Abstract
Carriers of the apolipoprotein A-IMilano (apoA-IM) variant, R173C, have reduced levels of plasma HDL but no increase in cardiovascular disease. Despite intensive study, it is not clear whether the removal of the arginine or the introduction of the cysteine is responsible for this altered functionality. We investigated this question using two engineered variations of the apoA-IM mutation: R173S apoA-I, similar to apoA-IM but incapable of forming a disulfide bond, and R173K apoA-I, a conservative mutation. Characterization of the lipid-free proteins showed that the order of stability was wild type≈R173K>R173S>R173C. Compared with wild-type apoA-I, apoA-IM had a lower affinity for lipids, while R173S apoA-I displayed intermediate affinity. The in vivo effects of the apoA-I variants were measured by injecting apoA-I-expressing adeno-associated virus into apoA-I-null mice. Mice that expressed the R173S variant again showed an intermediate phenotype. Thus, both the loss of the arginine and its replacement by a cysteine contribute to the altered properties of apoA-IM. The arginine is potentially involved in an intrahelical salt bridge with E169 that is disrupted by the loss of the positively charged arginine and repelled by the cysteine, destabilizing the helix bundle domain in the apoA-I molecule and modifying its lipid binding characteristics.
Keywords: high density lipoprotein, cholesterol, lipoprotein, protein structure
Apolipoprotein A-I (apoA-I), an amphipathic protein secreted by the liver and small intestine, is the major protein component of HDL (1). Plasma apoA-I and HDL cholesterol levels are inversely associated with the risk of cardiovascular disease (2). The anti-atherogenic properties of apoA-I are thought to arise from its central role in reverse cholesterol transport, the pathway by which excess cholesterol is effluxed from peripheral tissues and transported to the liver, where it is subsequently excreted from the body (2–4). The influence of apoA-I structure on its performance in reverse cholesterol transport is not well understood.
A powerful way to examine the structure-function relationship of a protein is to study the effects of genetic variants, especially ones that occur naturally and cause a phenotype in humans. In the case of apoA-I, the first known natural mutant is apoA-IM that is characterized by the mutation R173C (5). The introduction of a cysteine residue allows apoA-I to form apoA-IMilano (apoA-IM) homodimers or heterodimers with apoA-II (6). The apoA-IM mutation is located in the N-terminal helix bundle domain of the protein (7, 8). Naturally occurring variants of apoA-I between residues 121 and 186 are often associated with low apoA-I levels (9). Individuals heterozygous for the apoA-IM mutation have very low plasma apoA-I and HDL cholesterol levels as well as moderately elevated triglycerides (10). Low levels of apoA-IM in plasma are likely due to reduced LCAT activation (11), which can lead to higher catabolic turnover of apoA-I (12, 13). Despite a lipid profile that is usually associated with a high risk of premature cardiovascular disease, apoA-IM carriers display no increase in cardiovascular disease or events (10, 14, 15). This has led to speculation that apoA-IM is a gain-of-function mutation that has enhanced cardio-protective effects (16–22), while others believe that wild-type (WT) apoA-I and apoA-IM are functionally equivalent (23, 24). A clinical trial of repeated intravenous infusions of apoA-IM-phospholipid complexes demonstrated regression of existing atheromas after five weekly treatments (25, 26).
To date, it has not been determined how the R173C substitution is responsible for altering the functional aspects of the apoA-IM mutant. For instance, is it the removal of the arginine and the loss of positive charge at position 173 or the introduction of a cysteine residue with the ability to form disulfide bonds that is responsible? To investigate this question, we generated two engineered variants of the apoA-IM mutation, R173S apoA-I, a mutation structurally similar to apoA-IM but without the ability to form disulfide bonds, and R173K apoA-I, a conservative mutation that maintains the positive charge at position 173 of WT apoA-I. The physical properties of these engineered mutants and their effects on plasma lipid levels and HDL composition in vivo via virus-mediated gene transfer were compared with WT and R173C apoA-I. Adeno-associated virus (AAV) vectors that have been shown to be capable of long-term hepatic expression of WT apoA-I (27) in mice were used in these experiments.
MATERIALS AND METHODS
Preparation of apoA-I mutants
To express human WT apoA-I and engineered variants, the cDNA of interest was cloned into the multiple cloning sites of the pET32a (+) vector, and the target protein was expressed as a histidine-tagged fusion protein with the 109-amino-acid thioredoxin (Trx) at the N terminus (7, 28). The resulting plasmids were transformed into Escherichia coli strain BL21(DE3). These transformed cells were cultured in Luria-Bertani medium at 37°C, and expression of the fusion protein Trx-apoA-I was induced with isopropyl-β-d-thiogalactopyranoside for 3 h. After sonicating the bacterial pellet, the lysate was centrifuged and the supernatant loaded onto a nickel-chelating, histidine binding resin column (Novagen). The Trx-apoA-I fusion protein bound to the column was eluted, pooled, and dialyzed against 20 mM NH4HCO3. Subsequently, the fusion protein was complexed with dimyristoylphosphatidylcholine (DMPC) to prevent nonspecific cleavage and then cleaved with thrombin to release the Trx. The mixture was then lyophilized, delipidated, and dissolved in 6 M guanidine hydrochloride (GdnHCl) solution. Trx was separated from apoA-I by gel filtration chromatography on a Sephacryl S-300 column. Further purification (>95%) of the proteins was done by gel filtration with Superdex 75 and/or anion exchange chromatography with Q-Sepharose. Purity was confirmed by SDS-PAGE (data not shown). ApoA-IM was present in both monomeric and disulfide-linked forms; fractions corresponding to dimeric apoA-IM were pooled separately and confirmed to be >95% dimeric apoA-IM by SDS-PAGE (data not shown). All proteins were stored at −80°C in lyophilized form and before use were dissolved in the appropriate buffer containing 6 M GdnHCl and dialyzed extensively before use.
Circular dichroism spectroscopy
The α-helix contents of the apoA-I variants were determined by obtaining far-UV circular dichroism (CD) spectra from 184–260 at room temperature using a Jasco J-600 spectropolarimeter. The proteins were dissolved in 6 M GdnHCl (for monomeric apoA-IM, 1% β-mercaptoethanol was added to the GdnHCl solution to reduce C173 completely), dialyzed overnight against excess 10 mM sodium phosphate buffer (pH 7.4), and diluted to 25–50 μg/ml for obtaining the CD spectrum. For apoA-I/small unilamellar vesicle (SUV) mixtures, apoA-I was incubated for 1 h prior to the measurement with egg phosphatidylcholine (PC) SUV prepared by sonication as described before (29). The CD spectra were corrected by subtracting the baseline for an appropriate blank sample. The α-helix content was calculated from the molar ellipticity at 222 nm ([θ]222) using the equation: percentage α-helix = [(−[θ]222 +3000)/36000+3000] × 100 (30, 31). Thermal denaturation was monitored from the change in [θ]222 as the sample was heated at 1°/min over the temperature range 20–90°C, as described (32).
Isothermal denaturation studies
The effect of GdnHCl or urea concentration on the reversible unfolding of apoA-I secondary structure was monitored by CD spectroscopy using [θ]222 (31, 33) and by measuring the change in tryptophan fluorescence emission intensity at 335 nm with a Hitachi F-4500 fluorescence spectrophotometer using an excitation wavelength of 295 nm to avoid tyrosine fluorescence (33, 34). Proteins at a concentration of 50 μg/ml in Tris buffer (10 mM Tris-HCl, 150 mM NaCl, 0.02% NaN3, and 1 mM EDTA, pH 7.4) were incubated overnight at 4°C with GdnHCl or urea at various concentrations. The equilibrium constants (Kd) describing the unfolding of apoA-I at given denaturant concentrations were calculated as described before (35) using either [θ]222 or the change in tryptophan fluorescence emission intensity at 335 nm (7, 33). The free energy of denaturation, ΔG°D and the midpoint of denaturation were determined by the linear equation, ΔGD = ΔG°D − m[denaturant], where ΔGD = −RT ln Kd and m reflects the cooperativity of denaturation in the transition region (7, 35).
8-Anilino-1-naphthalensulfonic acid fluorescence measurements
The 8-anilino-1-naphthalensulfonic acid (ANS) fluorescence measurements were carried out with a Hitachi F-4500 fluorescence spectrophotometer. In all experiments, the apoA-I was freshly dialyzed from a 6 M GdnHCl solution into a Tris buffer (10 mM Tris-HCl, 150 mM NaCl, 0.02% NaN3, and 1 mM EDTA, pH 7.4) before use. The extent of ANS binding to hydrophobic sites on the apoA-I variants at 25°C was determined by measuring ANS fluorescence spectra recorded from 400 to 600 nm at an excitation wavelength of 395 nm, in the absence or presence of 50 μg/ml protein and an excess of ANS (250 μM) (7, 31).
DMPC clearance assay
The kinetics of solubilization of DMPC multilamellar vesicles by the apoA-I variants were measured by monitoring the decrease in absorbance at 325 nm, as described previously (36). The time courses of decrease in absorbance with time were fitted to a monoexponential decay equation, and the clearance rates were compared in terms of the product (rate constant X fraction of absorbance cleared at equilibrium) normalized to WT apoA-I (7).
Isothermal titration calorimetry measurements
Heats of apoA-I binding to egg PC SUV at 25°C were measured with a MicroCal MCS isothermal titration calorimeter (29). To ensure that the injected protein bound completely to the SUV surface, the PC-to-protein molar ratio was kept over 10,000. The decay rate constants for the heats of binding were obtained from fitting the titration curves to a one-phase exponential decay model.
Preparation of apoA-I AAV
WT apoA-I cDNA was mutated using the Quikchange site-directed mutagenesis kit from Stratagene. Specific primers were designed to mutate the WT apoA-I cDNA in the pAAVmcs plasmid (37). The plasmid containing the WT apoA-I cDNA served as the template for the mutagenic PCR reaction. After the PCR reaction, the sample was digested with Dpn I, an endonuclease specific for methylated and hemimethylated DNA, to digest the parental DNA template and select for mutation-containing synthesized DNA. The purified mutant plasmid was then transformed into XL1-Blue supercompetent cells. Several colonies were isolated and used to inoculate Luria-Bertani media containing 100 mg/ml ampicillin. The resulting plasmids were sequenced and restriction digested to confirm the presence of the intended mutation. The plasmid containing the mutant sequence was then submitted to the University of Pennsylvania Vector Core for use in creating the apoA-I AAV. All AAV were manufactured using triple-plasmid transfection of adherent human 293 cells using Ad helper, trans-packaging, and AAV vector plasmids. The AAV were purified from harvested lysates using ion exchange column chromatography as described previously (38). The human apoA-I AAV, and variants, contain the human apoA-I cDNA insert followed by a SV-40 poly(A) tail and are driven by the liver-specific human thyroglobulin promoter. The AAV were produced with a chimeric packaging construct in which the AAV2 rep gene was fused with the cap gene of AAV serotype 8 (37, 39).
Preparation and bleeding of WT apoA-I and apoA-I variant transgenic mice
ApoA-I −/− mice (8–12 weeks old, C57/Bl6 background; Jackson Laboratories) fed a chow diet were injected intraperitoneally with 1 × 1012 particles of apoA-IM AAV, R173S, R173K, WT apoA-I AAV, or LacZ AAV with a total of five mice per group. Blood was drawn from the retro-orbital plexus after 4 h of daytime fasting and allowed to clot for 1 h at 4°C. The blood was then spun (10,000 rpm, 7 min, 4°C) to isolate the serum. Total cholesterol, HDL cholesterol, phospholipid, and human apoA-I levels were measured on a Cobas Fara (Roche Diagnostics Systems) using Sigma Diagnostics reagents. HDL for compositional analysis was obtained by adding 40 μl of polyethylene glycol to 100 μl of serum and incubating at 4° for 30 min (40). Samples were spun down (10,000 rpm, 7 min, 4°C) and the serum collected and analyzed using the Cobas Fara. A pool of 120 μl of plasma from six mice was diluted 2-fold with fast-protein liquid chromatography column buffer (1 mM EDTA and 154 mM NaCl, pH 8.0). Two hundred microliters of diluted sample were run on a Superose 6 column at a flow rate of 0.4 ml/min, and 0.5 ml fractions were collected. All animal protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Electrophoresis and Western blotting
Plasma samples (1 μl) were resolved on 4–20% nondenaturing, Tris-glycine gradient gels (Invitrogen) or 4–12% SDS-PAGE Tris-glycine gels (Invitrogen) and transferred to a nitrocellulose membrane. A polyclonal goat anti-human apoA-I antibody (K45252G; Biodesign) was used as the primary antibody at a dilution of 1:5,000, while a peroxidase-conjugated donkey anti-goat IgG (705-035-147; Jackson Immuno Research) was used as the secondary antibody at a dilution of 1:25,000. Blots were developed using chemiluminescence. The HDL fraction (density = 1.073–1.21 g/ml) was isolated by ultracentrifugation (41) of pooled plasma samples from mice expressing either WT human apoA-I or one of the variants. The isolated HDL were then run on 8–25% SDS-PAGE Phastgels (GE Healthcare) and stained for protein with Coomassie blue.
RESULTS
Secondary structure of apoA-I variants
The secondary structures of apoA-I variants were analyzed by far-UV CD spectroscopy. The α-helix contents of lipid-free, monomeric apoA-IM and both R173K apoA-I and R173S apoA-I were no different than WT (Table 1); this finding is consistent with earlier comparisons of apoA-IM and WT apoA-I (42, 43). Interestingly, the dimeric form of lipid-free apoA-IM was significantly less α-helical than WT apoA-I, indicating that the presence of a disulfide bond at position 173 disrupts the WT secondary structure. The α-helicity in the lipid-bound state follows a similar pattern, with only the lipid-bound dimeric apoA-IM showing a reduction in α-helicity compared with WT apoA-I (Table 1). An earlier study by Calabresi et al. (44) showed that dimeric apoA-IM in both the lipid-free and lipid-bound states was more α-helical than WT apoA-I. This difference to our results is perhaps due to enhanced self-association at the higher concentration of dimeric apoA-IM that the authors used to measure α-helicity.
TABLE 1.
Physical properties of apoA-I variants
| ApoA-I Variants | Lipid-Free α-Helicity (%)a | Lipid-Bound α-Helicity (%)b | ANS Fluorescence Intensityc |
|---|---|---|---|
| WT | 44 ± 4 | 70 ± 4 | 1.0 |
| R173K | 44 ± 3 | 74 ± 1 | 1.1 |
| R173S | 44 ± 1 | 72 ± 3 | 1.2 |
| Milano (monomer) | 42 ± 4 | 65 ± 8 | 1.6 |
| Milano (dimer) | 33 ± 2d | 45 ± 6d | 1.6 |
Mean ± SD from at least four independent measurements.
Mean ± SD from three independent measurements. ApoA-I bound to egg PC SUV at a 60:1 (PC:apoA-I) molar ratio.
Values are ratios to WT apoA-I. Error is within 0.1.
P < 0.01 compared with WT apoA-I.
Thermal unfolding and GdnHCl and urea denaturation of apoA-I variants
Figure 1 shows the thermal denaturation curves of WT apoA-I, R173S apoA-I, and monomeric apoA-IM monitored by the change in molar ellipticity at 222 nm over the temperature range 20–90°C. In comparison to the denaturation curve of WT apoA-I, both R173S apoA-I and monomeric apoA-IM displayed a decrease in the cooperative nature of unfolding as indicated by the shallowness of their denaturation curves (Fig. 1). The midpoints of thermal denaturation are summarized in Table 2. The introduction of the apoA-IM mutation destabilized the protein in both the monomeric and dimeric forms; a lower free energy of unfolding of monomeric apoA-IM compared with WT apoA-I has been reported previously (43). The R173S mutation also destabilized apoA-I. The conservative mutation, R173K, had no effect on the stability of apoA-I as measured by thermal denaturation (data not shown).
Fig. 1.
Thermal unfolding of apoA-I variants monitored by changes in the molar ellipticity at 222 nm. Thermal denaturation was monitored by the change in molar ellipticity at 222 nm over the temperature range of 20–90°C of apoA-I solutions (50 μg/ml) in sodium phosphate buffer (pH 7.4). WT apoA-I (solid line), monomeric apoA-IMilano (dashed line), and R173S apoA-I (dotted line).
TABLE 2.
Stability of apoA-I variants
| ApoA-I Variants | Midpoint of Thermal Denaturation (°C)a | Midpoint of GdnHCl Denaturation(M)a | ΔG°D (kcal/mol)b | Midpoint of GdnHCl Denaturation (M)a | ΔG°D (kcal/mol)b | Midpoint of Urea Denaturation (M)a | ΔG°D (kcal/mol)b |
|---|---|---|---|---|---|---|---|
| Molar Ellipticity at 222 nm | Fluorescence Intensityc | ||||||
| WT | 60 | 1.0 | 3.9 ± 0.2 | 1.0 | 4.6 ± 0.3 | 3.0 | 6.2 ± 0.2 |
| R173K | 61 | 1.0 | 3.9 ± 0.2 | 1.1 | 4.5 ± 0.2 | 2.7d | 5.8 ± 0.2 |
| R173S | 56d | 0.9 | 3.3 ± 0.1d | 1.0 | 3.7 ± 0.1d | 2.2d | 4.1 ± 0.1d |
| Milano (monomer) | 53d,e | 0.8d | 2.4 ± 0.2d,e | 0.9 | 2.3 ± 0.1d,e | 1.6d,e | 1.8 ± 0.1d,e |
| Milano (dimer) | 56d,e | 0.8d | 0.9 ± 0.1d,e | 0.9d | 1.9 ± 0.1d,e | 2.0d,e | 2.2 ± 0.1d,e |
Average of at least four measurements which differed by <0.4%. ApoA-I variants in lipid-free state.
Free energy of denaturation listed as a mean ± SD, n = 3–5.
Change in Trp fluorescence intensity at 335 nm.
P < 0.01 compared with WT apoA-I.
P < 0.01 compared with each other.
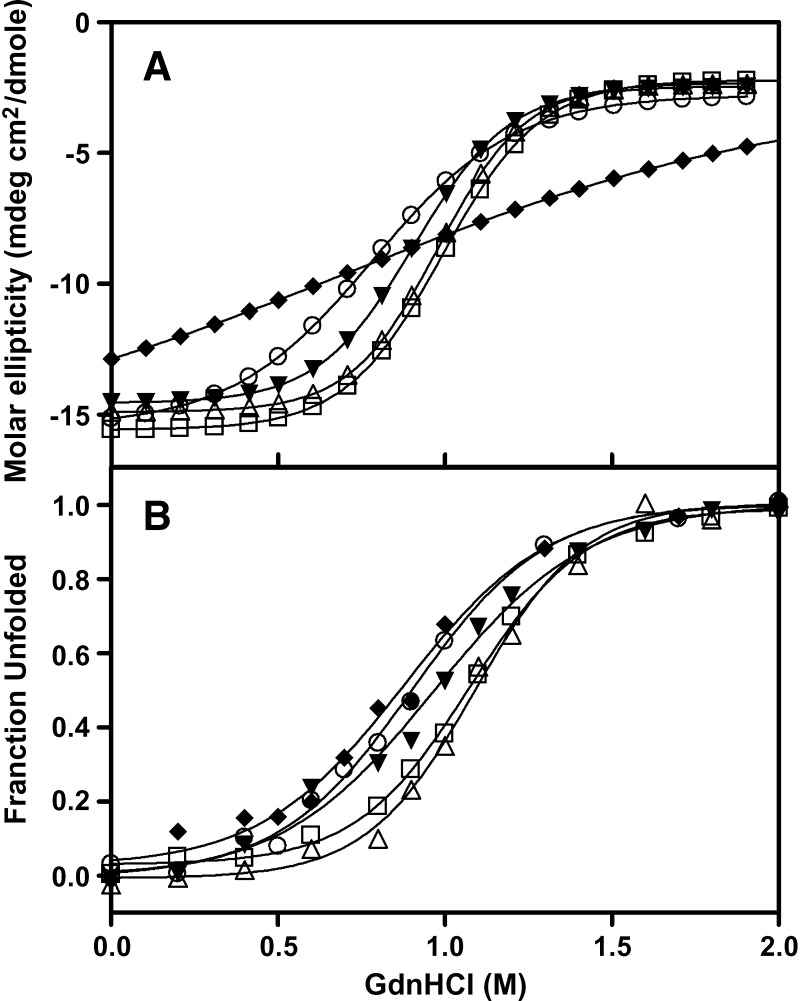
GdnHCl-induced denaturation profiles were monitored by the molar ellipticity at 222 nm as well as by decreases in tryptophan fluorescence emission intensity. These changes in tryptophan fluorescence measure the stability of the N-terminal helix bundle because apoA-I contains four tryptophan residues, all of which are located in the helix bundle domain, whereas molar ellipticity measures the unfolding of all α-helices in the protein molecule. In Fig. 2, the denaturation curves of both monomeric and dimeric apoA-IM as well as R173S apoA-I are shifted to lower concentrations of GdnHCl compared with WT apoA-I, indicating decreased structural stability. The dimeric apoA-IM displayed a decrease in the cooperative nature of unfolding as indicated by the shallowness of its denaturation curve (Fig. 2). The conformational stability, ΔG°D, and the midpoint of GdnHCl denaturation were determined from linear plots of the Gibbs free energy against the GdnHCl concentration and are listed in Table 2. The monomeric and dimeric apoA-IM mutant exhibited a significantly decreased ΔG°D and midpoint of GdnHCl denaturation compared with WT apoA-I, indicating a reduction in stability. Similar to the results for thermal unfolding, R173S apoA-I also demonstrated a decrease in ΔG°D and midpoint of GdnHCl denaturation, whereas R173K apoA-I was no different to WT apoA-I.
Fig. 2.
GdnHCl denaturation of the apoA-I variants monitored by changes in the molar ellipticity at 222 nm and Trp fluorescence intensity. GdnHCl denaturation measured by change in molar ellipticity at 222 nm (A) and the change in tryptophan fluorescence emission intensity at 335 nm (B). WT apoA-I (open squares), apoA-IMilano monomer (open circles), apoA-IMilano dimer (diamonds), R173S apoA-I (closed triangles), and R173K apoA-I (open triangles).
Urea-induced denaturation profiles were monitored by changes in tryptophan fluorescence emission intensity. GdnHCl denaturation measures only the contribution of hydrophobic or nonionic interactions to the stability of a protein due to the fact that electrostatic interactions between amino acids are masked by the ionic denaturant, whereas urea denaturation measures the contributions of both hydrophobic and electrostatic interactions (45). The conformational stability, ΔG°D, and the midpoint of urea denaturation were determined as described in Materials and Methods and are listed in Table 2. Similar to the results for thermal unfolding and GdnHCl denaturation, urea-induced denaturation indicates that R173S apoA-I was less stable than WT apoA-I and R173K apoA-I but more stable than monomeric and dimeric apoA-IM. Of particular interest was the observation that when electrostatic interactions were taken into account (urea denaturation), the monomeric form of apoA-IM was significantly less stable than dimeric form of apoA-IM (Table 2).
ANS binding
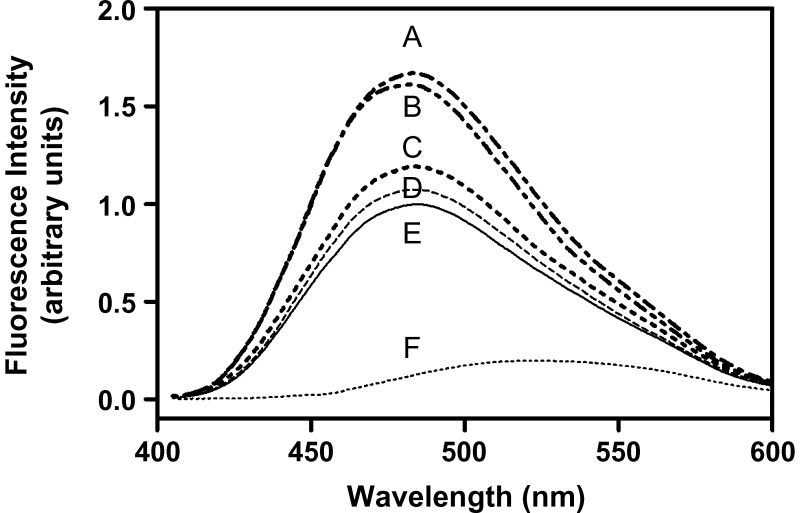
ANS binding studies were performed to compare the exposure of hydrophobic regions in the apoA-I variants to the aqueous environment. When compared with ANS fluorescence in the absence of a protein, WT apoA-I induced a 5-fold increase in fluorescence intensity (Fig. 3), indicating the presence of ANS accessible hydrophobic surface (7, 31, 46, 47). The apoA-IM mutation led to large increases in ANS fluorescence compared with WT apoA-I (Table 1), indicating that the inclusion of a cysteine at position 173 disturbs the tertiary structure of the protein, exposing more hydrophobic surface. The R173S mutation also increased the ANS fluorescence compared with WT apoA-I, but not to the same extent as apoA-IM. The ANS fluorescence with R173K apoA-I was similar to that of WT apoA-I.
Fig. 3.
ANS fluorescence spectra in the presence of apoA-I variants. The extent of ANS binding to hydrophobic sites on the apoA-I variants was determined by measuring ANS fluorescence spectra recorded from 400 to 600 nm at an excitation wavelength of 395 nm, in the absence or presence of 50 μg/ml protein and an excess of ANS. A: apoA-IMilano monomer; B: apoA-IMilano dimer; C: R173S apoA-I; D: R173K apoA-I; E: WT apoA-I; F: free ANS for comparison.
Interactions of apoA-I variants with lipid
We used a DMPC clearance assay to assess the abilities of the apoA-I variants to bind to, and solubilize, DMPC vesicles. As shown in Table 3, only the dimeric form of apoA-IM exhibited slower kinetics of solubilization than WT apoA-I, indicating that the presence of a disulfide bond linking two apoA-IM molecules at position 173 impairs the protein's ability to solubilize the DMPC vesicles. All other variants of apoA-I had the same ability as WT apoA-I to solubilize DMPC vesicles. Earlier studies have given conflicting results regarding the relative abilities of apoA-IM and WT apoA-I to solubilize DMPC vesicles. One group determined that monomeric apoA-IM cleared DMPC vesicles as efficiently as WT apoA-I (43), in agreement with our results. However, a second group determined that monomeric apoA-IM was more efficient at clearing DMPC vesicles than WT apoA-I and that dimeric apoA-IM was as efficient as WT apoA-I (42, 48). These discrepancies may be due to the fact that a single concentration of apoA-I was used, whereas our values were derived using a range of apoA-I concentrations.
TABLE 3.
DMPC clearance rate and ITC parameters of SUV binding of apoA-I variants
| ApoA-I Variants | DMPC Clearance Ratea | ΔH (kcal/mol)b | Decay rateb,c |
|---|---|---|---|
| WT | 1.0 | −92.6 ± 5.3 | 1.0 |
| R173K | 1.2 | −94.7 ± 5.7 | 1.0 |
| R173S | 1.1 | −88.1 ± 5.8d | 1.5 |
| Milano (monomer) | 1.0 | −70.6 ± 5.5d | 2.2 |
| Milano (dimer) | 0.5 | −64.2 ± 3.7d | 2.7 |
Relative rate of DMPC clearance compared with WT apoA-I. Average of 8 to 12 runs per sample.
Parameters of egg PC SUV binding from ITC, mean ± SD (n = 4–6).
Values are ratios to WT apoA-I; estimated error is within ± 0.05. The rate constant for WT apoA-I was 1.19 ± 0.03 min−1.
P < 0.01 compared with WT apoA-I.
To further characterize the interactions of apoA-I variants with lipid, we measured the heats of binding of apoA-I to egg PC SUV using isothermal titration calorimetry (ITC) (7, 49). Because there is no morphological change in the SUV particles when apoA-I binds, the heat change upon apoA-I binding contains contributions from protein-lipid interaction and conformational rearrangement of apoA-I (50). The binding enthalpies at low surface concentrations of the proteins are given in Table 3. The apoA-IM mutation resulted in a decreased binding enthalpy compared with WT apoA-I; the dimeric form of the protein showed a similar reduction to the monomeric form. The R173S apoA-I variant displayed a small but significant decrease in enthalpy compared with WT apoA-I, whereas the R173K mutation had no effect on the binding enthalpy. The exothermic enthalpy of binding is the major component of the favorable free energy of binding of apoA-I to SUV, and the increase in α-helix content associated with such binding is largely responsible for this enthalpy (29, 51). Thus, the reductions in enthalpy compared with WT apoA-I shown in Table 3 are a consequence of a smaller increase in α-helix content upon lipid interaction. The results of CD measurements confirmed that the increase in α-helix content upon SUV binding was less for apoA-IM than for WT apoA-I (Table 1).
Significant differences in the decay rates of the isothermal titration curves were observed between the apoA-I variants. As shown in Table 3, the decay rate was increased in the R173S apoA-I mutant and further increased for the monomeric and dimeric apoA-IM mutant. We also observed strong inverse correlations between the midpoint of thermal denaturation and the ITC decay rate as well as the midpoint of GdnHCl denaturation (Table 2) and the ITC decay rate (data not shown). These results suggest that the stability of the apoA-I molecule modulates its rate of conformational rearrangement on the surface of SUV particles.
In vivo apoA-I and plasma lipid levels
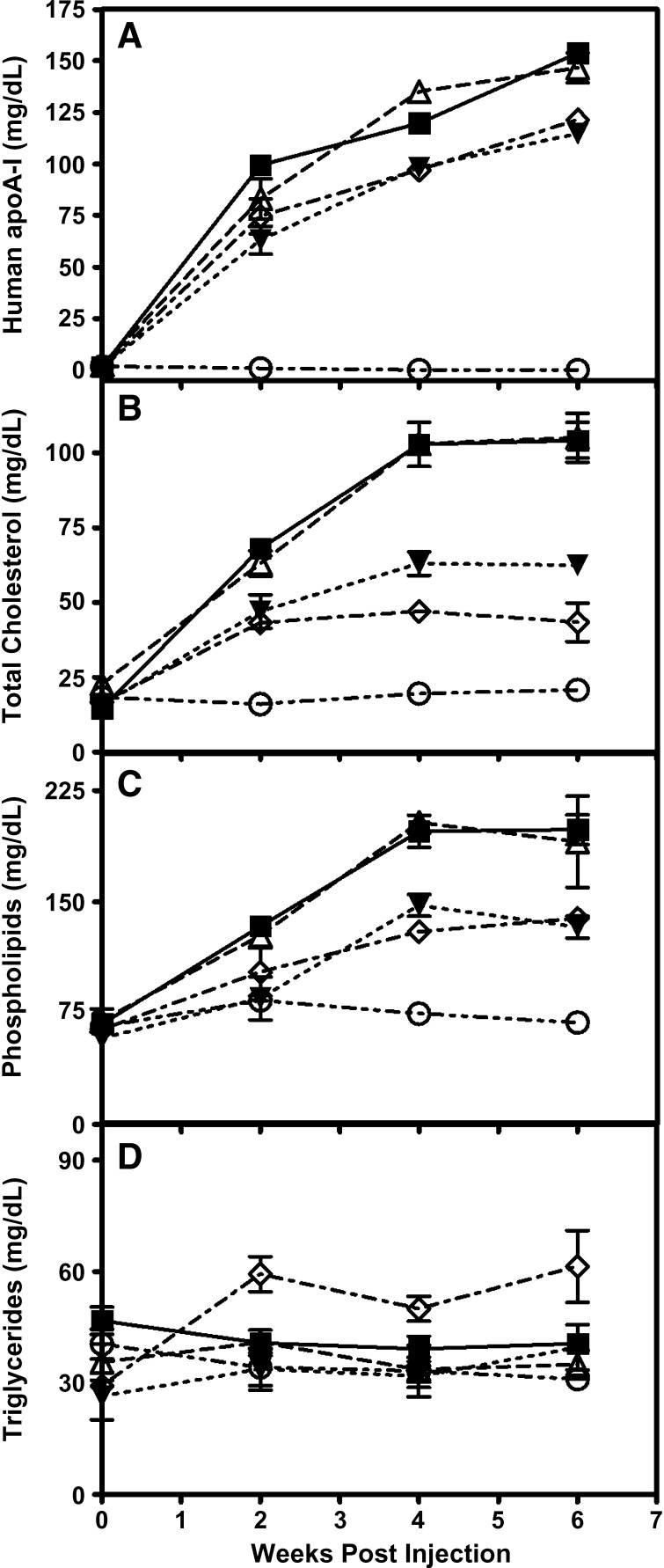
ApoA-I-null mice were injected intraperitoneally with 1 × 1012 viral particles of the appropriate AAV. Six weeks postinjection, plasma from mice expressing the LacZ control had no detectable human apoA-I and very low levels of HDL cholesterol (Table 4, Fig. 4). WT apoA-I-expressing mice had a plasma apoA-I level of 154 mg/dl and a HDL cholesterol level of 80 mg/dl, while apoA-IM-expressing mice had slightly lower plasma apoA-I values, with 43% ± 9% (n = 3) of the apoA-IM being in the dimeric state (Fig. 5). ApoA-IM-expressing mice also had substantially reduced levels of plasma lipids compared with WT apoA-I-expressing mice, with the exception of triglycerides, which were increased (Table 4). The increase in plasma triglycerides in the mice was comparable to the increase in triglycerides seen in the human apoA-IM carriers (52), but the cause of this is undetermined at this time. R173S apoA-I-expressing mice also had reduced HDL and total cholesterol levels compared with WT apoA-I-expressing mice, but higher levels than apoA-IM-expressing mice. R173S apoA-I-expressing mice had a triglyceride level and a percentage of cholesteryl ester in plasma similar to WT apoA-I-expressing mice. R173K apoA-I-expressing mice had similar lipid and apoA-I levels to mice expressing WT apoA-I. At 6 weeks, upon necropsy, hepatic apoA-I mRNA abundance in the WT, apoA-IM, R173S, and R173K apoA-I groups was not significantly different (data not shown). Plasma samples analyzed by FPLC (data not shown) gave HDL peaks that eluted at the same point with intensities proportional to the levels of HDL cholesterol (Table 4).
TABLE 4.
Plasma apoA-I and lipid levels
| AAVa | ApoA-I (mg/dl) | HDL Cholesterol (mg/dl) | Total Cholesterol (mg/dl) | Cholesteryl Ester (%) | Phospholipids (mg/dl) | Triglycerides (mg/dl) |
|---|---|---|---|---|---|---|
| WT apoA-I | 154 ± 4 | 80 ± 13 | 104 ± 13 | 73 ± 2 | 199 ± 23 | 41 ± 5 |
| apoA-IMilano | 121 ± 9b | 31 ± 2b | 44 ± 14b | 65 ± 2b | 139 ± 11b | 61 ± 22 |
| R173S apoA-I | 115 ± 10b | 48 ± 5b | 63 ± 8b | 71 ± 3 | 133 ± 17b | 40 ± 14 |
| R173K apoA-I | 147 ± 16 | 81 ± 13 | 105 ± 18 | 72 ± 2 | 190 ± 30 | 35 ± 9 |
| LacZ | 0 ± 0b | 14 ± 1b | 20 ± 3b | 66 ± 4b | 69 ± 11b | 31 ± 4 |
Respective AAV injected into apoA-I−/− mice with a dose of 1.0 × 1012 viral particles. All data taken from week 6 postinfection (except percentage cholesteryl ester, week 10) and represent a mean ± SD (n = 5).
P < 0.001 compared with WT.
Fig. 4.
Plasma apoA-I and lipid levels in mice expressing the human apoA-I variants. ApoA-I-null mice were infected with 1.0 × 1012 viral particles of AAV expressing: WT apoA-I (squares), apoA-IMilano (diamonds), R173S apoA-I (closed triangles), R173K apoA-I (open triangles), and LacZ as a control (circles). Mice were periodically bled after a 4-h fast and the plasma assayed for human apoA-I levels (A), cholesterol levels (B), phospholipids (C), and triglycerides (D).
Fig. 5.
Western blot analysis of WT and apoA-IM in mouse plasma. Seven weeks after AAV injection, apoA-I-null mice were bled and 1 μl of plasma was run on a 4–12% SDS-PAGE Tris-glycine gel. The samples were transferred to nitrocellulose and Western blotted for human apoA-I. Monomeric apoA-IM had an apparent molecular mass of approximately 28 kDa, while dimeric apoA-IM had a molecular mass of approximately 56 kDa. Nonreduced (A) and reduced (B) conditions.
HDL particle size and composition
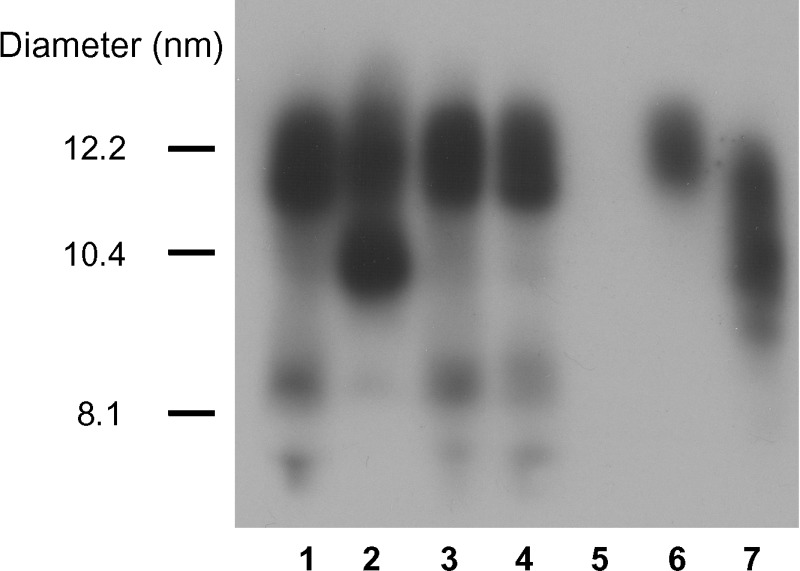
Western blot analysis of plasma using nondenaturing gradient gels revealed that mice expressing WT apoA-I, R173S apoA-I, and R173K apoA-I all produced a single HDL2-sized particle that was approximately 12 nm in diameter (Fig. 6). In contrast, apoA-IM-expressing mice produced equal amounts of two separate HDL particles, an HDL2-sized particle 12 nm in diameter, and a smaller HDL3-sized particle 10 nm in diameter. HDL compositions were obtained by treating plasma samples with polyethylene glycol to precipitate any apoB-containing lipoproteins. Subsequent analysis of the samples indicated that the HDL lipid compositions of all the variants were remarkably similar on a molar basis (Table 5) despite the difference in size of the apoA-IM HDL particle. Analysis of the protein composition of isolated HDL from WT apoA-I-expressing mice indicated that apoA-I was the only protein with no apoA-II or apoE detected (Fig. 7). For HDL isolated from apoA-IM-expressing mice, apoA-I accounted for approximately 95% of the total protein and a small amount of apoE was present (as analyzed by scanning densitometry using Image J version 1.31).
Fig. 6.
Particle size analysis of HDL from mice expressing the human apoA-I variants. Three weeks after AAV injection, apoA-I-null mice were bled and 1 μl of plasma was run on a 4–20% Tris-glycine nondenaturing gradient gel. The samples were transferred to nitrocellulose and Western blotted for human apoA-I. Lane 1, WT apoA-I; lane 2, apoA-IMilano; lane 3, R173S apoA-I; lane 4, R173K apoA-I; lane 5, LacZ; lane 6, human HDL2; lane 7, human HDL3.
TABLE 5.
Lipid compositions of HDL in mice expressing human ApoA-I variants
| HDLa | Phospholipid:apoA-I (mol:mol) | Free Cholesterol:apoA-I (mol:mol) | Cholesteryl Ester:apoA-I (mol:mol) | Triglyceride:apoA-I (mol:mol) |
|---|---|---|---|---|
| WT | 70 ± 24 | 9 ± 1 | 26 ± 9 | 1 ± 1 |
| Milano | 66 ± 14 | 11 ± 1 | 30 ± 10 | 2 ± 2 |
| R173S | 59 ± 13 | 6 ± 2 | 21 ± 4 | 1 ± 1 |
| R173K | 64 ± 11 | 7 ± 1 | 27 ± 9 | 1 ± 1 |
Respective AAV injected into apoA-I−/− mice with a dose of 1.0 × 1012 viral particles. All data taken from week 6 postinfection and represent a mean ± SD (n = 3–7).
Fig. 7.
Protein composition analysis of isolated WT and apoA-IM HDL. HDL was isolated from the plasma of mice expressing WT apoA-I and apoA-IM by ultracentrifugation and analyzed on a reducing 8–25% SDS-PAGE gel. Lane 1, WT apoA-I HDL; lane 2, apoA-IM HDL; lane 3, human apoA-I; lane 4, human apoA-II; lane 5, mouse apoE.
DISCUSSION
Heterozygous carries of the apoA-IM mutation have reduced plasma levels of apoA-I and HDL cholesterol but do not display the increase in cardiovascular disease that would be expected for such levels (10). This observation has led to intense interest in the apoA-IM mutation as a potential therapeutic agent to treat cardiovascular disease (25, 26). However, a fundamental understanding of why apoA-IM behaves as it does is lacking. To determine if the removal of the arginine at position 173 (and loss of positive charge) or the introduction of a cysteine residue (with the potential for disulfide bond formation) was responsible for the altered functional aspects of apoA-IM, we generated two engineered variants of apoA-IM with different residues at position 173. The first, R173S apoA-I, is a mutant structurally similar to apoA-IM but, unlike the sulfhydryl group of the cysteine residue in apoA-IM that has the ability to form disulfide bonds with other cysteine residues, the hydroxyl group of the serine in R173S apoA-I is unable to do so. The second mutation, R173K apoA-I, is a conservative mutation that maintains the positive charge at position 173 of WT apoA-I.
Effects of mutations at position 173 on apoA-I structure and lipid binding properties
Analysis of the X-ray crystal structure of lipid-free apoA-I (8) using the Protein Explorer 2.8 software (University of Massachusetts, www.proteinexplorer.org) indicates that arginine 173 forms an intrahelical salt bridge with glutamic acid 169 in the N-terminal helical bundle domain of the protein. Assuming that the α-helix spanning residue 173 is also present in a lipid-free apoA-I molecule in dilute solution, it follows that this ion pair between the two residues separated by a single turn of helix is likely to be maintained. Thus, the substitution of an uncharged serine residue for the positively charged arginine residue in the R173S apoA-I mutation would be expected to disrupt the salt bridge that is present in the WT protein and thereby destabilize the protein. On the other hand, maintenance of the positive charge by introduction of a lysine residue would be expected to have no effect on the interaction. Elimination of the positive charge by the R173S mutation alters the structure and stability of the protein, as indicated by reductions in the midpoints of thermal, GdnHCl, and urea denaturation (Table 2, Figs. 1 and 2) and increases in exposed hydrophobic regions measured by ANS fluorescence (Table 1, Fig. 3). Substituting a cysteine for the arginine in the apoA-IM mutation further destabilizes the protein. At physiological pH, the cysteine residue is slightly negatively charged (pKa = 8.3), unlike the arginine residue, which is positively charged (pKa = 12.5), or the serine residue, which has no charge (i.e., it is nonionizable). It can be inferred that the slightly negatively charged sulfhydryl group in the cysteine residue actively repels the negatively charged glutamic acid residue 169 that would form a salt bridge with the arginine at position 173 in the WT protein. This inference is validated by the fact that thermal denaturation and urea denaturation, both of which take into account electrostatic interactions, show that monomeric apoA-IM is less stable than dimeric apoA-IM (Table 2), indicating the presence of a destabilizing electrostatic interaction in monomeric apoA-IM that is removed when the covalent disulfide bond is formed in dimeric apoA-IM at position 173. However, when monomeric and dimeric apoA-IM are denatured by GdnHCl, which only measures the stability conferred by hydrophobic and nonionic interactions, monomeric apoA-IM is more stable than dimeric apoA-IM. The presence of the cysteine residue at position 173 significantly alters the structure and decreases the stability of the monomeric R173C variant compared with the R173S variant, as reflected by the changes in the denaturation parameters listed in Table 2. Further support for the involvement of position 173 in a stabilizing salt bridge in WT apoA-I comes from the observation that reversal of the charge by the mutation R173E reduces the midpoint of thermal denaturation to 51°C (data not shown), which is lower than the value for monomeric apoA-IM (Table 2). The above considerations of the electrostatic interactions of R173 are likely to also apply to apoA-I in the lipid-bound state. Thus, analysis using Protein Explorer 2.8 of the crystal structure of a truncated form of apoA-I, apoA-I Δ1-43, which adopts a lipid bound-like conformation (53), indicates that the intrahelix R173-E169 interaction also occurs in this state.
When apoA-I binds to lipid or lipoprotein particles, the C-terminal domain binds first accompanied by an increase in α-helix content in this region (54). Subsequently, α-helices in the N-terminal region of the apoA-I molecule interact with lipid (55, 56). In light of the two-domain tertiary structure of apoA-I (7, 8, 57), we have proposed a two-step mechanism for apoA-I binding to a lipid surface; the initial C-terminal domain binding is followed by opening of the helix bundle of the N-terminal domain and interaction of these α-helices with lipid (7, 54, 58). The apoA-IM mutation, and the engineered R173 variants, are located in the N-terminal domain of apoA-I and, as such, affect the ability of apoA-I to interact with lipid particles by influencing the stability of the α-helix bundle. Consistent with this concept, the decreased stability of the N-terminal helix bundle domain in the R173S and R173C apoA-I variants (Table 2) leads to enhanced rates of interaction of α-helices in this domain with the phospholipid particle surface (see the enhanced enthalpy decay rates listed in Table 3). Furthermore, the binding enthalpies listed in Table 3 indicate that the affinity of apoA-I for the surface of a stable egg PC SUV is reduced by nonconservative amino acid substitutions at position 173. Thus, the R173S and R173C variants are expected to bind less well to the phospholipid surface of HDL particles so that their presence in the lipid-free/poor pool of apoA-I is increased.
The lipid binding properties of apoA-I are also important for the formation of nascent HDL particles via the ABCA1 reaction. In this process, the solubilization of exovesiculated domains of cell plasma membrane is rate-limiting for the formation of discoidal HDL particles (59). Consequently, the effects of variations in the apoA-I molecule on the ability to solubilize phospholipid bilayers (as in DMPC multilamellar vesicles) are correlated with the ability to efflux cellular lipids via the ABCA1 pathway and create nascent HDL particles. Since the substitution of arginine at position 173 in the apoA-I molecule with lysine, serine, or cysteine has no effect on the ability of the proteins in the monomeric state to solubilize DMPC multilamellar vesicles (Table 3), it follows that these variants should all participate equally well in the ABCA1 reaction to form nascent HDL particles. Indeed, earlier work demonstrated that monomeric apoA-IM is as effective as WT apoA-I at forming HDL particles via the ABCA1 reaction (60). It is not known how dimeric apoA-IM participates in the ABCA1 reaction, but the fact that the dimeric form of apoA-IM had a 2-fold slower rate of clearance compared with WT apoA-I is probably due to steric hindrances imposed by the disulfide bond. In the DMPC clearance assay, apoA-I binds to the lipid surface, undergoes a conformational change, and then solubilizes a portion of the lipid (36). It follows that the rate of formation of nascent HDL particles containing dimeric apoA-IM via the ABCA1 reaction may be relatively slow in vivo, contributing to the reduction in plasma apoA-IM levels (Table 4). This effect occurs either because of steric hindrance to helix bundle opening associated with the presence of the disulfide bond or perhaps because of interference between the two molecules in the apoA-IM dimer as they bind to the lipid surface. In vivo, approximately 40% of apoA-IM generated via AAV-mediated gene transfer is in the dimeric state (Fig. 5) and presumably would have lipid interactions altered in this fashion.
Effects of mutations of R173 in apoA-I on HDL composition
AAVs were generated for each apoA-I variant and subsequently used to infect apoA-I-null mice. ApoA-I-null mice were used to maintain the simplicity of the system and allow the study of the apoA-IM mutation without the confounding factors of the presence of murine apoA-I, WT human apoA-I, or other potential heterodimer partners of apoA-IM, such as human apoA-II and apoE3; murine apoA-II and apoE do not contain cysteine residues and thus cannot form covalently bonded heterodimers with apoA-IM. Thus, this in vivo system was not intended to recreate the situation in human apoA-IM probands but rather give a model system to elucidate apoA-I structure-function relationships. Plasma apoA-I levels for WT apoA-I and the R173K variant were similar, but mice with the R173S and R173C variants had lower levels of apoA-I compared with WT apoA-I (Fig. 4, Table 4). Real-time PCR has shown that AAV induces equal amounts of the WT apoA-I and apoA-I variant mRNA in mouse livers (data not shown), indicating that production of WT apoA-I and the apoA-I variants is similar. As previously discussed, the formation of nascent HDL particles via the ABCA1 reaction is expected to be normal for all of the monomeric apoA-I variants studied, leading to the conclusion that the lower plasma apoA-I level of the R173S variant may be due to enhanced turnover of plasma apoA-I by clearance in the kidney (61–63). The reduced lipid affinity of the R173S and R173C apoA-I variants results in a larger pool of lipid-free apoA-I that is susceptible to rapid catabolism, as has been previously shown for apoA-IM (64). As discussed above, the ABCA1 reaction for dimeric apoA-IM may also be slower, contributing to the lower plasma apoA-IM levels.
The HDL lipid particle compositions for all of the apoA-I variants were very similar (Table 5) as were the protein compositions (Fig. 7). However, apoA-IM HDL had a bimodal size distribution with equal amounts of HDL2 and HDL3, whereas all other apoA-I variants formed primarily HDL2 (Fig. 6). The smaller apoA-IM HDL particle size is likely due to the presence of apoA-IM homodimer, which accounts for approximately 40% of apoA-IM mass in mice expressing apoA-IM via AAV-mediated gene transfer (Fig. 5) and not due to other apoproteins being present (Fig. 7). WT apoA-I can form HDL with two, three, four, or more apoA-I molecules per particle, while dimeric apoA-IM can only generate HDL particles with even numbers of apoA-I molecules (two, four, six, etc.), which is expected to constrain the HDL particle size possibilities. Consistent with this, previous work showed that apoA-IM restricts HDL particle size in vivo (64) and that this restriction is due to the formation of dimeric apoA-IM (65).
Whether or not alterations in HDL caused by the R173C mutation alter the anti-atherogenic properties of HDL compared with WT apoA-I is still controversial. Several recent mouse studies have reached conflicting results. An apoA-IM knockin mouse strain was crossed with an atherosclerosis-susceptible human apoB/apoA-II mouse model, and apoA-IM was found to have no protective advantage over WT apoA-I (23). However, a study in which bone marrow transplantation in apoA-I and apoE double-knockout mice using bone marrow that had been transduced with a retroviral vector expressing WT apoA-I or apoA-IM demonstrated that apoA-IM reduced atherosclerosis to a greater extent than WT apoA-I (19). However, when LDL receptor-deficient mice were infected with AAV expressing either WT apoA-I or apoA-IM and fed a Western diet for 8 weeks, there was no difference in lesion development (24).
In summary, the removal of the arginine residue and the introduction of the cysteine residue in the apoA-IM mutation contribute to the altered physical properties and in vivo metabolism of the protein. Both alterations involved in the apoA-IM mutation help to destabilize the protein and modify the interactions with lipids, resulting in lower plasma HDL cholesterol and apoA-I values in vivo, as well as an altered HDL particle size distribution.
Abbreviations
AAV, adeno-associated virus
apo, apolipoprotein
ANS, 8-anilino-1-naphthalensulfonic acid
CD, circular dichroism
DMPC, dimyristoylphosphatidylcholine
GdnHCl, guanidine hydrochloride
ITC, isothermal titration calorimetry
PC, phosphatidylcholine
SUV, small unilamellar vesicles
Trx, thioredoxin
WT, wild type
This work was supported by American Heart Association Grant 0625372U, National Institutes of Health Grant HL-22633, the Takeda Science Foundation, and the Suzuken Memorial Foundation.
Published, JLR Papers in Press, March 24, 2009.
References
- 1.Davidson W. S., and T. B. Thompson. 2007. The structure of apolipoprotein A-I in high density lipoproteins. J. Biol. Chem. 282 22249–22253. [DOI] [PubMed] [Google Scholar]
- 2.Lewis G. F., and D. J. Rader. 2005. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 96 1221–1232. [DOI] [PubMed] [Google Scholar]
- 3.Yancey P. G., A. E. Bortnick, G. Kellner-Weibel, M. De La Llera-Moya, M. C. Phillips, and G. H. Rothblat. 2003. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23 712–719. [DOI] [PubMed] [Google Scholar]
- 4.Curtiss L. K., D. T. Valenta, N. J. Hime, and K. A. Rye. 2006. What is so special about apolipoprotein AI in reverse cholesterol transport? Arterioscler. Thromb. Vasc. Biol. 26 12–19. [DOI] [PubMed] [Google Scholar]
- 5.Weisgraber K. H., S. C. Rall, T. P. Bersot, R. W. Mahley, G. Franceschini, and C. R. Sirtori. 1983. Apolipoprotein A-I milano - detection of normal A-I in affected subjects and evidence for a cysteine for arginine substitution in the variant A-I. J. Biol. Chem. 258 2508–2513. [PubMed] [Google Scholar]
- 6.Rocco A. G., L. Mollica, E. Gianazza, L. Calabresi, G. Franceschini, C. R. Sirtori, and I. Eberini. 2006. A model structure for the heterodimer apoA-I-Milano-apoA-II supports its peculiar susceptibility to proteolysis. Biophys. J. 91 3043–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito H., P. Dhanasekaran, D. Nguyen, P. Holvoet, S. Lund-Katz, and M. C. Phillips. 2003. Domain structure and lipid interaction in human apolipoproteins A-I and E, a general model. J. Biol. Chem. 278 23227–23232. [DOI] [PubMed] [Google Scholar]
- 8.Ajees A. A., G. M. Anantharamaiah, V. K. Mishra, M. M. Hussain, and H. M. K. Murthy. 2006. Crystal structure of human apolipoprotein A-I: insights into its protective effect against cardiovascular diseases. Proc. Natl. Acad. Sci. USA. 103 2126–2131. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Sorci-Thomas M. G., and M. J. Thomas. 2002. The effects of altered apolipoprotein A-I structure on plasma HDL concentration. Trends Cardiovasc. Med. 12 121–128. [DOI] [PubMed] [Google Scholar]
- 10.Sirtori C. R., L. Calabresi, G. Franceschini, D. Baldassarre, M. Amato, J. Johansson, M. Salvetti, C. Monteduro, R. Zulli, M. L. Muiesan, et al. 2001. Cardiovascular status of carriers of the apolipoprotein A-IMilano mutant - the Limone sul Garda study. Circulation. 103 1949–1954. [DOI] [PubMed] [Google Scholar]
- 11.Calabresi L., G. Franceschini, A. Burkybile, and A. Jonas. 1997. Activation of lecithin cholesterol acyltransferase by a disulfide-linked apolipoprotein A-I dimer. Biochem. Biophys. Res. Commun. 232 345–349. [DOI] [PubMed] [Google Scholar]
- 12.Roma P., R. E. Gregg, M. S. Meng, R. Ronan, L. A. Zech, G. Franceschini, C. R. Sirtori, and H. B. Brewer, Jr. 1993. In vivo metabolism of a mutant form of apolipoprotein A-I, apo A-IMilano, associated with familial hypoalphalipoproteinemia. J. Clin. Invest. 91 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Mendez O., E. Bruckert, G. Franceschini, N. Duhal, B. Lacroix, J. P. Bonte, C. Sirtori, J. C. Fruchart, G. Turpin, and G. Luc. 2000. Metabolism of apolipoproteins AI and AII in subjects carrying similar apoAI mutations, apoAI Milano and apoAI Paris. Atherosclerosis. 148 317–326. [DOI] [PubMed] [Google Scholar]
- 14.Gualandri V., G. Franceschini, C. R. Sirtori, G. Gianfranceschi, G. B. Orsini, A. Cerrone, and A. Menotti. 1985. AIMilano apoprotein identification of the complete kindred and evidence of a dominant genetic transmission. Am. J. Hum. Genet. 37 1083–1097. [PMC free article] [PubMed] [Google Scholar]
- 15.Gomaraschi M., D. Baldassarre, M. Amato, S. Eligini, P. Conca, C. R. Sirtori, G. Franceschini, and L. Calabresi. 2007. Normal vascular function despite low levels of high-density lipoprotein cholesterol in carriers of the apolipoprotein A-I-Milano mutant. Circulation. 116 2165–2172. [DOI] [PubMed] [Google Scholar]
- 16.Franceschini G., L. Calabresi, G. Chiesa, C. Parolini, C. R. Sirtori, M. Canavesi, and F. Bernini. 1999. Increased cholesterol efflux potential of sera from ApoA-IMilano carriers and transgenic mice. Arterioscler. Thromb. Vasc. Biol. 19 1257–1262. [DOI] [PubMed] [Google Scholar]
- 17.Calabresi L., M. Canavesi, F. Bernini, and G. Franceschini. 1999. Cell cholesterol efflux to reconstituted high-density lipoproteins containing the apolipoprotein A-IMilano dimer. Biochemistry. 38 16307–16314. [DOI] [PubMed] [Google Scholar]
- 18.Bielicki J. K., and M. N. Oda. 2002. Apolipoprotein A-IMilano and apolipoprotein A-IParis exhibit an antioxidant activity distinct from that of wild-type apolipoprotein A-I. Biochemistry. 41 2089–2096. [DOI] [PubMed] [Google Scholar]
- 19.Wang L., B. G. Sharifi, T. Pan, L. Song, A. Yukht, and P. K. Shah. 2006. Bone marrow transplantation shows superior atheroprotective effects of gene therapy with apolipoprotein A-I Milano compared with wild-type apolipoprotein A-I in hyperlipidemic mice. J. Am. Coll. Cardiol. 48 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parolini C., M. Marchesi, P. Lorenzon, M. Castano, E. Balconi, L. Miragoli, L. Chaabane, A. Morisetti, V. Lorusso, B. J. Martin, et al. 2008. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A-I-Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques - In vivo assessment by intravascular ultrasound and magnetic resonance imaging. J. Am. Coll. Cardiol. 51 1098–1103. [DOI] [PubMed] [Google Scholar]
- 21.Ibanez B., G. Vilahur, G. Cimmino, W. S. Speidl, A. Pinero, B. G. Choi, M. U. Zafar, C. G. Santos-Gallego, B. Krause, L. Badirnon, et al. 2008. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein A-I-Milano (ETC-216) administration - magnetic resonance imaging study in an experimental model of atherosclerosis. J. Am. Coll. Cardiol. 51 1104–1109. [DOI] [PubMed] [Google Scholar]
- 22.Marchesi M., E. A. Booth, G. Rossoni, R. A. Garcia, K. R. Hill, C. R. Sirtori, C. L. Bisgaier, and B. R. Lucchesi. 2008. Apolipoprotein A-I-Milano/POPC complex attenuates post-ischemic ventricular dysfunction in the isolated rabbit heart. Atherosclerosis. 197 572–578. [DOI] [PubMed] [Google Scholar]
- 23.Parolini C., G. Chiesa, E. Gong, S. Caligari, M. M. Cortese, T. Koga, T. M. Forte, and E. M. Rubin. 2005. Apolipoprotein A-I and the molecular variant apoA-IMilano: evaluation of the antiatherogenic effects in knock-in mouse model. Atherosclerosis. 183 222–229. [DOI] [PubMed] [Google Scholar]
- 24.Lebherz C., J. Sanmiguel, J. Wilson, and D. Rader. 2007. Gene transfer of wild-type apoA-I and apoA-I Milano reduce atherosclerosis to a similar extent. Cardiovasc. Diabetol. 6 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nissen S. E., T. Tsunoda, E. M. Tuzcu, P. Schoenhagen, C. J. Cooper, M. Yasin, G. M. Eaton, M. A. Lauer, W. S. Sheldon, C. L. Grines, et al. 2003. Effect of recombinant apoA-I milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 290 2292–2300. [DOI] [PubMed] [Google Scholar]
- 26.Nicholls S. J., E. M. Tuzcu, T. Crowe, I. Sipahi, P. Schoenhagen, S. Kapadia, S. L. Hazen, C. C. Wun, M. Norton, F. Ntanios, et al. 2006. Relationship between cardiovascular risk factors and atherosclerotic disease burden measured by intravascular ultrasound. J. Am. Coll. Cardiol. 47 1967–1975. [DOI] [PubMed] [Google Scholar]
- 27.Kitajima K., D. H. L. Marchadier, H. Burstein, and D. J. Rader. 2006. Persistent liver expression of murine apoA-1 using vectors based on adeno-associated viral vectors serotypes 5 and 1. Atherosclerosis. 186 65–73. [DOI] [PubMed] [Google Scholar]
- 28.Morrow J. A., K. S. Arnold, and K. H. Weisgraber. 1999. Functional characterization of apolipoprotein E isoforms overexpressed in Escherichia coli. Protein Expr. Purif. 16 224–230. [DOI] [PubMed] [Google Scholar]
- 29.Saito H., P. Dhanasekaran, D. Nguyen, E. Deridder, P. Holvoet, S. Lund-Katz, and M. C. Phillips. 2004. Alpha-helix formation Is required for high affinity binding of human apolipoprotein A-I to lipids. J. Biol. Chem. 279 20974–20981. [DOI] [PubMed] [Google Scholar]
- 30.Morrisett J. D., J. S. David, H. J. Pownall, and A. M. Gotto, Jr. 1973. Interaction of an apolipoprotein (Apolp-Alanine) with phosphatidylcholine. Biochemistry. 12 1290–1299. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M., P. Dhanasekaran, D. Nguyen, S. Ohta, S. Lund-Katz, M. C. Phillips, and H. Saito. 2006. Contributions of the N- and C-terminal helical segments to the lipid-free structure and lipid interaction of apolipoprotein A-I. Biochemistry. 45 10351–10358. [DOI] [PubMed] [Google Scholar]
- 32.Acharya P., M. L. Segall, M. Zaiou, J. Morrow, K. H. Weisgraber, M. C. Phillips, S. Lund-Katz, and J. Snow. 2002. Comparison of the stabilities and unfolding pathways of human apolipoprotein E isoforms by differential scanning calorimetry and circular dichroism. Biochim. Biophys. Acta. 1584 9–19. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka M., C. Vedhachalam, T. Sakamoto, P. Dhanasekaran, M. C. Phillips, S. Lund-Katz, and H. Saito. 2006. Effect of carboxyl-terminal truncation on structure and lipid interaction of human apolipoprotein E4. Biochemistry. 45 4240–4247. [DOI] [PubMed] [Google Scholar]
- 34.Eftink M. R. 2000. Use of fluorescence spectroscopy as thermodynamics tool. Methods Enzymol. 323 459–473. [DOI] [PubMed] [Google Scholar]
- 35.Sparks D. L., S. Lund-Katz, and M. C. Phillips. 1992. The charge and structural stability of apolipoprotein A-I in discoidal and spherical recombinant high density lipoprotein particles. J. Biol. Chem. 267 25839–25847. [PubMed] [Google Scholar]
- 36.Segall M. L., P. Dhanasekaran, F. Baldwin, G. M. Anantharamaiah, K. H. Weisgraber, M. C. Phillips, and S. Lund-Katz. 2002. Influence of apoE domain structure and polymorphism on the kinetics of phospholipid vesicle solubilization. J. Lipid Res. 43 1688–1700. [DOI] [PubMed] [Google Scholar]
- 37.Kitajima K., D. H. L. Marchadier, G. C. Miller, G. P. Gao, J. M. Wilson, and D. J. Rader. 2006. Complete prevention of atherosclerosis in ApoE-deficient mice by hepatic human ApoE gene transfer with adeno-associated virus serotypes 7 and 8. Arterioscler. Thromb. Vasc. Biol. 26 1852–1857. [DOI] [PubMed] [Google Scholar]
- 38.Sandalon Z., E. M. Bruckheimer, K. H. Lustig, L. C. Rogers, R. W. Peluso, and H. Burstein. 2004. Secretion of a TNFR: Fc fusion protein following pulmonary administration of pseudotyped adeno-associated virus vectors. J. Virol. 78 12355–12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao G., L. H. Vandenberghe, and J. M. Wilson. 2005. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 5 285–297. [DOI] [PubMed] [Google Scholar]
- 40.Asztalos B. F., M. de la Llera-Moya, G. E. Dallal, K. V. Horvath, E. J. Schaefer, and G. H. Rothblat. 2005. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J. Lipid Res. 46 2246–2253. [DOI] [PubMed] [Google Scholar]
- 41.Lund-Katz S., and M. C. Phillips. 1986. Packing of cholesterol molecules in human low-density-lipoprotein. Biochemistry. 25 1562–1568. [DOI] [PubMed] [Google Scholar]
- 42.Franceschini G., G. Vecchio, G. Gianfranceschi, D. Magani, and C. R. Sirtori. 1985. Apolipoprotein AI Milano. Accelerated binding and dissociation from lipids of a human apolipoprotein variant. J. Biol. Chem. 260 16321–16325. [PubMed] [Google Scholar]
- 43.Zhu X., G. Wu, W. Zeng, H. Xue, and B. Chen. 2005. Cysteine mutants of human apolipoprotein A-I: a study of secondary structural and functional properties. J. Lipid Res. 46 1303–1311. [DOI] [PubMed] [Google Scholar]
- 44.Calabresi L., G. Vecchio, R. Longhi, E. Gianazza, G. Palm, H. Wadensten, A. Hammarström, A. Olsson, A. Karlström, T. Sejlitz, et al. 1994. Molecular characterization of native and recombinant apolipoprotein A-IMilano dimer. The introduction of an interchain disulfide bridge remarkably alters the physicochemical properties of apolipoprotein A-I. J. Biol. Chem. 269 32168–32174. [PubMed] [Google Scholar]
- 45.Monera O. D., C. M. Kay, and R. S. Hodges. 1994. Protein denaturation with guanidine-hydrochloride or urea provides a different estimate of stability depending on the contributions of electrostatic interactions. Protein Sci. 3 1984–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beckstead J. A., B. L. Block, J. K. Bielicki, C. M. Kay, M. N. Oda, and R. O. Ryan. 2005. Combined N- and C-terminal truncation of human apolipoprotein A-I yields a folded, functional central domain. Biochemistry. 44 4591–4599. [DOI] [PubMed] [Google Scholar]
- 47.Rogers D. P., C. G. Brouillette, J. A. Engler, S. W. Tendian, L. Roberts, V. K. Mishra, G. M. Anantharamaiah, S. Lund-Katz, M. C. Phillips, and M. J. Ray. 1997. Truncation of the amino terminus of human apolipoprotein A-I substantially alters only the lipid-free conformation. Biochemistry. 36 288–300. [DOI] [PubMed] [Google Scholar]
- 48.Calabresi L., G. Vecchio, F. Frigerio, L. Vavassori, C. R. Sirtori, and G. Franceschini. 1997. Reconstituted high-density lipoproteins with a disulfide-linked apolipoprotein A-I dimer: evidence for restricted particle size heterogeneity. Biochemistry. 36 12428–12433. [DOI] [PubMed] [Google Scholar]
- 49.Saito H., P. Dhanasekaran, F. Baldwin, K. H. Weisgraber, S. Lund-Katz, and M. C. Phillips. 2001. Lipid binding-induced conformational change in human apolipoprotein E - evidence for two lipid-bound states on spherical particles. J. Biol. Chem. 276 40949–40954. [DOI] [PubMed] [Google Scholar]
- 50.Derksen A., D. Gantz, and D. M. Small. 1996. Calorimetry of apolipoprotein-A1 binding to phosphatidylcholine-triolein-cholesterol emulsions. Biophys. J. 70 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnulphi C., L. H. Jin, M. A. Tricerri, and A. Jonas. 2004. Enthalpy-driven apolipoprotein A-I and lipid bilayer interaction indicating protein penetration upon lipid binding. Biochemistry. 43 12258–12264. [DOI] [PubMed] [Google Scholar]
- 52.Franceschini G., C. R. Sirtori, E. Bosisio, V. Gualandri, G. B. Orsini, A. M. Mogavero, and A. Capurso. 1985. Relationship of the phenotypic-expression of the A-Imilano apoprotein with plasma-lipid and lipoprotein patterns. Atherosclerosis. 58 159–174. [DOI] [PubMed] [Google Scholar]
- 53.Borhani D. W., D. P. Rogers, J. A. Engler, and C. G. Brouillette. 1997. Crystal structure of truncated human apolipoprotein A-I suggests a lipid-bound conformation. Proc. Natl. Acad. Sci. USA. 94 12291–12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oda M. N., T. M. Forte, R. O. Ryan, and J. C. Voss. 2003. The C-terminal domain of apolipoprotein A-I contains a lipid-sensitive conformational trigger. Nat. Struct. Biol. 10 455–460. [DOI] [PubMed] [Google Scholar]
- 55.Rogers D. P., L. M. Roberts, J. Lebowitz, G. Datta, G. M. Anantharamaiah, J. A. Engler, and C. G. Brouillette. 1998. The lipid-free structure of apolipoprotein A-I: effects of amino-terminal deletions. Biochemistry. 37 11714–11725. [DOI] [PubMed] [Google Scholar]
- 56.Rogers D. P., L. M. Roberts, J. Lebowitz, J. A. Engler, and C. G. Brouillette. 1998. Structural analysis of apolipoprotein A-I: effects of amino- and carboxy-terminal deletions on the lipid-free structure. Biochemistry. 37 945–955. [DOI] [PubMed] [Google Scholar]
- 57.Saito H., S. Lund-Katz, and M. C. Phillips. 2004. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog. Lipid Res. 43 350–380. [DOI] [PubMed] [Google Scholar]
- 58.Kono M., Y. Okumura, M. Tanaka, D. Nguyen, P. Dhanasekaran, S. Lund-Katz, M. C. Phillips, and H. Saito. 2008. Conformational flexibility of the N-terminal domain of apolipoprotein A-I bound to spherical lipid particles. Biochemistry. 47 11340–11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vedhachalam C., P. T. Duong, M. Nickel, D. Nguyen, P. Dhanasekaran, H. Saito, G. H. Rothblat, S. Lund-Katz, and M. C. Phillips. 2007. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J. Biol. Chem. 282 25123–25130. [DOI] [PubMed] [Google Scholar]
- 60.Weibel G. L., E. T. Alexander, M. R. Joshi, D. J. Rader, S. Lund-Katz, M. C. Phillips, and G. H. Rothblat. 2007. Wild-type ApoA-I and the Milano variant have similar abilities to stimulate cellular lipid mobilization and efflux. Arterioscler. Thromb. Vasc. Biol. 27 2022–2029. [DOI] [PubMed] [Google Scholar]
- 61.Timmins J. M., J. Y. Lee, E. Boudyguina, K. D. Kluckman, L. R. Brunham, A. Mulya, A. K. Gebre, J. M. Coutinho, P. L. Colvin, T. L. Smith, et al. 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moestrup S. K., and L. B. Nielsen. 2005. The role of the kidney in lipid metabolism. Curr. Opin. Lipidol. 16 301–306. [DOI] [PubMed] [Google Scholar]
- 63.Lee J. Y., J. M. Timmins, A. Mulya, T. L. Smith, Y. W. Zhu, E. M. Rubin, J. W. Chisholm, P. L. Colvin, and J. S. Parks. 2005. HDLs in apoA-I transgenic Abca1 knockout mice are remodeled normally in plasma but are hypercatabolized by the kidney. J. Lipid Res. 46 2233–2245. [DOI] [PubMed] [Google Scholar]
- 64.Bielicki J. K., T. M. Forte, M. R. McCall, L. J. Stoltzfus, G. Chiesa, C. R. Sirtori, G. Franceschini, and E. M. Rubin. 1997. High density lipoprotein particle size restriction in apolipoprotein A-IMilano transgenic mice. J. Lipid Res. 38 2314–2321. [PubMed] [Google Scholar]
- 65.Favari E., M. Gomaraschi, I. Zanotti, F. Bernini, M. Lee-Rueckert, P. T. Kovanen, C. R. Sirtori, G. Franceschini, and L. Calabresi. 2007. A unique protease-sensitive high density lipoprotein particle containing the apolipoprotein A-I-Milano dimer effectively promotes ATP-binding cassette A1-mediated cell cholesterol efflux. J. Biol. Chem. 282 5125–5132. [DOI] [PubMed] [Google Scholar]