Abstract
Prokineticin-1 (PK1) is a recently described protein with a wide range of functions, including tissue-specific angiogenesis, modulation of inflammatory responses, and regulation of hemopoiesis. The aim of this study was to investigate the localization and expression of PK1 and PK receptor-1 (PKR1), their signaling pathways, and the effect of PK1 on expression of the inflammatory mediators cyclooxygenase (COX)-2 and IL-8 in third-trimester placenta. PK1 and PKR1 were highly expressed in term placenta and immunolocalized to syncytiotrophoblasts, cytotrophoblasts, fetal endothelium, and macrophages. PK1 induced a time-dependent increase in expression of IL-8 and COX-2, which was significantly reduced by inhibitors of Gq, cSrc, epidermal growth factor receptor (EGFR), and MAPK kinase. Treatment of third-trimester placenta with 40 nm PK1 induced a rapid phosphorylation of cSrc, EGFR, and ERK1/2. Phosphorylation of ERK1/2 in response to PK1 was dependent on sequential phosphorylation of cSrc and EGFR. Using double-immunofluorescent immunohistochemistry, PKR1 colocalized with IL-8 and COX-2 in placenta. These data suggest that PK1 may have a novel role as a mediator of the inflammatory response in placenta.
Prokineticin-1 (PK1) [also known as endocrine gland-derived vascular endothelial growth factor (VEGF)] is a recently described protein with a range of physiological and pathological functions (1). The cognate receptors for PK1 are two closely related G protein-coupled receptors, PK receptor (PKR)-1 and PKR2. These couple to either Gi or Gq (2-4), activating downstream signaling pathways that include calcium mobilization, stimulation of phosphoinositide turnover, and activation of MAPK.
PK1 is expressed in steroidogenic tissues including the placenta (5, 6), ovary (7, 8), and adrenal (3). It mediates tissue-specific vascular effects, which include capillary endothelial cell survival, proliferation, differentiation, and induction of fenestrae (3). In contrast to VEGF, PK1 has no effect on endothelial cells derived from aorta, umbilical vein, or cornea (5). More recently, mouse studies have demonstrated that PKR1 gene activation promotes angiogenesis in cultured cardiac endothelial cells without increasing VEGF levels (9). PK1 is also thought to have a role in immune regulation, affecting differentiation of human bone marrow cells into a distinct monocyte-derived cell population primed for release of proinflammatory cytokines (10). On stimulation with LPS, PK1-primed monocytes/macrophages demonstrate an exaggerated release of IL-12 and TNF-α and down-regulated production of IL-10 (10), thus favoring a proinflammatory T-helper cell type 1 response.
During the first trimester of pregnancy, PK1 and PKR1 are predominantly expressed in syncytiotrophoblast, with expression peaking from 8–10 wk gestation. PK1 is also expressed in specialized macrophages called Hofbauer cells in the placental villi from 6 wk of gestation. In contrast, PK2 is not expressed in trophoblast and expression of PKR2 is 80 times less than that of PKR1 (6). Supported by studies in the mouse (11), it has therefore been suggested that PK1 may have a role in trophoblast differentiation and placental angiogenesis during early pregnancy. Less is known about placental expression of PK1 and PKR1 in later pregnancy. Although PK1 expression has been demonstrated in normal third-trimester placenta, its immunolocalization and putative functions are not known (12). Moreover, the expression and immunolocalization of PKR1 in third-trimester placenta remain to be investigated.
We have recently found that PK1 induces up-regulation of IL-8 and cyclooxygenase (COX)-2 in a PKR1-overexpressing human endometrial epithelial cell line (13). Both IL-8 and COX-2 are important inflammatory and vascular mediators. IL-8 is a potent neutrophil chemoattractant and angiogenic factor, promoting endothelial cell chemotaxis (14), whereas COX-2 is the inducible isoform of the enzyme involved in the synthesis of prostaglandins from arachidonic acid (15). We therefore postulated that PK1 may, via stimulation of IL-8 and COX-2, be a novel mediator of the inflammatory response in term placenta.
The current study therefore had two objectives: first, to investigate the expression, localization, and signaling pathways of PK1 and PKR1 in third-trimester human placenta, and second, to study the effect of PK1 on the expression of IL-8 and COX-2.
Materials and Methods
Patients and tissue collection
Placentae (n = 32) and myometrium (full-thickness biopsy from lower uterine segment; n = 13) were collected at elective cesarean section (>37 wk gestation, uncomplicated singleton pregnancy). Nonpregnant myometrium (n = 6) was obtained from the uterine muscle layer distant from any pathology in women undergoing hysterectomy for uterine fibroids. Shortly after collection, tissue was placed in RNAlater (Applied Biosystems, Warrington, UK) for RNA extraction, fixed in 4% neutral buffered formalin, and wax embedded for immunohistochemistry or placed in RPMI (Sigma, Poole, UK) (containing 2 mm l-glutamine, 100 IU penicillin, and 100 μg/ml streptomycin) and transported to the laboratory for in vitro culture. Ethical approval was obtained from Lothian Local Research Ethics Committee, written informed consent was obtained from all subjects before tissue collection, and the study was conducted in accordance with the guidelines provided in The Declaration of Helsinki.
Immunohistochemistry
PK1 and PKR1 were localized by immunohistochemistry as previously described (16, 17). Briefly, 5-μm placental sections were dewaxed in xylene and rehydrated in graded ethanol. Antigen retrieval was performed by treating sections for 5 min in a pressure cooker in boiling 0.01 m citrate buffer (pH 6.0). Endogenous peroxidase activity was quenched with 10% (vol/vol) H2O2 in methanol at room temperature. Nonimmune swine serum (20% serum in Tris-buffered saline) was applied for 30 min before overnight incubation at 4 C with rabbit antihuman PK1 (1:1000; Phoenix Pharmaceuticals Inc., Belmont, CA) or rabbit antihuman PKR1 (1:500; Caltag Medsystems, Botolph Claydon, UK). An avidin-biotin peroxidase detection system was then applied (Dako Ltd., Cambridge, UK) with 3,3′-diaminobenzidine as the chromogen. Controls were incubated with isotype-matched IgG in place of primary antibody.
Confocal immunofluorescence microscopy
Colocalization of PKR1 with cytokeratin-18 (trophoblast marker), CD31 (blood vessel marker), CD68 (macrophage marker), CD3 (T-cell marker), CD20 (B-cell marker), IL-8, and COX-2 was performed by dual-immunofluorescence histochemistry. Sections were prepared and blocked in 5% normal chicken serum before incubating overnight at 4 C with antibodies directed against cytokeratin 18 (1:20), CD31 (1:100), CD3 (1:10), COX-2 (1:50; all Autogen Bioclear, Wiltshire, UK), CD68 (1:10), CD20 (1:20; both Dako), and IL-8 (1:10; R&D Systems Europe Ltd., Abingdon, UK). Subsequently, sections were incubated in appropriate biotinylated chicken secondary antibodies (1:200; all Autogen Bioclear), followed by incubation with the fluorochrome Avidin 488 Alexafluor (1:200 in PBS; Invitrogen, Paisley, UK). Sections were reblocked with 5% normal chicken serum and then incubated overnight at 4 C with rabbit antihuman PKR1 (1:500; Caltag Medsystems). Control sections were incubated with rabbit IgG. Finally, sections were washed, incubated for 10 min in TyrCy3 Red (1:50 in substrate; PE Healthcare, Amersham, UK), incubated with the nuclear counterstain To-Pro 2 (1:1000; Invitrogen) and mounted in Permafluor (Beckman Coulter, High Wycombe, UK). Sections were visualized and photographed using a laser-scanning microscope (LSM 510; Carl Zeiss, Jena, Germany) using a ×40 1.4 oil immersion lens. Control sections were incubated with normal IgG from the same host species.
Cell signaling experiments
To investigate PKR1 signaling pathways in placenta, after washing in PBS, finely chopped (2–3 mm) placental tissue was incubated overnight at 37 C in serum-free medium [DMEM/F-12 medium with l-glutamine (Invitrogen) containing 100 IU penicillin and 100 μg/ml streptomycin (PAA Laboratories, Middlesex, UK)]. Tissue explants were then incubated for 0, 5, 10, 20, and 30 min in the presence of 40 nm PK1 (Peprotech, London, UK), snap frozen in dry ice, and stored at −80 C for protein analysis. To dissect the signaling pathways further, after overnight incubation, tissue was preincubated for 1 h with vehicle or inhibitors of Gq (YM-254890, 1 μm), cSrc (PP2, 10 μm), epidermal growth factor receptor (EGFR) kinase (AG1478, 200 nm) or MAPK kinase (MEK) (PD98059, 50 μm; all obtained from Merck Chemicals, Nottingham, UK, except YM-254890 a kind gift from M. Taniguchi, Astellas Pharmaceuticals Inc., Tokyo, Japan) and then treated for 30 min with vehicle or 40 nm PK1. Doses of chemical inhibitors were determined empirically by titration. The IC50 values for inhibition of MEK with PD98059 is 50 μm (18). At this concentration, it is not known to inhibit other serine/threonine kinases. AG1478 is reported to be a selective inhibitor of EGFR kinase, used in the present study at 200 nm, well below the IC50 for inhibition of HER2-neu (IC50 > 100 μm) and platelet-derived growth factor receptor kinase (IC50 > 100 μm) (19). PP2 is a potent inhibitor of the c-Src family of nonreceptor tyrosine kinases, used at 10 μm, well below the IC50 values for inhibition of JAK2/ZAP-70 pathway (IC50 > 50 μm) (20). After treatment, tissue was frozen and stored until use. Frozen tissue fragments were lysed and proteins extracted in 750 μl lysis buffer [150 mm NaCl, 50 mm Tris-HCl (pH 7.4), 10 mm EDTA, 0.6% Nonidet-P40 substitute, and 10% glycerol containing protease inhibitors] in a tissue lyser (QIAGEN, Crawley, UK) and centrifuged for 20 min at 19,000 × g. Proteins were quantified by the method of Lowry (Bio-Rad, Hemel Hempstead, UK).
Western blot analysis
To investigate phosphorylation of ERK1/2, proteins (20–40 μg) were resolved on 4–12% Bis-Tris gels (Nupage; Invitrogen) and transferred onto polyvinylidene difluoride membrane (Millipore, Watford, UK). Membranes were blocked for 1 h at 25 C in Odyssey blocking buffer (Li-Cor Bioscience, Cambridge, UK) and incubated overnight at 4 C in blocking buffer containing rabbit anti-phospho-p42/44 ERK and mouse anti-p42 ERK (both obtained from Cell Signaling Technologies, New England Biolabs, Hertfordshire, UK; 1:1000). After washing in PBS containing 0.05% vol/vol Tween, membranes were incubated for 1 h at 25 C in the dark in antimouse IgG conjugated to IRDYE 800 (Tebu-bio, Peterborough, UK) and antirabbit IgG conjugated to Alexa Fluor 680 (Invitrogen) both at a dilution of 1:5000 in blocking buffer. Membranes were washed and proteins visualized and quantified using an Odyssey Infrared Imaging System (Li-Cor Bioscience). Relative density of immunoblots was calculated as phosphorylated ERK divided by the total ERK and was expressed as a fold change above vehicle controls.
For immunoprecipitation, equal amounts of protein were incubated overnight at 4 C with gentle rotation with an anti-phosphotyrosine antibody conjugated to agarose beads (20 μg for 1–2 mg protein; Autogen Bioclear, Wiltshire, UK). Beads were washed extensively with lysis buffer, and immune complexes were solubilized in Laemmli buffer [125 mm Tris-HCl (pH 6.8), 4% SDS, 5% 2-mercaptoethanol, 20% glycerol, and 0.05% bromophenol blue] and subjected to Western blotting with antibodies to phospho-EGFR (Autogen Bioclear) or phospho-cSrc (Merck Chemicals; both 1:1000 in blocking buffer). Membranes were incubated for 1 h in the dark in appropriate antibodies conjugated to Alexa Fluor 680 (1:5000 in blocking buffer; Invitrogen), and proteins were visualized by the Odyssey system as above. Relative densities of immunoblots were calculated as for phosphorylated ERK. The signal levels were normalized to light-chain IgG.
COX-2 and IL-8 gene expression experiments
To investigate the effect of PK1 on expression of COX-2 and IL-8, placental explants were preincubated in serum-free medium overnight, as for cell signaling experiments, and were then incubated for 2, 4, 6, 8, and 24 h in serum-free medium containing vehicle or 40 nm PK1. At the end of experiments, tissue was snap frozen in dry ice and stored at −80 C until subsequent RNA extraction. To investigate the role of the Gq, cSrc, EGFR, and ERK pathway in PK1-mediated expression of COX-2 and IL-8, tissue was serum starved overnight and then preincubated for 1 h with vehicle or inhibitors of Gq (YM-254890, 1 μm), cSrc (PP2, 10 μm), EGFR kinase (AG1478, 200 nm), or MEK (PD98059, 50 μm). After pre-treatment, tissue samples were divided into two and treated for 4 h with the same chemical inhibitors in the presence of either vehicle or 40 nm PK1. Tissue was then stored until subsequent RNA extraction. Although no direct measure of tissue integrity after culture was conducted in our studies (21), we measured RNA integrity as an indirect indicator of tissue viability using the Agilent system. In all our samples, the RNA integrity at the end of culture was comparable to that extracted from tissue shortly after collection. Moreover, there was no difference in RNA quality in samples subjected to the various treatment groups.
TaqMan quantitative RT-PCR
After treatment, tissue samples were disrupted using a tissue lyser (QIAGEN, Crawley, UK) in total RNA isolation reagent (TRIR; ABgene, Epsom, UK) or RNA lysis buffer (RNeasy system; QIAGEN). RNA samples were then extracted according to the manufacturer's instructions using phase-lock tubes (Eppendorf, Cambridge, UK). RNA samples were quantified and reverse transcribed as described previously (17). Quantitative PCR was performed using 2 μl cDNA in 50 μl of reaction mix containing 25 μl Master Mix (Applied Biosystems, Warrington, UK), forward and reverse primers, and FAM/TAMRA-labeled probe, together with VIC/TAMRA-labeled internal control reagents (ribosomal 18S). The sequences of primers and probes were as follows: IL-8, forward 5′-CTGGCCGTGGCTCTCTT-3′, reverse 5′-TTAGCACTCCTTGGCAAAACTG-3′, and probe 5′-FAM-CCTTCCTGATTTCTGCAGCTCTGTGTGAA-TAMRA-3′; COX-2, forward 5′-CCT TCCTCCTGTGCCTGATG-3′, reverse 5′-ACAATCTCATTTGAATCAGGAAGCT-3′, and probe 5′-FAM-TGCCCGACTCCCTTGGGTGTCATAMRA-3′; 18S, forward 5′-CGGCTACCACATCCAAGGAA-3′, reverse 5′-GCTGGAATTACCGCCTGCT-3′, and TAMRA probe 5′-VICTGCTGGCACCAGACTTGCCCTC-TAMRA-3′. PCRs were carried out using an ABI 7900HT (Applied Biosystems). Gene expression was normalized to RNA loading using ribosomal 18S as an internal standard. Results are expressed as relative to a positive RNA standard (cDNA obtained from single endometrial tissue) included in all reactions.
Statistics
All data are expressed as mean ± sem. Where appropriate, results were subjected to statistical analysis by one-way ANOVA with Tukey's multiple comparison test (GraphPad Prism version 4; GraphPad Software, San Diego, CA) and significance accepted when P < 0.05.
Results
PK1 and PKR1 are expressed in third-trimester placenta and pregnant and nonpregnant myometrium
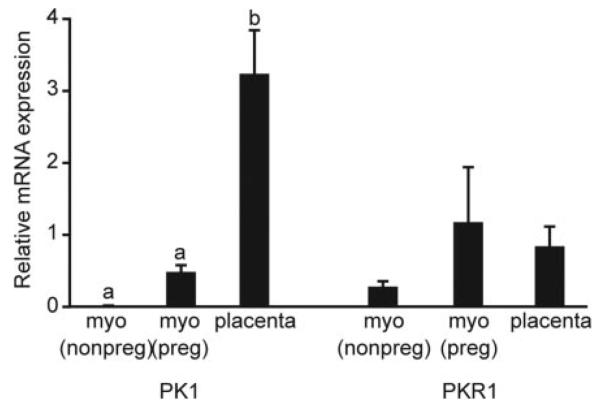
PK1 mRNA was detected in all placental (n = 16) and myometrial samples (pregnant and nonpregnant, n = 9 and n = 6, respectively) examined by quantitative RT-PCR. Relative expression of PK1 mRNA was significantly higher in placenta than pregnant or nonpregnant myometrium (3.24±0.6 vs. 0.49 ± 0.09 vs. 0.01 ± 0.003, respectively; P < 0.01). There was no significant difference in relative PKR1 mRNA expression between third-trimester placenta or pregnant or nonpregnant myometrium (0.85 ± 0.27 vs. 1.18 ± 0.76 vs. 0.29 ± 0.07, respectively; Fig. 1).
Fig. 1.
Relative expression of PK1 and PKR1 in third-trimester human placenta and pregnant (preg) and nonpregnant (nonpreg) myometrium (myo). Relative expression of PK1 and PKR1 mRNA were determined by real-time quantitative RT-PCR analysis in third-trimester placenta (n = 16), third-trimester myometrium (n = 9), and nonpregnant myometrium (n = 6). Relative expression of PK1 mRNA was significantly higher in placenta than pregnant or nonpregnant myometrium. There was no significant difference in relative PKR1 mRNA expression between third-trimester placenta and pregnant or nonpregnant myometrium. Data are expressed as mean ± SEM; b is significantly different from a (P < 0.01).
PK1 and PKR1 are immunolocalized to various cell types in third-trimester human placenta
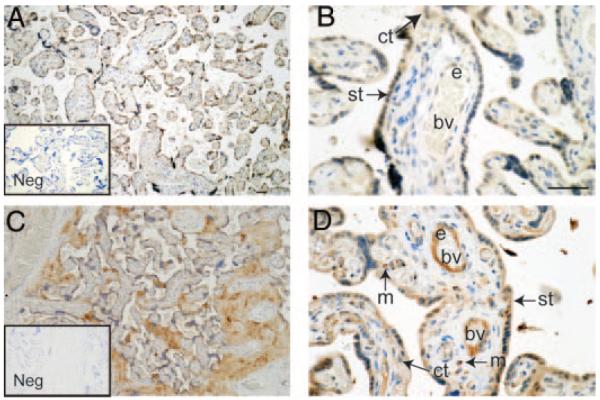
PK1 was immunolocalized to the syncytiotrophoblast layer of placental villi (Fig. 2, A and B) with additional immunostaining present in cytotrophoblasts and the endothelium of occasional fetal blood vessels (Fig. 2B). Similarly, PKR1 was localized to the syncytiotrophoblast layer (Fig. 2, C and D) and cytotrophoblast cells. PKR1 was consistently present in endothelium of fetal blood vessels and was also localized in macrophages (Hofbauer cells) present in the villous core (Fig. 2D). Immunostaining was absent from negative control sections (Fig. 2, A and C, insets).
Fig. 2.
Immunohistochemical localization of PK1 and PKR1 in third-trimester human placenta (n = 8). PK1 was immunolocalized to the syncytiotrophoblast (st) layer of placental villi, cytotrophoblasts (ct), and endothelium (e) of fetal blood vessels (bv) (A and B). PKR1 was immunolocalized to macrophages (m) in the villous core, syncytiotrophoblast (st), cytotrophoblasts (ct), and endothelium (e) (C and D). Immunohistochemistry negatives (Neg, insets) were incubated with isotype-matched IgG in place of primary antibody and displayed no immunoreactivity. Scale bars, 50 μm).
PKR1 colocalizes with cytokeratin, CD31, and CD68 in placenta
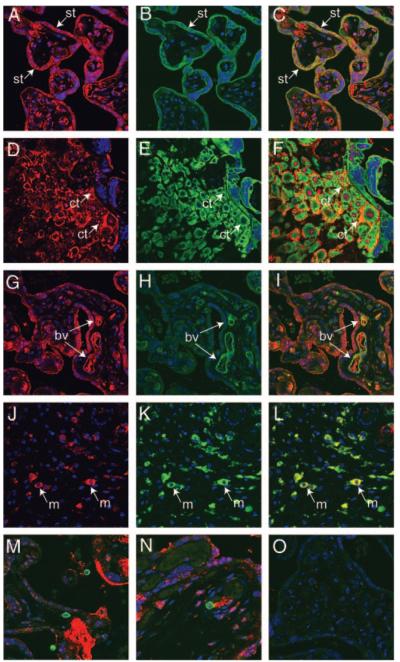
Immunofluorescent histochemistry and confocal microscopy were used to characterize the cell types associated with PKR1 immunoreactivity in third-trimester placenta. PKR1 and cytokeratin colocalized mainly to syncytiotrophoblast (Fig. 3, A–C). In addition, PKR1 and cytokeratin colocalized to occasional cytotrophoblast cells with positive staining occurring on the surface or between cells (Fig. 3, D–F). Some cytotrophoblast cells were positive only for cytokeratin and did not colocalize with PKR1. PKR1 colocalized with CD31 (blood vessel marker) in fetal vessels (Fig. 3, G–I) and CD68 (macrophage marker) in placental macrophages (Fig. 3, J–L). Occasional cells positive for CD3 (T-cell marker; Fig. 3M) and CD20 (B-cell marker; Fig. 3N) were seen, but they did not colocalize with PKR1.
Fig. 3.
Colocalization of PKR1 with cytokeratin, CD31, and CD68 in third-trimester human placenta (n = 4). PKR1 was colocalized with cytokeratin 18 (trophoblast marker), CD31 (blood vessel marker), CD68 (macrophage marker), CD3 (T-cell marker), and CD 20 (B-cell marker) by dual-immunofluorescence immunohistochemistry in third-trimester placenta. PKR1 (red, A and D) and cytokeratin 18 (green, B and E) were colocalized (yellow channel, merged, C and F) in syncytiotrophoblasts (st) and in some cytotrophoblasts (ct). PKR1 (red, G) and CD31 (green, H) were colocalized in blood vessel (bv) endothelium within villi (yellow channel, merged, I). PKR1 (red, J) colocalized with CD68 (green, K) to macrophages (m, yellow channel, merged, L). PKR1 did not colocalize with CD3 (M, merged) or CD20 (N, merged). Control sections were incubated with normal IgG from the same host species and displayed no immunoreactivity (O). Original magnification, ×40.
PK1 up-regulates placental expression of COX-2 and IL-8
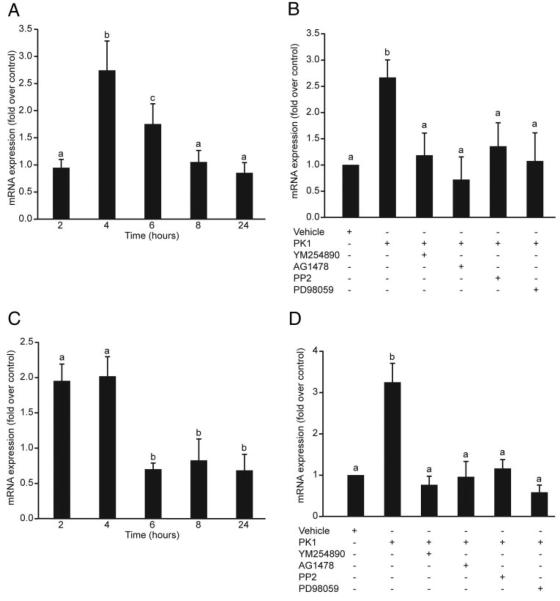
To determine the effect of PK1 on COX-2 and IL-8 expression in third-trimester placenta, placental explants, preincubated in serum-free medium, were then incubated with 40 nm PK1 for 2, 4, 6, 8, and 24 h. After 4 h incubation with 40 nm PK1, there was a significant fold increase in COX-2 mRNA expression in placenta compared with vehicle-treated placental explants (2.75 ± 0.54; Fig. 4A; P < 0.05). IL-8 mRNA expression was significantly increased at both 2 and 4 h after treatment with 40 nm PK1 (1.96 ± 0.24 at 2 h and 2.03 ± 0.27 at 4 h P < 0.05; Fig. 4C). Incubation of placental explants with 40 nm PK1 for 4 h in the presence of inhibitors of Gq, cSrc, EGFR, or MEK resulted in a significant fold decrease in expression of both COX-2 (P < 0.05; Fig. 4B) and IL-8 (P < 0.05; Fig. 4D) relative to placental tissue treated with 40 nm PK1 alone.
Fig. 4.
Relative expression of COX-2 and IL-8 in response to PK1 in third-trimester human placenta. Third-trimester placental explants were treated for 2, 4, 6, 8, and 24 h in the presence of vehicle or 40 nm PK1, and COX-2 and IL-8 expression was measured by real-time RT-PCR (n = 4). To investigate the signaling pathway, placental explants were treated for 4 h in the presence of vehicle or 40 nm PK1 and chemical inhibitors of Gq (YM254890, 1 μm), EGFR kinase (AG1478, 200 nm), cSrc (PP2, 10 μm), or MEK (PD98059, 50 μm), with COX-2 and IL-8 expression being measured as before (n = 3). A, There was a significant fold increase in COX-2 expression at 4 h in placenta treated with 40 nm PK1 compared with vehicle-treated control (P < 0.05); B, there was a significant fold decrease in COX-2 in explants treated with PK1 in the presence of chemical inhibitors relative to placenta treated with PK1 alone (P < 0.05); C and D, similarly, there was a significant fold increase in IL-8 expression at 2 and 4 h in placenta treated with PK1 compared with vehicle-treated control (P < 0.05; n = 4) (C) with expression being significantly reduced in the presence of chemical inhibitors (P < 0.05; n = 3) (D). Results are expressed as mean ± SEM; b is significantly different from a (P < 0.05), and c is not significantly different from a or b.
PKR1 induces ERK phosphorylation via cross-talk with EGFR
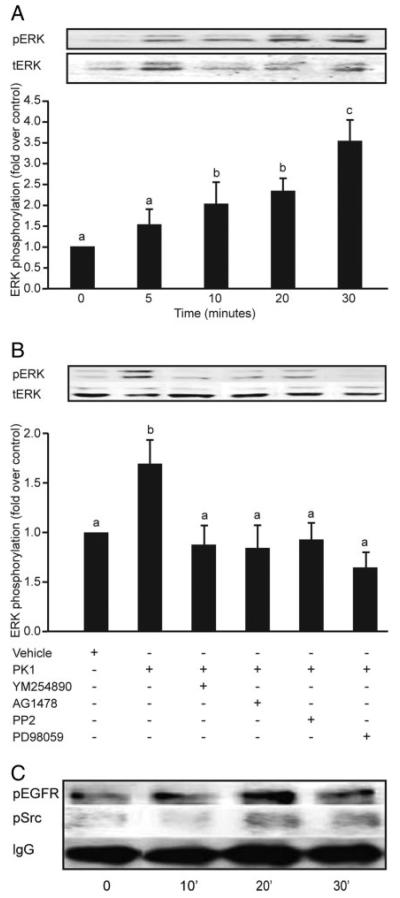
To confirm the pathways involved in PKR1 signaling in third-trimester placenta, placental explants were incubated with PK1 for 5, 10, 20, and 30 min and subsequently analyzed for phosphorylation of cSrc, EGFR, and ERK1/2. There was a significant time-dependent increase in phosphorylation of ERK1/2 that peaked at 30 min (Fig. 5A; 3.55 ± 0.5-fold increase compared with vehicle-treated explants). Coincubation of placental explants with PK1 and inhibitors of Gq, cSrc, EGFR, or MEK caused a significant decrease in ERK1/2 phosphorylation (Fig. 5B; P < 0.05). Treatment of placental explants with 40 nm PK1 also resulted in an approximately 2-fold increase in phosphorylation of cSrc and EGFR (Fig. 5C). However, the time to onset of phosphorylation of both proteins was variable between different placental explant cultures and ranged from 10–30 min.
Fig. 5.
Phosphorylation of ERK, EGF, and cSrc in response to PK1 in third-trimester human placenta. Placental explants were incubated with 40 nm PK1 for 5, 10, 20, and 30 min and subsequently analyzed for phosphorylation of ERK1/2, cSrc, and EGFR (n = 3). A, There was a time-dependent increase in phosphorylation of ERK1/2 that peaked at 30 min (c is significantly different from a but not b; P < 0.05). Explants treated for 30 min with vehicle or 40 nm PK1 in the presence of chemical inhibitors of Gq (YM254890), EGFR kinase (AG1478), cSrc (PP2), or MEK (PD98059) were then subsequently analyzed for ERK phosphorylation (n = 7). B, There was a significant decrease in ERK 1/2 phosphorylation in explants incubated with inhibitors compared with explants treated with PK1 alone (b is significantly different from a; P < 0.05). Placental explants were stimulated with 40 nm PK1 for 0, 10, 20, and 30 min, and lysates were immunoprecipitated with anti-phosphotyrosine antibody and then subjected to Western immunoblot analysis using antibodies against cSrc and EGFR (n = 4). Treatment with 40 nm PK1 resulted in a 2-fold increase in phosphorylation of Src and EGFR within 20 min of treatment. C, IgG was used to demonstrate equal sample loading (representative blot). Data are shown as mean ± SEM.
PKR1 colocalizes with COX-2 and IL-8 in placenta
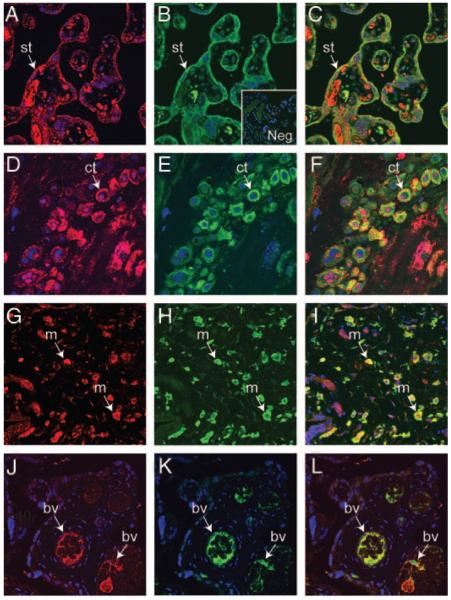
Immunofluorescent histochemistry and confocal microscopy were used to characterize colocalization of PKR1 with COX-2 or IL-8 in third-trimester placenta. PKR1 and COX-2 were colocalized in syncytiotrophoblast (Fig. 6, A–C), cytotrophoblast (Fig. 6, D–F) and macrophages (Fig. 6, G–I). IL-8 and PKR1 were predominately colocalized to fetal vascular endothelium (Fig. 6, J–L).
Fig. 6.
Colocalization of PKR1 with COX-2 and IL-8 in third-trimester human placenta (n = 4). PKR1 was colocalized with COX-2 and IL-8 by dual immunofluorescence in third-trimester placenta. PKR1 (red, A, D, and G) and COX-2 (green, B, E, and H) were colocalized in syncytiotrophoblasts (st), cytotrophoblasts (ct), and macrophages (m) in placenta (yellow, merged, C, F, and I, respectively). PKR1 (red, J) and IL-8 (green, K) were colocalized to fetal blood vessels (bv) within villi (yellow channel, L). Inset in B shows negative (Neg) control (original magnification ×40).
Discussion
This study demonstrates that PK1 and PKR1 are highly expressed in third-trimester placenta with PK1 up-regulating expression of IL-8 and COX-2 potentially via activation of PKR1 and cross-talk with EGFR. The latter finding is supported by double-immunofluorescent immunohistochemistry studies that show that PKR1 colocalizes with IL-8 and COX-2 in placenta. Together, these data suggest that PK1 may be a novel paracrine mediator of the inflammatory response in third-trimester placenta.
The data presented confirm that PK1 is expressed in third-trimester placenta (22, 23) and, in addition, demonstrate that its receptor PKR1 is also expressed in term placenta. The study also demonstrates that PK1 is localized to syncytiotrophoblast, cytotrophoblast, and fetal endothelium. This tissue distribution is similar to that described by Hoffmann et al. during the first trimester (6) in which expression and localization of PK1 was maximal in syncytiotrophoblast between 8 and 10 wk gestation. They proposed that PK1 may therefore have a role in trophoblast differentiation during the critical hypoxic period of placentation. The colocalization of PKR1 to macrophages demonstrated in this study suggests that PK1/PKR1 may have an additional novel role as a paracrine modulator of the immune response in placental macrophages.
This study also displays PK1 and PKR1 expression in myometrium. PKs were initially described as novel factors that mediated potent and sustained smooth muscle contractility in guinea pig ileum (24). However, subsequent studies have yielded conflicting results with PK1 reported to cause relaxation through a nitric oxide-mediated mechanism in murine proximal colon (25). This suggests that the specific cellular environment may determine different intracellular receptor coupling, thereby accounting for the phenotypic difference. More studies are required to investigate whether PK1 affects human myometrial contractility during pregnancy.
Previous studies have shown that PK1 signaling couples to Gq in transfected CHO cells (2, 26) and adrenal cortex capillary endothelial cells, resulting in cellular proliferation and migration in the latter cell type (3). To determine the intracellular signal transduction pathways activated by PK1 in placenta, chemical inhibitors of Gq, cSrc, EGFR, and MEK were used. PK1/PKR1 induced rapid sequential phosphorylation of cSrc, transactivation of EGFR, and phosphorylation of ERK1/2. Furthermore, phosphorylation of cSrc and EGFR in response to PK1 peaked within the time frame of maximal ERK1/2 phosphorylation. These data therefore suggest that in placenta, PK1 also signals via Gq coupling and EGFR transactivation.
Furthermore, we demonstrate that via EGFR transactivation, PK1 induces expression of IL-8 and COX-2 in third-trimester placenta. IL-8 (27) and COX-2 (28), together with prostaglandins and their receptors, are expressed in the human placenta throughout pregnancy (29, 30). IL-8 and COX-2 act synergistically to facilitate vascular permeability and inflammatory cell infiltration and activation (31). Moreover, both IL-8 and COX-2 are up-regulated in reproductive processes characterized by an inflammation including chorioamnionitis, preeclampsia, and parturition (32-35). We propose that PK1/PKR1 signaling may provide a common pathway by which an inflammatory response may be initiated within third-trimester placenta.
The EGF/EGFR loop is an important modulator of trophoblast biology. During the first trimester, EGFR activation modulates the proliferative, migratory, and invasive potential of extravillous trophoblast. This effect is both direct via binding of EGF (36) and indirect via receptor transactivation and subsequent up-regulation of factors including human chorionic gonadotropin, human placental lactogen (37), matrix metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 (38). During later pregnancy, the expression pattern of EGFR is altered in placentae of pregnancies complicated by preeclampsia and intrauterine growth restriction (39), supporting a role for EGFR in regulating placental biology throughout pregnancy. It has been previously hypothesized that PK1 may have an important role in trophoblast differentiation and invasion (11). Demonstration that PK1 signals via transactivation of EGFR, which is known to have an important role in trophoblast differentiation, supports this hypothesis and may provide a mechanism by which it may affect trophoblast biology in the third trimester.
This study further demonstrates that, via transactivation of EGFR, PK1 may be a novel mediator of the inflammatory response in placenta by up-regulating IL-8 and COX-2 expression. Enhanced production of IL-8 and COX-2 via EGFR-dependent mechanisms has previously been shown in severe asthma (40) and human colonic aberrant crypt foci (41), respectively. There is also evidence that EGFR may be up-regulated during inflammation, with increased expression during labor resulting in enhanced stimulation of prostanoid production and stimulation of uterine activity (42). Trans-activation of EGFR by PK1 with subsequent up-regulation of IL-8 and COX-2 may therefore provide a novel mechanism for initiation of an inflammatory response in placenta.
Many factors are thought to initiate inflammation. However, a growing body of evidence supports hypoxia as having a key role in initiating inflammatory reproductive processes (43, 44). PK1 is up-regulated by hypoxia in first-trimester trophoblast, probably via a hypoxia-inducible factor (HIF1α) binding site in its upstream promoter (6). EGFR (45), IL-8 (46), and COX-2 (47) are also up-regulated by HIF1α with placental expression increasing in conditions characterized by hypoxic stress including parturition (32), placental vascular disease (48), chorioamnionitis (34), and preeclampsia. We postulate that under hypoxic conditions, the role of PK1 in mediating an inflammatory response might be further enhanced via HIF-1α-mediated up-regulation in expression of PK1, EGFR, and its downstream effectors including IL-8 and COX-2. This response might be further augmented by a paracrine effect of PK1 on macrophages (10).
In conclusion, this study demonstrates a potential novel role for PK1 as a mediator of the inflammatory response in placenta. Further studies are required to investigate expression and regulation of PK1 and PKR1 in placenta under normoxic and hypoxic conditions in vitro and in physiological and pathological situations in vivo including parturition, placental insufficiency, and preeclampsia.
Acknowledgments
We acknowledge the assistance of Ms. Amie Bowler.
This work was funded by the Medical Research Council, a Moray Endowment Fellowship and an Action Medical Research Project Grant SP4024, PiggyBankKids. A.K. was funded by a Caledonian Fellowship.
Abbreviations
- COX
Cyclooxygenase
- EGFR
epidermal growth factor receptor
- HIF
hypoxia-inducible factor
- PK1
prokineticin-1
- PKR
PK receptor
- VEGF
vascular endothelial growth factor.
Footnotes
Disclosure Statement: F.C.D., S.B., A.E.K., and M.S. have nothing to disclose. H.N.J. consults for Ardana Biosciences.
References
- 1.Maldonado-Perez D, Evans J, Denison F, Millar RP, Jabbour HN. Potential roles of the prokineticins in reproduction. Trends Endocrinol Metab. 2007;18:66–72. doi: 10.1016/j.tem.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- 3.Lin R, LeCouter J, Kowalski J, Ferrara N. Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem. 2002;277:8724–8729. doi: 10.1074/jbc.M110594200. [DOI] [PubMed] [Google Scholar]
- 4.Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K. Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta. 2002;1579:173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 5.LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, Peale F, Gurney A, Hillan KJ, Ferrara N. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann P, Feige JJ, Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology. 2006;147:1675–1684. doi: 10.1210/en.2005-0912. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Frantz G, LeCouter J, Dillard-Telm L, Pham T, Draksharapu A, Giordano T, Peale F. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am J Pathol. 2003;162:1881–1893. doi: 10.1016/S0002-9440(10)64322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA, Duncan WC. Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab. 2005;90:427–434. doi: 10.1210/jc.2004-0843. [DOI] [PubMed] [Google Scholar]
- 9.Urayama K, Guilini C, Messaddeq N, Hu K, Steenman M, Kurose H, Ert G, Nebigil CG. The prokineticin receptor-1 (GPR73) promotes cardiomyocyte survival and angiogenesis. FASEB J. 2007;21:2980–2993. doi: 10.1096/fj.07-8116com. [DOI] [PubMed] [Google Scholar]
- 10.Dorsch M, Qiu Y, Soler D, Frank N, Duong T, Goodearl A, O'Neil S, Lora J, Fraser CC. PK1/EG-VEGF induces monocyte differentiation and activation. J Leukoc Biol. 2005;78:426–434. doi: 10.1189/jlb.0205061. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann P, Feige JJ, Alfaidy N. Placental expression of EG-VEGF and its receptors PKR1 (prokineticin receptor-1) and PKR2 throughout mouse gestation. Placenta. 2007;28:1049–1058. doi: 10.1016/j.placenta.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Chung JY, Song Y, Wang Y, Magness RR, Zheng J. Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2004;89:2484–2490. doi: 10.1210/jc.2003-031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans J, Catalano RD, Morgan K, Critchley HOD, Millar RP, Jabbour HN. Prokineticin 1 signaling and gene expression in early human pregnancy. Endocrinology. 2008 Mar 13; doi: 10.1210/en.2007-1633. 10.1210/en.2007-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi Y. Neutrophil infiltration and chemokines. Crit Rev Immunol. 2006;26:307–316. doi: 10.1615/critrevimmunol.v26.i4.20. [DOI] [PubMed] [Google Scholar]
- 15.Goppelt-Struebe M. Regulation of prostaglandin endoperoxide synthase (cyclooxygenase) isozyme expression. Prostaglandins Leukot Essent Fatty Acids. 1995;52:213–222. doi: 10.1016/0952-3278(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 16.Milne SA, Perchick GB, Boddy SC, Jabbour HN. Expression, localization, and signaling of PGE2 and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2001;86:4453–4459. doi: 10.1210/jcem.86.9.7856. [DOI] [PubMed] [Google Scholar]
- 17.Battersby S, Critchley HO, Morgan K, Millar RP, Jabbour HN. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2004;89:2463–2469. doi: 10.1210/jc.2003-032012. [DOI] [PubMed] [Google Scholar]
- 18.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calcium-dependent epidermal growth factor receptor trans-activation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273:8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 20.Salazar EP, Rozengurt E. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. J Biol Chem. 1999;274:28371–28378. doi: 10.1074/jbc.274.40.28371. [DOI] [PubMed] [Google Scholar]
- 21.Miller RK, Genbacev O, Turner MA, Aplin JD, Caniggia I, Huppertz B. Human placental explants in culture: approaches and assessments. Placenta. 2005;26:439–448. doi: 10.1016/j.placenta.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 22.LeCouter J, Lin R, Ferrara N. Endocrine gland-derived VEGF and the emerging hypothesis of organ-specific regulation of angiogenesis. Nat Med. 2002;8:913–917. doi: 10.1038/nm0902-913. [DOI] [PubMed] [Google Scholar]
- 23.LeCouter J, Lin R, Tejada M, Frantz G, Peale F, Hillan KJ, Ferrara N. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci USA. 2003;100:2685–2690. doi: 10.1073/pnas.0337667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 25.Hoogerwerf WA. Prokineticin 1 inhibits spontaneous giant contractions in the murine proximal colon through nitric oxide release. Neurogastroenterol Motil. 2006;18:455–463. doi: 10.1111/j.1365-2982.2006.00776.x. [DOI] [PubMed] [Google Scholar]
- 26.Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, Yamada T, Hinuma S, Inatomi N, Ohtaki T, Onda H, Fujino M. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun. 2002;293:396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- 27.Saito S, Kasahara T, Sakakura S, Umekage H, Harada N, Ichijo M. Detection and localization of interleukin-8 mRNA and protein in human placenta and decidual tissues. J Reprod Immunol. 1994;27:161–172. doi: 10.1016/0165-0378(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch E, Goldstein M, Filipovich Y, Wang H. Placental expression of enzymes regulating prostaglandin synthesis and degradation. Am J Obstet Gynecol. 2005;192:1836–1842. doi: 10.1016/j.ajog.2004.12.070. discussion 1842–1833. [DOI] [PubMed] [Google Scholar]
- 29.Grigsby PL, Sooranna SR, Adu-Amankwa B, Pitzer B, Brockman DE, Johnson MR, Myatt L. Regional expression of prostaglandin E2 and F2α receptors in human myometrium, amnion, and choriodecidua with advancing gestation and labor. Biol Reprod. 2006;75:297–305. doi: 10.1095/biolreprod.106.051987. [DOI] [PubMed] [Google Scholar]
- 30.Grigsby PL, Sooranna SR, Brockman DE, Johnson MR, Myatt L. Localization and expression of prostaglandin E2 receptors in human placenta and corresponding fetal membranes with labor. Am J Obstet Gynecol. 2006;195:260–269. doi: 10.1016/j.ajog.2006.01.082. [DOI] [PubMed] [Google Scholar]
- 31.Colditz IG. Effect of exogenous prostaglandin E2 and actinomycin D on plasma leakage induced by neutrophil-activating peptide-1/interleukin-8. Immunol Cell Biol. 1990;68(Pt 6):397–403. doi: 10.1038/icb.1990.53. [DOI] [PubMed] [Google Scholar]
- 32.Elliott CL, Kelly RW, Critchley HO, Riley SC, Calder AA. Regulation of interleukin 8 production in the term human placenta during labor and by antigestagens. Am J Obstet Gynecol. 1998;179:215–220. doi: 10.1016/s0002-9378(98)70275-3. [DOI] [PubMed] [Google Scholar]
- 33.Osman I, Young A, Jordan F, Greer IA, Norman JE. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J Soc Gynecol Investig. 2006;13:97–103. doi: 10.1016/j.jsgi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Griesinger G, Saleh L, Bauer S, Husslein P, Knofler M. Production of pro- and anti-inflammatory cytokines of human placental trophoblasts in response to pathogenic bacteria. J Soc Gynecol Investig. 2001;8:334–340. [PubMed] [Google Scholar]
- 35.Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J Soc Gynecol Investig. 2005;12:428–432. doi: 10.1016/j.jsgi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 2007;13:121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 37.Lai WH, Guyda HJ. Characterization and regulation of epidermal growth factor receptors in human placental cell cultures. J Clin Endocrinol Metab. 1984;58:344–352. doi: 10.1210/jcem-58-2-344. [DOI] [PubMed] [Google Scholar]
- 38.Qiu Q, Yang M, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction. 2004;128:355–363. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- 39.Faxen M, Nasiell J, Blanck A, Nisell H, Lunell NO. Altered mRNA expression pattern of placental epidermal growth factor receptor (EGFR) in pregnancies complicated by preeclampsia and/or intrauterine growth retardation. Am J Perinatol. 1998;15:9–13. doi: 10.1055/s-2007-993890. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton LM, Torres-Lozano C, Puddicombe SM, Richter A, Kimber I, Dearman RJ, Vrugt B, Aalbers R, Holgate ST, Djukanovic R, Wilson SJ, Davies DE. The role of the epidermal growth factor receptor in sustaining neutrophil inflammation in severe asthma. Clin Exp Allergy. 2003;33:233–240. doi: 10.1046/j.1365-2222.2003.01593.x. [DOI] [PubMed] [Google Scholar]
- 41.Cohen G, Mustafi R, Chumsangsri A, Little N, Nathanson J, Cerda S, Jagadeeswaran S, Dougherty U, Joseph L, Hart J, Yerian L, Tretiakova M, Yuan W, Obara P, Khare S, Sinicrope FA, Fichera A, Boss GR, Carroll R, Bissonnette M. Epidermal growth factor receptor signaling is up-regulated in human colonic aberrant crypt foci. Cancer Res. 2006;66:5656–5664. doi: 10.1158/0008-5472.CAN-05-0308. [DOI] [PubMed] [Google Scholar]
- 42.Gargiulo AR, Khan-Dawood FS, Dawood MY. Epidermal growth factor receptors in uteroplacental tissues in term pregnancy before and after the onset of labor. J Clin Endocrinol Metab. 1997;82:113–117. doi: 10.1210/jcem.82.1.3671. [DOI] [PubMed] [Google Scholar]
- 43.Matthiesen L, Berg G, Ernerudh J, Ekerfelt C, Jonsson Y, Sharma S. Immunology of preeclampsia. Chem Immunol Allergy. 2005;89:49–61. doi: 10.1159/000087912. [DOI] [PubMed] [Google Scholar]
- 44.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition: a review. Placenta. 2003;24(Suppl A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 45.Peng XH, Karna P, Cao Z, Jiang BH, Zhou M, Yang L. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1α signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem. 2006;281:25903–25914. doi: 10.1074/jbc.M603414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim KS, Rajagopal V, Gonsalves C, Johnson C, Kalra VK. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J Immunol. 2006;177:7211–7224. doi: 10.4049/jimmunol.177.10.7211. [DOI] [PubMed] [Google Scholar]
- 47.Cook-Johnson RJ, Demasi M, Cleland LG, Gamble JR, Saint DA, James MJ. Endothelial cell COX-2 expression and activity in hypoxia. Biochim Biophys Acta. 2006;1761:1443–1449. doi: 10.1016/j.bbalip.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Athayde N, Trudinger B. A proinflammatory cytokine response is present in the fetal placental vasculature in placental insufficiency. Am J Obstet Gynecol. 2003;189:1445–1451. doi: 10.1067/s0002-9378(03)00652-5. [DOI] [PubMed] [Google Scholar]