Abstract
The prevalence of cervical cancer in South African women is reported as being the highest in the world, occurring, on the average, in 60 of every 100,000 women. Cervical cancer is thus considered an important clinical problem in sub-Saharan Africa. Recent studies have suggested that epithelial tumors may be regulated by cyclooxygenase (COX) enzyme products. The purpose of this study was to determine whether cyclooxygenase-2 (COX-2) expression and PGE2 synthesis are up-regulated in cervical cancers. Real-time quantitative RT-PCR and Western blot analysis confirmed COX-2 ribonucleic acid and protein expression in all cases of squamous cell carcinoma (n = 8) and adenocarcinoma (n = 2) investigated. In contrast, minimal expression of COX-2 was detected in histologically normal cervix (n = 5). Immunohistochemical analyses localized COX-2 expression and PGE2 synthesis to neoplastic epithelial cells of all squamous cell (n = 10) and adenocarcinomas (n = 10) studied. Immunoreactive COX-2 and PGE2 were also colocalized to endothelial cells lining the microvasculature. Minimal COX-2 and PGE2 immunoreactivity were detected in normal cervix (n = 5). To establish whether PGE2 has an autocrine/paracrine effect in cervical carcinomas, we investigated the expression of two subtypes of PGE2 receptors, namely EP2 and EP4, by real-time quantitative RT-PCR. Expression of EP2 and EP4 receptors was significantly higher in carcinoma tissue (n = 8) than in histologically normal cervix (n = 5; P < 0.01). Finally, the functionality of the EP2/EP4 receptors was assessed by investigating cAMP generation after in vitro culture of cervical cancer biopsies and normal cervix in the presence or absence of 300 nmol/L PGE2. cAMP production was detected in all carcinoma tissue after treatment with exogenous PGE2 and was significantly higher in carcinoma tissue (n = 7) than in normal cervix (n = 5; P < 0.05). The fold induction of cAMP in response to PGE2 was 51.1 ± 12.3 in cervical carcinoma tissue compared with 5.8 ± 2.74 in normal cervix. These results confirm that COX-2, EP2, and EP4 expression and PGE2 synthesis are up-regulated in cervical cancer tissue and suggest that PGE2 may regulate neoplastic cell function in cervical carcinoma in an autocrine/paracrine manner via the EP2/EP4 receptors.
Cancer of the uterine cervix is one of the leading causes of cancer-related death in women world-wide. It is reported as being particularly common in less developed countries, including South and Central America, Southeast Asia, and sub-Saharan Africa (1-3) where 80% of the world’s cervical cancers occur (4). The prevalence of cervical cancer in South African women is reported to be the highest in the world, occurring, on the average, in 60 of every 100,000 women (3, 5, 6). Cancer of the cervix is the most common cancer in black (31.2%) and colored (22.9%) South African women, the second most common cancer in Asian women (8.9%), and the fourth most common cancer in white South African women (2.7%) (3, 7). The lifetime risk of developing cervical cancer is 1:34 for black women and 1:93 for white women (7). Three histological categories of epithelial tumors of the cervix are recognized by the WHO (8). These are squamous cell carcinoma, adenocarcinoma, and other less common types of epithelial tumors. The most common histological type of cervical carcinoma is squamous cell carcinoma, which accounts for 60-80% of all cervical cancers. Adenocarcinoma accounts for approximately 20% of invasive cervical carcinoma.
Cyclooxygenase (COX) enzymes, also called PG endoperoxide synthase, catalyze the rate-limiting step in the conversion of arachidonic acid to PGH2 and other eicosanoids, including PGE (9). There are at least two isoforms of the COX enzyme, COX-1 and COX-2 (10, 11). COX-1 is constitutively expressed in many tissues and cell types and generates PGs for normal physiological function (11). By contrast, the expression of COX-2 is rapidly induced after the stimulation of quiescent cells by growth factors, oncogenes, carcinogens, and tumor-promoting phorbol esters (10-12). PGE2 elicits its autocrine/paracrine effects on target cells through interaction with seven transmembrane G protein-coupled receptors, which belong to the rhodopsin family of serpentine receptors (13). Four main subtypes of PGE2 receptors have been identified (EP1, EP2, EP3, and EP4); these use alternate and, in some cases, opposing intracellular pathways (14). To date, the roles of the different PGE2 receptors, their divergent intracellular signaling pathways, as well as their target genes involved in mediating the effects of PGE2 on normal or neoplastically transformed cervical epithelium remain to be elucidated.
Recently, a relationship between COX-2, its synthesized product PGE2, and neoplastic transformation of epithelial cells has been established (15, 16). Transcription of COX-2 is up-regulated in numerous cancers, including colon, pancreas, esophagus, lung, prostate, and bladder (17-22). It has been proposed that COX-2 overexpression and PGE2 synthesis mediate neoplastic transformation of epithelial cells by increasing their proliferation rate, resistance to apoptosis, and invasiveness. These effects are mediated by suppressing the transcription of target genes that may be involved in cellular growth/transformation (e.g. p53) and adhesion (e.g. E-cadherin) (16, 23). Moreover, COX-2 and PGE2 promote cancer development and invasiveness by mediating the transcription of angiogenic factors that promote both the migration of endothelial cells and their arrangements into tubular structures (24, 25).
The present study was designed to investigate whether COX-2 expression and PGE2 synthesis are up-regulated in human squamous cell carcinomas and adenocarcinomas of the cervix. In addition, a possible autocrine/paracrine role for PGE2 in cervical carcinogenesis was assessed by investigating 1) the expression of EP2/EP4 receptors in cervical carcinoma tissue and 2) the effect of exogenous treatment of carcinoma tissue with PGE2 on cAMP turnover.
Materials and Methods
Tissue collection and processing
Cervical specimens were obtained at the time of surgery/biopsy from patients who were attending the Gynecologic Oncology Clinic at Groote Schuur Hospital (Cape Town, South Africa) and who had previously been diagnosed with invasive carcinoma of the cervix. Punch biopsies were taken from the lesion by an experienced gynecologist with a special interest in oncology. A portion of the biopsy was excised and fixed in formalin, followed by paraffin wax embedding for histopathological typing. The remaining portion was snap-frozen in either dry ice or liquid nitrogen and stored at -70 C for RT-PCR and Western blot analysis or was transported at 4 C for in vitro culture and PGE2 stimulation. Histologically normal cervical samples (N1-N25) were obtained from patients undergoing Wertheim’s hysterectomy for nonmalignant conditions. Pathological typing was defined according to the International Federation of Obstetricians and Gynecologists (26) staging upon physical examination. The extent of invasiveness and racial distribution of carcinoma biopsies (C1-C50) are presented in Table 1. The ages of the patients ranged from 29-81 yr, with a median age of 50.5 yr. The study was approved by the University of Cape Town research ethics committee, and informed consent was obtained from all patients before tissue collection. The data in this study were analyzed by ANOVA using StatView 5.0 (Abacus Concepts, Berkeley, CA).
TABLE 1.
Extent of invasiveness and racial distribution of cervical carcinoma biopsy samples of South African women
| Sample no. | Histological typing | FIGO stage | Race |
|---|---|---|---|
| C37, C46 | Squamous carcinoma | 1B; poorly differentiated | Black |
| C1-C3, C8, C11-C20, C39, C47 | Squamous carcinoma | 2B; well differentiated | Black |
| C4, C5, C44, C49 | Squamous carcinoma | 3B; well differentiated | Black |
| C9 | Adenocarcinoma | 1B; moderately differentiated | Black |
| C10, C21-C30, C38, C50 | Adenocarcinoma | 2B; well differentiated | Black |
| C6, C7, C31, C32, C34, C40-C42, C45 | Squamous carcinoma | 1B; poorly differentiated | Colored |
| C33, C43 | Squamous carcinoma | 1B; moderately differentiated | Colored |
| C35, C36, C48 | Squamous carcinoma | 2B; well differentiated | Colored |
Real-time quantitative PCR
Real-time quantitative RT-PCR was performed to assess COX-2, EP2, and EP4 expression. Ribonucleic acid (RNA) samples were extracted from cervical tissue (squamous cell carcinomas, C1-C8 and C31-C37; adenocarcinomas, C9, C10, and C38; normal cervix, N1-N5 and N11-N15) using Tri-Reagent (Sigma, Dorset, UK) according to the manufacturer’s protocol. RNA samples were reverse transcribed using MgCl2 (5.5 mmol/L), deoxy (d)-NTPs (0.5 mmol/L each), random hexamers (1.25 μmol/L), oligo(deoxythymidine) (1.25 μmol/L), ribonuclease inhibitor (0.4 U/μL), and multiscribe reverse transcriptase (1.25 U/μL; all from PE Applied Biosystems, Warrington, UK). The mix was aliquoted into individual tubes (16 μL/tube) and template RNA was added (4 μL/tube of 100 ng/μL RNA). Samples were incubated for 60 min at 25 C, 45 min at 48 C and then at 95 C for 5 min. A reaction mix was made containing Taqman buffer (5.5 mm MgCl2, 200 μm dATP, 200 μm dCTP, 200 μm dGTP, 400 μm dUTP), ribosomal 18S forward and reverse primers and probe (all at 50 nm), forward and reverse primers for COX-2, EP2 or EP4 receptor (300 nm), COX-2, EP2 or EP4 receptor probe (200 nm), AmpErase UNG (0.01 U/μL) and AmpliTaq Gold DNA Polymerase (0.025 U/μL; all from PE Biosystems). A volume of 48 μL of reaction mix was aliquoted into separate tubes for each complementary DNA sample and 2 μL/replicate of complementary DNA was added. After mixing 23 μL of sample were added to the wells on a PCR plate. Each sample was added in duplicate. A no template control (containing water) was included in triplicate. Wells were sealed with optical caps, and the PCR reaction was run on an ABI Prism 7700 using standard conditions. COX-2 and EP receptor primers and probe for quantitative PCR were designed using the PRIMER express program (PE Applied Biosystems). The sequences of the COX-2 primers and probe were as follows: forward, 5′-CCT TCC TCC TGT GCC TGA TG-3′; reverse, 5′-ACA ATC TCA TTT GAA TCA GGA AGC T-3′; and probe (FAM labeled), 5′-TGC CCG ACT CCC TTG GGT GTC A-3′. The sequences of the EP2 receptor primers and probe were as follows: forward, 5′-GAC CGC TTA CCT GCA GCT GTA C-3′; reverse, 5′-TGA AGT TGC AGG CGA GCA-3′; and probe (FAM labeled), 5′-CCA CCC TGC TGC TGC TTC TCA TTG TCT-3′. The sequences of the EP4 receptor primers and probe were as follows: forward: 5′-ACG CCG CCT ACT CCT ACA TG-3′; reverse, 5′-AGA GGA CGG TGG CGA GAA T-3′; and probe (FAM labeled), 5′-ACG CGG GCT TCA GCT CCT TCC T-3′. The ribosomal 18S primers and probe sequences were as follows: forward, 5′-CGG CTA CCA CAT CCA AGG AA-3′; reverse, 5′-GCT GGA ATT ACC GCG GCT-3′; and probe (VIC labeled), 5′-TGC TGG CAC CAG ACT TGC CCT C-3′. Expression of COX-2, EP2, and EP4 was normalized to RNA loading for each sample using the 18S ribosomal RNA as an internal standard. Relative gene expression in carcinoma tissue compared with normal cervix was calculated by dividing the expression in carcinoma tissue by the expression in normal cervix. The data are presented as the mean ± SEM.
Western blotting
COX-2 protein expression was assessed by Western blotting. Proteins were extracted from cervical tissue (squamous cell carcinomas, C1-C8; adenocarcinomas, C9 and C10; normal cervix, N1-N5) using Tri-Reagent (Sigma, St. Louis, MO) following the manufacturer’s instructions. A total of 100 μg protein were resuspended in 38 μL sample buffer [125 mmol/L Tris-HCl (pH 6.8), 4% SDS, 5% 2-mercaptoethanol, 20% glycerol, and 0.05% bromophenol blue], boiled for 5 min at 95 C, and run on a 10% SDS-polyacrylamide gel. Proteins were transferred onto polyvinylidene difluoride membrane (Millipore Corp., Watford, UK) and subjected to immunoblot analysis. Membranes were blocked for 1 h at 25 C in 5% skimmed milk powder diluted in washing buffer [50 mmol/L Tris-HCl, 150 mmol/L NaCl, and 0.05% (vol/vol) Tween-20]. Thereafter, membranes were incubated with goat anti-COX-2 primary IgG antibody (sc-1745, Autogenbioclear, Wiltshire, UK) at a dilution of 1:500 at4Cfor 18 h. Control samples were incubated with goat anti-COX-2 antibody preadsorbed to blocking peptide (sc-1745p, Autogenbioclear) according to the manufacturer’s protocol. Membranes were subsequently incubated for 1 h, respectively, with rabbit antigoat secondary IgG antibody conjugated to biotin (DAKO Corp., High Wycombe, UK; 1:500) and streptavidin-biotin-horseradish peroxidase complex (Amersham Pharmacia Biotech, Aylesbury, UK). Proteins were revealed by chemiluminescence (ECL Plus kit, Amersham Pharmacia Biotech) following the manufacturer’s instructions. The molecular mass of the COX-2 protein was approximately 72 kDa, as determined from the relative mobility on SDS-PAGE compared with the molecular mass standard.
Immunohistochemistry
The site of COX-2 expression and PGE2 synthesis was localized in cervical tissues by immunohistochemistry using archival cervical blocks (squamous cell carcinomas, C11-C20; adenocarcinomas, C21-C30; normal cervix, N5-N10) obtained from the Department of Anatomical Pathology, University of Cape Town (Cape Town, South Africa). Five-micron paraffin wax-embedded tissue sections were cut and mounted onto coated slides (TESPA, Sigma). Sections were dewaxed in xylene, rehydrated in graded ethanol, and washed in water followed by TBS (50 mm Tris-HCl and 150 mm NaCl, pH 7.4) and blocked for endogenous endoperoxidase (1% H2O2 in methanol). Antigen retrieval was performed by pressure cooking for 2 min in 0.01 mol/L sodium citrate, pH 6 (for COX-2 and PGE2). No antigen retrieval was performed for CD34 immunohistochemistry. Sections were blocked using 5% normal rabbit serum (for COX-2), 5% swine serum (for PGE2), or 5% normal goat serum (for CD34) diluted in TBS. Subsequently, the tissue sections were incubated with polyclonal goat anti-COX-2 antibody (sc-1745, Autogenbioclear) at a dilution of 1:400, rabbit anti-PGE2 antibody (supplied by Prof. R. W. Kelly, Medical Research Council Human Reproductive Sciences Unit, Edinburgh, UK) at a dilution of 1:100, or monoclonal mouse antihuman CD34 primary antibody (mca-547, Serotec, Oxford, UK) at a dilution of 1:25 at 4 C for 18 h. The rabbit antiserum that was raised against PGE2-complexed keyhole limpet hemocyanin has been previously characterized (27). Control tissue was incubated with 5% antisera (for CD34) or goat anti-COX-2 antibody preadsorbed to blocking peptide (sc-1745p, Autogenbioclear) according to the manufacturer’s protocol. Control tissue for PGE2 was incubated with rabbit anti-PGE2 antibody preadsorbed to excess exogenous PGE2. Briefly, the PGE2 antibody was incubated together with a 10-fold excess of exogenous PGE2 (Sigma) at 37 C for 2 h. Thereafter, the antibody-ligand mixture was diluted, and immunohistochemistry was performed as described above. After thorough washing with TBS, the tissue sections probed with the goat antihuman COX-2 and rabbit anti-PGE2 primary antibodies were incubated with biotinylated rabbit antigoat secondary IgG antibody (for COX-2; DAKO Corp.) or swine antirabbit secondary IgG antibody (for PGE2; DAKO Corp.) at a dilution of 1:500 at 25 C for 40 min. Thereafter, the tissue sections were incubated with streptavidin-biotin peroxidase complex (DAKO Corp.) at 25 C for 20 min. Tissue sections probed with the mouse antihuman CD34 antibody were developed using a mouse EnVision Kit (DAKO Corp.) according to the manufacturer’s instructions. Color reaction was developed by incubation with 3,3′-diaminobenzidine (DAKO Corp.). The tissue sections were counterstained in aqueous hematoxylin, followed by sequential dehydration using graded ethanol and xylene, before mounting and coverslipping.
PGE2 stimulation and cAMP measurement
Determination of basal cAMP levels in cervical tissues
Initially, basal cAMP levels were measured in cervical tissue (squamous cell carcinomas, C39-C44; normal cervix, N16-N20; Fig. 5A). Carcinoma and normal cervical tissues were obtained on the day of surgery/biopsy, sectioned finely, and divided equally into three aliquots. The tissue was transported at 4 C and then incubated in 35-mm tissue culture dishes containing 2 mL DMEM (Sigma), 10% FCS, 0.3 mg/mL l-glutamine, 100 IU penicillin, and 100 μg streptomycin for 1.5 h. One aliquot of tissue was snap-frozen to determine the basal cAMP concentration in the tissue at the time of collection. The other two aliquots were incubated overnight at 37 C in humidified 5% CO2 in the presence or absence of 3 μg/mL indomethacin (a dual COX enzyme inhibitor). Subsequently, tissue sections were harvested by centrifugation at 2000 × g. The supernatant was discarded, and the tissue was homogenized in 0.1 mol/L HCl. The cAMP concentration was quantified by ELISA using a cAMP kit (Biomol, Affiniti, Exeter, UK) according to the manufacturer’s protocol and normalized to the protein concentration of the homogenate. Protein concentrations were determined using protein assay kits (Bio-Rad Laboratories, Inc., Hemel Hempstead, UK).
Fig. 5.

A, Basal cAMP levels (picomoles of cAMP per mg protein) in cervical tissues (mean ± SEM of squamous cell carcinomas, C39-C43, and normal cervix, N15-N20). Basal cAMP levels were determined shortly after biopsy (T0) and after overnight (O/N) culture in the absence (-) or presence (+) of indomethacin. B, cAMP response (picomoles of cAMP per mg protein) in squamous cell carcinoma (C44-C49), adenocarcinoma (C50), and normal cervix (N21-N25). Cervical tissues were treated with indomethacin overnight and either stimulated with 300 nmol/L PGE2 (□) or 50 μmol/L forskolin (■; positive control) or left unstimulated ( ).
).
cAMP production in cervical tissues in response to exogenous PGE2
Cervical tissues (squamous carcinoma, C45-C49; adenocarcinoma, C50; normal cervix, N21-N25) were sectioned finely, divided equally into three aliquots, and incubated overnight in DMEM (Sigma), containing 10% FCS, 0.3 mg/mL l-glutamine, 100 IU penicillin, 100 μg streptomycin, and 3 μg/mL indomethacin. After overnight incubation, samples were incubated in the same medium containing isobutylmethylxanthine (Sigma) to a final concentration of 1 mmol/L for 30 min at 37 C and then stimulated with 0 nmol/L PGE2, 300 nmol/L PGE2, or 50 μmol/L forskolin (forskolin treatment in sample C45 was excluded due to the small size of the biopsy) for 5 min. Tissue sections were harvested by centrifugation at 2000 × g. The supernatant was discarded, and the tissue homogenized in 0.1 mol/L HCl. cAMP and protein concentrations were determined as described above.
Results
Expression of COX-2 in cervical carcinomas was investigated using real-time quantitative RT-PCR (Fig. 1A) and Western blot analysis (Fig. 1B). Expression of COX-2 was significantly up-regulated in all cases of squamous cell carcinoma and adenocarcinoma investigated. COX-2 expression as assessed by quantitative RT-PCR was 150.8 ± 43.18-fold greater in cervical carcinoma tissues than in normal cervical tissue (P < 0.05). Western blot analysis on these cervical carcinomas revealed immunoreactive bands of approximately 72 kDa. Minimal levels of COX-2 transcript was detected in normal cervical tissue by quantitative RT-PCR, and no COX-2 protein was detected in any of the normal cervical samples. Preadsorbing the primary antibody with the blocking peptide abolished the COX-2 signal in the carcinoma samples, thus confirming the specificity of detection of the 72-kDa COX-2 protein in the carcinoma samples.
Fig. 1.

A, Relative expression of COX-2 RNA in cervical squamous cell carcinoma (C1-C8), adenocarcinoma (C9 and C10), and normal cervix (N1-N5), as determined by real-time quantitative RT-PCR. B, Western blot analysis of 100 μg total protein isolated from human cervical carcinoma tissue. The proteins were loaded onto a 10% SDS-gel, electrophoresed, and subsequently transferred to a polyvinylidene difluoride membrane. The immunoblot was probed with antibody raised against the C-terminus of human COX-2. A specific band of approximately 72 kDa was detected in all squamous (C1-C8) and adenocarcinoma (C9 and C10). No signal was detected in normal cervical tissue (a representative sample is shown). Moreover, preadsorbing the antibody with the blocking peptide (B) abolished the COX-2 signal in all carcinoma samples (a representative sample is shown).
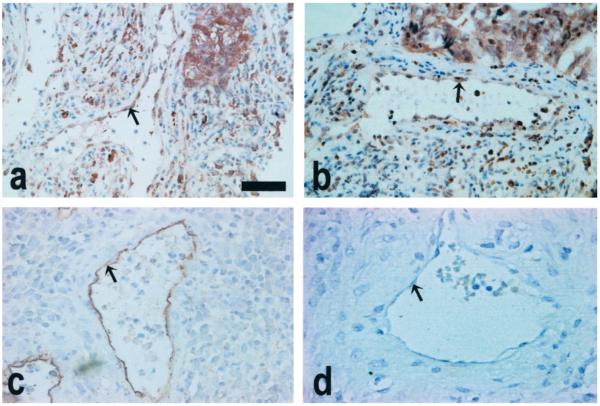
The site of COX-2 expression and PGE2 synthesis in the carcinoma tissue was investigated by immunohistochemistry. Immunoreactive COX-2 and PGE2 were up-regulated in all carcinoma samples. COX-2 and PGE2 were localized to the neoplastically transformed squamous epithelium in squamous cell carcinoma (Fig. 2, A and B, respectively), and to neoplastically transformed columnar epithelium lining the endocervical canal and the glandular epithelium of the endocervical glands in adenocarcinomas (Fig. 2, C and D, respectively). In addition, COX-2 and PGE2 immunostaining was observed in endothelial cells lining the vasculature in all squamous cell carcinoma and adenocarcinoma sections investigated (Fig. 3, A and B). To confirm that COX-2 expression and PGE2 synthesis were localized to the endothelial cells of blood vessels, immunohistochemistry was performed on tissue sections using antibodies raised against the CD34 endothelial cell marker. The pattern of expression with CD34 was identical to that observed with COX-2 and PGE2, thus confirming that COX-2 expression and PGE2 synthesis are localized to the endothelial cell layer of blood vessels in human cervical carcinoma (Fig. 3C). Negligible staining was observed in the stromal compartment of all carcinoma tissue investigated. Moreover, little or no staining for COX-2 and PGE2 was observed in the normal cervical tissues (Fig. 2, E and F, respectively). Preadsorbing the antibody with the blocking peptide (COX-2-negative control) or incubating sections with PGE2 preincubated with exogenous PGE2 ligand (PGE2-negative control) abolished the COX-2 and PGE2 signals in all carcinoma samples (insets in figures show negative controls for COX-2 and PGE2 performed on serial sections). No CD34 staining was observed in sections incubated with nonimmune serum in place of primary antibody (Fig. 3D).
Fig. 2.
COX-2 expression and PGE2 synthesis are detected in epithelial cells of squamous cell carcinoma (A and B, respectively) and columnar and glandular epithelium of adenocarcinomas (C and D, respectively). Minimal COX-2 and PGE2 signal was detected in normal cervical tissue (E and F, respectively). Insets are serial sections that were stained with preadsorbed COX-2 or PGE2 serum, respectively (negative controls). Scale bar, 100 μm.
Fig. 3.
COX-2 (A) expression and PGE2 (B) synthesis are detected in endothelial cells (arrowed) of all carcinoma tissues. Vascular endothelial cells in cervical cancer tissues were localized using antibodies raised against the human CD34 endothelial cell marker (C). D, A representative section incubated with nonimmune goat serum (CD34-negative control). Scale bar, 50 μm.
The expression of two subtypes of PGE2 receptors, namely EP2 and EP4, was investigated by real-time quantitative RT-PCR in cervical carcinoma and normal cervix (Fig. 4). Expression of both receptors was significantly up-regulated in all carcinoma tissues compared with that in normal cervix (P < 0.01). The relative expressions of EP2 and EP4 receptor in carcinoma tissue were 14.5 ± 3.2- and 106 ± 25.8-fold (respectively) greater than that in normal cervix. To assess the activity of the EP2/EP4 receptors in the cervical tissue, basal levels of cAMP were determined at the time of tissue collection and after overnight incubation in the absence or presence of 3 μg/mL indomethacin (Fig. 5A). The cAMP concentration immediately after tissue excision was significantly higher in carcinoma compared with normal cervix (77.9 ± 30.9 vs. 32.5 ± 8.7 pmol cAMP/mg protein; P < 0.05). cAMP concentrations in carcinoma tissue after overnight incubation in the absence of indomethacin was similar to that detected in the tissue at the time of excision (64.2 ± 5.1 pmol cAMP/mg protein), but was significantly reduced when the tissue was cultured in the presence of indomethacin (2.59 ± 0.64 pmol cAMP/mg protein). In normal cervical tissue, levels of cAMP were significantly reduced after overnight incubation in the absence or presence of indomethacin (11.96 ± 1.35 and 4.0 ± 0.7 pmol cAMP/mg protein, respectively; P < 0.05). Subsequently, we determined the effect of exogenous PGE2 and forskolin treatment on cAMP production in carcinoma and normal cervical tissues (Fig. 5B). Stimulation of cervical carcinoma tissue with 300 nmol/L PGE2 or 50 μmol/L forskolin (positive control) yielded a greater cAMP response than in normal cervical tissue treated in the same manner. Overall, the inductions of cAMP generation after PGE2 and forskolin stimulation were 51.1 ± 12.3- and 55.3 ± 15.84-fold, respectively, in cancer tissue and 5.8 ± 1.68- and 9.18 ± 1.59-fold, respectively, in normal cervix (P < 0.01).
Fig. 4.

Relative expression of EP2 (□) and EP4 (■) receptors in cervical squamous cell carcinoma (C31-C37), adenocarcinoma (C38) and normal cervix (N11-N15) as determined by real-time quantitative RT-PCR.
Discussion
This study confirms up-regulation of COX-2 expression and PGE2 production in squamous cell carcinoma and adenocarcinoma of the human cervix, as demonstrated by real-time quantitative RT-PCR, Western blot analysis, and immunohistochemistry. These data suggest a similar pattern of expression of COX-2 in cancer of the cervix as demonstrated in other carcinomas (17-22). In addition, in this study increased COX-2 expression is associated with increased synthesis of PGE2, as both COX-2 and PGE2 colocalized in neoplastic epithelial cells and endothelial cells of the microvasculature. Previous studies have suggested that PGE2 is the predominant PG synthesized from arachidonic acid by COX-2 (28). The exact role of up-regulated COX-2 and PGE2 in cervical cancers remains to be elucidated. In other model systems, enhanced synthesis of PGE2 resulting from up-regulated COX-2 induces malignant change in epithelial cells through immunosuppression (9), inhibiting apoptosis (23), increasing metastatic potential of epithelial cells (29), and promoting angiogenesis (24, 25). COX-2 and PGE2 control the process of angiogenesis in tumors either directly or indirectly. In an in vitro model, overexpression of COX-2 and PGE2 in colon epithelial cells enhances the expression of angiogenic factors that act on endothelial cells, resulting in enhanced cell migration and microvascular tube formation (24). More recently, it was suggested that COX-2 and PGE2 produced by endothelial cells may also directly regulate the process of angiogenesis (25). The arrangement of rat aortic endothelial cells into tubular structures is reduced after treatment with selective COX-2 inhibitors, and this effect is partially reversed by cotreatment with PGE2 (25). Hence, it is feasible to suggest that in cervical carcinomas the process of angiogenesis is regulated by COX-2 and PGE2 through an epithelial-endothelial and/or endothelial-endothelial cell interaction. This is supported by our data demonstrating COX-2 expression and PGE2 synthesis in neoplastic epithelial cells as well as endothelial cells.
PGE2 acts on target cells through interaction with G protein-coupled receptors. To date, several of these receptors have been cloned (termed EP1-EP4) that use alternate intracellular signaling pathways (14). In this study we investigated a possible autocrine/paracrine role for synthesized PGE2 in neoplastic cervical carcinoma tissue. For this we assessed the expression and functionality of two subtypes of PGE2 receptors, namely EP2 and EP4, which mediate their effects on target cells via the protein kinase A pathway by activating adenylate cyclase and increasing intracellular cAMP levels via Gsα (13). In vitro studies have suggested that cAMP is the primary secondary messenger in regulating COX activity, as cAMP activity accompanies a concomitant increase in COX activity (9). The data presented in this study confirm up-regulation of expression of EP2 and EP4 receptors compared with normal cervical tissue. This is associated with elevated basal cAMP concentrations in carcinoma tissue compared with normal cervix. Treatment of cervical tissue with the COX enzyme inhibitor indomethacin significantly reduced the cAMP concentration. This suggests that the elevated basal cAMP concentration in the carcinoma tissue is mediated by COX enzyme products. Moreover, treatment of cervical carcinoma tissue with exogenous PGE2 or forskolin after overnight incubation with the COX enzyme inhibitor indomethacin resulted in a rapid cAMP response that was greater in carcinoma tissue than in normal cervical tissue. Taken together, these data confirm that PGE2 synthesized in cervical carcinoma tissue mediates an autocrine/paracrine effect via interaction with EP2/EP4 receptors. It is possible that other receptor subtypes may also be associated with PGE2 function in the cervical carcinoma tissue. Due to limitations in the sizes of the biopsies obtained at surgery, it was not possible to investigate other intracellular signaling pathways that may be associated with PGE2 function in cervical cancers (14).
COX-2 inhibitors exhibit dramatic antineoplastic activity in a number of tumor model systems investigated to date, including colon cancer cells implanted into nude mice, tumor production in APC mutant mice, and carcinogen-induced tumors in rats (30-32). This is mediated partially by reducing PGE2 synthesis in the COX-2-overexpressing cells, which, in turn, down-regulates the survival, metastatic, and angiogenic potentials of the cancerous tissue (23, 24, 29). This has prompted the suggestion that the inhibition of PGE2 secretion by the application of COX-2 inhibitors may have an effect on growth and invasiveness of various carcinomas (24, 25, 29, 30). Such treatments may also be of benefit in regulating the growth of cervical carcinoma. Treatment of cervical carcinoma with NSAIDs will suppress endogenous expression of COX-2 and synthesis of PGE2, which may act in an autocrine/paracrine manner via the EP2/EP4 receptors. However, it is important to emphasize that in sexually active women the use of selective COX-2 inhibitors may be of partial therapeutic benefit. In these women, the growth and invasiveness of neoplastic cells may be under the direct influence of PGE2 present in seminal plasma. The PG concentration in seminal plasma is 10,000 times higher than that at the site of inflammation, and PGE is the predominant type of PG detected (33). Future studies to elucidate the relative contributions of endogenous and seminal plasma PGs on the phenotypic behavior of neoplastically transformed cervical epithelial and endothelial cells may assist in implementing improved therapy for women with cervical carcinomas.
Acknowledgments
The authors acknowledge Dr. L. Galio, Ms. N. Ally, Ms. S. Boddy, and Ms. S. Dickson for advice and technical assistance.
References
- 1.Aareleid T, Pukkala E, Thomson H, Hakama M. Cervical cancer incidence and mortality trends in Finland and Estonia: a screened vs. an unscreened population. Eur J Cancer. 1993;5:745–749. doi: 10.1016/s0959-8049(05)80359-4. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N, Bosch FX, de Sanjose S, et al. The causal link between human papillomavirus and invasive cervical cancer: a population-based case-control study in Colombia and Spain. Int J Cancer. 1992;52:743–749. doi: 10.1002/ijc.2910520513. [DOI] [PubMed] [Google Scholar]
- 3.Sitas F, Carrara H, Terblanche M, Madhoo J. Screening for cancer of the cervix in South Africa. S Afr Med J. 1997;87:620–622. [PubMed] [Google Scholar]
- 4.Beral V, Hermon C, Munoz N, Devesa SS. Cervical cancer. Vol. 19. Cold Spring Harbor: Cold Spring Harbor Press; 1994. [PubMed] [Google Scholar]
- 5.Cronje HS, Trumpelmann MD, Divall PD, Scott LL, Middlecote BD, De Wet JI. Cervical cytological services in the Orange Free State. Demographic characteristics. S Afr Med J. 1989;76:615–618. [PubMed] [Google Scholar]
- 6.Learmonth GM, Durcan CM, Beck JD. The changing incidence of cervical intra-epithelial neoplasia. S Afr Med J. 1990;77:637–639. [PubMed] [Google Scholar]
- 7.Sitas F, Madhoo J, Wessie J. Incidence of histologically diagnosed cancer in South Africa, 1993-1995. National Cancer Registry of South Africa, South African Institute for Medical Research; 1998. [Google Scholar]
- 8.Scully RE, Bonfiglio TA, Kurman RJ, Silverberg SG, Wilkinson EJ. Histological typing of female genital tract tumors. Berlin: Springer-Verlag; 1994. [Google Scholar]
- 9.DeWitt DL. Prostaglandin endoperoxide synthase:regulation of enzyme expression. Biochim Biophys Acta. 1991;1083:121–134. doi: 10.1016/0005-2760(91)90032-d. [DOI] [PubMed] [Google Scholar]
- 10.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- 12.Subbaramaiah K, Telang N, Ramonetti JT, et al. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 1996;56:4424–4429. [PubMed] [Google Scholar]
- 13.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 14.Ashby B. Co-expression of prostaglandin receptors with opposite effects: a model for homeostatic control of autocrine and paracrine signaling. Biochem Pharmacol. 1998;55:239–246. doi: 10.1016/s0006-2952(97)00241-4. [DOI] [PubMed] [Google Scholar]
- 15.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 16.Subbaramaiah K, Altorki N, Chung WJ, Mestre JR, Sampat A, Dannenberg AJ. Inhibition of cyclooxygenase-2 gene expression by p53. J Biol Chem. 1999;274:10911–10915. doi: 10.1074/jbc.274.16.10911. [DOI] [PubMed] [Google Scholar]
- 17.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tucker ON, Dannenberg AJ, Yang EK, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 19.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 20.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- 21.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Overexpression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Mohammed SI, Knapp DW, Bostwick DG, et al. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res. 1999;59:5647–5650. [PubMed] [Google Scholar]
- 23.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 24.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 25.Jones MK, Wang H, Peskar BM, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 26.FIGO . T.N.M. atlas. 3rd Ed. Heidelberg: Springer-Verlag; 1992. [Google Scholar]
- 27.Kelly RW, Graham BJ, O’Sullivan MJ. Measurement of PGE2 as the methyl oxime by radioimmunoassay using a novel iodinated label. Prostaglandins Leukot Essent Fatty Acids. 1989;37:187–91. doi: 10.1016/0952-3278(89)90084-7. [DOI] [PubMed] [Google Scholar]
- 28.Brock TG, McNish RW, Peters-Golden M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J Biol Chem. 1999;274:11660–11666. doi: 10.1074/jbc.274.17.11660. [DOI] [PubMed] [Google Scholar]
- 29.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc Δ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 31.Sheng H, Shao J, Kirkland SC, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 33.Templeton AA, Cooper I, Kelly RW. Prostaglandin concentrations in the semen of fertile men. J Reprod Fertil. 1978;52:147–150. doi: 10.1530/jrf.0.0520147. [DOI] [PubMed] [Google Scholar]