Abstract
This study uses the mouse to explore the role of ABCA1 in the movement of this cholesterol from the peripheral organs to the endocrine glands for hormone synthesis and liver for excretion. The sterol pool in all peripheral organs was constant and equaled 2,218 and 2,269 mg/kg, respectively, in abca1+/+ and abca1−/− mice. Flux of cholesterol from these tissues equaled the rate of synthesis plus the rate of LDL-cholesterol uptake and was 49.9 mg/day/kg in control animals and 62.0 mg/day/kg in abca1−/− mice. In the abca1+/+ animals, this amount of cholesterol moved from HDL into the liver for excretion. In the abca1−/− mice, the cholesterol from the periphery also reached the liver but did not use HDL. Fecal excretion of cholesterol was just as high in abac1−/− mice (198 mg/day/kg) as in the abac1+/+ animals (163 mg/day/kg), although the abac1−/− mice excreted relatively more neutral than acidic sterols. This study established that ABCA1 plays essentially no role in the turnover of cholesterol in peripheral organs or in the centripetal movement of this sterol to the endocrine glands, liver, and intestinal tract for excretion.
Keywords: ATP binding cassette transporter A1, Tangier disease, low density lipoprotein transport, high density lipoprotein transport, cholesterol synthesis, cholesterol absorption, reverse cholesterol transport, steroid hormones
Every cell in the body is surrounded by a plasma membrane of unique physicochemical characteristics. This membrane is essentially a bilayer of various phospholipids, sphingolipids, and glycolipids interdigitated with unesterified cholesterol molecules. The rigid, hydrophobic ring structure of the sterol interacts through hydrophobic bonding with the fatty acid chains of the phospholipids to condense the bilayer and establish a very hydrophobic region ∼3 nm thick that runs through the center of the membrane (1, 2). Thus, the typical cell membrane is highly permeable to water but essentially impermeable to protons, electrolytes, and hydrophilic molecules. Also imbedded in the membrane is a variety of signaling and transport proteins that allow the cell to maintain, in a highly selective manner, the metabolic processes essential for life. These functions are all critically dependent upon the presence of the cholesterol molecule in the bulk phase of the plasma membrane. As a consequence, rates of cholesterol synthesis are very high in the growing organs of the developing fetus and newborn animal (3–5). Any mutation that limits the availability of this critical molecule during development leads either to intrauterine death of the embryo or to major developmental abnormalities in the newborn (6–8). Even after maturity is reached and organs attain their adult size, there is continuous replacement, or “turnover,” of cholesterol in the membranes of cells. This renewal process is apparently so important that virtually every cell in the body has invested in the complex biochemical machinery necessary to convert acetyl-CoA into cholesterol (9, 10). Lesser amounts of sterol are also acquired through the receptor-mediated and bulk phase uptake of lipoproteins like LDL (11, 12). However, as the pool of cholesterol in every tissue is essentially constant over a lifetime, and as no cell can degrade the sterol nucleus, it necessarily follows that each day an amount of cholesterol equal to that synthesized and acquired from LDL must leave the tissue and be transported to the endocrine glands and, more importantly, to the liver for excretion from the body. This turnover process is so active that in a small animal like the mouse, with a very high metabolic rate, nearly 7% (∼150 mg/day/kg) of the whole body pool is replaced each day, while the human, with a much lower metabolic rate, turns over about 0.7% (∼15 mg/day/kg) of the body pool daily (13).
Some insights into the molecular events controlling this turnover process came from studies in patients with Tangier disease, a genetic illness characterized by a marked reduction in the level of circulating apolipoprotein AI (apoAI) and cholesterol carried in HDL (HDL-C) and by tissue infiltration of lipid-laden macrophages (14). With the discovery that this disease was apparently caused by mutations in the transport protein ABCA1 (15–18), a fairly straightforward paradigm was developed to describe the putative events characterizing so-called “reverse cholesterol transport.” In this scheme, ABCA1 was envisioned as reducing the activation energy for the desorption of cholesterol from the outer leaflet of the plasma membrane by transferring the sterol, along with phospholipids, directly onto apoAI-rich, nascent HDL particles circulating in the pericellular fluid. These nascent particles would then mature in the circulation and, ultimately, deliver their cargo of cholesterol to the endocrine tissues and liver using pathways mediated by scavenger receptor class B type I, cholesteryl ester transfer protein (CETP), and the LDL receptor (LDLR) (19–21). Importantly, this model implied that plasma membrane cholesterol turnover was driven by events external to the cell and that the velocity of this process would be proportional to the levels of both ABCA1 in the membrane and apoAI in the pericellular fluid. If true, in the absence of ABCA1 activity, there should be sterol accumulation and downregulation of cholesterol synthesis and cholesterol carried in LDL (LDL-C) uptake in all peripheral organs, decreased flux of cholesterol from the periphery to the endocrine glands and liver, and, finally, decreased biliary and fecal sterol excretion.
While this was an attractive hypothesis for explaining cholesterol turnover, recent experimental measurements have yielded results contrary to these predictions. For example, in the abca1−/− mouse, an animal model manifesting the same abnormalities in apoAI and HDL-C metabolism as seen in Tangier patients (22, 23), biliary cholesterol secretion, and fecal sterol output were apparently normal and not reduced (24–26). Furthermore, measurements of HMG-CoA reductase activity suggested that sterol synthesis in several peripheral organs was actually increased and not suppressed (24). From very limited data, it appeared that patients with Tangier disease also excreted essentially normal amounts of fecal sterols (personal observation by Prof. Gerd Assmann). Thus, these experimental observations suggested that ABCA1 might not play an important role in the massive flux of cholesterol that moves daily out of the peripheral organs. Because of the critical importance of this pathway for understanding overall sterol homeostasis in the whole animal and human, these studies were undertaken to quantitate each step in this pathway for the movement of cholesterol from the peripheral organs to the sites of excretion. Using mice lacking ABCA1 function, the size of the cholesterol pool and the rate of cholesterol export were measured in each organ in vivo. These rates were compared with independent measurements of the amounts of sterol that were delivered to the endocrine glands and liver from HDL and to the amount of cholesterol excreted from the body in the feces as either acidic or neutral sterols. These quantitative measurements establish, as suggested by the earlier publications, that turnover of cholesterol in the peripheral organs and the centripetal flow of this sterol to the sites of excretion are unaffected by whether ABCA1 is functioning or not.
MATERIAL AND METHODS
Animals and diets
The mice used in these experiments were derived from animals kindly provided by Dr. Trudy A. Christiansen-Weber (R.W. Johnson Pharmaceutical Research Institute, San Diego, CA) (23). These mice were supplied to us as heterozygotes on a C57BL/6:129/Ola background. They were then used to generate abca1−/− mice that were, in turn, bred with C57BL/6:129/Sv wild-type mice. This was done over 10 generations so that, ultimately, the mutation was carried on a predominantly C57BL/6:129/Sv background. The genotypes were identified using PCR analysis as described (23). All animals were housed in plastic colony cages in rooms with alternating periods of light and dark. After weaning and genotyping by the end of the third week, the animals were fed ad libitum a low cholesterol (0.02%, w/w) pelleted diet (No. 7001; Harlan-Teklad, Madison, WI) until they were studied at 3 months of age. All experiments were carried out during the fed state near the end of the dark phase and used female animals. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical School.
Isolation and radiolabeling of LDL and HDL
Mouse plasma was harvested from both male and female mice lacking LDLR activity (ldlr−/−) that had been maintained on the low-cholesterol basal diet. The LDL and HDL fractions were isolated by preparative ultracentrifugation in the density ranges of 1.020–1.055 and 1.063–1.21 g/ml, respectively. The LDL preparation was then radiolabeled in the protein moiety with either 125I-tyramine cellobiose or with 131I (27–29). The apoE-containing HDL contaminating this LDL fraction was removed by passing the lipoprotein solution over a heparin sepharose CL-6B column (Pharmacia Biotech, Uppsala, Sweden) (30). After dialysis, these radiolabeled preparations were passed through a 0.45 μm Millex-HA filter immediately prior to injection into the recipient animals. HDL-C was labeled with either the intracellularly trapped [1α, 2α(n)-3H]cholesteryl oleyl ether or [cholesteryl-4-14C]oleate by exchange from donor liposomes as described (31–33). Freshly collected plasma from cholesteryl ester transfer protein transgenic mice was used as a source of CETP. The labeled HDL was reisolated by ultracentrifugation (d = 1.07–1.21 g/ml) and dialyzed against saline. Both labeled LDL and HDL preparations were used within 48 h of preparation.
Measurement of LDL and HDL clearance rates in vivo
Mice were anesthetized, and a catheter was inserted into the jugular vein. After awakening, each animal was given a bolus of 125I-tyramine cellobiose-labeled LDL followed by a continuous infusion of the same preparation at a rate calculated to maintain a constant specific activity in the plasma (34). Ten minutes before termination of the 4 h infusion period, a bolus of 131I-labeled LDL was administered to each animal. The mice were exsanguinated at the end of the 4 h infusions, and all tissues were removed. The remaining carcass was cut into small pieces. Tissue and plasma samples were then assayed for their content of 125I and 131I (28, 35). A similar procedure was used for HDL clearance measurements. These animals were administered a priming dose of [3H]cholesteryl oleyl ether-labeled HDL followed by a continuous infusion of the same radiolabeled lipoprotein for 4 h. Animals were exsanguinated 10 min after intravenously injecting [14C]cholesteryl oleate-labeled HDL. Plasma, tissue samples, and the remaining carcass were saponified in alcoholic KOH, and sterols were extracted and assayed for their 3H and 14C content. The rates of clearance of LDL and HDL by various tissues were expressed as the microliter of plasma cleared of its LDL or HDL content per hour per gram wet weight (μl/h/g) (34). Using the steady-state plasma concentrations of cholesterol in the LDL and HDL fractions, these clearance values were also used to calculate the amount of total cholesterol and cholesteryl ester, respectively, taken up into each organ from LDL and HDL. These values were expressed as the milligram of sterol taken up per day per kilogram of body weight (mg/day/kg).
Measurement of cholesterol synthesis rates in vivo
Each animal was injected intraperitoneally with approximately 40 mCi of [3H]water. One hour later, the animals were anesthetized and exsanguinated. The tissues and remaining carcass were saponified, and the digitonin-precipitable sterols were isolated as described (27). The rates of sterol synthesis in each of these tissues were then expressed as the nmol of [3H]water incorporated into digitonin-precipitable sterols each hour per gram wet weight of tissue (nmol/h/g). These rates of incorporation of [3H]water into sterols by tissues were also converted to an equivalent number of milligrams of cholesterol synthesized, assuming 0.69 3H atoms were incorporated into the sterol molecule for each carbon atom (36, 37). These rates were expressed as the milligrams of cholesterol synthesized per day per kilogram of body weight (mg/day/kg).
Measurement of intestinal cholesterol absorption, bile acid pool size, and composition
Fractional (%) cholesterol absorption was measured by a fecal dual-isotope ratio method using [4-14C]cholesterol (Perkin-Elmer Life Sciences) and [5,6-3H]sitostanol (American Radiolabeled Chemicals, St. Louis, MO) as described (38). Bile acid pool size was determined using a HPLC method as the total bile acid content of the small intestine, gall bladder, and liver combined (38). Pool size was expressed as milligram per kilogram of body weight (mg/kg).
Measurement of fecal acidic and neutral and total sterol excretion
Stools were collected from individual animals over a 72 h period. These were dried, weighed, and ground. The rates of fecal bile acid and neutral sterol excretion were measured by an enzymatic method and gas-liquid chromatography, respectively (38), and are expressed as milligrams of sterol excreted each day per kilogram of body weight (mg/day/kg). The total sterol excretion was calculated as the sum of the acidic and neutral sterol outputs for each animal.
Measurement of plasma and tissue cholesterol concentrations and plasma steroid hormone concentrations
The total plasma cholesterol concentration was measured enzymatically (Kit No. 1127771; Boehringer Mannheim, Indianapolis, IN). Plasma triacylglycerol concentrations were measured using Infinity Triglycerides Liquid Stable reagent (ThermoTrace, Noble Park, Australia) (39). Plasma lipoproteins were separated by fast-protein liquid chromatography using a Sepharose 6 column (33, 35). The cholesterol content in each fraction was quantitated enzymatically. The organs and remaining carcass were saponified, and their sterols were extracted with petroleum ether. The total cholesterol concentration was determined by gas-liquid chromatography (40). Plasma corticosterone, estradiol, progesterone, and testosterone concentrations were determined by a commercial facility (Endocrine Services Laboratory, Oregon Regional Primate Research Center, Beaverton, OR).
Calculations
While the various rate constants for cholesterol synthesis and uptake were all usually expressed per hour per gram wet weight of tissue, these same data were also expressed per day per organ and were then normalized to a constant body weight of 1 kg. Thus, all values are also shown in various figures as milligrams of cholesterol synthesized or taken up by each organ each day per kilogram of body weight (mg/day/kg). The data in all of these experiments are presented as the mean ± 1 SEM. The unpaired Student's t-test was used to compare various sets of data, and an asterisk indicates a value that is significantly different (P < 0.05) from its corresponding control value.
RESULTS
These studies were designed to measure both the absolute rates of cholesterol flux out of the individual organs into the blood each day as well as the absolute rates of fecal sterol excretion from the whole animal. As cholesterol turnover in organs like the liver and intestine is very different from turnover in more distal organs like the kidney and lung, the various tissues of the body were classified into two, presumably functionally different, groups of organs. Tissues like muscle, lung, kidney, and central nervous system, that presumably depend upon the movement of cholesterol from the cells of these organs to HDL for delivery to the liver, are referred to as peripheral tissues. Organs like liver, small intestine, and other parts of the gastrointestinal tract that can excrete sterol directly into the intestinal lumen, are referred to as central tissues. The most relevant measurement, therefore, for these studies was to quantitate the absolute rates of cholesterol flux from the individual peripheral tissues to the plasma in the presence and absence of ABCA1 function.
Plasma lipoprotein cholesterol concentrations
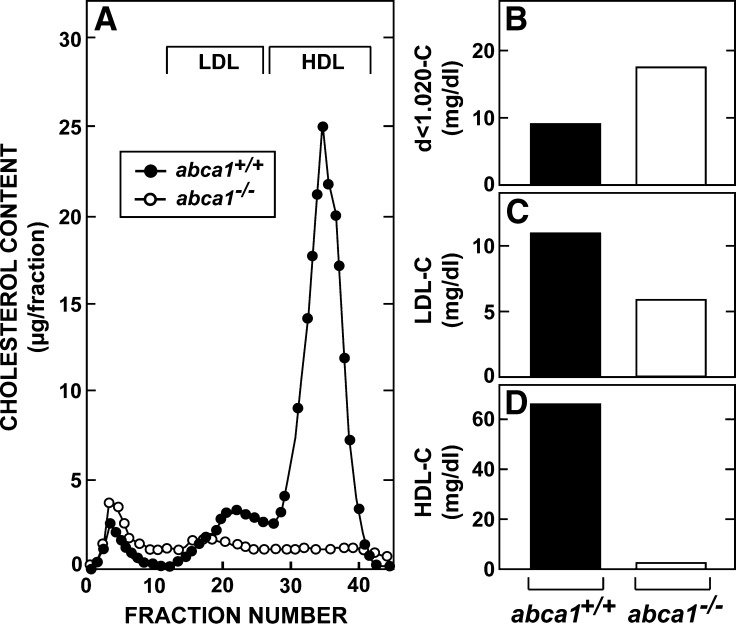
Preliminary studies revealed similar abnormalities in lipid metabolism in both male and female abca1−/− mice. However, because of the quantitative nature of these studies, all measurements of cholesterol flux were carried out in female animals of the same age. At 3 months of age, these abca1−/− mice showed the expected abnormalities in circulating lipid levels (22, 23). The total plasma cholesterol concentration averaged 85 ± 6 mg/dl in the abca1+/+ animals but only 25 ± 3 mg/dl in the abca1−/− mice. The plasma triacylglycerol concentrations in these same two groups equaled 51 ± 6 and 118 ± 23 mg/dl, respectively. As shown in Fig. 1A, analysis of lipoprotein levels in pooled plasma by HPLC revealed a marked reduction in HDL-C and a smaller reduction in LDL-C in the animals lacking ABCA1 function. In contrast, the level of cholesterol carried in larger, less dense particles was modestly increased in the abca1−/− mice. These findings were confirmed when the lipoproteins in these pooled samples were separated by ultracentrifugation. The cholesterol carried in the d < 1.020 g/ml fraction increased from 9 mg/dl in the abca1+/+ mice to 17 mg/dl in the abca1−/− animals (Fig. 1B), while the LDL-C concentration fell from 11 to 6 mg/dl (Fig. 1C). Most strikingly, the HDL-C concentration was only 2 mg/dl in the abca1−/− mice compared with 66 mg/dl in the control animals (Fig. 1D).
Fig. 1.
Plasma lipoprotein cholesterol concentrations in 3-month-old female abca1+/+ and abc1−/− mice maintained on a low-cholesterol diet. Plasma was pooled from groups of six to eight mice of each genotype and fractionated by either HPLC (A) or by ultracentrifugation (B–D).
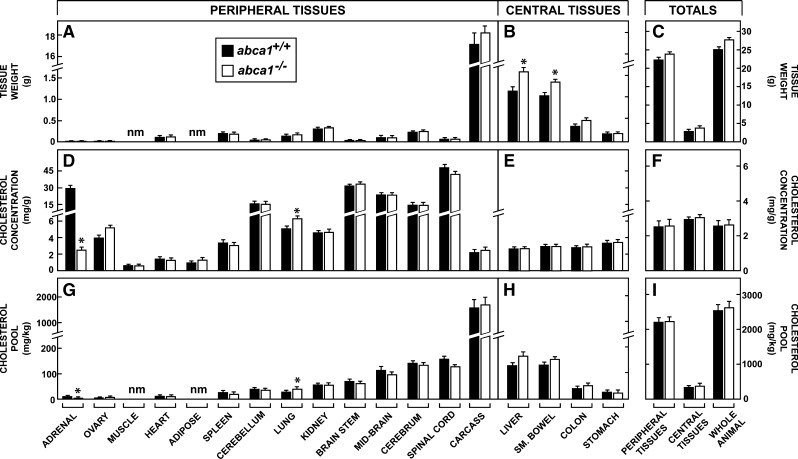
Organ weights and tissue cholesterol pools
At 3 months of age, the female abca1−/− mice had achieved about the same mean body weight (27.6 g) as the abca1+/+ control animals (25.1 g). Of note, the weights of individual peripheral tissues were not different in the two genotypes, as shown in Fig. 2A. However, several of the central tissues, including the liver and small bowel, were heavier (Fig. 2B). As a result, these central organs made up a slightly greater proportion of total body weight in the abca1−/− animals (14%) than in the control mice (11%) (Fig. 2C). Nevertheless, the relevant compartment of peripheral tissues made up the great majority (86–89%) of the whole animal weights in both groups. The concentration of cholesterol, expressed as milligrams per gram wet weight, varied from values of only ∼1.0 mg/g in tissues like muscle to >20 mg/g in organs like adrenal and various parts of the central nervous system (Fig. 2D). Importantly, however, these concentrations were virtually identical in nearly every tissue, as were the mean concentrations for all of the organs in the central and peripheral compartments (Fig. 2F) in the abca1+/+ and abca1−/− mice. Only the lung revealed a small increase in sterol concentration, while the adrenal showed the expected marked reduction, as has been reported in other conditions in the mouse where the HDL-C level was very low (41).
Fig. 2.
Organ weights, cholesterol concentrations, and cholesterol pools in 3-month-old female abca1+/+ and abca1−/− mice maintained on a low-cholesterol diet. These organs have been divided into peripheral tissues, which largely must deliver their cholesterol to HDL for transport to the liver for excretion, and central tissues, which can potentially deliver their cholesterol directly into the gastrointestinal tract for excretion. The absolute weight of each tissue is shown (A, B) as well as the total weight of the peripheral and central organs (C). The tissue identified as carcass is what remains when all other organs have been removed and consists largely of muscle, adipose tissue, skin, and bone, including marrow. The concentration of cholesterol in each of these tissues is expressed as milligrams per gram wet weight (D, E), and the average concentrations for all of the peripheral and central tissues and for the whole animal (F) are also illustrated. These concentration values were multiplied by the respective organ weights to obtain the size of the cholesterol pool present in each tissue. These values, in turn, were normalized to a constant body weight of 1 kg so that the size of the cholesterol pool present in each tissue is expressed as milligrams per kilogram of body weight (G, H). These latter values were summed to yield the cholesterol pools present in the peripheral and central tissues and in the whole animal (I). The sizes of the cholesterol pools in muscle and adipose tissue were not measured individually, but these are included in the carcass. The abbreviation “nm” means not measured. The asterisk identifies those values that are significantly different (P < 0.05) from the corresponding values in the abca1+/+ mice. Each column represents the mean ± 1 SEM of data from six to seven animals.
These concentration values, along with organ and whole animal weights, were used to calculate the absolute size of the cholesterol pool present in each tissue expressed as the mg of sterol present in each organ in a 1 kg animal. These absolute pools varied from only about 7 mg/kg in the adrenal to 1545 mg/kg in the carcass of the control mice (Fig. 2G, H). Importantly, except for the lung, in no tissue of the peripheral compartment were these cholesterol pools significantly expanded in the absence of ABCA1 function. Thus, in both the abca1+/+ and abca1−/− mice, where the whole animal pools equaled 2,552 and 2,668 mg/kg, respectively, the total pool of cholesterol in all of the tissues of the peripheral compartment equaled about 86% of the total body pools (Fig. 2I). Clearly, loss of ABCA1 function had not led to expansion of the cholesterol pools in these tissues of the periphery.
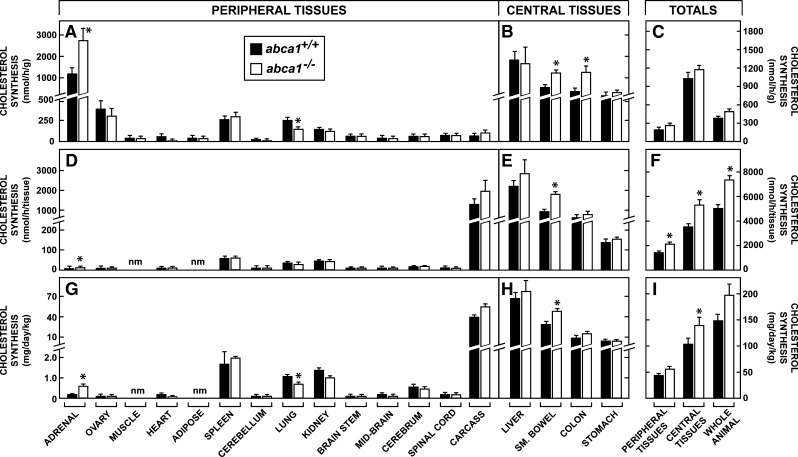
Rates of cholesterol synthesis in the peripheral organs
It was conceivable, however, that these pools were kept constant by either reducing the rate of cholesterol synthesis or the rate of LDL-C uptake, or both, in the face of diminished sterol output from these organs in the abca1−/− mice. To examine this possibility, rates of sterol synthesis were next measured in vivo. In general, rates of cholesterol synthesis were much higher in the central organs (Fig. 3B) than in the peripheral tissues (Fig. 3A). This difference reflects the much higher rates of cholesterol turnover known to occur in cells of the liver and gastrointestinal tract. The rates of synthesis per gram of tissue in the peripheral compartment varied from only 14 nmol/h in muscle to 1183 nmol/h in adrenal in the abca1+/+ mice, and, with the exception of the lung, there was no significant suppression of sterol synthesis in any tissue with deletion of ABCA1 function. In several of the central organs, the rates of synthesis were actually slightly elevated in the abca1−/− animals (Fig. 3B). Notably, synthesis in the adrenal gland was elevated 3-fold. This was anticipated and reflected the adaptive response of the gland to reduced HDL-C uptake in these abca1−/− animals. When these rates were adjusted for the small differences in organ weights, and recalculated in terms of the rate of synthesis per whole tissue, similar trends were evident. Rather than being suppressed, synthesis was marginally increased in the carcass and several tissues of the central compartment (Fig. 3D, E) so that total synthesis in the peripheral and central tissues, as well as in the whole animal, was modestly increased in the absence of ABCA1 function (Fig. 3F).
Fig. 3.
Rates of cholesterol synthesis in 3-month-old female abca1+/+ and abca1−/− mice maintained on a low-cholesterol diet. Rates of synthesis were measured in vivo and expressed as the nmol of [3H]water incorporated into sterols per hour per gram of tissue (A, B). These data were averaged to yield the mean rates of synthesis in the peripheral and central tissues and in the whole animal (C). These values were multiplied by the appropriate organ weights in each animal to give the rates of synthesis in each whole tissue (D, E), and these were summed to give total rates of synthesis in the peripheral and central organs and in the whole animal (F). These rates were then extended to 24 h, adjusted to a constant body weight of 1 kg, and converted to milligrams of cholesterol synthesized per day per kilogram (G, H). The values in the individual tissues were summed to give absolute rates of cholesterol synthesis in the peripheral and central compartments and in the whole animal (I). The abbreviation “nm” means not measured. The asterisk identifies those values that are significantly different (P < 0.05) from the corresponding values in the abca1+/+ mice. Each column represents the mean ± 1 SEM of data from six to seven animals.
Finally, these values were adjusted for the small differences in whole animal weights, and the rates of [3H]water incorporation into sterol were converted to milligrams of cholesterol synthesized per day per kilogram of body weight. In the peripheral compartment, these rates varied from <1.0 mg/day/kg in tissues like ovary and adrenal to >35 mg/day/kg in the carcass (Fig. 3G). Again, with the exception of the lung, these rates of synthesis were essentially identical in the abca1+/+ and abca1−/− mice. The absolute rates of synthesis in the central tissues were much higher than those in the peripheral compartment but, again, were nearly the same in the two genotypes (Fig. 3H). Thus, in the control mice, total synthesis in the peripheral organs equaled 44.5 mg/day/kg (29% of whole body synthesis), while the whole animal synthesized 150 mg/day/kg (Fig. 3I). In the abca1−/− animals, these peripheral organs synthesized 58.2 mg/day/kg (also 29% of whole body synthesis), while the whole animal synthesized 199 mg/day/kg. Of note, while the tissues of the peripheral compartment accounted for ∼88% of whole body weight, they were responsible for only 29% of whole animal synthesis. Clearly, loss of ABCA1 function had not suppressed synthesis in essentially any of these tissues.
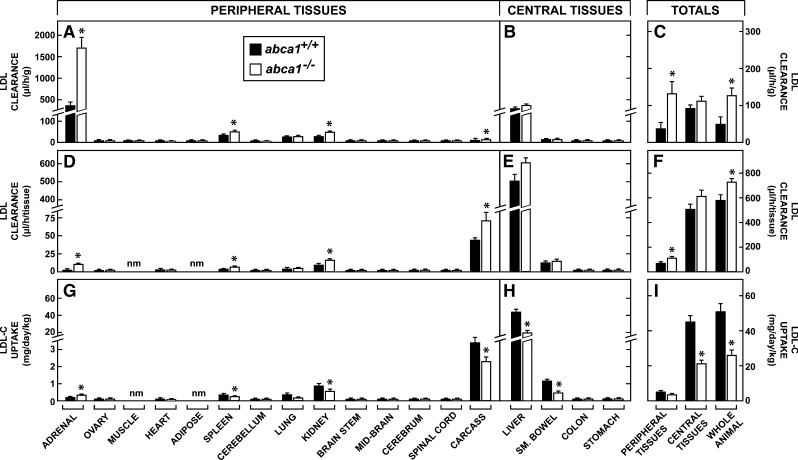
Rates of LDL-C uptake in the peripheral and central tissues
It remained possible, however, that cholesterol acquisition through lipoprotein uptake was suppressed in the abca1−/− mice. The next study, therefore, quantitated the rates of receptor-mediated and bulk-phase endocytosis of cholesterol carried in LDL in 3-month-old female mice. In the peripheral organs, the rates of LDL clearance varied from ∼1.0 μl/h/g in muscle and various parts of the central nervous system to 429 μl/h/g in the adrenal (Fig. 4A) in abca1+/+ animals. As expected, the liver in the central compartment had high rates of clearance (358 μl/h/g) per gram of tissue (Fig. 4B). In the peripheral compartment, these clearance rates were either the same or higher in the various organs of the abca1−/− mice, so that the mean rates of clearance in all of the tissues of the peripheral and central compartments, as well as in the whole animal, were elevated in the absence of ABCA1 function (Fig. 4C). After correcting for organ size, the liver was responsible for most LDL-C clearance (Fig. 4D, E) and, alone, accounted for 85% and 82% of whole animal LDL-C degradation, respectively, in the abca1+/+ and abca1−/− mice (Fig. 4F).
Fig. 4.
Rates of LDL clearance and LDL-C uptake in 3-month-old female abca1+/+ and abca1−/− mice maintained on a low-cholesterol diet. Rates of LDL clearance were measured as the microliter of plasma cleared of LDL per hour per gram (A, B), and these values were averaged to give the mean rates of clearance in the peripheral and central tissues (C). These values were then multiplied by the appropriate organ weights in each animal to give the rates of clearance in each whole tissue (D, E), and these were summed to give total rates of clearance in the peripheral and central tissues (F). Using the total cholesterol concentration in the LDL fraction in the plasma of these two genotypes, these rates were extended to 24 h and adjusted to a constant body weight of 1 kg to yield the milligrams of LDL-C taken up into these tissues each day per kilogram (G, H). These values were summed to give the absolute rates of LDL-C uptake into the peripheral and central tissues and by the whole animal (I). The abbreviation “nm” means not measured. The asterisk identifies those values that are significantly different (P < 0.05) from the corresponding values in the abca1+/+ mice. Each column represents the mean ± 1 SEM of data from 6 to 10 animals.
Since the receptor-mediated component of LDL clearance is saturable, the higher clearance rates seen in some tissues of the abca1−/− mice probably reflected the lower LDL-C concentration in the plasma (Fig. 1C), rather than an actual increase in lipoprotein uptake. When these clearance values were multiplied by the concentration of LDL-C in the two groups of animals and adjusted to a constant body weight, this proved to be the case. In the abca1+/+ mice, whole animal LDL-C uptake equaled 50.5 mg/day/kg, and 86% of this took place in the liver (43.8 mg/day/kg) (Fig. 4H, I). Whole animal LDL-C degradation equaled only 24.6 mg/day/kg in the abca1−/− animals, however, so that uptake was lower in some tissues of these mice (Fig. 4G). Notably, in the adrenal, LDL-C uptake increased from 0.21 mg/day/kg to 0.37 mg/day/kg, indicating that this organ actually had increased LDLR activity in the face of the low circulating HDL-C levels. Thus, these studies revealed that the LDL-C production rate, which had to equal the LDL-C degradation rate, was significantly lower in the abca1−/− animals (24.6 mg/day/kg) compared with the control mice (50.5 mg/day/kg). Nevertheless, uptake of LDL-C in all of the tissues of the peripheral compartment was so small (5.4 mg/day/kg versus 3.8 mg/day/kg in the abca1+/+ and abca1−/− animals, respectively) that these differences contributed little to alterations in overall tissue cholesterol turnover.
Total cholesterol flux out of the peripheral compartment
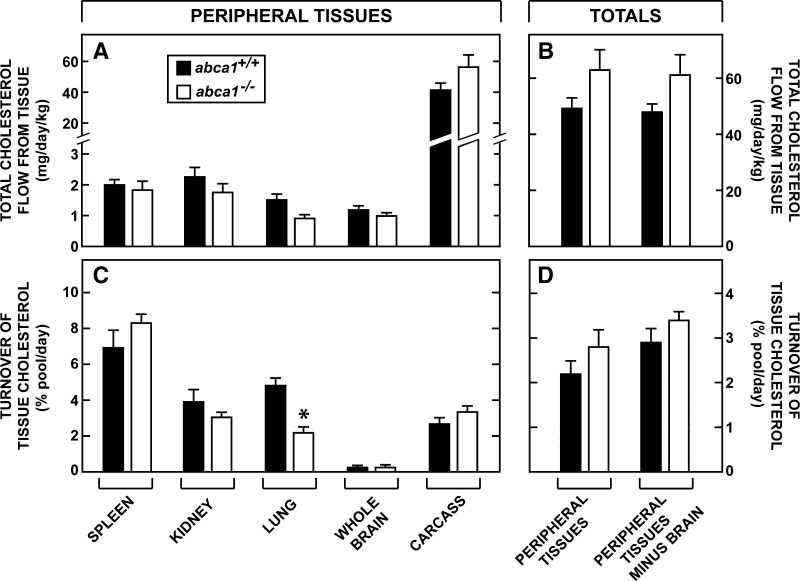
With these data available, it was possible to calculate the magnitude of the cholesterol flux out of all of the peripheral tissues each day. Since the cholesterol pools in these organs were constant, the amount of cholesterol leaving these tissues must equal the amount of sterol synthesized (Fig. 3G) plus the amount taken up as LDL-C (Fig. 4G) each day. The sum of these two values for the larger organs and for all of the tissues in the peripheral compartment combined are shown in Fig. 5A and B. The flux of cholesterol out of cerebellum, brainstem, midbrain, cerebrum, and spinal cord combined was <1.3 mg/day/kg in both the abca1+/+ and abca1−/− mice (Fig. 5A). These rates were higher in the spleen and kidney and equaled about 2 mg/day/kg. However, the major flux of cholesterol came from the large tissues of the carcass, which equaled 41.5 mg/day/kg and 55.5 mg/day/kg, respectively, in the abca1+/+ and abca1−/− mice (Fig. 5A). Most importantly, the total flux of cholesterol from all tissues of the peripheral compartment equaled 49.9 mg/day/kg in the control mice and 62.0 mg/day/kg in the animals lacking ABCA1 function (Fig. 5B).
Fig. 5.
Total flow of cholesterol from the peripheral tissues. The rates of total sterol acquisition from synthesis (Fig. 3G) and LDL-C uptake (Fig. 4G) were summed to obtain the amount of cholesterol that must leave the major peripheral organs each day (A). The total flow of cholesterol from all peripheral organs, as well as the flow from the peripheral tissues minus the brain, were also calculated (B). Each value was divided by the size of the respective cholesterol pool in that tissue to yield the daily turnover rate (C). Similarly, the mean turnover of cholesterol in all of the tissues of the periphery and in the organs of the periphery excluding the brain, was also calculated (D). The asterisk identifies those values that are significantly different (P < 0.05) from the corresponding values in the abca1+/+ mice. Each column represents the mean ± 1 SEM.
Using these flux rates and the pool sizes (Fig. 2G) determined in each organ, the rates of cholesterol turnover in every tissue of the peripheral compartment could be calculated. The lowest rate of turnover was found in the brain (<0.2% of the pool each day), while these rates were higher in the carcass (∼3%), kidney (∼3.5%), and spleen (∼7.5%) (Fig. 5C). With the exception of the lung, these turnover rates were the same in the two genotypes so that overall turnover in all of the organs of the peripheral compartment equaled 2.2% per day and 2.8% per day in the abca1+/+ and abca1−/− mice, respectively (Fig. 5D). Because the large sterol pool in the brain with a very slow turnover distorted these overall calculations, turnover of cholesterol in the tissues of the peripheral compartment was also calculated after excluding the tissues of the central nervous system. Under these conditions, cholesterol turnover in the peripheral organs equaled 2.8% per day in the abca1+/+ mice and 3.4% per day in the abca1−/− animals (Fig. 5D). Thus, there was no alteration in the flux of cholesterol from the peripheral organs or in the turnover of tissue cholesterol pools in these organs after loss of ABCA1 function. The only exception was the lung, where turnover of the pool decreased from 4.8% per day to 2.2% per day with loss of ABCA1 activity (Fig. 5C). While not shown in Fig. 5, turnover in the central organs, as expected, was much higher and exceeded 20% per day in most tissues.
Net excretion of cholesterol through steroid hormone formation
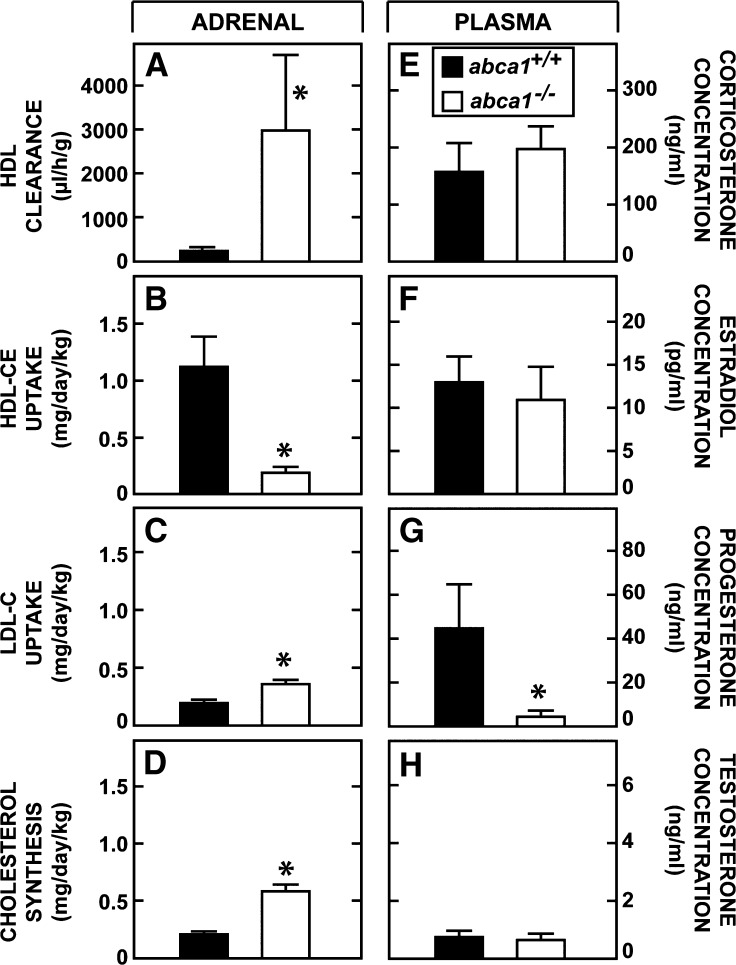
The cholesterol leaving these peripheral organs must ultimately be excreted from the body either after conversion to steroid hormones or, more importantly, through the biliary excretion of neutral and acidic sterols. The next experiment quantitated the flow of sterol to the adrenal and other endocrine organs in the presence and absence of ABCA1 function. The rate of selective cholesteryl ester clearance into the adrenal through the activity of scavenger receptor class B type I equaled 260 μl/h/g and 3005 μl/h/g, respectively, in the abca1+/+ and abca1−/− mice (Fig. 6A). When multiplied by the concentration of cholesteryl ester in plasma HDL in each of these groups of animals, the rates of cholesterol uptake into the adrenal equaled 1.13 mg/day/kg in the control mice but only 0.21 mg/day/kg in the abca1−/− animals (Fig. 6B). However, as quantitated above, in response to this diminished uptake of HDL-C, the uptake of LDL-C increased from 0.21 to 0.37 mg/day/kg (Fig. 6C), while the rate of local synthesis increased from 0.22 to 0.60 mg/day/kg (Fig. 6D) in the abca1+/+ and abca1−/− mice, respectively. Thus, because of the marked reduction in HDL-C in the abca1−/− animals, the uptake of cholesterol from this source was reduced by 0.92 mg/day/kg, which was not fully compensated for by the increase in sterol acquisition of 0.54 mg/day/kg brought about by increased rates of synthesis and LDL-C uptake. This observation presumably explained the finding that plasma corticosterone levels were maintained (Fig. 6E), yet the large pool of reserve cholesteryl esters could not be preserved (Fig. 2D) in the adrenal of the abca1−/− mice. Plasma estradiol (Fig. 6F) and testosterone (Fig. 6H) levels also were normal, while the plasma progesterone (Fig. 6G) concentration was lower in the animals lacking ABCA1 function. Thus, there was less cholesterol being converted to hormones through the endocrine glands in the abca1−/− animals, but this effect was small and the result of the marked reduction in HDL-C concentration and so was related only secondarily to loss of ABCA1 function.
Fig. 6.
Rates of cholesterol utilization by the adrenal and plasma steroid hormone levels. Rates of HDL clearance were measured in the adrenal (A), and using the concentration of cholesteryl esters in the HDL fraction of these two genotypes, these values were used to calculate the rate of HDL-CE uptake by this gland (B). For comparison, the rates of LDL-C uptake (from Fig. 4G) and cholesterol synthesis (from Fig. 3G) are shown in C and D, respectively. The plasma concentration of four different steroid hormones is also shown (E–H). The asterisk identifies those values that are significantly different (P < 0.05) from the corresponding values in the abca1+/+ mice. Each column represents the mean ± 1 SEM of data from 6 to 10 animals in the first set of data (A–D) and from 8 to 15 mice in the second group (E–H).
Net excretion of cholesterol through the liver
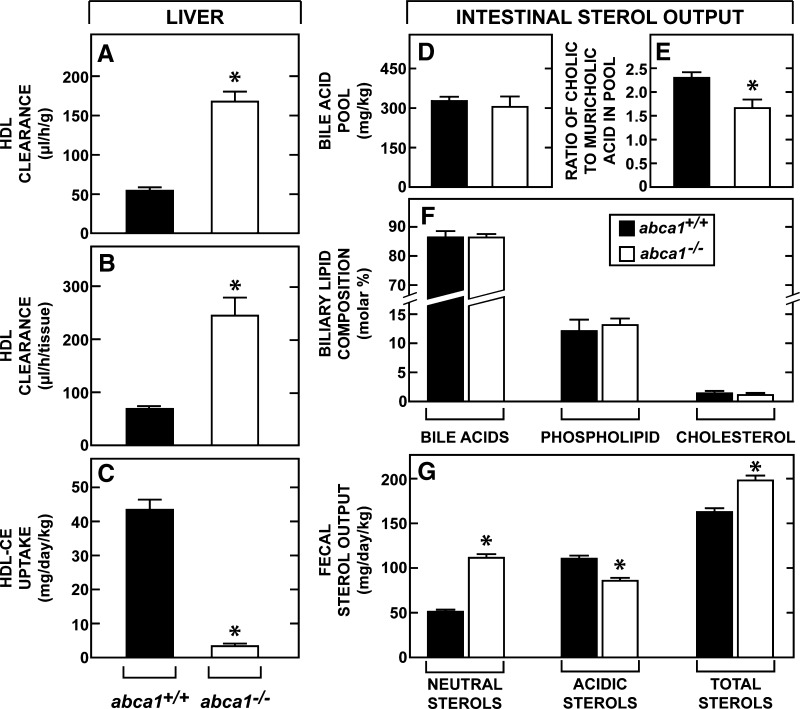
Quantitatively, sterol excretion through the liver is far more important than that used by the endocrine glands. Therefore, the next studies measured the rate of cholesterol movement from the peripheral organs through HDL to the liver. The clearance of cholesteryl ester from HDL into the liver equaled 53 μl/h/g in the control mice and increased to 168 μl/h/g in the abca1−/− animals (Fig. 7A). After correcting for organ weight, these clearance values were 72 μl/h and 248 μl/h (Fig. 7B), respectively, in these two genotypes. When adjusted to the concentration of cholesteryl ester in the plasma HDL fractions of these two groups, the absolute rate of cholesterol uptake into the liver of the abca1+/+ mice equaled 44.8 mg/day/kg but was only 3.9 mg/day/kg (Fig. 7C) in the abca1−/− mice. Thus, in the control animals, the rate of movement of cholesterol out of the peripheral compartment (49.9 mg/day/kg) corresponded closely to the amount of sterol delivered to the liver from HDL. In the abca1−/− animals, however, there was a very large discrepancy between the mass of sterol leaving the peripheral compartment (62.0 mg/day/kg) and the amount of cholesterol delivered to the liver by HDL.
Fig. 7.
Rates of HDL-CE clearance and uptake by the liver, bile acid pool size and composition, and rates of fecal sterol excretion. Rates of hepatic HDL clearance were measured (A, B) and, along with the concentration of cholesteryl esters in the HDL fraction of these two genotypes, were used to calculate the rates of HDL-CE uptake by the liver (C). The amount of bile acid present in the liver, biliary tract, and entire small bowel combined was measured and is presented as the bile acid pool size (D). The ratio of cholic acid to muricholic acid in this pool was also determined (E). The molar ratios of bile acid, phospholipid, and cholesterol in bile obtained from the gallbladder was quantitated (F). In a separate group of animals, feces were collected for determination of the daily output of both neutral and acidic sterols (G). The asterisk identifies those values that are significantly different (P < 0.05) from the corresponding values in the abca1+/+ mice. Each column represents the mean ± 1 SEM of data in 6 to 10 animals in each group in the first set of data (A–C), five animals in the second set (D, E), four animals in the third (F), and 10 animals in the last set (G).
Therefore, the critical issue was whether this large amount of sterol from the peripheral compartment actually reached the liver for excretion. In the abca1−/− mice, the size of the bile acid pool found in the biliary system and small bowel was slightly smaller in the abca1−/− animals (308 mg/kg) than in the control mice (330 mg/kg) (Fig. 7D), and the ratio of cholic acid to muricholic acid was lower (Fig. 7E). Both of these findings suggested that the rate of bile acid synthesis was slightly lower in the absence of ABCA1 function. Nevertheless, the molar ratios of bile acid, phospholipid, and cholesterol in gallbladder bile were not different in the two genotypes (Fig. 7F). Most importantly, output of fecal neutral sterols equaled 52 mg/day/kg in the control mice, but 112 mg/day/kg in the abca1−/− animals, while acidic sterol output in these two groups equaled 111 and 86 mg/day/kg, respectively (Fig. 7G). Thus, total sterol excretion through the gastrointestinal tract equaled 163 mg/day/kg in the abca1+/+ mice and 198 mg/day/kg in the abca1−/− animals. These values corresponded closely to the rates of whole animal synthesis (Fig. 3I) plus the small amounts of cholesterol in the diet (∼30 mg/day/kg) and confirmed that the sterol turned over in the peripheral compartment had reached the liver and been excreted in the abca1−/− animals despite the fact that this flux apparently did not take place through HDL. Finally, fractional cholesterol absorption was modestly lower in the abca1−/− animals (39 ± 4%) compared with that in the control mice (58 ± 4%). However, this finding was difficult to interpret since, on the one hand, it might have reflected an actual reduction in sterol absorption in the abca1−/− mice or, on the other, it may have only been the result of greater dilution of the specific activity of the intraluminal cholesterol pool that resulted from the higher rate of sterol excretion in these animals (Fig. 7G).
DISCUSSION
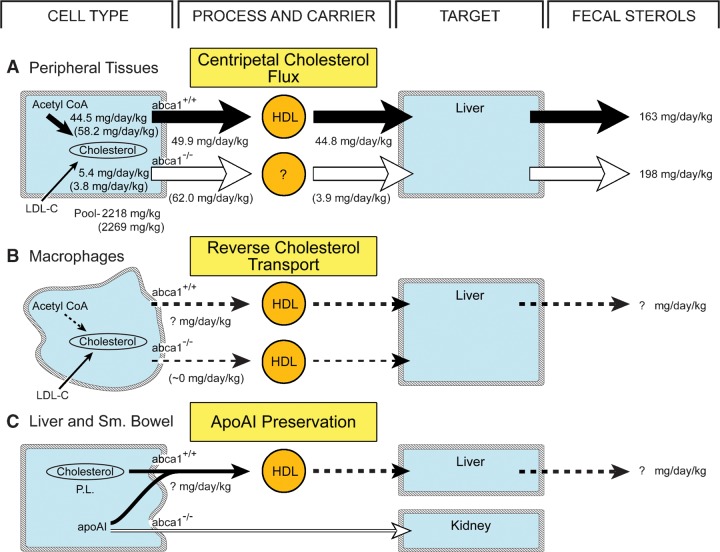
These experiments provide direct, quantitative data to support the conclusion that ABCA1 plays virtually no role in normal turnover of cholesterol in the plasma membranes of parenchymal cells in the peripheral organs. As illustrated in Fig. 8A, as most of the sterol used in this turnover comes from de novo synthesis (44.5 mg/day/kg), and not from the uptake of LDL-C (5.4 mg/day/kg) (Figs. 3I, 4I), this process is appropriately referred to as the centripetal flux of cholesterol from the peripheral tissues to the sites of utilization and excretion in the endocrine glands and liver. Nearly all of this flux (44.8 mg/day/kg) (Fig. 7C) is accounted for by movement of cholesteryl ester from HDL to the liver, while much smaller amounts are also taken up by the endocrine tissues (not shown in Fig. 8A). This major flux, along with additional amounts of cholesterol synthesized in the central tissues and taken in from the diet, fully accounts for the acidic and neutral sterols appearing in the feces (163 mg/day/kg) of the abca1+/+ mice (Fig. 7G). In the absence of ABCA1 activity, neither the centripetal flux of cholesterol out of the peripheral tissues (62.0 mg/day/kg) nor the rate of fecal sterol excretion (198 mg/day/kg) is reduced even though there is a marked reduction in the rate of cholesteryl ester movement through HDL to the liver (3.9 mg/day/kg). How the remaining large amount of cholesterol reaches the liver in the abca1−/− animals is not currently known, although VLDL may be involved in this process since the VLDL concentrations are elevated in the abca1−/− mouse compared with controls.
Fig. 8.
Three major pathways in which ABCA1 and HDL might be involved. The first (A) represents the centripetal movement of cholesterol out of the parenchymal cells of the peripheral organs during plasma membrane sterol turnover. The contribution of synthesis and LDL-C uptake to this normal turnover is shown for both the abca1+/+ (numbers without parentheses) and abca1−/− (numbers in parentheses) animals. Also shown are the rates of cholesteryl ester movement from HDL to the liver and the rates of total sterol excretion in the feces. The second pathway (B) represents reverse cholesterol transport from primarily macrophages in the periphery. These flux rates are very small and essentially unmeasurable. Finally, the third pathway (C) illustrates the role of ABCA1 in transferring cholesterol and phospholipids from cells of the liver and small bowel to newly synthesized apoAI in order to prevent its rapid degradation and excretion by the kidneys. Again, this flux is presumably very small.
However, this result is consistent with earlier reports that the concentration of HDL circulating in the plasma also plays no role in determining the rate of sterol movement from the peripheral tissues to the liver. When the concentration of HDL is varied over a wide range by variably expressing CETP in the mouse, centripetal cholesterol flux is constant (42). In the mouse that lacks apoAI and, therefore, has very low HDL levels, the flow of sterol from the peripheral organs to the liver, again, is unchanged (41). Finally, in a naturally occurring situation, the guinea pig normally synthesizes most of the cholesterol turned over in the organs of the periphery and transports this sterol to the liver for excretion. This flux of sterol takes place despite the fact that this species has little detectable HDL circulating in the plasma (43–45). Taken together, these many observations establish that neither ABCA1 activity nor the level of circulating HDL determines the rate of plasma membrane cholesterol turnover in the major peripheral organs of the body.
Other data do support a role for ABCA1 in promoting cholesterol movement out of tissue cells of the macrophage lineage (Fig. 8B) (46, 47). As most of this sterol is presumably derived from the uptake of cholesterol carried in LDL, this process is appropriately referred to as reverse cholesterol transport (19, 20). When ABCA1 function is deleted, this sterol accumulates as cholesteryl ester, and these cells appear as lipid-laden macrophages in organs like the lung, tonsils, spleen, lymph nodes, and intestine (22, 48–50). Of note, alveolar macrophages make up a large percentage of cells in the normal lung (51). It is likely, therefore, that this process of reverse cholesterol transport was actually identified in this organ in this study in that, in the abca1−/− mice, the lung manifested a small increase in its cholesterol pool (Fig. 2G), suppression of cholesterol synthesis (Fig. 3G), and significant reduction in cholesterol turnover (Fig. 5C). However, while there are about 1 × 108 macrophages in the adult mouse (52), this number represents only a small fraction of the cells present throughout the body. Thus, the magnitude of this reverse cholesterol transport is probably extremely small and essentially unmeasurable with the techniques used in this study, particularly in the face of the massive centripetal flow of sterol out of the peripheral parenchymal cells. This conclusion is further supported by the important observations that the plasma HDL-C concentration is not increased by restoration of abca1+/+ bone marrow cells in the abca1−/− mouse (47) and is not decreased by deletion of ABCA1 function in the macrophages of the abca1+/+ animal (53). Thus, there is a process of reverse cholesterol transport from macrophages to the liver, but this flux is too small to be identified in these experiments.
Another fundamentally important role of ABCA1 is to protect newly synthesized apoAI from premature degradation (Fig. 8C). ApoAI is synthesized in both the liver and small bowel and forms the essential backbone of circulating HDL (54, 55). However, unless immediately lipidated, this protein is rapidly removed from the circulation by the kidneys (56). This important process is apparently carried out by ABCA1 expressed in both the liver and intestine where small amounts of cholesterol and, particularly, phospholipid are transferred to the apoAI molecule to form nascent HDL particles. These nascent particles then enter the circulation and presumably pick up much larger amounts of sterol from the peripheral organs for transport back to the liver. This protective effect of the phospholipid and cholesterol is an important determinant of the steady-state concentration of apoAI, and, therefore, HDL, in the plasma. Specific deletion of ABCA1 function in either the liver or small bowel leads to significantly lower concentrations of circulating HDL-C (57, 58). However, the actual amount of cholesterol transferred from the liver or intestine to apoAI to carry out this protective function is likely also to be very small. Most of the cholesterol present as HDL-C in the plasma presumably comes from the peripheral organs. It would obviously make no physiological sense to move large quantities of sterol from the liver and intestine to HDL particles that, in turn, would be immediately cleared back into these same organs for excretion. The fact that the amount of cholesteryl ester moving from HDL to the endocrine glands and liver in the abca1+/+ animals (∼46 mg/day/kg) (Fig. 8A) essentially equals the flux of sterol from all of the peripheral organs indicates that the amount of cholesterol entering the HDL-C pool from the liver and intestine must be very small and essentially undetectable. Nevertheless, this role of ABCA1 in protecting the apoAI synthesized in the liver and intestine is of critical importance to the metabolism of this lipoprotein. In the absence of this function, the marked abnormalities in HDL turnover seen in the abca1−/− mouse and in humans with Tangier disease occurs.
Apart from these important functions of ABCA1 in protecting apoAI and in removing cholesterol from macrophages, these studies provide additional information on three other aspects of cholesterol metabolism in these animals. First, the abca1−/− mice have lower plasma LDL-C concentrations and elevated triacylglycerol levels, as do patients with Tangier disease. In this study, the LDL-C production rate in the abca1−/− mice was only about half (24.6 mg/day/kg) of that found in the control mice (50.5 mg/day/kg) (Fig. 4I), suggesting that this was the cause of the lower circulating LDL-C level. While this low production rate could have resulted from either reduced hepatic secretion of cholesterol carried in VLDL or a decrease in the proportion of VLDL metabolized to LDL, the fact that the plasma concentrations of lipoproteins in the d < 1.020 g/ml fraction and triacylglycerol were both elevated suggests that the latter explanation is correct. Conceivably, the very low HDL levels seen in these mice in some manner alter the rate of VLDL conversion to LDL.
Second, ABCA1 presumably functions on the plasma membrane to carry out its roles in removing cholesterol from the macrophage and in transferring sterol to apoAI from the liver and intestinal cells. Other data, however, have suggested that this protein cycles into the late endosomal/lysosomal compartment of cells (59) and may also actually mediate cholesterol movement from this organelle into the cytosolic compartment (60, 61). Nearly every cell takes up lipoproteins containing either apoB100 or apoE through receptor-mediated and bulk-phase endocytosis and processes the cholesteryl ester in these particles to unesterified cholesterol in the lysosome (11). This unesterified sterol is then transferred to the metabolically active pool of cholesterol in the cytosol through the concerted actions of two proteins, Niemann-Pick type C1 (NPC1) and NPC2 (62–64). Mutations that inactivate either NPC1 or NPC2 lead to an age-dependent accumulation of unesterified cholesterol and an increase in the rate of cholesterol synthesis in nearly every tissue in the body and to a massive expansion of the whole animal cholesterol pool (65, 66). Importantly, none of these metabolic changes occurred in the abca1−/− mice (Figs. 2, 3), indicating that ABCA1 does not play a detectable role in the intracellular movement of sterol from the endosomal/lysosomal compartment to the cytosol.
Third, there are now four syndromes where disordered intracellular cholesterol metabolism leads to tissue infiltration and activation of macrophages. Mutations of lysosomal acid lipase cause the accumulation of cholesteryl esters in lysosomes of these cells (67), while unesterified cholesterol accumulates in this organelle with mutations in NPC1 or NPC2. The macrophages of the abca1−/− mice accumulate cholesteryl ester in the cytosolic compartment. In all of these situations, there is apparently activation of the macrophages with the production of many proinflammatory factors, such as tumor necrosis factor-α and interleukin-6 (53, 68). It would be of considerable interest, therefore, to explore the potential role of these activated macrophages in the parenchymal cell death seen in the arterial wall in Tangier disease, the liver and brain in NPC disease, and in various organs in Wolman disease. It may be of fundamental importance to understanding the pathogenesis of these diseases to elucidate how disordered, intracellular sterol metabolism leads to this activation and proinflammatory protein production in macrophages.
In summary, while ABCA1 plays a critical role in preserving apoAI and in removing cholesterol from macrophages, it appears to play no role in controlling the major centripetal flux of sterol from the peripheral organs to the endocrine glands and the liver. It seems likely that the concept that movement of cholesterol through the cell membrane is controlled by this protein and by the concentration of HDL in the pericellular fluid is incorrect. The process of cholesterol turnover in the plasma membrane may be so important to the integrity of the cell that it is controlled by events within the cell rather than by the lipoproteins present in the pericellular fluid. However, what process overcomes the activation energy for desorption of the sterol molecule from the outer leaflet of the membrane and what vehicle moves this cholesterol to the sites of degradation in the absence of HDL remain to be elucidated.
Acknowledgments
The authors express their appreciation to Elizabeth Moore, Amanda Fletcher, Brian Cady, Stephen Ostermann, Monti Schneiderman, and Sean Campbell for their excellent technical assistance and to Kerry Foreman for expert preparation of the manuscript. The authors are also indebted to Dr. Christiansen-Weber for providing us with the breeding stock of abca1+/− mice and to Prof. Gerd Assmann for providing unpublished data on fecal sterol output in a patient with Tangier disease.
Abbreviations
apoAI, apolipoprotein AI
CETP, cholesteryl ester transfer protein
HDL-C, cholesterol carried in high density lipoprotein
LDL-C, cholesterol carried in low density lipoprotein
LDLR, low density lipoprotein receptor
NPC, Niemann-Pick type C
This work was supported by U.S. Public Health Service Grant R01 HL09610 (J.M.D. and S.D.T.) and by a grant from the Moss Heart Fund (J.M.D.).
Published, JLR Papers in Press, March 12, 2009.
References
- 1.Simons K., and E. Ikonen. 2000. How cells handle cholesterol. Science. 290 1721–1726. [DOI] [PubMed] [Google Scholar]
- 2.Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. D. Watson. 1989. The plasma membrane. In Molecular Biology of the Cell. M. Robertson, editor. Garland Publishing, New York. 275–340.
- 3.Dietschy J. M., T. Kita, K. E. Suckling, J. L. Goldstein, and M. S. Brown. 1983. Cholesterol synthesis in vivo and in vitro in the WHHL rabbit, an animal with defective low density lipoprotein receptors. J. Lipid Res. 24 469–480. [PubMed] [Google Scholar]
- 4.Belknap W. M., and J. M. Dietschy. 1988. Sterol synthesis and low density lipoprotein clearance in vivo in the pregnant rat, placenta, and fetus. J. Clin. Invest. 82 2077–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quan G., C. Xie, J. M. Dietschy, and S. D. Turley. 2003. Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res. Dev. Brain Res. 146 87–98. [DOI] [PubMed] [Google Scholar]
- 6.Salen G., S. Shefer, A. K. Batta, G. S. Tint, G. Xu, A. Honda, M. Irons, and E. R. Elias. 1996. Abnormal cholesterol biosynthesis in the Smith-Lemli-Opitz syndrome. J. Lipid Res. 37 1169–1180. [PubMed] [Google Scholar]
- 7.Irons M., E. R. Elias, G. S. Tint, G. Salen, R. Frieden, T. M. Buie, and M. Ampola. 1994. Abnormal cholesterol metabolism in the Smith-Lemli-Opitz syndrome: report of clinical and biochemical findings in four patients and treatment in one patient. Am. J. Med. Genet. 50 347–352. [DOI] [PubMed] [Google Scholar]
- 8.Ohashi K., J. Osuga, R. Tozawa, T. Kitamine, H. Yagyu, M. Sekiya, S. Tomita, H. Okazaki, Y. Tamura, N. Yahagi, et al. 2003. Early embryonic lethality caused by targeted disruption of the 3-hydroxy-3-methylglutaryl-CoA reductase gene. J. Biol. Chem. 278 42936–42941. [DOI] [PubMed] [Google Scholar]
- 9.Turley S. D., D. K. Spady, and J. M. Dietschy. 1997. Identification of a metabolic difference accounting for the hyper- and hyporesponder phenotypes of cynomolgus monkey. J. Lipid Res. 38 1598–1611. [PubMed] [Google Scholar]
- 10.Li H., S. D. Turley, B. Liu, J. J. Repa, and J. M. Dietschy. 2008. GM2/GD2 and GM3 gangliosides have no effect on cellular cholesterol pools or turnover in normal or NPC1 mice. J. Lipid Res. 49 1816–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu B., C. Xie, J. A. Richardson, S. D. Turley, and J. M. Dietschy. 2007. Receptor-mediated and bulk-phase endocytosis cause macrophage and cholesterol accumulation in Niemann-Pick C disease. J. Lipid Res. 48 1710–1723. [DOI] [PubMed] [Google Scholar]
- 12.Brown M. S., and J. L. Goldstein. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science. 232 34–47. [DOI] [PubMed] [Google Scholar]
- 13.Dietschy, J. M. 2009. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol. Chem. In press. [DOI] [PMC free article] [PubMed]
- 14.Assmann G., O. Simantke, H-E. Schaefer, and E. Smootz. 1977. Characterization of high density lipoproteins in patients heterozygous for Tangier disease. J. Clin. Invest. 60 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks-Wilson A., M. Marcil, S. M. Clee, L-H. Zhang, K. Roomp, M. van Dam, L. Yu, C. Brewer, J. A. Collins, H. O. F. Molhuizen, et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22 336–345. [DOI] [PubMed] [Google Scholar]
- 16.Bodzioch M., E. Orsó, J. Klucken, T. Langmann, A. Böttcher, W. Diederich, W. Drobnik, S. Barlage, C. Büchler, M. Porsch-Özcürümez, et al. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22 347–351. [DOI] [PubMed] [Google Scholar]
- 17.Rust S., M. Rosier, H. Funke, J. Real, Z. Amoura, J-C. Piette, J-F. Deleuze, H. B. Brewer, N. Duverger, P. Denèfle, et al. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22 352–355. [DOI] [PubMed] [Google Scholar]
- 18.Lawn R. M., D. P. Wade, M. R. Garvin, X. Wang, K. Schwartz, J. G. Porter, J. J. Seilhamer, A. M. Vaughan, and J. F. Oram. 1999. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Invest. 104 R25–R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tall A. R., N. Wang, and P. Mucksavage. 2001. Is it time to modify the reverse cholesterol transport model? J. Clin. Invest. 108 1273–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groen A. K., R. P. J. O. Elferink, H. J. Verkade, and F. Kuipers. 2004. The ins and outs of reverse cholesterol transport. Ann. Med. 36 135–145. [DOI] [PubMed] [Google Scholar]
- 21.Attie A. D., J. P. Kastelein, and M. R. Hayden. 2001. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J. Lipid Res. 42 1717–1726. [PubMed] [Google Scholar]
- 22.McNeish J., R. J. Aiello, D. Guyot, T. Turi, C. Gabel, C. Aldinger, K. L. Hoppe, M. L. Roach, L. J. Royer, J. de Wet, et al. 2000. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl. Acad. Sci. USA. 97 4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christiansen-Weber T. A., J. R. Voland, Y. Wu, K. Ngo, B. L. Roland, S. Nguyen, P. A. Peterson, and W-P. Fung-Leung. 2000. Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am. J. Pathol. 157 1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drobnik W., B. Lindenthal, B. Lieser, M. Ritter, T. Christiansen Weber, G. Liebisch, U. Giesa, M. Igel, H. Borsukova, C. Büchler, et al. 2001. ATP-binding cassette transporter A1 (ABCA1) affects total body sterol metabolism. Gastroenterology. 120 1203–1211. [DOI] [PubMed] [Google Scholar]
- 25.Groen A. K., V. W. Bloks, R. H. J. Bandsma, R. Ottenhoff, G. Chimini, and F. Kuipers. 2001. Heptobiliary cholesterol transport is not impaired in Abca1-null mice lacking HDL. J. Clin. Invest. 108 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosters A., R. J. J. M. Frijters, F. G. Schaap, E. Vink, T. Plösch, R. Ottenhoff, M. Jirsa, I. M. De Cuyper, F. Kuipers, and A. K. Groen. 2003. Relation between hepatic expression of ATP-binding cassette transporters G5 and G8 and biliary cholesterol secretion in mice. J. Hepatol. 38 710–716. [DOI] [PubMed] [Google Scholar]
- 27.Turley S. D., D. K. Spady, and J. M. Dietschy. 1995. Role of liver in the synthesis of cholesterol and the clearance of low density lipoproteins in the cynomolgus monkey. J. Lipid Res. 36 67–79. [PubMed] [Google Scholar]
- 28.Osono Y., L. A. Woollett, J. Herz, and J. M. Dietschy. 1995. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J. Clin. Invest. 95 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glass C., R. C. Pittman, D. B. Weinstein, and D. Steinberg. 1983. Dissociation of tissue uptake of cholesterol ester from that of apoprotein A-I of rat plasma high density lipoprotein: selective delivery of cholesterol ester to liver, adrenal, and gonad. Proc. Natl. Acad. Sci. USA. 80 5435–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisgraber K. H., and R. W. Mahley. 1980. Subfractionation of human high density lipoproteins by heparin-Sepharose affinity chromatography. J. Lipid Res. 21 316–325. [PubMed] [Google Scholar]
- 31.Hough J. L., and D. B. Zilversmit. 1984. Comparison of various methods for in vitro cholesteryl ester labeling of lipoproteins from hypercholesterolemic rabbits. Biochim. Biophys. Acta. 792 338–347. [DOI] [PubMed] [Google Scholar]
- 32.Pittman R. C., T. P. Knecht, M. S. Rosenbaum, and C. A. Taylor, Jr. 1987. A nonendocytotic mechanism for the selective uptake of high density lipoprotein-associated cholesterol esters. J. Biol. Chem. 262 2443–2450. [PubMed] [Google Scholar]
- 33.Woollett L. A., and D. K. Spady. 1997. Kinetic parameters for high density lipoprotein apoprotein AI and cholesteryl ester transport in the hamster. J. Clin. Invest. 99 1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spady D. K., D. W. Bilheimer, and J. M. Dietschy. 1983. Rates of receptor-dependent and -independent low density lipoprotein uptake in the hamster. Proc. Natl. Acad. Sci. USA. 80 3499–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie C., S. D. Turley, and J. M. Dietschy. 1999. Cholesterol accumulation in tissues of the Niemann-Pick type C mouse is determined by the rate of lipoprotein-cholesterol uptake through the coated-pit pathway in each organ. Proc. Natl. Acad. Sci. USA. 96 11992–11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turley S. D., J. M. Andersen, and J. M. Dietschy. 1981. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J. Lipid Res. 22 551–569. [PubMed] [Google Scholar]
- 37.Dietschy J. M., and D. K. Spady. 1984. Measurement of rates of cholesterol synthesis using tritiated water. J. Lipid Res. 25 1469–1476. [PubMed] [Google Scholar]
- 38.Schwarz M., D. W. Russell, J. M. Dietschy, and S. D. Turley. 1998. Marked reduction in bile acid synthesis in cholesterol 7α-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J. Lipid Res. 39 1833–1843. [PubMed] [Google Scholar]
- 39.Repa J. J., S. D. Turley, G. Quan, and J. M. Dietschy. 2005. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. J. Lipid Res. 46 779–789. [DOI] [PubMed] [Google Scholar]
- 40.Turley S. D., M. W. Herndon, and J. M. Dietschy. 1994. Reevaluation and application of the dual-isotope plasma ratio method for the measurement of intestinal cholesterol absorption in the hamster. J. Lipid Res. 35 328–339. [PubMed] [Google Scholar]
- 41.Jolley C. D., L. A. Woollett, S. D. Turley, and J. M. Dietschy. 1998. Centripetal cholesterol flux to the liver is dictated by events in the peripheral organs and not by the plasma high density lipoprotein or apolipoprotein A-I concentration. J. Lipid Res. 39 2143–2149. [PubMed] [Google Scholar]
- 42.Osono Y., L. A. Woollett, K. R. Marotti, G. W. Melchior, and J. M. Dietschy. 1996. Centripetal cholesterol flux from extrahepatic organs to the liver is independent of the concentration of high density lipoprotein-cholesterol in plasma. Proc. Natl. Acad. Sci. USA. 93 4114–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spady D. K., and J. M. Dietschy. 1983. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J. Lipid Res. 24 303–315. [PubMed] [Google Scholar]
- 44.Chapman M. J. 1986. Comparative analysis of mammalian plasma lipoproteins. Methods Enzymol. 128 70–143. [DOI] [PubMed] [Google Scholar]
- 45.Li J. R., D. M. Dinh, and B. A. Kottke. 1979. Alteration of biliary ursodeoxycholic acid in guinea pig during early stages of cholestyramine feeding. Steroids. 34 705–715. [DOI] [PubMed] [Google Scholar]
- 46.Bortnick A. E., G. H. Rothblat, G. Stoudt, K. L. Hoppe, L. J. Royer, J. McNeish, and O. L. Francone. 2000. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J. Biol. Chem. 275 28634–28640. [DOI] [PubMed] [Google Scholar]
- 47.Haghpassand M., P-A. K. Bourassa, O. L. Francone, and R. J. Aiello. 2001. Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels. J. Clin. Invest. 108 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer E. J., T. J. Triche, L. A. Zech, L. A. Stein, M. M. Kemeny, M. F. Brennan, and H. B. Brewer, Jr. 1983. Massive omental reticuloendothelial cell lipid uptake in Tangier disease after splenectomy. Am. J. Med. 75 521–526. [DOI] [PubMed] [Google Scholar]
- 49.Hobbs H. H., and D. J. Rader. 1999. ABC1: connecting yellow tonsils, neuropathy, and very low HDL. J. Clin. Invest. 104 1015–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldorf D. S., R. I. Levy, and D. S. Fredrickson. 1967. Cutaneous cholesterol ester deposition in Tangier disease. Arch. Dermatol. 95 161–165. [PubMed] [Google Scholar]
- 51.Wright J. R., and D. C. Youmans. 1995. Degradation of surfactant lipids and surfactant protein A by alveolar macrophages in vitro. Am. J. Physiol. 268 L772–L780. [DOI] [PubMed] [Google Scholar]
- 52.Lee S. H., P. M. Starkey, and S. Gordon. 1985. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J. Exp. Med. 161 475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu X., J-Y. Lee, J. M. Timmins, J. M. Brown, E. Boudyguina, A. Mulya, A. K. Gebre, M. C. Willingham, E. M. Hiltbold, N. Mishra, et al. 2008. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 283 22930–22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu A-L., and H. G. Windmueller. 1979. Relative contributions by liver and intestine to individual plasma apolipoproteins in the rat. J. Biol. Chem. 254 7316–7322. [PubMed] [Google Scholar]
- 55.Glickman R. M., and P. H. R. Green. 1977. The intestine as a source of apolipoprotein A1. Proc. Natl. Acad. Sci. USA. 74 2569–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horowitz B. S., I. J. Goldberg, J. Merab, T. M. Vanni, R. Ramakrishnan, and H. N. Ginsberg. 1993. Increased plasma and renal clearance of an exchangeable pool of apolipoprotein A-I in subjects with low levels of high density lipoprotein cholesterol. J. Clin. Invest. 91 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timmins J. M., J-Y. Lee, E. Boudyguina, K. D. Kluckman, L. R. Brunham, A. Mulya, A. K. Gebre, J. M. Coutinho, P. L. Colvin, T. L. Smith, et al. 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brunham L. R., J. K. Kruit, J. Iqbal, C. Fievet, J. M. Timmins, T. D. Pape, B. A. Coburn, N. Bissada, B. Staels, A. K. Groen, et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neufeld E. B., A. T. Remaley, S. J. Demosky, J. A. Stonik, A. M. Cooney, M. Comly, N. K. Dwyer, M. Zhang, J. Blanchette-Mackie, S. Santamarina-Fojo, et al. 2001. Cellular localization and trafficking of the human ABCA1 transporter. J. Biol. Chem. 276 27584–27590. [DOI] [PubMed] [Google Scholar]
- 60.Chen W., N. Wang, and A. R. Tall. 2005. A PEST deletion mutant of ABCA1 shows impaired internalization and defective cholesterol efflux from late endosomes. J. Biol. Chem. 280 29277–29281. [DOI] [PubMed] [Google Scholar]
- 61.Subramanian K., and W. E. Balch. 2008. NPC1/NPC2 function as a tag team duo to mobilize cholesterol. Proc. Natl. Acad. Sci. USA. 105 15223–15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carstea E. D., J. A. Morris, K. G. Coleman, S. K. Loftus, D. Zhang, C. Cummings, J. Gu, M. A. Rosenfeld, W. J. Pavan, D. B. Krizman, et al. 1997. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 277 228–231. [DOI] [PubMed] [Google Scholar]
- 63.Naureckiene S., D. E. Sleat, H. Lackland, A. Fensom, M. T. Vanier, R. Wattiaux, M. Jadot, and P. Lobel. 2000. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 290 2298–2301. [DOI] [PubMed] [Google Scholar]
- 64.Infante R. E., M. L. Wang, A. Radhakrishnan, H. J. Kwon, M. S. Brown, and J. L. Goldstein. 2008. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc. Natl. Acad. Sci. USA. 105 15287–15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie C., S. D. Turley, P. G. Pentchev, and J. M. Dietschy. 1999. Cholesterol balance and metabolism in mice with loss of function of Niemann-Pick C protein. Am. J. Physiol. 276 E336–E344. [DOI] [PubMed] [Google Scholar]
- 66.Liu B., S. D. Turley, D. K. Burns, A. M. Miller, J. J. Repa, and J. M. Dietschy. 2009. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc. Natl. Acad. Sci. USA. 106 2377–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du H., M. Duanmu, D. Witte, and G. A. Grabowski. 1998. Targeted disruption of the mouse lysosomal acid lipase gene: long-term survival with massive cholesteryl ester and triglyceride storage. Hum. Mol. Genet. 7 1347–1354. [DOI] [PubMed] [Google Scholar]
- 68.Repa J. J., H. Li, T. C. Frank-Cannon, M. A. Valasek, S. D. Turley, M. G. Tansey, and J. M. Dietschy. 2007. Liver X receptor activation enhances cholesterol loss from the brain, decreases neuroinflammation, and increases survival of the NPC1 mouse. J. Neurosci. 27 14470–14480. [DOI] [PMC free article] [PubMed] [Google Scholar]