Abstract
The nuclear liver X receptor (LXR) regulates multiple aspects of cholesterol, triacylglycerol (TG), and carbohydrate metabolism. Activation of LXR induces the expression of genes encoding enzymes involved in de novo lipogenesis (DNL) resulting in hepatic steatosis in mice. Pharmacological LXR activation has also been reported to improve insulin sensitivity and glucose homeostasis in diabetic rodents. The effects of pharmacological LXR ligands on insulin's action on hepatic lipid metabolism are not known. We evaluated secretion of VLDL during a hyperinsulinemic euglycemic clamp in mice treated with the LXR-ligand T0901317. In untreated mice, hyperinsulinemia reduced the availability of plasma NEFA for VLDL-TG synthesis, increased the contribution of DNL to VLDL-TG, reduced VLDL particle size, and suppressed overall VLDL-TG production rate by approximately 50%. Upon T0901317 treatment, hyperinsulinemia failed to reduce VLDL particle size or suppress VLDL-TG production rate, but the contribution of DNL to VLDL-TG was increased. In conclusion, the effects of LXR activation by T0901317 on lipid metabolism can override the normal control of insulin to suppress VLDL particle secretion.
Keywords: apolipoprotein B, de novo lipogenesis, FoxO1, LXR, microsomal triglyceride transfer protein, stable isotopes, sterol regulatory element-binding protein-1c, T0901317
The liver X receptor (LXR) α and β are oxysterol-sensing nuclear receptors which, upon activation, induce the transcription of genes encoding proteins involved in reverse cholesterol transport (1). Among these genes is the one encoding the cholesterol transporter ATP binding cassette A1 (ABCA1), a protein involved in efflux of cholesterol by macrophages to nascent HDL particles (2–5). In the rodent liver, LXRs also regulate the transcription of Cyp7a1 (6, 7), which encodes the rate-limiting enzyme in the conversion of cholesterol into bile acids. As a result, pharmacological LXR ligands are beneficial in the prevention of atherosclerosis, as has been shown in mice deficient for the LDL receptor (Ldlr−/−) (8, 9) and apolipoprotein E (Apoe−/− mice) (8).
However, the application of an LXR agonist as an anti-atherosclerotic drug is hampered by its detrimental effects on fatty acid metabolism. In vivo studies have shown that pharmacological LXR activation results in significant accumulation of triacylglycerols (TG) in the liver (10–12). This fatty liver results from the induction of genes involved in de novo lipogenesis (DNL) and the genes encoding sterol regulatory element-binding protein-1c (SREBP-1c), acetyl-CoA carboxylase-1 (ACC1), and fatty acid synthase (FAS), all of which are established LXR targets (12–16). Upon translocation to the nucleus, SREBP-1c itself independently stimulates transcription of genes involved in DNL (17). Moreover, transcription of the gene encoding carbohydrate-response element-binding protein (ChREBP) is enhanced upon LXR activation (18). Acc1 and Fas are also independently activated by ChREBP (19, 20).
The pharmacological LXR agonist T0901317 has also been shown to stimulate the secretion of large, TG-rich VLDL particles from liver (10). VLDL synthesis and secretion is a two-step process that takes place in distinct compartments of the liver cell, both of which involve the action of the microsomal triglyceride transfer protein (MTP). First, apolipoprotein B (apoB) molecules become lipidated to form a small pre-VLDL particle in the rough endoplasmic reticulum (ER) (21). Next, this pre-VLDL particle is transported to the smooth ER for further lipidation, moved to the cellular membrane, and then released from the cell. Thus, the observed production of large, TG-rich VLDL particles might very well be the result of increased MTP activity, even though Mtp mRNA levels may not be increased by T0901317 treatment (10).
With respect to glucose metabolism, LXR activation is thought to have anti-diabetic actions presumably via transcriptional reduction of the liver gluconeogenic gene encoding phosphoenolpyruvate carboxykinase (PEPCK) (22, 23). In contrast, in vivo studies using the hyperinsulinemic euglycemic clamp showed that the LXR agonist GW3965 slightly improved peripheral, but not hepatic, insulin sensitivity in leptin-deficient ob/ob mice (11). The insulin-induced metabolic clearance rate of glucose was improved in GW3965-fed mice, but the treatment failed to enhance insulin's suppression of hepatic glucose production.
In normal mice, acute insulin infusion reduces VLDL-TG production rate (24), albeit more insulin is needed to suppress VLDL secretion than hepatic glucose production (25). Studies performed in vitro have shown that insulin is able to inhibit VLDL release via acceleration of apoB degradation (26), and as a result, insulin reduces the number of VLDL particles secreted. Moreover, insulin inhibits the transcription of the gene Mtp (27, 28), probably via Akt-mediated phosphorylation and deactivation of the forkhead transcription factor FoxO1 (29). Reduction in MTP activity would reduce transfer of TG to nascent apoB, which could result in smaller particles formed and less TG secreted. In contrast, one could argue that an increased availability of hepatic TGs would stimulate hepatic VLDL production. However, various observations suggest that increased hepatic TG content per se does not stimulate hepatic VLDL production. For instance, ob/ob mice show severe hepatic steatosis and increased DNL but do not have increased VLDL-TG production under basal conditions (30). Indeed, hyperinsulinemia could not suppress VLDL-TG secretion in ob/ob mice. An inability to secrete VLDL, however, does result in hepatic steatosis, as has been shown in mice with absent liver Mtp (31). In humans, production of TG-rich VLDL particles has been observed in hyperinsulinemic patients, and metabolic studies have demonstrated that insulin fails to suppress the secretion of large, TG-rich VLDL particles in participants with elevated liver fat and type 2 diabetes (32).
Thus, both elevations in insulin and excess TG availability may impact VLDL-TG secretion and VLDL particle size. The effect of insulin to reduce VLDL-TG secretion may be counter-balanced by both TG synthesis and expanded hepatic TG stores, which may drive VLDL particle assembly and secretion. The present study was designed to test the strength of insulin's suppression of particle secretion against the propensity of an LXR agonist to cause steatosis and increase TG release rates. Using a clamp in combination with stable isotope infusion demonstrated that the LXR-mediated effects to stimulate VLDL secretion could not be dampened even under the extended, controlled conditions of a hyperinsulinemic euglycemic clamp.
MATERIALS AND METHODS
Animals and diets
All experiments were performed in male C57BL/6J mice and were approved by the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center. The animals were fed Teklad Mouse/Rat diet 7002 (Harlan Teklad Premier Laboratory Diets) supplemented with or without 0.025% w/w T0901317 for six days. On the morning of the seventh day, mice were fasted for 5 h and subsequently subjected to one of the following experiments: 1) collection of basal plasma and liver samples; 2) a hyperinsulinemic euglycemic clamp; 3) VLDL-TG production rate determination under hyperinsulinemic euglycemic conditions; or 4) determination of the contribution of newly made fatty acids to VLDL-TG under clamp and control conditions using stable isotope infusion.
Collection of basal plasma and liver samples
For basal plasma and liver data, mice were killed by isoflurane overdose, and their blood and livers were collected. Plasma was isolated by centrifugation. Plasma and liver cholesterol and TG concentrations were measured as described (33). Total RNA was prepared from individual mouse livers, and real-time RT-PCR was performed as described (33). Specific primers for each gene were designed by using primer express software (PE Biosystems, Foster City, CA). The relative amounts of all mRNAs were calculated by using the Comparative CT method (User Bulletin no. 2, PE Applied Biosystems). Cyclophilin mRNA was used as the invariant control. The primers for real-time PCR were described previously (34, 35).
Hyperinsulinemic euglycemic clamp
In the hyperinsulinemic euglycemic clamp studies, mice were anesthetized with sodium pentobarbital and infused with either a basal solution or with the insulin and glucose solution via the right jugular vein. The basal solution contained 0.5% BSA and was infused at a rate of 0.45 ml/h. The insulin infusion contained 220 mU/ml insulin, 40 μg/ml somatostatin, and 1% BSA infused with a rate of 0.135 ml/h. The glucose solution was a 15% glucose solution; the infusion rate depended on the blood glucose levels measured. Before the start of the infusion and every 15 min thereafter, blood glucose levels were measured with a handheld glucose meter in a small blood drop collected from the tail vein. In the mice infused with the insulin and glucose solution, the infusion rate of the glucose solution was adjusted to maintain euglycemic conditions. After 3 h of infusion, the mice were killed by cardiac puncture, and their blood and livers were collected.
VLDL-TG production rate determination under hyperinsulinemic euglycemic conditions
The same infusion protocol as described for the hyperinsulinemic euglycemic clamp experiments was used. After 1.5 h of infusion, 12.5 mg Triton WR-1339 in 100 μl PBS was injected in the left jugular vein. Small blood samples were collected from the tail before and 30, 60, and 90 min after Triton WR-1339 injection. A large blood sample was collected by cardiac puncture 90 min after Triton WR-1339 injection. The collected blood samples were used for TG measurements as described (33), and the VLDL-TG production rate was calculated from the slope of the plasma TG versus time curve.
Plasma VLDL was isolated by density gradient ultracentrifugation. Plasma (300 μl) was adjusted to 1 ml with a NaCL/KBr solution (density 1.006 g/ml), centrifuged at 100,000 rpm for 150 min and the top layer (VLDL) was isolated. VLDL-TG concentration was measured as described (33). VLDL phospholipids and total and free cholesterol were determined using commercially available kits (Wako Chemicals, Richmond, VA). The VLDL particle size was calculated using the equation according to Fraser (36) and Harris et al. (37) in which the diameter (nm) = 60 × [(0.211 × TG/PL) + 0.27)]. Per condition, volumes of VLDL containing equal amounts of TG were pooled, and lipids were extracted with methanol and cold ether. The remaining VLDL proteins were dissolved in SDS loading buffer (50 mM Tris-chloride, pH 6.8; 5.5% β-mercaptoethanol; 7% SDS; 2.5% sodium myristyl sulfate; 0.1% bromophenol blue; 10% glycerol), boiled for 10 min and subjected to electrophoresis on 4.5% SDS gels. ApoB100 and apoB48 were determined using antibodies against human apoB raised in rabbit and horseradish peroxidase-conjugated anti-rabbit from donkey (Amersham Pharmacia Bioscience, GE Health Care Life Sciences, Piscataway, NJ) and SuperSignal West Pico Chemiluminescent Substrate System (Pierce, Rockford, IL).
Determination of the contribution of DNL-derived fatty acids to VLDL-TG under hyperinsulinemic euglycemic conditions
The same infusion protocol as described for the hyperinsulinemic euglycemic clamp experiments was used. To the basal solution, 0.4 mg/ml [1-13C]acetate was added. To the insulin solution a greater amount of [1-13C]acetate (1.2 mg/ml) was added given the approximately 3-fold difference in infusion rate between the two solutions. The mice were killed by cardiac puncture after 3 h of infusion, and their blood was collected.
Plasma VLDL was isolated as described above. VLDL lipids were isolated, TG separated, and fatty acid methyl esters prepared as previously described (38). GC-MS analysis was performed (39) using palmitic acid (16:0) as the marker of fatty acid synthesis. Comparable ion peak areas between standards and biological samples were achieved by either adjustment of the volume injected or by dilution or concentration of the sample. By tracking the variously labeled methyl-palmitate isotopomers (M0, M1, and M2) in VLDL-TG using GC-MS, information regarding the relative contribution of DNL used for VLDL-TG synthesis was obtained using mass isotopomer distribution analysis (MIDA) (39).
Microsomal triglyceride transfer protein activity assay
The activity of the MTP was determined as described (40) with slight modifications. Briefly, liver tissue was homogenized in ice-cold assay buffer (10 mM Tris, 150 mM NaCl2, 2 mM EDTA, pH 7.4) with protease inhibitors (Complete, Roche Diagnostics, Mannheim, Germany) and sonicated. Protein concentrations were measured using the BCA method (Pierce) and adjusted with assay buffer to get approximate 1.5 μg/μl. In a black fluorescence 96-well plate, 10 μl of homogenate was added to each well with 5 μl donor vesicles, 5 μl acceptor vesicles (from the Roar MTP Activity Assay Kit, Roar Biomedical, New York, NY), and 180 μl assay buffer. After 3–6 h of incubation at 37°C, the fluorescence (excitation 465 nm, emission 535 nm) was measured and corrected for the blank measurement.
Immunoblotting procedures
Nuclear SREBP-1 and FoxO1 levels were measured in nuclear fractions from pieces of frozen liver that were homogenized in 1.5 ml homogenization buffer (20 mM Tris-chloride, pH 7.4; 2 mM MgCl2; 0.25 M sucrose; 10 mM Na-EDTA; 10 mM Na-EGTA) supplemented with protease inhibitors (5 mM dithiothreitol; 0.1 mM leupeptin; 1 mM phenylmethylsulfonyl fluoride; 0.5 mM Pefabloc; 5 μg/ml pepstatin A; 25 μg/ml N-acetyl-leu-leu-norleucinal; 10 μg/ml aprotinin). The liver homogenate was centrifuged at 2,500 g for 5 min at 4°C. The pellet was washed in 1 ml of homogenization buffer and collected by centrifugation at 1,000 g for 5 min at 4°C. The nuclear pellet was resuspended in 0.3 ml of buffer (20 mM HEPES-KOH, pH 7.6; 2.5% glycerol; 0.42 M NaCl; 1.5 mM MgCl2; 1 mM Na-EDTA; 1 mM Na-EGTA) supplemented with the protease inhibitors described above. The suspension was rotated at 4°C for 1 h and centrifuged at 20,000 g for 20 min at 4°C to collect the nuclear extract proteins in the supernatant. Akt phosphorylation was measured in liver lysates made in buffer (30 mM Tris·Cl, pH 7.4; 2.5 mM EDTA, pH 8.0; 150 mM NaCl; 0.5 mM Na3VO4; 5 mM NaF; 5 mM MgCl2; 1.3 M glycerol) and protease inhibitors (Complete, Roche Diagnostics) and cleared by centrifugation.
The protein concentrations of the nuclear extracts and hepatic lysates were measured with the BCA Kit (Pierce). Equal amounts of protein from nuclear extracts of 4 mice per group were pooled. The pooled sample was mixed with 4 × SDS loading buffer (0.15 M Tris-chloride, pH 6.8; 12% SDS; 0.02% bromophenol blue; 30% glycerol; 6% β-mercaptoethanol) and equal amounts of protein per sample were subjected to SDS-PAGE and immunoblot analysis (41). SREBP-1, FoxO1, FoxO1-Ser245P, total Akt, Akt-Ser473P, MTP, the loading controls cAMP-responsive element-binding protein (CREB), and actin were determined using antibodies against mouse SREBP-1 raised in rabbit (42); anti-FoxO1 and anti–FoxO1-Ser256P raised in rabbit (Abcam, Cambridge, UK); anti-Akt and anti–Akt-S473P raised in rabbit (Cell Signaling, Beverly, MA); anti-MTP raised in mouse (BD Biosciences, San Jose, CA); anti-CREB raised in rabbit (Zymed, San Francisco, CA); and anti-actin raised in rabbit (Sigma, St. Louis, MO). Finally, horseradish peroxidase–conjugated anti-rabbit from donkey (Amersham Pharmacia Bioscience) and SuperSignal West Pico Chemiluminescent Substrate System (Pierce) were used.
Statistics
Unless otherwise state, data are presented as mean ± SD. Differences between groups were tested using a general linear model with corrections for multiple comparisons; P < 0.05 was considered significant when two groups were compared; P < 0.008 was considered significant when four groups were compared.
RESULTS
Increased liver TG and MTP activity following T0901317 treatment
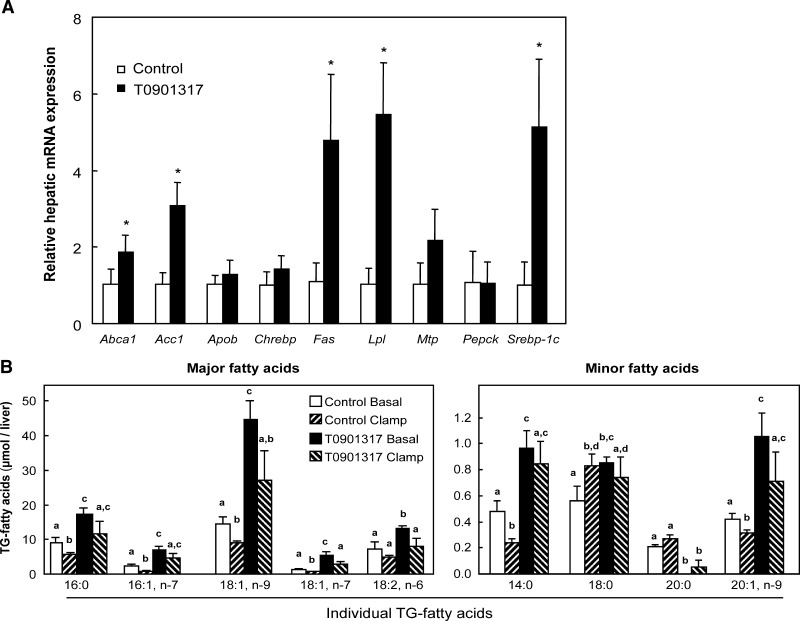
The administration of the LXR agonist T0901317 to mice for one week resulted in increased concentrations of plasma cholesterol and liver TG (Table 1), similar to that published previously with LXR agonists (10, 12, 43). The expression of the LXR target genes Abca1, Srebp-1c, Acc1, and Fas was significantly increased upon T0901317 treatment (Fig. 1A). In contrast to a previous publication (18), expression of Chrebp was not increased upon LXR activation in liver. Expression of lipoprotein lipase (Lpl) was increased approximately 6-fold. LXR activation only slightly induced Mtp mRNA levels but had no effect on Apob expression. This latter observation, in combination with the results of our previous study in which T0901317 treatment resulted in the secretion of large, TG-rich VLDL particles (10), prompted us to measure hepatic MTP activity. We found that total hepatic MTP activity was significantly increased following one week of T0901317 administration (Table 1). The composition and content of fatty acids in the liver-TG pool reflected the ability of T0901317 to increase both fatty acid synthesis and the desaturation enzymes (Fig. 1B, Control Basal versus T0901317 Basal).
TABLE 1.
Plasma and liver parameters of 5-h fasted mice fed a control or 0.025% w/w T0901317 diet for one week
| Control | T0901317 | |
|---|---|---|
| Plasma triacylglycerols (mg/dl) | 79.5 ± 5.1 | 84.6 ± 5.1 |
| Plasma cholesterol (mg/dl) | 90.8 ± 14.0a | 126 ± 9.4b |
| Plasma NEFA (mEq/l) | 0.558 ± 0.090 | 0.547 ± 0.098 |
| Blood glucose (mg/dl) | 103 ± 14 | 114 ± 12 |
| Plasma insulin (ng/ml) | 1.15 ± 0.54 | 1.44 ± 0.37 |
| Liver weight (% of bodyweight) | 5.2 ± 0.4a | 5.9 ± 0.4b |
| Liver triacylglycerols (mg/g) | 8.0 ± 4.7a | 25.4 ± 7.1b |
| Liver cholesterol (mg/g) | 2.4 ± 0.2 | 2.1 ± 0.2 |
| Liver proteins (mg/g) | 184 ± 15 | 192 ± 8a |
| Liver MTP activity (pmol/μg protein) | 4.3 ± 2.4 | 6.5 ± 0.7 |
| Liver MTP activity (μmol/liver) | 1.01 ± 0.55a | 1.82 ± 0.35b |
Values are averages ± SD; n = 4; values that do not share a superscript symbol are significantly different.
Fig. 1.
Hepatic gene expression and content of liver TG fatty acids. A: Hepatic gene expression of 5-h–fasted mice fed a control or a 0.025% w/w T0901317 diet for one week. Results were normalized to cyclophilin with data from mice fed the control diet defined as “1”. Values are averages ± SD; n = 4; * = significant different from control. Abca1, ATP-binding cassete-A1; Acc1, acetyl-CoA carboxylase-1; Apob, apolipoprotein B; Chrebp, carbohydrate response element binding protein; Fas, fatty acid synthase; Lpl, lipoprotein lipase; Mtp, microsomal triglyceride transfer protein; Pepck, phosphoenol pyruvate carboxykinase; Srebp-1c, sterol regulatory element-binding protein-1c. B: Comparison of clamp and LXR-mediated effects to change liver TG fatty acid content. Values are averages ± SEM; n = 4–5 animals per group. Bars without similar symbols are significantly different.
Effect of T0901317 on insulin sensitivity of glucose metabolism
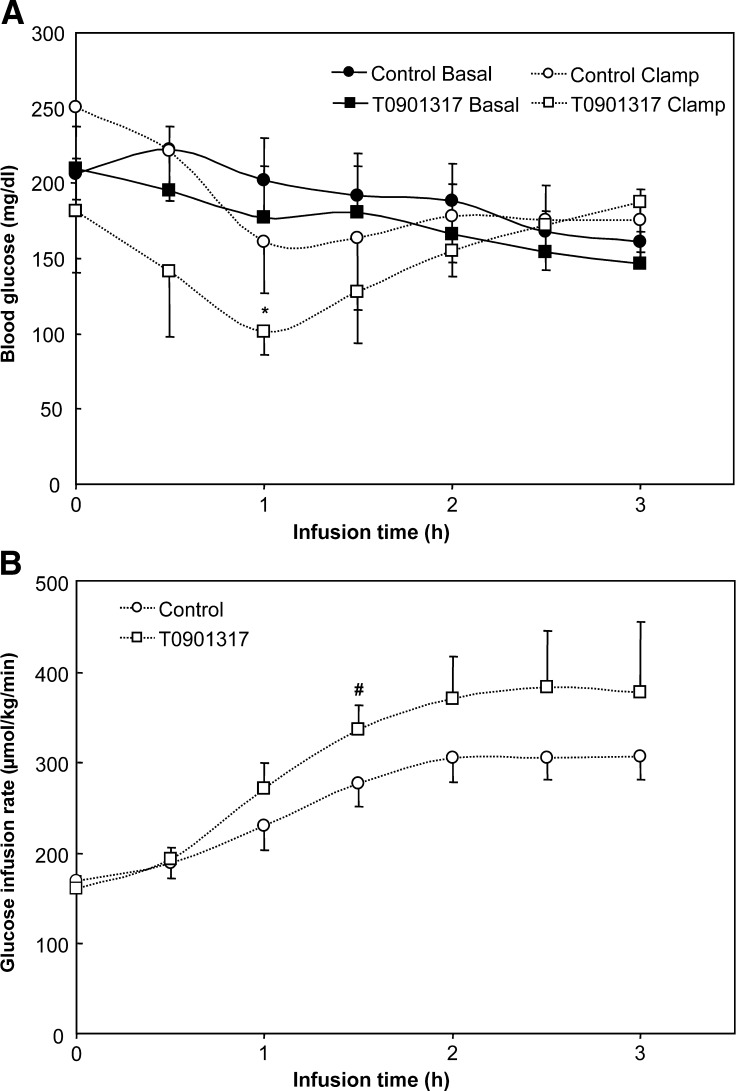
Previous reports have shown effects of LXR activation on glucose metabolism in diabetic rodents (23–25). In the present study, we tested the effect of T0901317 on insulin-mediated changes in VLDL parameters. In the first set of experiments, a 3-h hyperinsulinemic euglycemic clamp was performed. Apart from the 1 h time point, the blood glucose concentrations of the clamped mice were comparable to those of basal-infused mice (Fig. 2A). The glucose infusion rates to maintain euglycemic values tended to be higher in the T0901317-treated mice (Fig. 2B). As shown in Table 2, hyperinsulinemia did not affect MTP activity but significantly reduced liver-TG content, which likely resulted from loss of plasma NEFA entering the liver. Under clamp conditions, NEFA fell to undetectable levels (Table 2).
Fig. 2.
Blood glucose (A) and glucose infusion rates (B) during a 3-h basal infusion or hyperinsulinemic euglycemic clamp in fasted mice fed a control or T0901317-supplemented diet for one week. Values are averages ± SD; n = 4; * = significant different from control basal; # = significant different from control clamp.
TABLE 2.
Plasma and liver parameters after a 3-h basal (saline infusion) or hyperinsulinemic euglycemic clamp in mice fed a control or 0.025% w/w T0901317 diet for one week
| Control |
T0901317 |
|||
|---|---|---|---|---|
| Basal | Clamp | Basal | Clamp | |
| Plasma triacylglycerols (mg/dl) | 136.4 ± 18.7a | 74.5 ± 29.3a,b | 83.0 ± 70.7a,b | 63.7 ± 26.3b |
| Plasma cholesterol (mg/dl) | 69.2 ± 6.5 | 61.4 ± 6.2 | 81.7 ± 11.2 | 81.8 ± 10.7 |
| Plasma NEFA (mEq/l) | 0.343 ± 0.126a | NDb | 0.500 ± 0.257a | NDb |
| Plasma insulin (ng/ml) | 1.64 ± 1.06 | 40.7 ± 19.4 | 3.87 ± 1.34 | 19.7 ± 13.4 |
| Liver weight (% of bodyweight) | 5.4 ± 0.4 | 5.8 ± 0.5 | 5.9 ± 0.6 | 6.1 ± 0.5 |
| Liver triacylglycerols (mg/g) | 8.8 ± 2.4a | 6.2 ± 0.9a | 26.5 ± 3.0b | 22.6 ± 7.6a,b |
| Liver cholesterol (mg/g) | 2.5 ± 0.2 | 2.3 ± 0.1 | 2.3 ± 0.2 | 2.2 ± 0.2 |
| Liver proteins (mg/g) | 191 ± 14a,b,c | 167 ± 8a,b | 212 ± 9a,c | 192 ± 22a,b,c |
| Liver MTP activity (pmol/μg protein/h) | 1.14 ± 0.19 | 1.15 ± 0.19 | 1.38 ± 0.18 | 1.34 ± 0.12 |
| Liver MTP activity (μmol/liver/h) | 0.24 ± 0.02a,b | 0.24 ± 0.06a,b | 0.39 ± 0.05c | 0.34 ± 0.03b,c |
Food was removed from the mice 5 h before the start of the infusions. Values are averages ± SD; n = 4; ND, not detectable; values that do not share a superscript symbol are significantly different.
Hyperinsulinemia enhances LXR-mediated nuclear SREBP-1 translocation
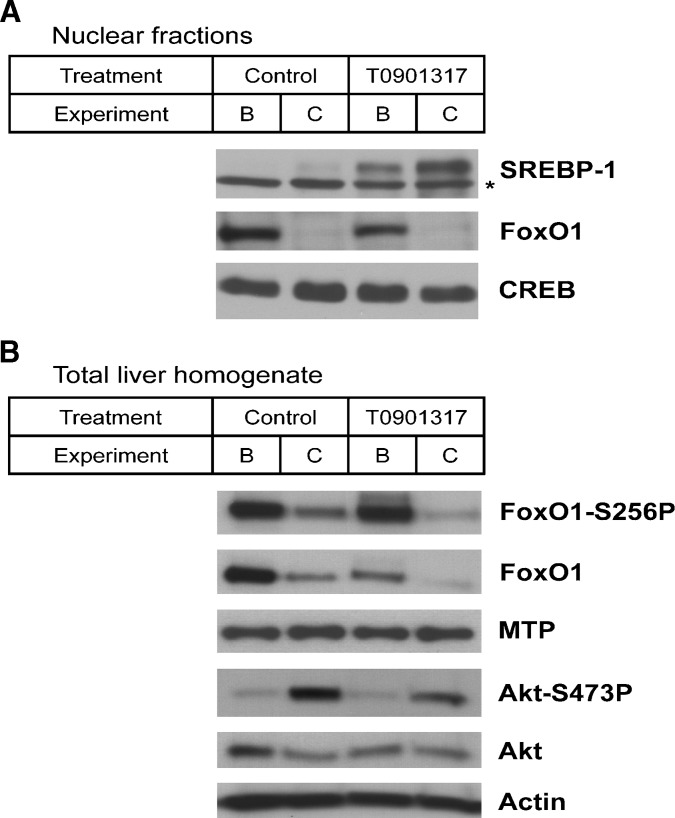
Upon fasting, when insulin levels are low, the expression of DNL genes is decreased. In mice that overexpress hepatic SREBP-1c, this fasting-induced effect on gene expression is prevented (44), supporting SREBP-1c as a key regulator of the hepatic insulin response. SREBP-1c transcription and activation are regulated by insulin (45–47), and insulin reduces the turnover rate of nuclear SREBP-1c (48). In the clamp studies, hyperinsulinemia also increased the hepatic nuclear SREBP-1 content (Fig. 3A). Moreover, the nuclear SREBP-1 content markedly increased upon T0901317 treatment, and this effect was further enhanced by hyperinsulinemia.
Fig. 3.
Immunoblot analysis of the mature SREBP-1, FoxO1, and CREB in nuclear liver fractions (A) and total and Ser256-phosphorylated FoxO1, MTP, total, and Ser473 phosphorylated Akt, and actin in whole liver (B). Livers were harvested after a 3-h basal infusion or hyperinsulinemic euglycemic clamp in fasted mice fed a control or T0901317-supplemented diet for one week. Results are from pooled liver fractions (n = 4). B, Basal infusion; C, hyperinsulinemic euglycemic clamp. The asterisk (*) indicates a nonspecific band.
Recently, it has been shown that insulin reduces transcription of Mtp via phosphorylation of the transcription factor FoxO1 and translocation of the factor from the nucleus to the cytosol (29). We found reduced nuclear FoxO1 protein upon clamping (Fig. 3A), and the total hepatic FoxO1, with/without phosphorylation, was also decreased in the hyperinsulinemic-euglycemic state. The MTP protein content, however, was not affected by T0901317 treatment or hyperinsulinemia (Fig. 3B). Akt phosphorylation was determined following the hyperinsulinemic euglycemic clamp as a control, and as expected, Akt phosphorylation was increased. Unexpectedly, the total amount of FoxO1 protein content was reduced upon LXR activation.
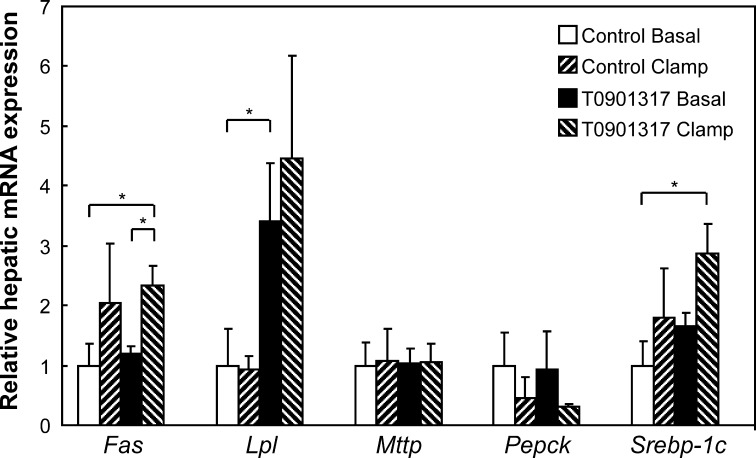
Next, we studied the combined effects of LXR activation and hyperinsulinemia on the expression of several genes. Figure 4 shows that in the experimental protocol used (i.e., sedated mice subjected to a 3-h infusion protocol), the effects of insulin on gene lipogenic expression seem to override the effects of the LXR activation. For instance, hyperinsulinemia induced the expression of Fas and Srebp-1c, but the effects of T0901317 on these genes were severely blunted (compare Fig. 4 with Fig. 1), which may have been due to sedation and the infusion protocol. The effects on Lpl expression, in contrast, are mainly affected by T0901317 not by hyperinsulinemia. Hyperinsulinemia suppressed the expression of Pepck as expected, although not statistically significant. The expression of Mtp was affected by neither T0901317 nor hyperinsulinemia.
Fig. 4.
Hepatic gene expression upon a 3-h basal infusion or hyperinsulinemic euglycemic clamp in fasted mice fed a control or T0901317-supplemented diet for one week. Results were normalized to cyclophilin with data from mice fed the control diet receiving the basal infusion is defined as “1”. Values are averages ± SD; n = 4; * = significant different. Fas, fatty acid synthase; Lpl, lipoprotein lipase; Mtp, microsomal triglyceride transfer protein; Pepck, phosphoenol pyruvate carboxykinase; Srebp-1c, sterol regulatory element-binding protein-1c.
Reduced insulin sensitivity of VLDL secretion upon T0901317 treatment
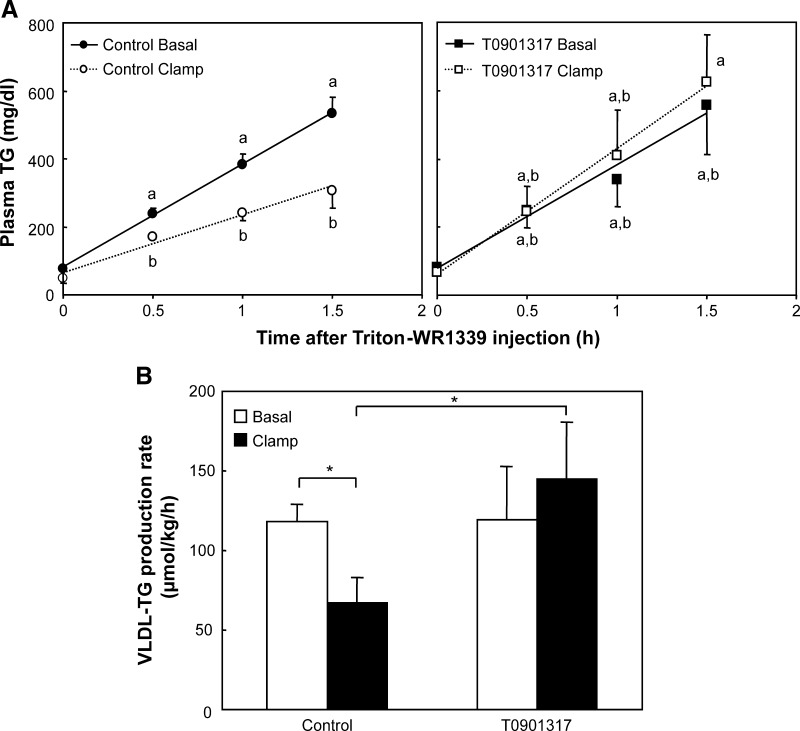
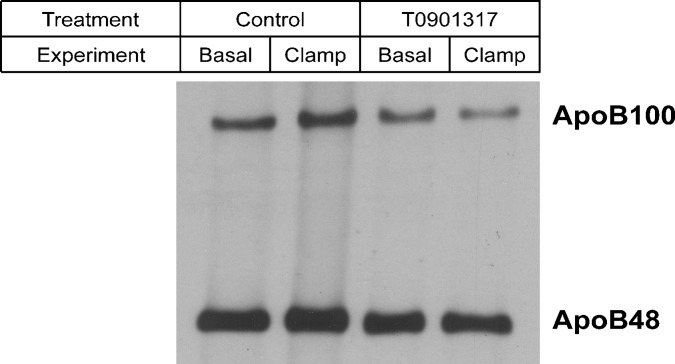
To study the acute effect of insulin on VLDL-TG production, the hyperinsulinemic euglycemic clamp was repeated on another set of mice. After 1.5 h of infusion, the mice were given an injection of Triton WR-1339 to assess TG secretion rates (Fig. 5A). The hyperinsulinemic conditions suppressed VLDL-TG production rate in control mice but not in T0901317-treated mice (Fig. 5B). Analysis of nascent VLDL particles showed that they were larger and more TG-rich in T0901317-treated mice compared with control mice (Table 3). Hyperinsulinemia reduced VLDL particle size in control mice but not in the T0901317-treated mice, an effect in accordance with the unaffected VLDL-TG production rates. The results depicted in Table 3 were in line with the VLDL-apoB content (Fig. 6). When isolated VLDL containing similar TG amounts were used for immunoblotting, we found that the clamping protocol increased the amount of apoB in untreated mice, suggesting that hyperinsulinemia reduced the VLDL particle size. Compared with control mice, the amount of apoB per VLDL-TG was reduced in T0901317-treated mice, suggesting increased VLDL particle size. Hyperinsulinemic conditions did not increase the VLDL-apoB versus VLDL-TG ratio in T0901317-treated mice.
Fig. 5.
A: Plasma triacylglycerol (TG) concentrations in samples obtained after injection of Triton WR-1339 during the last 1.5 h of a 3-h basal infusion or hyperinsulinemic euglycemic clamp in fasted mice fed a control or T0901317-supplemented diet for one week. Values that do not share a symbol are significantly different for that time point. B: VLDL-TG production rate in μmol/kg/h calculated from the plasma TG versus time curves. Values are averages ± SD; n = 5; * = significant difference.
TABLE 3.
Relative lipid composition of nascent VLDL particles upon a 3-h basal infusion or hyperinsulinemic euglycemic clamp in fasted mice fed a control or T0901317-supplemented diet for one week
| Control |
T0901317 |
|||
|---|---|---|---|---|
| Basal | Clamp | Basal | Clamp | |
| Free cholesterol (%) | 11.4 ± 1.2a,b | 14.1 ± 1.4a,b | 10.4 ± 0.8a | 10.8 ± 0.6a |
| Cholesteryl esters (%) | 1.0 ± 0.4 | 2.5 ± 1.0 | 0.9 ± 0.5 | 1.6 ± 1.0 |
| Phospholipids (%) | 13.1 ± 0.6a | 14.3 ± 0.7a | 11.2 ± 0.5b | 11.5 ± 0.8b |
| Triacylglycerol (%) | 74.6 ± 1.5a | 69.0 ± 2.2 | 77.5 ± 1.9a | 76.2 ± 1.1a |
| Surface to core ratio | 0.324 ± 0.029a,b | 0.398 ± 0.039a,b | 0.275 ± 0.020a | 0.286 ± 0.14a |
| Calculated particle size (nm) | 88.7 ± 4.9a | 77.6 ± 4.4 | 104.2 ± 6.1b | 100.6 ± 6.2a,b |
VLDL particles were isolated by ultracentrifugation from plasma retrieved 1.5 h after Triton WR-1339 injection. The percents of lipids are the molar percents of the total lipids in the VLDL. The surface-to-core ratio is the molar ratio free cholesterol and phospholipids versus triacylglycerol and cholesteryl esters. The particle size is calculated using the equation in which the diameter (nm) = (60 × {[0.211 x (mol of triacylglycerol)/(mol of phospholipids)] + 0.27)}). Values are averages ± SD; n = 5; values which do not share a superscript symbol are significantly different.
Fig. 6.
Immunoblot analysis of apoB in nascent VLDL particles upon a 3-h basal infusion or hyperinsulinemic euglycemic clamp in fasted mice fed a control or T0901317-supplemented diet for one week. VLDL particles were isolated as described in Materials and Methods. For each condition, VLDL containing equal amounts of TG were pooled, delipidated with ice-cold ether, dissolved in sample-loading buffer, and subjected to 4.5% SDS-PAGE for immunoblot analysis of apoB using a polyclonal antibody.
Increased contribution of fatty acids derived from de novo lipogenesis to VLDL upon hyperinsulinemia with an additional effect of T0901317
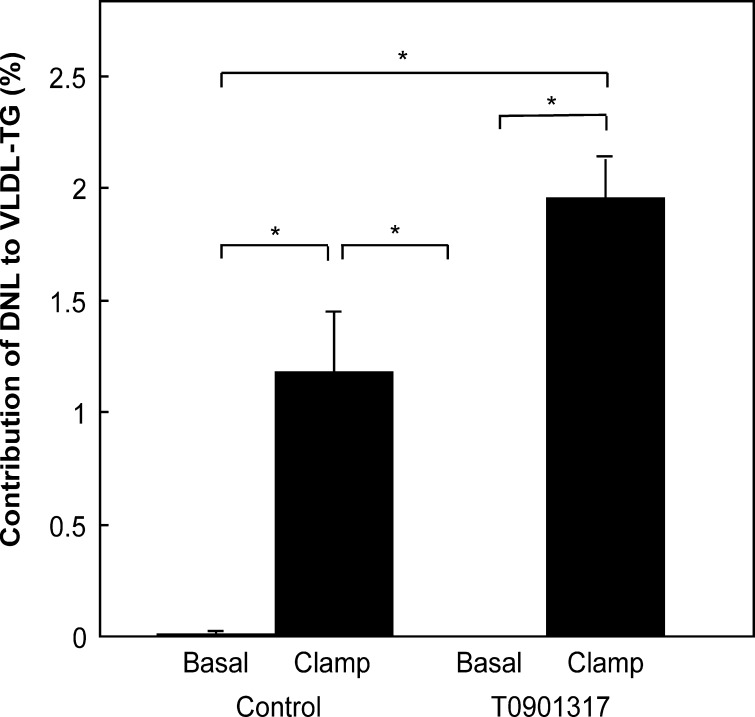
To determine whether increased DNL was in part responsible for the enhanced VLDL-TG production rate in the T0901317-treated mice, the hyperinsulinemic euglycemic clamping experiment was repeated with [1-13C]acetate added to the infusate. As shown in Fig. 7, the presence of DNL fatty acids was below detection in VLDL-TG under basal conditions, as expected in fasted animals. During the hyperinsulinemic euglycemic clamp, the contribution of DNL-fatty acids to VLDL-TG increased. Furthermore, in the clamped T0901317-treated mice, the contribution of DNL fatty acids was even greater, supporting increased DNL as a driver of increased VLDL-TG production in hyperinsulinemic T0901317-treated mice.
Fig. 7.
Contribution of de novo lipogenesis to palmitate in VLDL-TG upon a 3-h basal infusion or hyperinsulinemic euglycemic clamp in fasted mice fed a control or T0901317-supplemented diet for one week. [1-13C]acetate was added to the basal and insulin solution. Plasma VLDL was isolated by ultracentrifugation, TG separated using TLC, and fatty acid methyl esters prepared as described in Materials and Methods. GC-MS analysis was performed in which palmitic acid was used as the marker of fatty acid synthesis. De novo lipogenesis was calculated as described in Materials and Methods. Values are averages ± SD; n = 4; * = significant difference.
DISCUSSION
In the present study, we tested the effects of pharmacological LXR activation by T0901317 on insulin regulation of VLDL production in mice. We show that LXR activation overcame the ability of insulin to suppress VLDL-TG secretion. The enhanced VLDL production was due, in part, to increased hepatic MTP activity and the increased contribution of fatty acids generated via DNL to the VLDL-TG pool.
The consequences of LXR activation on hepatic lipid metabolism have been demonstrated previously (10–12). Using T0901317, LXR-induced lipogenesis and hepatic steatosis were accompanied by the secretion of large, TG-rich VLDL particles (10). Prior to the present studies, it was unknown why LXR stimulation resulted in larger VLDL particles being secreted. The increased synthesis of fatty acids and, subsequently, TGs was generally thought to be responsible for larger particles, but the present study shows for the first time that enhanced MTP activity might also contribute to this effect (Table 1).
We also investigated the role of insulin on nuclear FoxO1 translocation. As expected, we found that insulin reduced the nuclear FoxO1 content (Fig. 3A), which might be due to decreased total FoxO1 in liver (Fig. 3B). This latter observation requires more detailed investigation. LXR activation tended to lower the total FoxO1 concentration, and under these conditions, repression by FoxO1 would not be thought to be responsible for the slightly increased Mtp mRNA levels with T0901317 treatment (Fig. 1A) as suggested by Kamagate et al. (29). In addition, the effects of insulin on nuclear FoxO1 content (Fig. 3A) also did not decrease the hepatic mRNA levels of the FoxO1 target gene Mtp (Fig. 4). It was also shown that transgenic mice expressing constitutively active hepatic FoxO1 had decreased mRNA levels of Srebp-1c and its target genes (49). Kamei et al. (50) reported that transgenic mice overexpressing FoxO1 specifically in skeletal muscle had decreased muscle Srebp-1c mRNA levels. In vitro experiments have shown that FoxO1 suppresses the binding of LXR to the Srebp-1c promoter, and the authors therefore concluded that FoxO1 antagonizes the LXR-mediated induction of Srebp-1c expression in skeletal muscle. Zhang et al. (49) speculated that FoxO1 might impair the function of LXR. Conversely, our study suggests that LXR activation reduced FoxO1 protein content in the liver. The interplay among LXR, FoxO1, SREBP-1c, and lipid metabolism will be a key area for further study.
Upon LXR activation, VLDL particles were larger (Table 3 and Fig. 6), a change consistent with past findings (10). Analysis of the nuclear SREBP-1 abundance (Fig. 3) in combination with the VLDL-TG production rate measurements under hyperinsulinemic euglycemic conditions (Fig. 5) led to new insights. Hyperinsulinemia increased the hepatic nuclear SREBP-1 content in both control and T0901317-treated mice, pointing toward unaffected insulin sensitivity of this component of hepatic lipid metabolism. This is despite the T0901317-mediated hepatic steatosis (Tables 1 and 2), a condition normally associated with insulin resistance (51, 52).
In control mice, we demonstrated a striking reduction in liver-TG content under clamp conditions (Table 2 and Fig. 1B). To our knowledge, this is the first time this effect has been quantitated. This observation supports the dominant contribution of NEFAs as substrate for liver-TG synthesis, as shown before (53). Further, the reduction in VLDL-TG secretion observed during the clamp was likely due to both loss of NEFA as substrate for TG synthesis and a reduction in particle assembly. LXR treatment elevated liver TGs and MTP activity such that insulin only induced a weak suppression of VLDL-TG secretion. This conserved insulin sensitivity of lipid synthesis was remarkable in that it coincided with the observation that VLDL-TG production itself was less suppressed upon acute hyperinsulinemia in T0901317 treated mice (Fig. 6). Increased SREBP-1 translocation might be an underlying cause for the continued VLDL production during hyperinsulinemia. More newly made fatty acids were incorporated into the VLDL upon hyperinsulinemia in T0901317-treated mice (Fig. 7). The increased DNL and TG production, in combination with—or as the cause of—enhanced MTP activity, resulted in continued VLDL-TG production under hyperinsulinemic conditions.
In conclusion, the present results confirm the strong effect of LXR-mediated lipid synthesis to stimulate VLDL-TG secretion in mice. Upon LXR activation, the mice displayed dyslipidemic VLDL characteristics: larger VLDL particle secretion resistant to insulin-mediated inhibition. These findings suggest that the normal function of insulin to suppress VLDL secretion can be overridden by greater lipid availability. Further development of the LXR activators will need to balance these effects to result in net benefit with respect to hepatic insulin sensitivity.
Acknowledgments
The authors would like to thank Jay D. Horton and Folkert Kuipers for critical reading of the manuscript, and Daniel Smith, Lauren Koob, Michael Adams, and Vidya Vaidyanathan for skillful technical assistance.
Abbreviations
ApoB, apolipoprotein B
DNL, de novo lipogenesis
ER, endoplasmic reticulum
LXR, liver X receptor
MIDA, mass isotopomer distribution analysis
MTP, microsomal triglyceride transfer protein
TG, triacylglycerol
This work was supported by a grant from the Ter Meulen Fund, Royal Netherlands Academy of Arts and Science, The Netherlands.
References
- 1.Repa J. J., and D. J. Mangelsdorf. 2002. The liver X receptor gene team: potential new players in atherosclerosis. Nat. Med. 8 1243–1248. [DOI] [PubMed] [Google Scholar]
- 2.Repa J. J., S. D. Turley, J. M. A. Lobaccaro, J. Medina, L. Li, K. Lustig, B. Shan, R. A. Heyman, J. M. Dietschy, and D. J. Mangelsdorf. 2000. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 289 1524–1529. [DOI] [PubMed] [Google Scholar]
- 3.Costet P., Y. Luo, N. Wang, and A. R. Tall. 2000. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 275 28240–28245. [DOI] [PubMed] [Google Scholar]
- 4.Venkateswaran A., B. A. Laffitte, S. B. Joseph, P. A. Mak, D. C. Wilpitz, P. A. Edwards, and P. Tontonoz. 2000. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc. Natl. Acad. Sci. USA. 97 12097–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz K., R. M. Lawn, and D. P. Wade. 2000. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem. Biophys. Res. Commun. 274 794–802. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann J. M., S. A. Kliewer, L. B. Moore, T. A. Smith-Oliver, B. B. Oliver, J. L. Su, S. S. Sundseth, D. A. Winegar, D. E. Blanchard, T. A. Spencer, et al. 1997. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 272 3137–3140. [DOI] [PubMed] [Google Scholar]
- 7.Peet D. J., S. D. Turley, W. Ma, B. A. Janowski, J. M. A. Lobaccaro, R. E. Hammer, and D. J. Mangelsdorf. 1998. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 93 693–704. [DOI] [PubMed] [Google Scholar]
- 8.Joseph S. B., E. McKilligin, L. Pei, M. A. Watson, A. R. Collins, B. A. Laffitte, M. Chen, G. Noh, J. Goodman, G. N. Hagger, et al. 2002. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA. 99 7604–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terasaka N., A. Hiroshima, T. Koieyama, N. Ubukata, Y. Morikawa, D. Nakai, and T. Inaba. 2003. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 536 6–11. [DOI] [PubMed] [Google Scholar]
- 10.Grefhorst A., B. M. Elzinga, P. J. Voshol, T. Plösch, T. Kok, V. W. Bloks, F. H. van der Sluijs, L. M. Havekes, J. A. Romijn, H. J. Verkade, et al. 2002. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipopoprotein particles. J. Biol. Chem. 277 34182–34190. [DOI] [PubMed] [Google Scholar]
- 11.Grefhorst A., T. H. van Dijk, A. Hammer, F. H. van der Sluijs, R. Havinga, L. M. Havekes, J. A. Romijn, P. H. Groot, D. J. Reijngoud, and F. Kuipers. 2005. Differential effects of pharmacological liver X receptor activation on hepatic and peripheral insulin sensitivity in lean and ob/ob mice. Am. J. Physiol. Endocrinol. Metab. 289 E829–E838. [DOI] [PubMed] [Google Scholar]
- 12.Schultz J. R., H. Tu, A. Luk, J. J. Repa, J. C. Medina, L. Li, S. Schwendner, S. Wang, M. Thoolen, D. J. Mangelsdorf, et al. 2000. Role of LXR in control of lipogenesis. Genes Dev. 14 2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Repa J. J., G. Liang, J. Ou, Y. Bashmakov, J. M. A. Lobaccaro, I. Shimomura, B. Shan, M. S. Brown, J. L. Goldstein, and D. J. Mangelsdorf. 2000. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 14 2819–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshikawa T., H. Shimano, M. Amemiya-Kudo, N. Yahagi, A. H. Hasty, T. Matsuzaka, H. Okazaki, Y. Tamura, Y. Iizuka, K. Ohashi, et al. 2001. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Mol. Cell. Biol. 21 2991–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horton J. D., I. Shimomura, M. S. Brown, R. E. Hammer, J. L. Goldstein, and H. Shimano. 1998. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J. Clin. Invest. 101 2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph S. B., B. A. Lafitte, P. H. Patel, M. A. Watson, K. E. Matsukuma, R. Walczak, J. L. Collins, T. F. Osborne, and P. Tontonoz. 2002. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 277 11019–11025. [DOI] [PubMed] [Google Scholar]
- 17.Brown M. S., J. Ye, R. B. Rawson, and J. L. Goldstein. 2000. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 100 391–398. [DOI] [PubMed] [Google Scholar]
- 18.Cha J. Y., and J. J. Repa. 2007. The liver X receptor (LXR) and hepatic lipogenesis: the carbohydrate-response element-binding protein is a target gene of LXR. J. Biol. Chem. 282 743–751. [DOI] [PubMed] [Google Scholar]
- 19.Ishii S., K. Iizuka, B. C. Miller, and K. Uyeda. 2004. Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc. Natl. Acad. Sci. USA. 101 15597–15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iizuka K., R. K. Bruick, G. Liang, J. D. Horton, and K. Uyeda. 2004. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. USA. 101 7281–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon D. A., and H. Jamil. 2000. Progress towards understanding the role of microsomal triglyceride transfer protein in apolipoprotein-B lipoprotein assembly. Biochim. Biophys. Acta. 1486 72–83. [DOI] [PubMed] [Google Scholar]
- 22.Cao G., Y. Liang, C. L. Broderick, B. A. Oldham, T. P. Beyer, R. J. Schmidt, Y. Zhang, K. R. Stayrook, C. Suen, K. A. Otto, et al. 2003. Antidiabetic action of a liver X receptor agonist mediated by inhibition of hepatic gluconeogenesis. J. Biol. Chem. 278 1131–1136. [DOI] [PubMed] [Google Scholar]
- 23.Laffitte B. A., L. S. Chao, J. Li, R. Walczak, S. Hummasti, S. B. Joseph, A. Castrillo, D. C. Wilpitz, D. J. Mangelsdorf, J. L. Collins, et al. 2003. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc. Natl. Acad. Sci. USA. 100 5419–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grefhorst A., J. Hoekstra, T. G. Derks, D. M. Ouwens, J. F. Baller, R. Havinga, L. M. Havekes, J. A. Romijn, and F. Kuipers. 2005. Acute hepatic steatosis in mice by blocking β-oxidation does not reduce insulin sensitivity of very low density lipoprotein production. Am. J. Physiol. Gastrointest. Liver Physiol. 289 G592–G598. [DOI] [PubMed] [Google Scholar]
- 25.den Boer M. A., P. J. Voshol, F. Kuipers, J. A. Romijn, and L. M. Havekes. 2006. Hepatic glucose production is more sensitive to insulin-mediated inhibition than hepatic VLDL-triglyceride production. Am. J. Physiol. Endocrinol. Metab. 291 E1360–E1364. [DOI] [PubMed] [Google Scholar]
- 26.Fisher E. A., M. Pan, X. Chen, X. Wu, H. Wang, H. Jamil, J. D. Sparks, and K. J. Williams. 2001. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J. Biol. Chem. 276 27855–27863. [DOI] [PubMed] [Google Scholar]
- 27.Wetterau J. R., M. C. Lin, and H. Jamil. 1997. Microsomal triglyceride transfer protein. Biochim. Biophys. Acta. 1345 136–150. [DOI] [PubMed] [Google Scholar]
- 28.Hagan D. L., B. Kienzle, H. Jamil, and N. Hariharan. 1994. Transcriptional regulation of human and hamster microsomal triglyceride transfer protein genes. Cell type-specific expression and response to metabolic regulators. J. Biol. Chem. 269 28737–28744. [PubMed] [Google Scholar]
- 29.Kamagate A., S. Qu, G. Perdomo, D. Su, D. H. Kim, S. Slusher, M. Meseck, and H. H. Dong. 2008. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Invest. 118 2347–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiegman C. H., R. H. J. Bandsma, M. Ouwens, F. H. van der Sluijs, R. Havinga, T. Boer, D. J. Reijngoud, J. A. Romijn, and F. Kuipers. 2003. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes. 52 1081–1089. [DOI] [PubMed] [Google Scholar]
- 31.Minehira K., S. G. Young, C. J. Villanueva, L. Yetukuri, M. Oresic, M. K. Hellerstein, R. V. Farese, J. D. Horton, F. Preitner, B. Thorens, et al. 2008. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J. Lipid Res. 49 2038–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adiels M., J. Westerbacka, A. Soro-Paavonen, A. M. Häkkinen, S. Vehkavaara, M. J. Caslake, C. Packard, S. O. Olofsson, H. Yki-Järvinen, M. R. Taskinen, et al. 2007. Acute suppression of VLDL1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia. 50 2356–2365. [DOI] [PubMed] [Google Scholar]
- 33.Shimano H., J. D. Horton, R. E. Hammer, I. Shimomura, M. S. Brown, and J. L. Goldstein. 1996. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J. Clin. Invest. 98 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang G., J. Yang, J. D. Horton, R. E. Hammer, J. L. Goldstein, and M. S. Brown. 2002. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 277 9520–9528. [DOI] [PubMed] [Google Scholar]
- 35.Yang J., J. L. Goldstein, R. E. Hammer, Y. A. Moon, M. S. Brown, and J. D. Horton. 2001. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc. Natl. Acad. Sci. USA. 98 13607–13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraser R. 1970. Size and lipid composition of chylomicrons of different Svedberg units of flotation. J. Lipid Res. 11 60–65. [PubMed] [Google Scholar]
- 37.Harris W. S., B. E. Hustvedt, E. Hagen, M. H. Green, G. Lu, and C. A. Drevon. 1997. N-3 fatty acids and chylomicron metabolism in the rat. J. Lipid Res. 38 503–515. [PubMed] [Google Scholar]
- 38.Timlin M. T., and E. J. Parks. 2005. Temporal pattern of de novo lipogenesis in the postprandial state in healthy men. Am. J. Clin. Nutr. 81 35–42. [DOI] [PubMed] [Google Scholar]
- 39.Donnelly K. L., C. I. Smith, S. J. Schwarzenberg, J. Jessurun, M. D. Boldt, and E. J. Parks. 2005. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Améen C., U. Edvardsson, A. Ljungberg, L. Asp, P. Åkerblad, A. Tuneld, S. O. Olofsson, D. Lindén, and J. Oscarsson. 2005. Activation of peroxisome proliferator-activated receptor α increases the expression and activity of microsomal triglyceride transfer protein in the liver. J. Biol. Chem. 280 1224–1229. [DOI] [PubMed] [Google Scholar]
- 41.Park S. W., Y. A. Moon, and J. D. Horton. 2004. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J. Biol. Chem. 279 50630–50638. [DOI] [PubMed] [Google Scholar]
- 42.Engelking L. J., H. Kuriyama, R. E. Hammer, J. D. Horton, M. S. Brown, J. L. Goldstein, and G. Liang. 2004. Overexpression of Insig-1 in the livers of transgenic mice inhibits SREBP processing and reduces insulin-stimulated lipogenesis. J. Clin. Invest. 113 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plösch T., T. Kok, V. W. Bloks, M. J. Smit, R. Havinga, G. Chimini, A. K. Groen, and F. Kuipers. 2002. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J. Biol. Chem. 277 33870–33877. [DOI] [PubMed] [Google Scholar]
- 44.Horton J. D., Y. Bashmakov, I. Shimomura, and H. Shimano. 1998. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl. Acad. Sci. USA. 95 5987–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hegarty B. D., A. Bobard, I. Hainault, P. Ferré, P. Bossard, and F. Foufelle. 2005. Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory element-binding protein-1c. Proc. Natl. Acad. Sci. USA. 102 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleischmann M., and P. B. Iynedjian. 2000. Regulation of sterol regulatory-element binding protein 1 gene expression in liver: role of insulin and protein kinase B/cAkt. Biochem. J. 349 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yabe D., R. Komuro, G. Liang, J. L. Goldstein, and M. S. Brown. 2003. Liver specific mRNA for insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc. Natl. Acad. Sci. USA. 100 3155–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yellaturu C. R., X. Deng, L. M. Cagen, H. G. Wilcox, E. A. Park, R. Raghow, and M. B. Elam. 2005. Posttranslational processing of SREBP-1 in rat hepatocytes is regulated by insulin and cAMP. Biochem. Biophys. Res. Commun. 332 174–180. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W., S. Patil, B. Chauhan, S. Guo, D. R. Powell, J. Le, A. Klotsas, R. Matika, X. Xiao, R. Franks, et al. 2006. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 281 10105–10117. [DOI] [PubMed] [Google Scholar]
- 50.Kamei Y., S. Miura, T. Suganami, F. Akaike, S. Kanai, S. Sugita, A. Katsumata, H. Aburatani, T. G. Unterman, O. Ezaki, et al. 2008. Regulation of SREBP1c gene expression in skeletal muscle: role of retinoid X receptor/liver X receptor and forkhead-O1 transcription factor. Endocrinology. 149 2293–2305. [DOI] [PubMed] [Google Scholar]
- 51.Angulo P. 2002. Nonalcoholic fatty liver disease. N. Engl. J. Med. 346 1221–1231. [DOI] [PubMed] [Google Scholar]
- 52.Kumar K. S., and P. F. Malet. 2000. Nonalcoholic steatohepatitis. Mayo Clin. Proc. 75 733–739. [DOI] [PubMed] [Google Scholar]
- 53.Barrows B. R., and E. J. Parks. 2006. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J. Clin. Endocrinol. Metab. 91 1446–1452. [DOI] [PubMed] [Google Scholar]