubMed] [Google Scholar]
- Smith OP Milling, Battersby S, Sales KJ, Critchley HO, Jabbour HN. Prostacyclin receptor up-regulates the expression of angiogenic genes in human endometrium via cross talk with epidermal growth factor receptor and the extracellular signaling receptor kinase 1/2 pathway. Endocrinology. 2006;147(4):1697–1705. doi: 10.1210/en.2005-1073. [DOI] [PubMed] [Google Scholar]
- Milne SA, Perchick GB, Boddy SC, Jabbour HN. Expression, localization, and signaling of PGE(2) and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2001;86(9):4453–4459. doi: 10.1210/jcem.86.9.7856. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- Pickles VR. A plain-muscle stimulant in the menstruum. Nature. 1957;180(4596):1198–1199. doi: 10.1038/1801198a0. [DOI] [PubMed] [Google Scholar]
- Prentice A. Fortnightly review. Medical management of menorrhagia. BMJ. 1999;319(7221):1343–1345. doi: 10.1136/bmj.319.7221.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A. Health burden of menstrual disorders. In: O’Brien S, MacLean A, editors. Disorders of the menstrual cycle. RCOG Press; London: 2000. pp. 171–186. [Google Scholar]
- Rees MC, Canete-Soler R, Bernal A Lopez, Turnbull AC. Effect of fenamates on prostaglandin E receptor binding. Lancet. 1988;2(8610):541–542. doi: 10.1016/s0140-6736(88)92660-8. [DOI] [PubMed] [Google Scholar]
- Rees MC, Dunnill MS, Anderson AB, Turnbull AC. Quantitative uterine histology during the menstrual cycle in relation to measured menstrual blood loss. Br J Obstet Gynaecol. 1984;91(7):662–666. doi: 10.1111/j.1471-0528.1984.tb04827.x. [DOI] [PubMed] [Google Scholar]
- Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74(2–3):143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Reid PC, Mukri F. Trends in number of hysterectomies performed in England for menorrhagia: examination of health episode statistics, 1989 to 2002–3. BMJ. 2005;330(7497):938–939. doi: 10.1136/bmj.38376.505382.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales KJ, Jabbour HN. Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction. 2003;126(5):559–567. doi: 10.1530/rep.0.1260559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales KJ, Maudsley S, Jabbour HN. Elevated prostaglandin EP2 receptor in endometrial adenocarcinoma cells promotes vascular endothelial growth factor expression via cyclic 3′,5′-adenosine monophosphate-mediated transactivation of the epidermal growth factor receptor and extracellular signal-regulated kinase 1/2 signaling pathways. Mol Endocrinol. 2004;18(6):1533–1545. doi: 10.1210/me.2004-0022. [DOI] [PubMed] [Google Scholar]
- Sanz MJ, Cortijo J, Morcillo EJ. PDE4 inhibitors as new anti-inflammatory drugs: effects on cell trafficking and cell adhesion molecules expression. Pharmacol Ther. 2005;106(3):269–297. doi: 10.1016/j.pharmthera.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Smith SK, Abel MH, Kelly RW, Baird DT. Prostaglandin synthesis in the endometrium of women with ovular dysfunctional uterine bleeding. Br J Obstet Gynaecol. 1981;88(4):434–442. doi: 10.1111/j.1471-0528.1981.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo MM. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(delta 716) knockout mice. Nat Med. 2001;7(9):1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Ullrich V, Zou MH, Bachschmid M. New physiological and pathophysiological aspects on the thromboxane A(2)-prostacyclin regulatory system. Biochim Biophys Acta. 2001;1532(1–2):1–14. doi: 10.1016/s1388-1981(01)00126-3. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Weeks AD, Duffy SR, Walker JJ. A double-blind randomised trial of leuprorelin acetate prior to hysterectomy for dysfunctional uterine bleeding. Br J Obstet Gynaecol. 2000;107(3):323–328. doi: 10.1111/j.1471-0528.2000.tb13226.x. [DOI] [PubMed] [Google Scholar]
- Willman EA, Collins WP, Clayton SG. Studies in the involvement of prostaglandins in uterine symptomatology and pathology. Br J Obstet Gynaecol. 1976;83(5):337–341. doi: 10.1111/j.1471-0528.1976.tb00839.x. [DOI] [PubMed] [Google Scholar]