Abstract
Background
The impact of the pneumococcal conjugate vaccine (PCV-7) on antibiotic resistance among pneumococcal strains causing invasive pneumococcal disease (IPD) has varied in different locales in the United States. We assessed trends in IPD including trends for IPD caused by penicillin non-susceptible strains before and after licensure of PCV-7 and the impact of the 2008 susceptibility breakpoints for penicillin on the epidemiology of resistance.
Methodology/Principal Findings
We performed a retrospective review of IPD cases at Morgan Stanley Children's Hospital of NewYork-Presbyterian, Columbia University Medical Center. Subjects were ≤18 years of age with Streptococcus pneumoniae isolated from sterile body sites from January 1995–December 2006. The rate of IPD from 1995–1999 versus 2002–2006 significantly decreased from 4.1 (CI95 3.4, 4.8) to 1.7 (CI95 1.3, 2.2) per 1,000 admissions. Using the breakpoints in place during the study period, the proportion of penicillin non-susceptible strains increased from 27% to 49% in the pre- vs. post-PCV-7 era, respectively (p = 0.001), although the rate of IPD caused by non-susceptible strains did not change from 1995–1999 (1.1 per 1,000 admissions, CI95 0.8, 1.5) when compared with 2002–2006 (0.8 per 1,000 admissions, CI95 0.6, 1.2). In the multivariate logistic regression model controlling for the effects of age, strains causing IPD in the post-PCV-7 era were significantly more likely to be penicillin non-susceptible compared with strains in the pre-PCV-7 era (OR 2.46, CI95 1.37, 4.40). However, using the 2008 breakpoints for penicillin, only 8% of strains were non-susceptible in the post-PCV-7 era.
Conclusions/Significance
To date, there are few reports that document an increase in the relative proportion of penicillin non-susceptible strains of pneumococci causing IPD following the introduction of PCV-7. Active surveillance of pneumococcal serotypes and antibiotic resistance using the new penicillin breakpoints is imperative to assess potential changes in the epidemiology of IPD.
Introduction
Invasive pneumococcal disease (IPD) caused by strains non-susceptible to penicillin became increasingly prevalent during the mid-1990's. In 1998, the Center for Disease Control and Prevention reported that 24% of strains associated with IPD were non-susceptible to penicillin and that serotypes 6B, 9V, 14, 19F, and 23F accounted for 78% of penicillin non-susceptible strains [1]. In 2000, the heptavalent pneumococcal conjugate vaccine (PCV-7) was approved by the FDA to reduce the burden of pneumococcal disease, and in August 2000, the American Academy of Pediatrics recommended that all children younger than 2 years of age and high-risk children 2–5 years of age receive PCV-7 [2]. By 2006, 87% of American children 19–35 months of age had received 3 or more doses of PCV-7 [3] and numerous studies have documented a reduction in the incidence of IPD [4]–[6].
However, the impact of PCV-7 on the proportion of penicillin non-susceptible strains causing IPD has varied [5], [7]–[8]. Notably, these studies did not assess changes in the rates of invasive disease caused by such strains. In Utah, the proportion of penicillin non-susceptible strains decreased from 34% in the pre-PCV-7 era to 22% in the post-PCV-7 era [5]. In Dallas, no change in penicillin susceptibility was noted between 1999 and 2005 [7] and in Philadelphia, a 14% relative increase in the proportion of penicillin non-susceptible strains was observed following PCV-7 approval [8]. We assessed trends in hospital admissions for IPD before and after licensure of PCV-7 at our children's hospital in New York City. We also examined the epidemiology of antimicrobial susceptibility, including the impact of the 2008 susceptibility breakpoints for penicillin [9].
Methods
Study Design and Study Site
We reviewed cases of IPD admitted to the Morgan Stanley Children's Hospital of NewYork-Presbyterian, Columbia University Medical Center located in New York City from January 1, 1995 to December 31, 2006. The Institutional Review Board of Columbia University approved this study with a waiver of documentation of informed consent.
Study Subjects and Study Isolates
The electronic data warehouse was queried for patients ≤18 years of age with a positive culture for S. pneumoniae isolated from blood, cerebrospinal fluid (CSF), pleural fluid, joint fluid, bone, and/or abscesses during the study period. If more than one culture from an individual patient was positive for S. pneumoniae, only the first was included in the analysis, and if both blood and CSF cultures were positive, the strain from CSF was included.
Antimicrobial Susceptibilities
Antimicrobial susceptibilities as determined by the Clinical Laboratory and Standards Institute (formerly the National Committee on Clinical Laboratory Standards) in use during the study period [10] were compared to those introduced in 2008 [9]. During the study period, the breakpoints in use for penicillin for CSF and non-CSF isolates were comparable: susceptible ≤0.06, intermediate ≥0.1–1, and resistant ≥2 µg/ml. In 2008, the breakpoints were changed for both CSF isolates: susceptible ≤0.06 and resistant ≥0.12 µg/ml and for non-CSF isolates: susceptible ≤2, intermediate 4, and resistant ≥8 µg/ml. Breakpoints for 3rd generation cephalosporins did not change; CSF isolates: susceptible ≤0.5, intermediate 1, and resistant ≥2 µg/ml and non-CSF isolates: susceptible ≤1, intermediate 2, and resistant ≥4 µg/ml.
Statistical Analysis
Categorical variables were summarized as proportions. Pre- (1995–2000) and post- (2001–2006) PCV-7 variables were compared using the Chi-squared test and continuous variables were compared using the two-sample t-test. Multivariate logistic regression was used to estimate the rate change for penicillin susceptibility between the pre- and post-PCV-7 eras, controlling for age as a possible confounding variable. As PCV-7 was introduced in August 2000, 2000–2001 were considered to be transition years and thus the rate of IPD and the rate of IPD caused by penicillin non-susceptible strains was compared from 1995–1999 versus 2002–2006.
Results
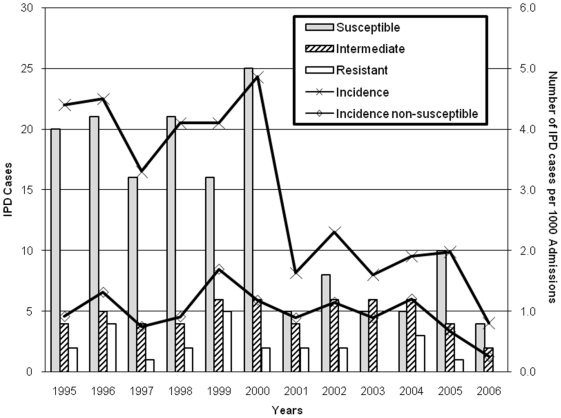
During the 12-year study period, 242 cases of IPD occurred of which 169 (70%) cases occurred from 1995–2000. Overall, bacteremia (n = 215) and meningitis (n = 17) were the most common diagnoses, and 56% of IPD cases occurred in boys. The rate of IPD from 1995–1999 vs. 2002–2006 significantly decreased (p<0.001) from 4.1 (CI95 3.4, 4.8) to 1.7 (CI95 1.3, 2.2) per 1,000 admissions as shown in the Figure 1.
Figure 1. Invasive pneumococcal disease at a NYC Children's Hospital, 1995–2006.
The number of cases of invasive pneumococcal disease (IPD) caused by penicillin resistant, intermediate, and susceptible strains of S. pneumoniae is shown (Left-hand Y axis). The rate of IPD per 1000 admissions and the rate of IPD caused by penicillin non-susceptible strains per 1000 admissions are shown (Right-hand Y-axis).
Children with IPD were younger in the pre-PCV-7 era than children in the post-PCV-7 era (mean age 2.8 versus 4.6 years, respectively, p = 0.001). In the pre-PCV-7 era, children ≤2 years of age were less likely to be infected with non-susceptible strains (breakpoints in use during the study period) than older children (OR 0.4, CI95 0.2, 0.8, p = 0.01) as 19 (20%) of 95 children ≤2 years of age versus 26 (37.7%) of 69 children >2 years of age were infected with non-susceptible strains. In the post-PCV-7 era, there was no association between age and non-susceptible strains as 15 (43%) of 35 children ≤2 years of age versus 21 (55%) of 38 children >2 years of age were infected with non-susceptible strains (p = 0.29).
As interpreted by the breakpoints in use during the study period, the MIC50 (median MIC) for penicillin in the pre-PCV-7 era was 0.06 µg/ml and in the post-PCV-7 era was 0.094 µg/ml, while the MIC90 (90th percentile MIC) in the pre-PCV-7 era was 1.0 µg/ml and in the post-PCV-7 era was 2.0 µg/ml. In the pre-PCV-7 era, 45 (27%) of 164 tested strains were non-susceptible to penicillin (MIC ≥0.1 µg/ml) while in the post-PCV-7 era, 36 (49%) of 73 strains were non-susceptible (p = 0.001). In the multivariate logistic regression model controlling for the effects of age, strains of IPD in the post-PCV-7 era were significantly more likely to be penicillin non-susceptible than strains in the pre-PCV-7 era (OR 2.5, CI95 1.4, 4.4, p = 0.003). However, the rate of IPD caused by non-susceptible strains did not change from 1995–1999 (1.1 per 1,000 admissions, CI95 0.8, 1.5) when compared with 2002–2006 (0.8 per 1,000 admissions, CI95 0.6, 1.2).
As expected, the breakpoints introduced in 2008 changed the epidemiology of penicillin resistance. A comparison of the proportion of penicillin non-susceptible strains using the previous and current breakpoints for non-CSF and CSF isolates from the pre- and post-PCV-7 era is shown in Table 1. Among the 220 non-CSF isolates from the study period, the proportion of penicillin-resistant strains would decrease from 10% (22/220) using previous breakpoints to 1.4% (3/220) using current breakpoints (p<0.001). Similarly, the proportion of intermediately susceptible non-CSF isolates would decrease from 24% (53/220) to 3% (6/220) [p<0.001]. In contrast, among the 17 CSF isolates from the study period, the proportion of resistant strains would not significantly change; there were 2 (12%) resistant strains using previous breakpoints vs. 6 (35%) resistant strains using current breakpoints (p = 0.106). Thus, in the post-PCV-7 era, there were only 4 non-CSF and 2 CSF isolates that were non-susceptible to penicillin using current breakpoints.
Table 1. Penicillin Susceptibility among Non-CSF and CSF Isolates in the Pre and Post PCV-7 Eras using Previous1 and Current2 Breakpoints*.
| Breakpoint Interpretations | Non-CSF Isolates Pre-PCV-7 Era3 | Non-CSF Isolates Post-PCV-7 Era | CSF Isolates Pre-PCV-7 Era | CSF Isolates Post-PCV-7 Era | ||||
| Previous breakpoints | Current breakpoints | Previous breakpoints | Current breakpoints | Previous breakpoints | Current breakpoints | Previous breakpoints | Current breakpoints | |
| Susceptible | 110(73%) | 146(97%) | 35(51%) | 65(94%) | 9(69%) | 9(69%) | 2(50%) | 2(50%) |
| Intermediate | 27(18%) | 3(2%) | 26(38%) | 3(4%) | 2(15.5%) | 0 | 2(50%) | 0 |
| Resistant | 14(9%) | 2(1%) | 8(11%) | 1(1%) | 2(15.5%) | 4(31%) | 0 | 2(50%) |
| Total | 151 | 151 | 69 | 69 | 13 | 13 | 4 | 4 |
Abbreviations: 7-Valent Pneumococcal Conjugate Vaccine (PCV-7); Cerebrospinal fluid (CSF).
Breakpoints used prior to 2008.
Breakpoints introduced in 2008.
32 of the susceptible non-CSF isolates were processed using Kirby-Bauer and therefore did not have an MIC result and 5 non-CSF isolates from 1995 were missing susceptibility results.
There was no significant change in susceptibility to 3rd generation cephalosporin agents (p = 0.66) in the pre- vs. post-PCV-7 eras; only 3.0% (4/132 tested strains) and 1.4% (1/70 tested strains) were non-susceptible to these agents, respectively. All tested strains (n = 208) were susceptible to vancomycin during the study period. In the post-PCV-7 era, all tested strains (n = 67) were susceptible to levofloxacin. In contrast, resistance to erythromycin increased from 6.7% (7/105 tested strains) in the pre-PCV-7 era to 29.6% (21/71 tested strains) in the post-PCV-7 era (p<0.001).
Discussion
In our patient population, the incidence of IPD before and following PCV-7 licensure was similar to previous reports [4], [5], [7]. Prior studies have also documented an increase in the mean age of patients with IPD in the post-PCV-7 era [4], [5], [7]. However, as our children's hospital serves as a referral center, the rates presented are not population-based and our findings may not be generalizable to other clinical and geographic settings. Furthermore, we could not assess the efficacy of PCV-7 vaccine on actual cases of IPD.
To our knowledge, when compared with other studies performed throughout the U.S. (Table 2), this is one of the few reports to document an increase in the relative proportion of penicillin non-susceptible strains following the introduction of PCV-7. However, most previous reports have not addressed changes in the rate of IPD caused by non-susceptible pneumococcal strains and generally have not used the penicillin breakpoints recently introduced by the CLSI [9]. As we have shown, use of these new breakpoints would most likely decrease the proportion of non-susceptible strains isolated from non-CSF sources and increase the proportion of non-susceptible strains isolated from the CSF. Furthermore, we demonstrated that younger children were less likely to be infected with non-susceptible strains in the pre-PCV-7 era. We speculate that the shift to IPD in older children during the post-PCV-7 era could have influenced the penicillin non-susceptibility profile between the pre- and post-PCV-7 eras as older children could have had more exposure to antibiotics and thus be more likely to be infected with non-susceptible pneumococci.
Table 2. Comparison of Studies Describing the Impact of PCV-7 on Invasive Pneumococcal Disease in Children.
| Ref | Study Sites (Years of Study) | Subjects Age | IPD Cases or Isolates (N) | Change in proportion of PCN non-susceptible strains pre vs. post PCV-7 licensure | Vaccine-Related Serotype change | Non-Vaccine-Related Serotype change |
| This study | New York, NY (1995–2006) | ≤18 years | 242 cases | 22% increase (p = 0.001) | Not available | Not available |
| 4 | Selected sites in US1 (1999 vs 2004) | ≤2 years2 | 858 isolates | 81% decrease (CI95 (80%, 82%)) | 98% decrease (CI95 (96%, 99%)) | 150% increase |
| 5 | Intermountain Health Care System3 (1996–2000 vs 2001–2003) | <18 years | 234 cases | 12% decrease (p = 0.04) | 23% decrease (p<0.001) | 20% increase (p<0.001) |
| 7 | Dallas, TX (1999–2005) | ≤17 years | 398 cases | No change (p = 0.687) | Not available | 64% increase (p<0.001) |
| 8 | Philadelphia, PA (1999–2000 vs 2001–2005) | ≤17 years | 188 cases bacteremia | 14% increase (p = 0.019) | 40% decrease | 18% increase |
| 11 | Alaska (1995–2006) | <2 years | 349 cases | 6% decrease (p<0.001) | 97% decrease | 130% increase among Native children from 2001–2003 vs 2004–20064 |
| 12 | Cleveland, OH (1979–2004) | <5 years2 | Not available | Not available | 92% decrease (p<0.001) | 64% decrease (p = 0.028) |
| 13 | Selected sites in US1 (1997–2004) | ≤90 days | 146 cases | 75% decrease, PCV-7 serotypes (p<0.001) No change, non-PCV-7 serotypes | 31% decrease | 31% increase |
Abbreviations used in table: Reference (Ref) 7-Valent Pneumococcal Conjugate Vaccine (PCV-7); Invasive Pneumococcal Disease (IPD); Penicillin (PCN).
Included: San Francisco county, CA; Connecticut; 20 counties of Georgia, including Atlanta; Baltimore, Maryland; 7 counties surrounding St. Paul, Minnesota; 7 counties surrounding Rochester, New York; 3 counties surrounding Portland, Oregon; 5 urban counties, Tennessee.
This study presented data for all ages, but selected pediatric age strata were presented as indicated.
Included: Utah, Idaho, Montana, Wyoming, and parts of Arizona and Nevada.
No change in non-vaccine serotypes from 1995–2000 vs 2001–2003 among either Alaskan Native or Non-Native children.
While our findings may be due to pneumococcal serotype replacement or emergence of a new serotype in the post-PCV-7 era, such as been shown in diverse locations in the U.S., [Table 2] we were unable to assess this possibility because we did not systematically study serotypes.
Monitoring the burden of IPD in the United States is needed to continue to assess the efficacy of the PCV-7 vaccine as well as the efficacy of newer, more extensive valent PCVs. Surveillance must include the antimicrobial susceptibility and pneumococcal serotypes associated with IPD. While our results suggest the continued need for empiric treatment of penicillin non-susceptible strains, particularly if meningitis is suspected, epidemiologic trends for antimicrobial susceptibility should be studied with the new penicillin breakpoints.
Acknowledgments
The authors thank Adam Ratner for several helpful discussions of the manuscript and John Lawrence for his information technology support. The authors also gratefully acknowledge the contributions of Ms. Shing Lee and Mr. Jimmy K. Duong of the Biostatistics Consulting Services and the Irving Institute for Clinical and Translational Research at Columbia University Medical Center.
Footnotes
Competing Interests: As indicated in the acknowledgements section, the authors had helpful conversations with Dr. Adam Ratner, who is also in the same pediatric infectious diseases division as Dr. Lisa Saiman and Ann Smith.
Funding: The authors have no support or funding to report.
References
- 1.Whitney CG, Farley MM, Hadler J, Harrison LH, Lexau C, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Eng J Med. 2000;343:1917–1924. doi: 10.1056/NEJM200012283432603. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics. Committee on Infectious Diseases. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics. 2000;106:362–366. doi: 10.1542/peds.106.2.362. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among children aged 19–35 months- United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:880–885. [PubMed] [Google Scholar]
- 4.Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Eng J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 5.Byington CL, Samore MH, Stoddard GJ, Barlow S, Daly J, et al. Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups. Clin Infect Dis. 2005;41:21–29. doi: 10.1086/430604. [DOI] [PubMed] [Google Scholar]
- 6.Shah SS, Ratner AJ. Trends in pneumococcal disease-associated hospitalizations. Clin Infect Dis. 2006;42:e1–5. doi: 10.1086/498745. Epub 2005 Nov 23. [DOI] [PubMed] [Google Scholar]
- 7.Messina AF, Katz-Gaynor K, Barton T, Ahmad N, Ghaffar F, et al. Impact of pneumococcal conjugate vaccine on serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Dallas, TX, children from 1999 through 2005. Pediatr Infect Dis J. 2007;26:461–467. doi: 10.1097/INF.0b013e31805cdbeb. [DOI] [PubMed] [Google Scholar]
- 8.Steenhoff AP, Shah SS, Ratner AJ, Patil SM, McGowan K. Emergence of vaccine-related pneumococcal serotypes as a cause of bacteremia. Clin Infect Dis. 2006;42:907–914. doi: 10.1086/500941. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute (CLSI) Wayne, PA: Clinical Laboratory and Standards Institute; 2008. Performance standards for antimicrobial susceptibility testing. M100-S18. [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Wayne, PA: Clinical Laboratory and Standards Institute; 2007. Performance standards for antimicrobial susceptibility testing. Volume 27 #1 M100-S17. [Google Scholar]
- 11.Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–1792. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs MR, Good CE, Beall B, Bajaksouzian S, Windau AR, et al. Changes in serotype and antimicrobial susceptibility of invasive Streptococcus pneumoniae strains in Cleveland: a quarter century of experience. J Clin Microbiol. 2008;46:982–990. doi: 10.1128/JCM.02321-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295:1668–1674. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]