Abstract
Background
Different classes of small RNAs (sRNAs) refine the expression of numerous genes in higher eukaryotes by directing protein partners to complementary nucleic acids, where they mediate gene silencing. Plants encode a unique class of sRNAs, called trans-acting small interfering RNAs (tasiRNAs), which post-transcriptionally regulate protein-coding transcripts, as do microRNAs (miRNAs), and both sRNA classes control development through their targets. TasiRNA biogenesis requires multiple components of the siRNA pathway and also miRNAs. But while 21mer siRNAs originating from transgenes can mediate silencing across several cell layers, miRNA action seems spatially restricted to the producing or closely surrounding cells.
Principal Findings
We have previously described the isolation of a genetrap reporter line for TAS3a, the major locus producing AUXIN RESPONS FACTOR (ARF)-regulating tasiRNAs in the Arabidopsis shoot. Its activity is limited to the adaxial (upper) side of leaf primordia, thus spatially isolated from ARF-activities, which are located in the abaxial (lower) side. We show here by in situ hybridization and reporter fusions that the silencing activities of ARF-regulating tasiRNAs are indeed manifested non-cell autonomously to spatially control ARF activities.
Conclusions/Significance
Endogenous tasiRNAs are thus mediators of a mobile developmental signal and might provide effective gene silencing at a distance beyond the reach of most miRNAs.
Introduction
Different aspects of development in plants and animals are regulated by endogenous sRNAs, most of which belong to the class of miRNAs [1]. Plant genomes in addition encode another group of sRNAs called tasiRNAs, which are also involved in gene silencing, but whose biosynthesis is distinct from that of miRNAs [2]. miRNAs originate from longer single-stranded precursor RNAs, which are directly processed into functional sRNA duplexes by a Dicer endonuclease, DICER-LIKE 1 (DCL1) in Arabidopsis. tasiRNA synthesis, however, first requires functional miRNAs, which bind to and trigger cleavage of a non-coding TAS precursor RNA. Subsequent synthesis of a complementary strand to the TAS precursor forms a double-stranded template for Dicer-mediated tasiRNA production. Genetic screens have identified several players required for tasiRNA biosynthesis, including RNA-DEPENDENT RNA POLYMERASE 6 (RDR6), which mediates double-strand formation [3], [4], and DCL4, which processes the double-strand in ∼21mer intervals into tasiRNAs [5], [6], [7]. ARGONAUTE 7/ZIPPY (AGO7) and DOUBLE-STRANDED RNA BINDING PROTEIN 4 (DRB4) function specifically in the synthesis of tasiRNAs from the three TAS3 loci (TAS3a-c) with roles in the initial miRNA-binding step and as a partner of DCL4 respectively (Fig. 1a) [8], [9].
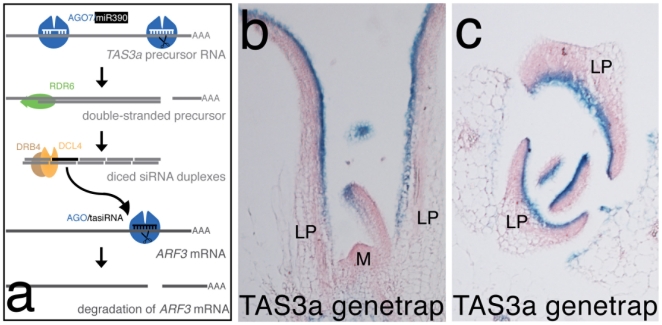
Figure 1. A genetrap inserted at the TAS3a locus reports activity in the adaxial L1 and L2 layers.
(a) Schematic of tasiRNA biosynthesis from the TAS3 locus. Longitudinal (b) and transverse (c) sections through the shoot apex visualize the activity of a genetrap inserted upstream of the TAS3a locus (GT19682) in the adaxial domain of leaf primordia. “M” marks the shoot meristems, “LP” young leaf primordia.
tasiRNAs and miRNAs have a similar mode of action: they base-pair to target mRNAs and post-transcriptionally control target mRNA and/or protein accumulation. The use of tasiRNAs, whose synthesis requires a much larger set of components, and not miRNAs might thus be unanticipated. A possible explanation would be the use of tasiRNAs to mediate silencing non-cell autonomously across cell boundaries, since many miRNAs have been reported to perform their repressive function cell-autonomously [10], [11], [12]; tasiRNAs however require DCL4 and RDR6 for their production, which have previously been implicated in the production of siRNAs from transgenes, whose silencing effects are mobile and spread across several cell layers within the plant [13].
Tretter et al. have recently generated an artificial transgene-based system to produce siRNAs from a tasiRNA-like precursor and found non-cell autonomous silencing in various tissues mediated by those siRNAs [12].
We have investigated endogenous tasiRNAs from the TAS3 loci, which function in the determination of leaf shape in Arabidopsis thaliana [3], [4] and also in maize where different factors involved in tasiRNA biosynthesis have been localized in a polar fashion in leaf primordia [14], [15]. We asked whether tasiRNA-mediated gene silencing involves a mobile developmental silencing signal. Our results suggest that tasiRNAs, like transgene-derived siRNAs, manifest their effects non-cell autonomously, and thus propose a specialized role for tasiRNAs in the post-transcriptional control of developmental regulators, which distinguishes itself from that of miRNAs by the range of silencing activities.
Results and Discussion
Accumulation of TAS3 precursor RNA is restricted to the adaxial L1 and L2 layers in leaf primordia
Mutations in genes encoding the tasiRNA biosynthesis components lead to similar phenotypic consequences that are generally weak, including the slight downward curling of rosette leaves and the precocious initiation of trichomes on the abaxial (lower) side of young leaves, also known as accelerated vegetative phase change [16]. These phenotypes are also observed in a tas3a insertion mutant, suggesting that TAS3a is the major TAS locus involved in leaf development [8].
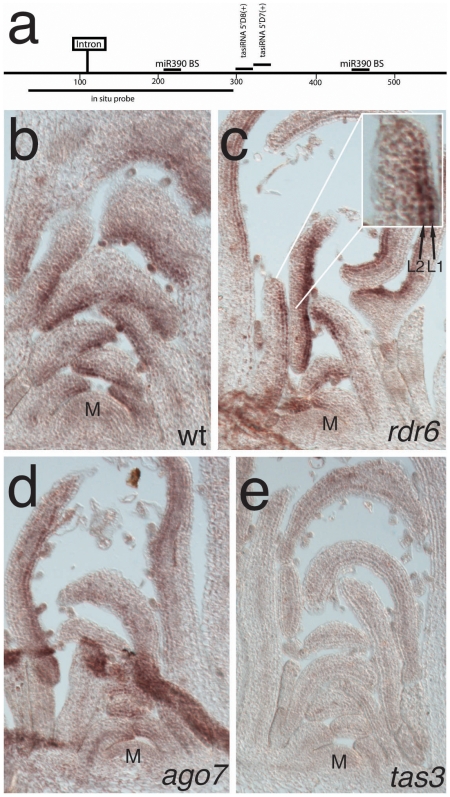
We have previously described the isolation of a genetrap reporter line for TAS3a whose activity is limited to the adaxial (upper) side of leaf primordia [17] (Fig. 1b, c). In accordance with these genetrap staining patterns, we reproducibly detected TAS3a precursor RNA exclusively in the adaxial L1 and L2 layers by in situ hybridization on serial longitudinal sections through young wild-type leaf primordia (Fig. 2b). Staining was strongest in the distal part of older primordia (> stage 4) and excluded from the shoot apical meristem. Similar TAS3a accumulation patterns were observed in mutants of the tasiRNA pathway rdr6 and ago7 (Fig. 2c–d), but no signal was detected in tas3a null mutants (Fig. 2e). We were not able to detect TAS3b precursor RNA in leaf primordia, neither on sections nor in whole-mount samples (data not shown), consistent with a predominant role for TAS3a in generating tasiRNAs after leaf primordia have been initiated. A putative role for TAS3c was very unlikely due to its very low capability of producing tasiRNAs [18].
Figure 2. The TAS3a precursor accumulates in the adaxial L1 and L2 layer.
(a) Schematic of the TAS3a precursor transcript. miR390 binding sites (BS) and tasiRNAs complementary to ARF3 and ARF4 transcripts are shown above the bar, the fragment amplified to generate an antisense probe for in situ hybridization is indicated below the bar. TAS3a precursor transcripts accumulate exclusively in the adaxial L1 and L2 layer (compare inset), most strongly in the distal tips, as shown by in situ hybridization on wt (b), rdr6 (c), and ago7 (d) longitudinal sections through the shoot apex of short-day grown plants, while no signal is detected in tas3a insertion mutants (e). “M” indicates the shoot meristem.
These results indicate that the non-coding RNA precursor transcript of ARF-regulating tasiRNAs accumulates specifically on the adaxial side of leaf primordia, and is refined to the L1 and L2 cell layers within cells which are well separated from the abaxial domain that is specified by the ARF target genes [19].
ARF3-GUS fusion proteins over-accumulate outside the adaxial L1/L2 layer in the absence of tasiRNA-mediated regulation
Targets of tasiRNAs include the AUXIN-RESPONSE FACTOR proteins ARF2, ARF3/ETTIN and ARF4, which mediate cellular responses to the phytohormone auxin [20], [21], [22]. Both ARF3 and ARF4 promote the specification of the abaxial leaf fate [19]. ARF4 mRNA is restricted to the abaxial side, while ARF3 mRNA is detected rather ubiquitously in young leaf primordia by in situ hybridization [19].
ARF3-GUS fusion proteins, expressed under their own promoter [23] accumulate only weakly in wild-type leaves (Fig. 3a), however when silent mutations are introduced in the tasiRNA binding sites within the coding region (mARF3), ARF3-GUS protein accumulates very strongly not only in the adaxial L1 and L2 cell layer, where the precursor of tasiRNAs is produced, but throughout the leaf and in the meristem (Fig. 3b). These findings imply (1) a potent regulation of ARF3 accumulation mediated by tasiRNAs, and (2) the activity of a non-cell autonomous factor that normally prevents high levels of ARF3-GUS accumulation outside the domain of TAS3 origin.
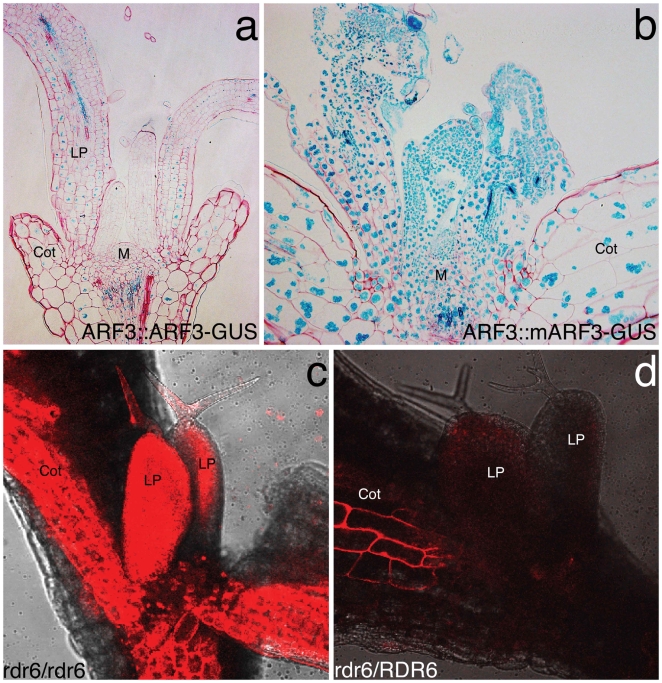
Figure 3. Silencing effects of tasiRNAs are manifested non-cell autonomously.
(a) ARF3-GUS protein expressed from its native promoter accumulates only weakly in leaf primordia and is absent from the shoot apical meristem (M). When insensitive to tasiRNA-mediated regulation (mARF3-GUS), much stronger GUS signals are detected throughout primordia as well as the shoot apical meristem with identical staining conditions (b). A ubiquitously expressed tasiRNA-responsive dsRed-sensor is active in most tissues in the absence of tasiRNAs in an rdr6 mutant background (c), but becomes greatly reduced throughout primordia (LP) and cotyledons (Cot) in the presence of a wild-type RDR6 copy in heterozygotes, i.e. in the presence of tasiRNAs (d). Exposure times in (c) and (d) were the same.
ARF-targeting tasiRNAs exert their silencing effects throughout leaf primordia
To assay for non-cell autonomous spreading of tasiRNA-mediated gene silencing within the shoot apex, we developed a ubiquitously expressed dsRed “sensor” transgene that contains a target site for ARF-regulating tasiRNAs in the 3′UTR with mismatches that resemble the endogenous tasiRNA binding site “A” in the ARF3 transcript [20]. The absence of dsRed fluorescence in a cell would be indicative of tasiRNA accumulation and activity.
Transgenes were introduced into plants homozygous for the recessive rdr6-3 mutation [24], in which tasiRNAs are absent such that sensor proteins accumulated near-ubiquitously (Fig. 3c). Three independent strongly expressing lines were backcrossed to the parental Ler strain to monitor the spatial distribution of tasiRNA-mediated silencing activity in F1 plants, which do express a functional copy of RDR6 and thus accumulate tasiRNAs. As shown in Fig. 3d, dsRed fluorescence was strongly reduced in young leaf primordia in rdr6 heterozygotes, both on the adaxial and abaxial sides, suggesting that a silencing factor originating from the TAS3 transcript was indeed trafficking far out of the adaxial L1 and L2 layers and into most cells of young leaf primordia.
A major concern when silencing transgenes is the RDR6-dependent generation of secondary small RNAs from the primary target (in this case dsRed), which reinforce silencing both locally and in closely surrounding cells. Multiple successive cycles can thus spread the silencing signal throughout the plant [25]. Further, secondary siRNA for many primary miRNA targets are known [26] and can be detected in ARF3 by deep sequencing [27]. Consistent with an exclusive role of (primary) tasiRNAs, and not secondary siRNAs in dsRed silencing, we never detected complete erasure of dsRed activity in RDR6 heterozygous plants: fluorescence remained easily detectable in a patch of epidermal cells in the cotyledons (Fig. 3d) and in epidermal cells of root meristems (Supplemental Fig. S1) as well as fully differentiated root cells close to the hypocotyl (not shown).
Therefore we propose that the mobile silencing signal that restricts the accumulation of ARF3 manifests its effects primarily through tasiRNAs. This notion is further supported by our observations that mARF3-GUS over-accumulates ectopically outside the domain of TAS3a expression (Fig. 3a and b), since secondary siRNAs originating from the endogenous ARF3 transcript would be present in both wt- and mutARF3-GUS and silence ARF3-GUS efficiently if they were the critical silencing effector. The endogenous ARF3 gene may play some role, however, in suppressing the phenotypic effects of ARF3 C- terminal truncations that lack the tasiRNA site [19].
The nature of the mobile component remains unknown. tasiRNAs themselves, due to their small size, serve as an attractive possibility, but the close association of small RNAs with Argonaute proteins needs to be taken into consideration as well, and further research is required to find out if tasiRNAs, or an unstable precursor difficult to detect by in situ hybridization, can directly cross cell boundaries.
Taken together, our data suggests that not only transgene-derived siRNAs [13], but also endogenous tasiRNAs, both of which are produced by DCL4 can manifest their silencing activities non-cell autonomously. The activity of many miRNAs on the other hand, which are mostly produced by DCL1 and often function in a similar developmental context, seems spatially restricted in its silencing potential to the producing or closely surrounding cells [10], [11], [12], [28]. The use of tasiRNAs, which involve a large number of components for their synthesis, rather than miRNAs, might thus directly relate to the range of their biological effects.
Materials and Methods
Plant material and growth conditions
All mutants used in this study have been described previously. For in situ hybridization, we used mutants in the Col-0 background: rdr6–15 [29], zip-1, referred to as ago7 throughout the manuscript [21], and tas3a [8]. The genetrap insertion line GT19682 is in Ler background [17], as is the rdr6–3 allele [24], which was transformed with the tasiRNA sensor construct (see below).
Transgenic Plants
ARF3::ARF3-GUS and ARF3::rARF3-GUS plants have been described [23]. The tasiRNA sensor was generated by introducing the target site “A” found in ARF3 (same as site “B” found in ARF4) [20] into the 3′UTR of dsRed using the primers 5′-atggcctcctccgagaacg and 5′-taaggatccttcttgaccttgcaagaccctaatctacaggaacaggtggtggcggccctcg (stop codon of dsRed underlined, target site in bold). The product was placed downstream of the 35S promoter in the binary vector pBIN61 and introduced into Ler and rdr6–3 plants with standard techniques.
GUS staining and histology
GUS staining was carried out as described [30]. Staining were performed for 16 h at 37°C. Samples in Figure 3 were stained in parallel and for the same time. In situ hybridizations were carried out as described [30]. Plants were grown in a short day incubator (8 h light, 16 h dark) at 23°C for 30 days, fixed and dehydrated by hand, and embedded into wax with an automated embedding system (Thermo Shandon Excelsior ES Tissue Processor). Sections were 10 µm. The TAS3a probe excluded the regions that produce the mature ARF-targeting tasiRNAs (Supplementary Figure S1), and was amplified from Arabidopsis Col-0 cDNA with the following oligonucleotides (T3 and T7 sites in capital letters): forward (T3): AATTAACCCTCACTAAAGGgagagaagagctcccatggatgaa, reverse (T7): TAATACGACTCACTATAGGGAGAagagaataatgaaatgcatcatctag. In vitro transcription was carried out with a DIG RNA labeling kit from Roche using T7 RNA polymerase.
Light microscopy
Histological sections were imaged with a Leica DMRB compound Microscope equipped with Qimaging MicroPublisher 5.0RTV digital camera.
Confocal imaging
Plants were grown on vertical ½ MS agar plates without antibiotics for 5 days in continuous light at 21°C, mounted in water on glass slides and imaged using a Zeiss LSM510 confocal laser-scanning microscope.
Supporting Information
Accumulation of the tasiRNA sensor in root meristems. (a) The tasiRNA sensor is detected throughout the root meristem, most strongly in the outer cell layers, in the absence of tasiRNAs in homozygous rdr6-3 mutants, but becomes restricted to the epidermis in the presence of tasiRNAs in RDR6 heterozygotes, i.e. in the presence of tasiRNAs (b).
(0.20 MB TIF)
Acknowledgments
We thank Mary Byrne for initiating our histological work on tasiRNAs, and John Xue for help at earlier stages, Sasha Goldshmidt for help with microscopy, and Lisa Bianco for assistance with automated embedding. We thank Dan Chitwood for sharing unpublished data and helpful discussions, and Hervé Vaucheret, Jim Carrington and the Arabidopsis Biological Resource Center for seeds.
NOTE ADDED IN PROOF
While our manuscript was under revision Chitwood et al. (Chitwood DH, Nogueira FT, Howell MD, Montgomery TA, Carrington JC, Timmermans MC [2009] Pattern formation via small RNA mobility. Genes Dev. 23(5)) reported a similar study and proposed the cell-to-cell mobility of ARF-regulating tasiRNAs.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the National Science Foundation (DBI-0421604) and the Robertson Research Fund to R.M.; a starting grant from the European Research Council (ERC, Frontiers of RNAi, 210890) to O.V.; an ANR grant (ANR06 GPLA 011) to A.M. and M.C.; a grant from the European Union integrated project SIROCCO (Silencing RNAs: Organisers and Coordinators of Complexity in Eukaryotic Organisms; LSHG-CT-2006-037900) to D.G; an EMBO long-term fellowship (ALTF 864-2005) and a postdoctoral fellowship from DuPont to R.S.; a European Union Marie Curie fellowship (041419) to V.R.F.; and a research fellowship from the DFG to M.B. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Baulcombe D. RNA silencing. Trends in Biochemical Sciences. 2005;30:290–293. doi: 10.1016/j.tibs.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Vaucheret H. MicroRNA-dependent trans-acting siRNA production. Science's Stke [Electronic Resource]: Signal Transduction Knowledge Environment. 2005;2005:pe43. doi: 10.1126/stke.3002005pe43. [DOI] [PubMed] [Google Scholar]
- 3.Peragine A, Yoshikawa M, Wu G, Albrecht HL, Poethig RS. SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes & Development. 2004;18:2368–2379. doi: 10.1101/gad.1231804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vazquez F, Vaucheret H, Rajagopalan R, Lepers C, Gasciolli V, et al. Endogenous trans-acting siRNAs regulate the accumulation of Arabidopsis mRNAs. Molecular Cell. 2004;16:69–79. doi: 10.1016/j.molcel.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Gasciolli V, Mallory AC, Bartel DP, Vaucheret H. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting siRNAs. Current Biology. 2005;15:1494–1500. doi: 10.1016/j.cub.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Xie Z, Allen E, Wilken A, Carrington JC. DICER-LIKE 4 functions in trans-acting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12984–12989. doi: 10.1073/pnas.0506426102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshikawa M, Peragine A, Park MY, Poethig RS. A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes & Development. 2005;19:2164–2175. doi: 10.1101/gad.1352605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adenot X, Elmayan T, Lauressergues D, Boutet S, Bouche N, et al. DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Current Biology. 2006;16:927–932. doi: 10.1016/j.cub.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Nakazawa Y, Hiraguri A, Moriyama H, Fukuhara T. The dsRNA-binding protein DRB4 interacts with the Dicer-like protein DCL4 in vivo and functions in the trans-acting siRNA pathway. Plant Molecular Biology. 2007;63:777–785. doi: 10.1007/s11103-006-9125-8. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez JP, Pekker I, Goldshmidt A, Blum E, Amsellem Z, et al. Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell. 2006;18:1134–1151. doi: 10.1105/tpc.105.040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parizotto EA, Dunoyer P, Rahm N, Himber C, Voinnet O. In vivo investigation of the transcription, processing, endonucleolytic activity, and functional relevance of the spatial distribution of a plant miRNA. Genes & Development. 2004;18:2237–2242. doi: 10.1101/gad.307804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tretter EM, Alvarez JP, Eshed Y, Bowman JL. Activity range of Arabidopsis small RNAs derived from different biogenesis pathways. Plant Physiol. 2008;147:58–62. doi: 10.1104/pp.108.117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunoyer P, Himber C, Voinnet O. DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nature Genetics. 2005;37:1356–1360. doi: 10.1038/ng1675. [DOI] [PubMed] [Google Scholar]
- 14.Nogueira FT, Chitwood DH, Madi S, Ohtsu K, Schnable PS, et al. Regulation of small RNA accumulation in the maize shoot apex. PLoS Genet. 2009;5:e1000320. doi: 10.1371/journal.pgen.1000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogueira FT, Madi S, Chitwood DH, Juarez MT, Timmermans MC. Two small regulatory RNAs establish opposing fates of a developmental axis. Genes Dev. 2007;21:750–755. doi: 10.1101/gad.1528607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poethig RS, Peragine A, Yoshikawa M, Hunter C, Willmann M, et al. The function of RNAi in plant development. Cold Spring Harbor Symposia on Quantitative Biology. 2006;71:165–170. doi: 10.1101/sqb.2006.71.030. [DOI] [PubMed] [Google Scholar]
- 17.Garcia D, Collier SA, Byrne ME, Martienssen RA. Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Current Biology. 2006;16:933–938. doi: 10.1016/j.cub.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 18.Howell MD, Fahlgren N, Chapman EJ, Cumbie JS, Sullivan CM, et al. Genome-wide analysis of the RNA-DEPENDENT RNA POLYMERASE6/DICER-LIKE4 pathway in Arabidopsis reveals dependency on miRNA- and tasiRNA-directed targeting. Plant Cell. 2007;19:926–942. doi: 10.1105/tpc.107.050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pekker I, Alvarez JP, Eshed Y. Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell. 2005;17:2899–2910. doi: 10.1105/tpc.105.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen E, Xie Z, Gustafson AM, Carrington JC. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121:207–221. doi: 10.1016/j.cell.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Hunter C, Willmann MR, Wu G, Yoshikawa M, de la Luz Gutierrez-Nava M, et al. Trans-acting siRNA-mediated repression of ETTIN and ARF4 regulates heteroblasty in Arabidopsis. Development. 2006;133:2973–2981. doi: 10.1242/dev.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams L, Carles CC, Osmont KS, Fletcher JC. A database analysis method identifies an endogenous trans-acting short-interfering RNA that targets the Arabidopsis ARF2, ARF3, and ARF4 genes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9703–9708. doi: 10.1073/pnas.0504029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahlgren N, Montgomery TA, Howell MD, Allen E, Dvorak SK, et al. Regulation of AUXIN RESPONSE FACTOR3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Current Biology. 2006;16:939–944. doi: 10.1016/j.cub.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Xu L, Wang H, Yuan Z, Cao X, et al. The Putative RNA-dependent RNA polymerase RDR6 acts synergistically with ASYMMETRIC LEAVES1 and 2 to repress BREVIPEDICELLUS and MicroRNA165/166 in Arabidopsis leaf development. Plant Cell. 2005;17:2157–2171. doi: 10.1105/tpc.105.033449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Himber C, Dunoyer P, Moissiard G, Ritzenthaler C, Voinnet O. Transitivity-dependent and -independent cell-to-cell movement of RNA silencing. EMBO Journal. 2003;22:4523–4533. doi: 10.1093/emboj/cdg431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronemus M, Vaughn MW, Martienssen RA. MicroRNA-targeted and small interfering RNA-mediated mRNA degradation is regulated by argonaute, dicer, and RNA-dependent RNA polymerase in Arabidopsis. Plant Cell. 2006;18:1559–1574. doi: 10.1105/tpc.106.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lister R, O'Malley RC, Tonti-Filippini J, Gregory BD, Berry CC, et al. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133:523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell. 2006;18:1121–1133. doi: 10.1105/tpc.105.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen E, Xie Z, Gustafson AM, Sung GH, Spatafora JW, et al. Evolution of microRNA genes by inverted duplication of target gene sequences in Arabidopsis thaliana. Nat Genet. 2004;36:1282–1290. doi: 10.1038/ng1478. [DOI] [PubMed] [Google Scholar]
- 30.Weigel D, Glazebrook J. Arabidopsis: A laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Accumulation of the tasiRNA sensor in root meristems. (a) The tasiRNA sensor is detected throughout the root meristem, most strongly in the outer cell layers, in the absence of tasiRNAs in homozygous rdr6-3 mutants, but becomes restricted to the epidermis in the presence of tasiRNAs in RDR6 heterozygotes, i.e. in the presence of tasiRNAs (b).
(0.20 MB TIF)