Abstract
This study was designed to establish the mechanism responsible for the increased apolipoprotein (apo) A-II levels caused by the cholesteryl ester transfer protein inhibitor torcetrapib. Nineteen subjects with low HDL cholesterol (<40 mg/dl), nine of whom were also treated with 20 mg of atorvastatin daily, received placebo for 4 weeks, followed by 120 mg of torcetrapib daily for the next 4 weeks. Six subjects in the nonatorvastatin cohort participated in a third phase, in which they received 120 mg of torcetrapib twice daily for 4 weeks. At the end of each phase, subjects underwent a primed-constant infusion of [5,5,5-2H3]l-leucine to determine the kinetics of HDL apoA-II. Relative to placebo, torcetrapib significantly increased apoA-II concentrations by reducing HDL apoA-II catabolism in the atorvastatin (−9.4%, P < 0.003) and nonatorvastatin once- (−9.9%, P = 0.02) and twice- (−13.2%, P = 0.02) daily cohorts. Torcetrapib significantly increased the amount of apoA-II in the α-2-migrating subpopulation of HDL when given as monotherapy (27%, P < 0.02; 57%, P < 0.003) or on a background of atorvastatin (28%, P < 0.01). In contrast, torcetrapib reduced concentrations of apoA-II in α-3-migrating HDL, with mean reductions of −14% (P = 0.23), −18% (P < 0.02), and −18% (P < 0.01) noted during the atorvastatin and nonatorvastatin 120 mg once- and twice-daily phases, respectively. Our findings indicate that CETP inhibition increases plasma concentrations of apoA-II by delaying HDL apoA-II catabolism and significantly alters the remodeling of apoA-II-containing HDL subpopulations.
Keywords: high density lipoproteins, lipoprotein kinetics, Torcetrapib, HDL subspecies
Low levels of HDL cholesterol are inversely associated with coronary heart disease (CHD) risk. HDLs contain two major proteins, apolipoprotein (apo) A-I and apoA-II, which constitute ∼70 and 20% of total HDL protein mass, respectively. In human plasma, HDL can be classified on the basis of apolipoprotein composition into lipoproteins containing apoA-I but no apoA-II [lipoprotein A-I (LpA-I)] and lipoproteins containing both apoA-I and apoA-II (LpA-I:A-II) (1). ApoA-II has a higher lipid affinity than apoA-I and, based on in vitro studies, is able to displace apoA-I from lipoprotein particles (2, 3). A number of studies have shown that apoA-II can influence the metabolism of HDL by altering the activity of lipolytic enzymes and lipid transfer proteins. The presence of apoA-II on HDL particles has been shown to inhibit the ability of endothelial lipase to influence the metabolism of HDL in vivo (4). Similarly, the lipid transfer activities of both cholesteryl ester transfer protein (CETP) and phospholipid transfer protein are partially inhibited by apoA-II (5, 6). It has also been reported that LCAT activity is reduced in HDL reconstituted with apoA-II relative to HDL reconstituted with apoA-I (7). Conflicting results have been reported regarding the effect of apoA-II on hepatic lipase, with evidence to support both stimulatory (8) and inhibitory (9, 10) effects.
CETP is a plasma glycoprotein that plays a critical role in HDL metabolism by mediating the exchange of cholesteryl ester from HDL for triglyceride in apoB-containing lipoproteins, which include LDL and VLDL. We previously reported that partial inhibition of CETP activity in humans with torcetrapib significantly increased steady-state concentrations of apoA-I by reducing its clearance rate (11), an effect that was thought to be beneficial. Since that time, torcetrapib's development was halted due to an imbalance in all-cause mortality in CHD patients (12). The failure of this first in class CETP inhibitor has caused speculation as to whether the adverse effects of torcetrapib might be molecule or mechanism based. However, evidence to support the concept that the failure of torcetrapib was due to a compound-specific, rather than a class, effect is beginning to accumulate. In addition to having adverse effects on systolic blood pressure, it is now known that torcetrapib increased serum aldosterone levels and altered serum electrolyte concentrations in some subjects (12). Moreover, experiments in preclinical models have further revealed that the increased blood pressure caused by torcetrapib is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone (13). Taken together with the apparent lack of this side effect with other CETP inhibitors, such as anacetrapib and dalcetrapib (14), there is renewed optimism for CETP as a therapeutic target. With this in mind, it is important to define the mechanisms responsible for the lipoprotein changes associated with CETP inhibitor therapy.
With regard to cardiovascular disease risk reduction, the potential benefit of increasing apoA-II levels remains controversial. Although epidemiological evidence supports an inverse relationship between apoA-II levels and atheroscelerotic risk in humans (15–17), no clinical evidence of coronary atherosclerosis was found in the only known cases of apoA-II deficiency in humans, two Japanese sisters with inherited apoA-II deficiency (18). Moreover, it has also been shown that apoA-II can inhibit the remodeling of HDL in vitro (19), which could potentially influence the ability of HDL to promote reverse cholesterol transport. In this report, we describe the results of a study that was designed to establish the mechanism by which apoA-II levels are increased by CETP inhibition and to determine the effect of CETP inhibition on concentrations of apoA-II-containing HDL particles in patients with low baseline levels of HDL.
METHODS
Subjects
Subjects were recruited at two medical centers, Tufts-Medical Center, Boston, MA and the University of Pennsylvania School of Medicine, Philadelphia, PA. Patients were eligible for this trial if they met the following criteria: an age of 18–70 years, an HDL cholesterol of <40 mg/dl, triglycerides of <400 mg/dl, an LDL cholesterol level of <160 mg/dl, and a body mass index between 18 and 35 kg/m2. Subjects having an LDL cholesterol of >160 mg/dl were considered for the atorvastatin arm of this study provided that they met all other criteria, including that of an LDL cholesterol of <160 mg/dl once stabilized on 20 mg atorvastatin. Exclusion criteria are described elsewhere in detail (20). The study protocol was approved by the Human Investigation Review Committee of each institution, and informed written consent was obtained from each participant.
Experimental design
This was a single-blind, placebo-controlled, fixed sequence study designed to examine the effects of torcetrapib on lipoprotein metabolism in subjects with low HDL cholesterol. A total of 19 subjects were enrolled in this trial, with nine subjects in the atorvastatin arm and 10 in the nonatorvastatin arm. A detailed description of the study design has been reported previously (20). Briefly, the study consisted of an introductory period of 2 to 4 weeks, during which time subjects were screened and, if necessary (LDL cholesterol >160 mg/dl), stabilized on atorvastatin 20 mg. All subjects next received placebo for 4 weeks, followed by torcetrapib 120 mg once a day for an additional 4 weeks. Six subjects from the nonatorvastatin cohort also participated in a third phase, in which they received torcetrapib 120 mg twice daily for 4 weeks. At the end of each 4-week phase, subjects underwent a primed-constant infusion of deuterated leucine, while fed 20 identical small meals, to determine the kinetics of HDL apoA-II (21). At 11 AM (0 h), [5,5,5-2H3]l-leucine, 10 μmol/kg body weight, was injected as a bolus IV over 1 min and then by continuous infusion, 10 μmol·kg body wt−1·h−1, over a 15 h period. Blood samples, 20 ml, were collected at 0, 30, 35, and 45 min and 1, 1.5, 2, 3, 4, 6, 9, 12, 14, and 15 h, and HDL particles were isolated by sequential ultracentrifugation.
Determination of plasma LpA-I and LpA-I:A-II concentrations
Blood samples were collected from subjects, after a 12–14 h fast, into tubes containing 0.1% EDTA. Plasma was isolated by centrifugation at 2500 rpm, 4°C, for 20 min. LpA-I was measured by rocket electrophoresis using the Hydragel LpA-I particle assay from Sebia (Norcross, GA). LpA-I:A-II concentration was calculated by subtracting LpA-I from the total plasma apoA-I concentration, as previously described (22).
Analysis of apoA-II-containing HDL subpopulations by two-dimensional lipoprotein electrophoresis
The distribution of apoA-II-containing HDL subpopulations in the plasma was determined using nondenaturing two-dimensional agarose-polyacrylamide gel electrophoresis as reported previously (23). The concentration of apoA-II within each HDL subpopulation was calculated by multiplying the percentage of distribution of the apoA-II-containing HDL subpopulation with the plasma concentration of apoA-II.
Quantitation and isolation of HDL apoA-II
Plasma apoA-II concentrations at each kinetic time point were measured on a Hitachi 911 autoanalyzer using an immunoturbidimetric assay, reagents, and calibrators from Wako Diagnostics (Richmond, VA). HDL particles (d = 1.063 to 1.21 g/ml) were isolated from 5 ml of plasma by sequential density ultracentrifugation. ApoA-II was isolated from HDL by preparative SDS-PAGE using a 6–30% linear gradient in a Tris-glycine buffer system, as previously described (21, 24). The protein was transferred to a Westran S polyvinyl difluoride membrane (25) (Schleicher and Schuell BioScience) using a Tris-glycine-methanol buffer system and visualized with Coomassie brilliant blue R-250.
Sample hydrolysis, derivatization, and determination of isotopic enrichment
ApoA-II bands were excised from the polyvinyl difluoride membranes and hydrolyzed in 12 N HCl at 110°C for 24 h. After removal of the HCl by evaporation, the amino acids were converted to N-propyl ester, N-heptafluorobutyramide derivatives and the isotopic enrichment determined as previously described (26).
Kinetic analysis
The kinetic parameters of HDL apoA-II were determined as previously described (24). Briefly, the SAAMII program was used to fit the model to the observed tracer data using a weighted-least-squares approach to determine the best fit. The VLDL apoB-100 enrichment plateau was used as the maximal level of apoA-II enrichment. ApoA-II pool size (PS) was calculated by multiplying the plasma apoA-II concentration (mg/dl) by plasma volume (0.45 l/kg body weight). ApoA-II production rate (PR) was calculated using the formula PR (mg·kg−1·d−1) = [fractional catabolic rate (FCR; pools/d) × apoA-II concentration (mg/dl) × plasma volume (0.45 l/kg body weight)]/ body weight (kg).
Statistical analyses
The normality of end points was assessed by the Shapiro-Wilk goodness-of-fit test (27), in addition to visual examination of histograms and box plots. Paired t-tests were used to assess differences between the placebo and drug phases within a given group, whereas two-sample t-tests were used to detect statistically significant differences between the atorvastatin and nonatorvastatin groups (SAS System for Windows, release 8.02; SAS Institute, Cary, NC). Percentage change relative to placebo was computed on an individual subject basis and summarized descriptively by cohort. All data in the text and tables are presented as means ± SD.
RESULTS
Effects of torcetrapib on plasma concentrations of apoA-II and LpA-I and LpA-I:A-II particles
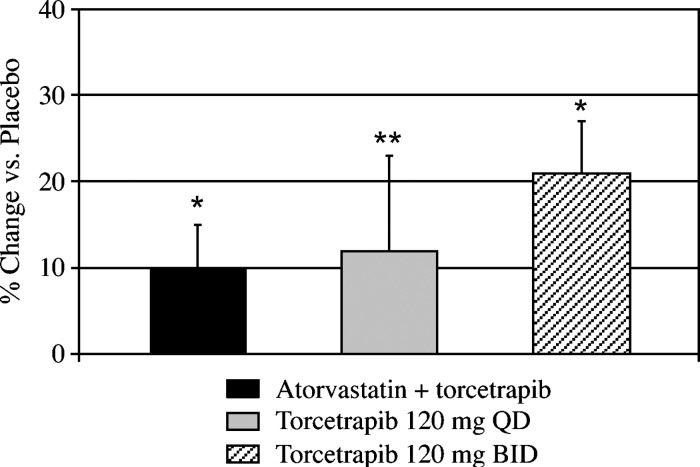
As reported previously (20), partial inhibition of CETP activity with torcetrapib significantly increased plasma concentrations of HDL cholesterol and apoA-I, either when given as monotherapy or in combination with atorvastatin. Plasma concentrations of apoA-II were also significantly increased by torcetrapib (Fig. 1). Relative to placebo, torcetrapib increased plasma apoA-II levels by a mean of 10% (P < 0.001), from 30 ± 4 to 33 ± 4 mg/dl, in the atorvastatin cohort, by 12% (P = 0.01), from 29 ± 2 to 33 ± 5 mg/dl, in the 120 mg torcetrapib once daily cohort, and by 21% (P < 0.001), from 30 ± 1 to 36 ± 3 mg/dl, in the 120 mg torcetrapib twice daily cohort.
Fig. 1.
Effect of torcetrapib on the percentage of change in plasma concentrations of apoA-II versus placebo. Relative to placebo, torcetrapib increased plasma apoA-II levels by a mean of 10%, from 30 ± 4 to 33 ± 4 mg/dl, in the atorvastatin group, by 12%, from 29 ± 2 to 33 ± 5 mg/dl, in the 120 mg torcetrapib once daily group, and by 21%, from 30 ± 1 to 36 ± 3 mg/dl, in the 120 mg torcetrapib twice daily group. *P < 0.001 and **P = 0.01 for the comparison of absolute concentrations of apoA-II with the placebo phase. QD, once daily; BID, twice daily.
The effects of torcetrapib on concentrations of LpA-I and LpA-I:A-II particles are shown in Table 1. Torcetrapib significantly increased LpA-I levels by 40% in the atorvastatin cohort, by 36% in the 120 mg once daily cohort, and by 73% in the 120 mg twice daily cohort. LpA-I:A-II concentrations were increased to a lesser extent than were LpA-I concentrations. Torcetrapib increased the mean level of LpA-I:A-II (mg/dl) from 81 ± 14 to 86 ± 13 (6%, P = 0.19) in the atorvastatin cohort, from 79 ± 6 to 86 ± 9 (9%, P = 0.01) in the 120 mg once daily cohort, and from 79 ± 6 to 95 ± 8 (20%, P < 0.03) in the 120 mg twice daily cohort.
TABLE 1.
Effects of torcetrapib on concentrations of LpA-I and LpA-I:A-II particles
| Atorvastatin+Torcetrapib |
Torcetrapib |
||
|---|---|---|---|
| Parameter | (120 mg once daily) | (120 mg once daily) | (120 mg twice daily) |
| LpA-I (mg/dl) | |||
| Placebo | 24 ± 10 | 31 ± 7 | 33 ± 8 |
| Torcetrapib | 34 ± 16c | 41 ± 8a | 6 ± 14a |
| Change (%) | 39.7 | 36.4 | 73.3 |
| LpA-I:A-II (mg/dl) | |||
| Placebo | 81 ± 14 | 79 ± 6 | 79 ± 6 |
| Torcetrapib | 86 ± 13 | 86 ± 9b | 95 ± 8c |
| Change (%) | 6.3 | 9.1 | 20.3 |
Data are expressed as mean ± SD. aP < 0.001, bP = 0.01, and cP < 0.03 for comparison with placebo phase.
Effects of torcetrapib on apoA-II-containing HDL subpopulations
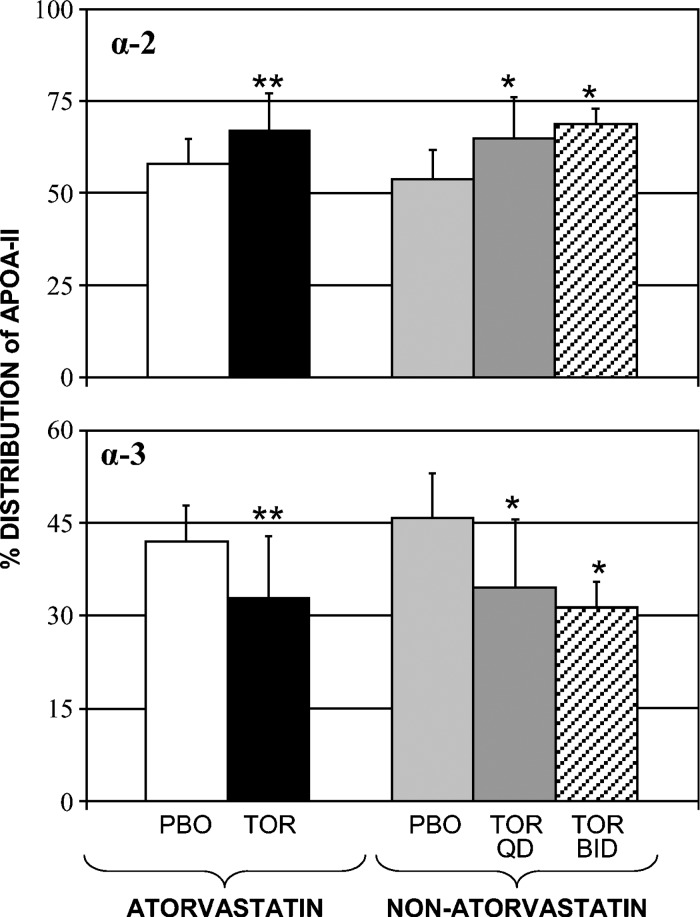
The effects of torcetrapib on concentrations of apoA-II-containing HDL subpopulations are provided in Table 2. Relative to placebo, torcetrapib 120 mg once daily increased the amount of apoA-II in the α-2 subpopulation of HDL in both the atorvastatin (27%, P < 0.02) and nonatorvastatin (28%, P < 0.01) cohorts, while an increase of 57% (P < 0.003) was observed in the 120 mg twice daily group. In contrast, torcetrapib reduced concentrations of apoA-II in α-3-migrating HDL, with mean reductions of −14% (P = 0.23), −18% (P < 0.02), and −18% (P < 0.01) noted during the atorvastatin and nonatorvastatin 120 mg once daily and twice daily phases, respectively. As shown in Fig. 2, the preceding changes in absolute concentrations of apoA-II within α-2 and α-3 HDL were, in turn, associated with significant differences in the percentage of distribution of apoA-II between these HDL subpopulations. Relative to placebo, the percentage of distribution of apoA-II within α-2 increased from 58 ± 6 to 67 ± 10% (17%, P < 0.04) in the atorvastatin cohort, from 54 ± 7 to 65 ± 11% (16%, P < 0.01) in the 120 mg once daily cohort, and from 53 ± 8 to 69 ± 4% (31%, P < 0.01) in the 120 mg twice daily cohort. Conversely, torcetrapib significantly reduced the percentage of distribution of apoA-II within α-3 in each of the cohorts. The 120 mg once daily dose of torcetrapib reduced the percentage of distribution of apoA-II within α-3 by −21% in both the atorvastatin (P = 0.04) and nonatorvastatin (P < 0.01) cohorts, while a reduction of −30% was observed for the torcetrapib 120 mg twice daily cohort (P < 0.01).
TABLE 2.
Effects of torcetrapib on concentrations of apoA-II-containing HDL subpopulations
| Atorvastatin+Torcetrapib |
Torcetrapib |
||
|---|---|---|---|
| Parameter | (120 mg once daily) | (120 mg once daily) | (120 mg twice daily) |
| α-2 (mg/dl) | |||
| Placebo | 17.5 ± 2.9 | 16.4 ± 3.1 | 15.8 ± 2.0 |
| Torcetrapib | 21.8 ± 3.8c | 21.3 ± 5.6b | 24.6 ± 1.5a |
| Change (%) | 26.7 | 27.7 | 56.6 |
| α-3 (mg/dl) | |||
| Placebo | 12.9 ± 2.7 | 13.0 ± 2.4 | 14.2 ± 2.5 |
| Torcetrapib | 11.2 ± 4.9 | 10.9 ± 2.5c | 11.4 ± 2.1b |
| Change (%) | −14.1 | −18.3 | −18.2 |
Data are presented as mean ± SD. aP < 0.003, bP < 0.01, and cP < 0.02 for comparison with placebo phase.
Fig. 2.
Effect of torcetrapib on the percentage of distribution of apoA-II among HDL subpopulations (mean ± SD). Relative to placebo, the percentage of distribution of apoA-II within the α-2 subpopulation of HDL increased from 58 ± 6 to 67 ± 10% (17%, P < 0.04) in the atorvastatin cohort, from 54 ± 7 to 65 ± 11% (16%, P < 0.01) in the 120 mg torcetrapib once daily cohort, and from 53 ± 8 to 69 ± 4% (31%, P < 0.01) in the 120 mg torcetrapib twice daily cohort. Conversely, torcetrapib significantly reduced the percentage of distribution of apoA-II within the α-3 subpopulation of HDL in each of the cohorts. *P < 0.01 and **P = 0.04 for comparison with placebo phase. QD, once daily; BID, twice daily; PBO, placebo; TOR, torcetrapib.
Effects of torcetrapib on HDL apoA-II kinetic parameters
HDL apoA-II kinetic parameters at the end of the placebo and drug phases are presented in Table 3. Relative to placebo, torcetrapib 120 mg once daily increased HDL apoA-II PS by 11 ± 13% (P < 0.03) in the atorvastatin cohort and by 12 ± 6% (P < 0.001) in the nonatorvastatin cohort. The relatively higher HDL apoA-II PS values that were observed for the nonatorvastatin cohort, as compared with the atorvastatin cohort, during the placebo and torcetrapib 120 mg once daily phases were not significantly different. The 120 mg twice daily dose of torcetrapib increased HDL apoA-II PS to the greatest extent (25 ± 7%, P < 0.001).
TABLE 3.
Effects of torcetrapib on HDL apoA-II kinetic parameters
| Atorvastatin+Torcetrapib |
Torcetrapib |
||
|---|---|---|---|
| Parameter | (120 mg once daily) | (120 mg once daily) | (120 mg twice daily) |
| ApoA-II PS (mg) | |||
| Placebo | 968 ± 175 | 1018 ± 165 | 1000 ± 135 |
| Torcetrapib | 1067 ± 194c | 1143 ± 208b | 1236 ± 134b |
| Change (%) | 10.8 | 12.4 | 25.3 |
| P value | 0.03 | 0.0005 | 0.0005 |
| ApoA-II FCR (pools/d) | |||
| Placebo | 0.207 ± 0.036 | 0.228 ± 0.080 | 0.201 ± 0.028 |
| Torcetrapib | 0.187 ± 0.035d | 0.206 ± 0.088 | 0.177 ± 0.027a |
| Change (%) | −9.4 | −9.9 | −13.2 |
| P value | 0.0025 | 0.02 | 0.02 |
| ApoA-II PR (mg·kg·d−1) | |||
| Placebo | 2.27 ± 0.35 | 2.62 ± 0.63 | 2.55 ± 0.42 |
| Torcetrapib | 2.31 ± 0.57 | 2.65 ± 0.74 | 2.79 ± 0.59 |
| Change (%) | 0.9 | 5.7 | 9.0 |
| P value | 0.66 | 0.93 | 0.15 |
Data are presented as mean ± SD. aP = 0.00002, bP < 0.001, cP = 0.025, and dP < 0.04 for comparison with placebo phase.
In the atorvastatin cohort, analysis of the HDL apoA-II kinetic data revealed that the torcetrapib-induced increases in HDL apoA-II PS were primarily due to reductions in the HDL apoA-II FCR. Relative to placebo, HDL apoA-II FCR was reduced by a mean of −9%, from 0.207 ± 0.036 to 0.187 ± 0.036 pools/day (P < 0.003), in the nine subjects that received combination therapy. When given as monotherapy, torcetrapib 120 mg once daily significantly reduced HDL apoA-II clearance by −10%, from 0.228 ± 0.080 to 0.206 ± 0.088 pools/day (P = 0.02), while the twice daily cohort had a 13% reduction in FCR, from 0.201 ± 0.028 to 0.177 ± 0.027 pools/day (P = 0.02). Torcetrapib did not significantly alter HDL apoA-II production rate in any of the treatment groups.
DISCUSSION
Because of its critical role in HDL metabolism, CETP represents an attractive target for HDL-raising therapies. We have previously reported that torcetrapib, a potent inhibitor of CETP activity, significantly increased plasma concentrations of apoA-II in patients with low HDL cholesterol levels (20). Given the key role that apoA-II plays in HDL metabolism, the present study was designed to establish the mechanism responsible for the increased apoA-II levels caused by CETP inhibition and to determine the effect of CETP inhibition on concentrations of apoA-II-containing HDL particles.
Analysis of the HDL apoA-II kinetic data revealed that the increases in apoA-II pool size observed in subjects treated with torcetrapib were due to reductions in HDL apoA-II clearance rate. Torcetrapib did not significantly alter apoA-II production rate in any of the cohorts. These results are similar to what we previously reported for torcetrapib's effects on HDL apoA-I (11), a finding consistent with the concept that CETP inhibition influences the metabolism of both LpA-I:A-II and LpA-I only HDL particles. Our findings are consistent with those of Ikewaki et al. (28), who reported that the elevated concentrations of apoA-I and apoA-II observed in patients with CETP deficiency were solely due to delayed catabolism of each protein. The delayed catabolism of HDL apoA-I and apoA-II observed in CETP deficiency and with small molecule CETP inhibition is likely due to increased HDL core lipid content and, in turn, increased particle size, as small HDL particles are cleared more rapidly than larger HDL (29, 30).
The majority of apoA-I in the plasma has α-mobility. By two-dimensional lipoprotein electrophoresis, α-migrating HDL particles can be further classified into α-1, α-2, and α-3, with particle sizes of 11.2, 9.51, and 7.12 nm, respectively (31). In healthy subjects, apoA-II is present only in the α-2 and α-3 subpopulations of HDL, with the α-1 subpopulation containing only apoA-I (23). In contrast, we previously reported that three out of five subjects with heterozygous CETP deficiency had apoA-II in α-1 HDL (32). Thus, one of our aims was to examine the effects of CETP inhibition with torcetrapib on the distribution and concentration of apoA-II among α-migrating HDL subpopulations. In all but one subject, where apoA-II was found in α-1 HDL during the 120 mg twice daily dose phase, we found that torcetrapib did not alter the normal distribution of apoA-II (data not shown). However, torcetrapib did significantly alter the distribution and concentration of apoA-II among α-2 and α-3 HDL. In subjects treated with torcetrapib on a background of atorvastatin, we observed significant increases in the amount of apoA-II in α-2 HDL in response to torcetrapib, while concentrations of apoA-II in the relatively smaller-sized α-3 HDL subpopulation were not altered. In subjects that received torcetrapib as monotherapy, significant dose-dependent increases in apoA-II α-2 HDL concentrations were observed, as well as significant reductions in the apoA-II content of the α-3 subpopulation of HDL, with similar reductions noted in both groups (i.e., not dose dependent). These findings are consistent with in vitro observations where apoA-II prevented preβ-migrating, lipid-poor apoA-I from dissociating from HDLs that were being remodeled by CETP (19). CETP inhibition likely reduces the remodeling of α-1 and α-2 HDL, possibly through inhibitory effects of apoA-II on phospholipases (endothelial lipase and hepatic lipase), which, in turn, may influence the association of apoA-I and apoA-II with these particles.
In evaluating the available data, it can be argued that neither a compelling “pro” nor “con” case can be made for the role of increased apoA-II in atherosclerotic risk reduction. This is due to the fact that apoA-II appears to have both beneficial and deleterious effects on lipoprotein metabolism. For example, a potential inhibitory role for apoA-II in the first step of the reverse cholesterol transport pathway, cellular cholesterol efflux, has been suggested (33). In contrast, de Beer et al. (34) have reported that apoA-II enhances a different step in the reverse cholesterol transport pathway, namely, the scavenger receptor class B type 1-mediated uptake of cholesteryl esters. In terms of the results of the present study, the HDL subpopulation profile induced by torcetrapib therapy, increased apoA-I in the α-1 subpopulation of HDL (11), increased apoA-II in the α-2 subpopulation of HDL, and reduced apoA-II content in the α-3 subpopulation of HDL, would be predicted to reduce CHD risk based on our previous findings in the Veterans Affairs HDL Intervention Trial cohort (35). Further support for this concept comes from a recent study by Birjmohun et al. (36), who found that apoA-II was associated with reduced risk of developing coronary artery disease in apparently healthy subjects.
In conclusion, our results provide new insight into the mechanisms by which CETP inhibition influences HDL metabolism in humans. These data indicate that torcetrapib has similar effects on concentrations of apoA-II in the α-2- and α-3-migrating subpopulations of HDL, when given either as monotherapy or on a background of atorvastatin. Torcetrapib significantly increases the apoA-II content of α-2 HDL but reduces that of apoA-II in α-3 HDL, an HDL subpopulation profile consistent with reduced CHD risk. In all cohorts, the increases in plasma apoA-II concentrations were solely due to delayed HDL apoA-II catabolism, with no changes observed in HDL apoA-II production rates.
Acknowledgments
The authors thank the nursing and dietary staff of each clinical research center as well as Jennifer Dykhouse, Aisha Wilson, Rodrigo Ferreira, and Judith R. McNamara for technical assistance. The authors are also grateful to Drs. Gregory G. Dolnikowski and P. Hugh Barrett for their expert advice.
Abbreviations
apo, apolipoprotein
CETP, cholesteryl ester transfer protein
CHD, coronary heart disease
FCR, fractional catabolic rate
LpA-I, lipoprotein A-I
PR, production rate
PS, pool size
This work was supported, in part, by the Department of Clinical Research, Medicinal Products Research and Development, Pfizer. Support was also provided by the General Clinical Research Center of New England Medical Center, which is funded by the National Center for Research Resources of the National Institutes of Health (M01-RR00054), and the General Clinical Research Center of the University of Pennsylvania (M01-RR00040). M.E.B. and E.J.S. were supported, in part, by National Institutes of Health Grant R01-HL60935 from the National Heart, Lung, and Blood Institute.
Published, JLR Papers in Press, February 11, 2009.
References
- 1.Cheung M. C., and J. J. Albers. 1982. Distribution of high density lipoprotein particles with different apoprotein composition: particles with apoA-I and apoA-I and particles with apoA-I but not apoA-II. J. Lipid Res. 23 747–753. [PubMed] [Google Scholar]
- 2.Massey J. B., M. F. Rohde, W. B. Van Winkle, A. M. Gotto, Jr., and H. J. Pownall. 1981. Physical properties of lipid-protein complexes formed by the interaction of dimyristoylphosphatidylcholine and human high-density apolipoprotein A-II. Biochemistry. 20 1569–1574. [DOI] [PubMed] [Google Scholar]
- 3.Rye K-A., and P. J. Barter. 1994. The influence of apolipoproteins on the structure and function of spheroidal, reconstituted high-density lipoproteins. J. Biol. Chem. 269 10298–10303. [PubMed] [Google Scholar]
- 4.Broedl U. C., W. Jin, I. V. Fuki, J. S. Millar, and D. J. Rader. 2006. Endothelial lipase is less effective at influencing HDL metabolism in vivo in mice expressing apoA-II. J. Lipid Res. 47 2191–2197. [DOI] [PubMed] [Google Scholar]
- 5.Lagrost L., L. Persegol, C. Lallemant, and P. Gambert. 1994. Influence of apolipoprotein composition of high density lipoprotein particles on cholesteryl ester transfer protein activity. J. Biol. Chem. 269 3189–3197. [PubMed] [Google Scholar]
- 6.Pussinen P. J., M. Jauhiainen, and C. Enholm. 1997. ApoA-I/A-II molar ratio in the HDL particle influences phospholipid transfer protein-mediated HDL interconversion. J. Lipid Res. 38 12–21. [PubMed] [Google Scholar]
- 7.Durbin D. M., and A. Jonas. 1997. The effect of apolipoprotein A-II on the structure and function of apolipoprotein A-I in a homogeneous reconstituted high density lipoprotein particle. J. Biol. Chem. 272 31333–31339. [DOI] [PubMed] [Google Scholar]
- 8.Jahn C. E., J. C. Osborne, E. J. Schaefer, and H. B. Brewer. 1981. In vitro activation of the enzymic activity of hepatic lipase by apoA-II. FEBS Lett. 131 366–368. [DOI] [PubMed] [Google Scholar]
- 9.Zhong S., I. J. Goldberg, C. Bruce, E. Rubin, J. L. Breslow, and A. Tall. 1994. Human apoA-II inhibits the hydrolysis of HDL triglyceride and the decrease of HDL size induced by hypertriglyceridemia and cholesteryl ester transfer protein in transgenic mice. J. Clin. Invest. 94 2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hime N. J., P. J. Barter, and K. A. Rye. 2001. Evidence that apolipoprotein A-I facilitates hepatic lipase mediate phospholipid hydrolysis in reconstituted HDL containing apolipoprotein A-II. Biochemistry. 40 5496–5505. [DOI] [PubMed] [Google Scholar]
- 11.Brousseau M. E., M. R. Diffenderfer, J. S. Millar, C. Nartsupha, B. F. Asztalos, F. K. Welty, M. L. Wolfe, M. Rudling, I. Bjorkhem, B. Angelin, et al. 2005. Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler. Thromb. Vasc. Biol. 25 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barter P. J., M. Caulfield, M. Eriksson, S. M. Grundy, J. J. Kastelein, M. Komajda, J. Lopez-Sendon, L. Mosca, J. C. Tardif, D. D. Waters, et al.: ILLUMINATE Investigators. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357 2109–2122. [DOI] [PubMed] [Google Scholar]
- 13.Forrest M. J., D. Bloomfield, R. J. Briscoe, P. N. Brown, A. M. Cumiskey, J. Ehrhart, J. C. Hershey, W. J. Keller, X. Ma, H. E. McPherson, et al. 2008. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br. J. Pharmacol. 154 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishna R., M. S. Anderson, A. J. Bergman, B. Jin, M. Fallon, J. Cote, K. Rosko, C. Chavez-Eng, R. Lutz, D. M. Bloomfield, et al. 2007. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomized placebo-controlled phase I studies. Lancet. 370 1907–1914. [DOI] [PubMed] [Google Scholar]
- 15.Buring J. E., G. T. O'Connor, S. Z. Goldhaber, B. Rosner, P. N. Herbert, C. B. Blum, J. L. Breslow, and C. H. Hennekens. 1992. Decreased HDL2 and HDL3 cholesterol, Apo A-I and Apo A-II, and increased risk of myocardial infarction. Circulation. 85 22–29. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien T., T. T. Nguyen, B. J. Hallaway, D. Hodge, K. Bailey, D. Holmes, and B. A. Kottke. 1995. The role of lipoprotein A-I and lipoprotein A-I/A-II in predicting coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 15 228–231. [DOI] [PubMed] [Google Scholar]
- 17.Blanco-Vaca F., J. C. Escolà-Gil, J. M. Martín-Campos, and J. Julve. 2001. Role of apoA-II in lipid metabolism and atherosclerosis: advances in the study of an enigmatic protein. J. Lipid Res. 42 1727–1739. [PubMed] [Google Scholar]
- 18.Deeb S. S., K. Takata, R. L. Peng, G. Kajiyama, and J. J. Albers. 1990. A splice-junction mutation responsible for familial apolipoprotein A-II deficiency. Am. J. Hum. Genet. 46 822–827. [PMC free article] [PubMed] [Google Scholar]
- 19.Rye K-A., K. Wee, L. K. Curtiss, D. J. Bonnet, and P. J. Barter. 2003. Apolipoprotein A-II inhibits high density lipoprotein remodeling and lipid-poor apolipoprotein A-I formation. J. Biol. Chem. 278 22530–22536. [DOI] [PubMed] [Google Scholar]
- 20.Brousseau M. E., E. J. Schaefer, M. L. Wolfe, L. T. Bloeden, A. G. Digenio, R. W. Clark, J. P. Mancuso, and D. J. Rader. 2004. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N. Engl. J. Med. 350 1505–1515. [DOI] [PubMed] [Google Scholar]
- 21.Cohn J. S., D. A. Wagner, S. D. Cohn, J. S. Millar, and E. J. Schaefer. 1990. Measurement of very low density and low density lipoprotein apolipoprotein (apo) B-100 and high density lipoprotein apoA-I production in human subjects using deuterated leucine: effect of fasting and feeding. J. Clin. Invest. 85 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra H. J., H. Mezdour, N. Ghalim, J. M. Bard, and J. C. Fruchart. 1990. Differential electroimmunoassay of human LpA-I lipoprotein particles on ready-to-use plates. Clin. Chem. 36 1431–1435. [PubMed] [Google Scholar]
- 23.Asztalos B. F., M. Lefevre, T. A. Foster, R. Tulley, M. Windhauser, L. Wong, and P. S. Roheim. 1997. Normolipidemic subjects with low HDL cholesterol levels have altered HDL subpopulations. Arterioscler. Thromb. Vasc. Biol. 17 1885–1893. [DOI] [PubMed] [Google Scholar]
- 24.Velez-Carrasco W., A. H. Lichtenstein, Z. Li, G. G. Dolnikowski, S. Lamon-Fava, F. K. Welty, and E. J. Schaefer. 2000. Apolipoprotein A-I and A-II kinetic parameters as assessed by endogenous labeling with [2H3]leucine in middle-aged and elderly men and women. Arterioscler. Thromb. Vasc. Biol. 20 801–806. [DOI] [PubMed] [Google Scholar]
- 25.Dwyer K. P., P. H. Barrett, D. Chan, J. I. Foo, G. F. Watts, and K. D. Croft. 2002. Oxazolinone derivative of leucine for GC-MS: a sensitive and robust method for stable isotope kinetic studies of lipoproteins. J. Lipid Res. 43 344–349. [PubMed] [Google Scholar]
- 26.Welty F. K., A. H. Lichtenstein, P. H. Barrett, G. G. Dolnikowski, J. M. Ordovas, and E. J. Schaefer. 1997. Production of apolipoprotein B-67 in apolipoprotein B-67/B-100 heterozygotes: technical problems associated with leucine contamination in stable isotope studies. J. Lipid Res. 38 1535–1543. [PubMed] [Google Scholar]
- 27.Shapiro S. S., and M. B. Wilk. 1965. An analysis of variance test for normality (complete samples). Biometrika. 52 591–611. [Google Scholar]
- 28.Ikewaki K., D. J. Rader, T. Sakamoto, M. Nishiwaki, N. Wakimoto, J. R. Schaefer, T. Ishikawa, T. Fairwell, L. A. Zech, H. Nakamura, et al. 1993. Delayed catabolism of apolipoproteins A-I and A-II in human cholesteryl ester transfer protein deficiency. J. Clin. Invest. 92 1650–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinton E. A., S. Eisenberg, and J. L. Breslow. 1991. Increased apo A-I and apo A-II fractional catabolic rate in patients with low high density lipoprotein-cholesterol levels with or without hypertriglyceridemia. J. Clin. Invest. 87 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horowitz B. S., I. J. Goldberg, J. Merab, T. M. Vanni, R. Ramakrishnan, and H. N. Ginsberg. 1993. Increased plasma and renal clearance of an exchangeable pool of apolipoprotein A-I in subjects with low levels of high density lipoprotein cholesterol. J. Clin. Invest. 91 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asztalos B. F., C. H. Sloop, L. Wong, and P. S. Roheim. 1993. Two-dimensional electrophoresis of lipoproteins: recognition of new apoA-I-containing subpopulations. Biochim. Biophys. Acta. 1169 291–300. [DOI] [PubMed] [Google Scholar]
- 32.Asztalos B. F., K. V. Horvath, K. Kajinami, C. Nartsupha, C. E. Cox, M. Batista, E. J. Schaefer, A. Inazu, and H. Mabuchi. 2004. Apolipoprotein composition of HDL in cholesteryl ester transfer protein deficiency. J. Lipid Res. 45 448–455. [DOI] [PubMed] [Google Scholar]
- 33.Lagrost L., C. Dengremont, A. Athias, C. de Geitere, J. C. Fruchart, C. Lallemant, P. Gambert, and G. Castro. 1995. Modulation of cholesterol efflux from Fu5AH hepatoma cells by the apolipoprotein content of high density lipoprotein particles. Particles containing various proportions of apolipoproteins A-I and A-II. J. Biol. Chem. 270 13004–13009. [DOI] [PubMed] [Google Scholar]
- 34.de Beer M. C., D. M. Durbin, L. Cai, N. Mirocha, A. Jonas, N. R. Webb, F. C. de Beer, and D. R. van Der Westhuyzen. 2001. Apoliporotein A-II modulates the binding and selective uptake of reconstituted high density lipoprotein by scavenger receptor BI. J. Biol. Chem. 276 15832–15839. [DOI] [PubMed] [Google Scholar]
- 35.Asztalos B. F., D. Collins, L. A. Cupples, S. Demissie, K. V. Horvath, H. E. Bloomfield, S. J. Robins, and E. J. Schaefer. 2005. Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arterioscler. Thromb. Vasc. Biol. 25 2185–2191. [DOI] [PubMed] [Google Scholar]
- 36.Birjmohun R. S., G. M. Dallinga-Thie, J. A. Kuivenhoven, E. S. Stroes, J. D. Otvos, N. J. Wareham, R. Luben, J. J. Kastelein, K. T. Khaw, and S. M. Boekholdt. 2007. Apolipoprotein A-II is inversely associated with risk of future coronary artery disease. Circulation. 116 2029–2035. [DOI] [PubMed] [Google Scholar]