Abstract
Telomerase activity appears to be associated with cell immortalization and malignant progression. Understanding how telomerase activity is regulated in vivo is important not only for understanding the molecular biology of telomerase but also for the potential clinical application of anticancer drugs. This study evaluated telomerase activity and quantified the expression of human telomerase reverse transcriptase (hTERT) mRNA and human telomerase RNA (hTR) using a real-time reverse transcriptase-polymerase chain reaction (RT-PCR) method before and after the exposure of cisplatin and 5-fluorouracil (5-FU) in two head and neck squamous cell carcinoma (HNSCC) cell lines. Two human HNSCC cell lines (PNUH-12 and SNU-899) were studied. Cell cytotoxicity, the change of telomerase activity, and hTERT mRNA and hTR expression by 5-FU and cisplatin exposure were assessed by MTT assay, TRAP assay, and real-time RT-PCR, respectively. In two cell lines, after cisplatin exposure, the telomerase activity and hTERT mRNA expression decreased, but hTR expression increased according to the concentration of drug. However, in both cell lines, the telomerase activity and hTR did not show any significant change after 5-FU treatment, but the expression of hTERT mRNA decreased. These results suggest that there may be other important regulating mechanism except hTERT mRNA as the regulation factor of telomerase activity in HNSCC cell lines.
Keywords: Telomerase; Telomerase Reverse Transcriptase; Telomerase RNA, Cisplatin; 5-Fluorouracil
INTRODUCTION
Telomerase, a ribonucleoprotein enzyme that consists of RNA and protein, can synthesize telomeres. Normally, some telomerase is found in germ cells and hemopoietic cells, but not in normal somatic cells. However, malignant cells gain the ability to proliferate indefinitely because telomerase is usually active in them (1). Telomerase activity has been studied as a cancer-diagnosing indicator and a prognostic factor that helps to predict the progress of malignant tumors (2, 3).
Telomerase consists of three important components of human telomerase RNA (hTR), telomerase-associated protein 1 (TP1), and human telomerase reverse transcriptase (hTERT) (4, 5). The hTERT mRNA is a catalytic subunit protein of telomerase, which harbors the enzymatic activity of telomerase (4). A very strong correlation between the presence of hTERT mRNA and telomerase activity has been generally accepted, although direct evidence is scarce. hTR and TP1 are broadly expressed in both cancers and in normal tissues and their expression is not significantly correlated with telomerase activity (6-8).
An understanding of how telomerase activity is regulated in vivo is important not only to understand the molecular biology of telomerase, but also because of its potential as a possible target molecule for anticancer drugs. A combined regimen of cisplatin and 5-fluorouracil (5-FU) is used mainly for head and neck malignant tumors including hypopharyngeal cancer and laryngeal carcinoma (9). An understanding of the mechanism of inhibiting telomerase activity with these drugs would provide valuable information on how telomerase is regulated in vivo. This study evaluated the telomerase activity and quantified the expression of hTERT mRNA and hTR using a real-time reverse transcriptase-polymerase chain reaction (RT-PCR) method before and after the exposure of cisplatin and 5-FU in head and neck cancer cell lines.
MATERIALS AND METHODS
Cancer cell lines
The laryngeal carcinoma cell line (SNU-899) was purchased from the Korean Cell Line Bank (10). PNUH-12 cells originating from hypopharyngeal squamous cell carcinoma were established at the Department of Otolaryngology, College of Medicine, Pusan National University (11). PNUH-12 cells, a p53 mutant-type (one point mutation at the 78th base, C to G, in exon 7) human hypopharyngeal squamous cell carcinoma cell line, were maintained as a monolayer in Dulbecco's Modified Eagle medium (DMEM, GIBCO BRL, Grand Island, NY, U.S.A.) containing 10% (v/v) fetal bovine serum, 100 units/mL penicillin and 100 mg/mL streptomycin and were incubated at 37℃ in a humidified atmosphere of 5% CO2.
Anticancer drugs
The study used cisplatin (Dong-A, Pharmaceutical Co., Seoul, Korea) and 5-FU (Choongwae Pharmaceutical Co., Seoul, Korea), which are widely used for the treatment of malignant head and neck cancer.
Cell cytotoxicity assay (MTT Assay)
Cytotoxicities to cisplatin and 5-FU were assessed by MTT (3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. Briefly, 200 µL of cell suspension (1×104 cells) was added to each well of 96-well flat-bottom microtitre plates (Costar, Cambrige, Massachusetts, U.S.A.), and each plate was incubated for 24 hr at 37℃ in a humidified 5% CO2 atmosphere. After incubation, drugs were made up in the medium, and various concentrations were added to the plates at a volume of 200 µL per well. The plates were incubated for 48 hr with the drugs. Following incubation, 100 µL of MTT working solution (2 mg/mL, Sigma Chemical Co., St. Louise, MO, U.S.A.) was added to each well and incubated for 4 hr at 37℃. The medium was carefully discarded, and the formazan crystals were dissolved in 100 µL dimethylsulphoxide. The absorbance of each well was measured with a microculture plate reader (Emax) at 570 nm. Absorbance values were expressed as a percentage of untreated controls and an inhibition concentration 50 (IC50) was calculated. The IC50 value represents the mean of at least four independent experiments.
Measurements of telomerase activity and analysis by the TRAP method
The activity of telomerase was measured and examined with using a TRAPEZE Telomerase Detection Kit (Intergen Co. NY, U.S.A.) using the TRAP assay by Kim et al. (1). Cells before and after treatment with anticancer drugs were put in a microcentrifuge tube and 100 µL of 1X CHAPS lysis buffer (10 mM Tris-HCl, pH 7.5, 1 mM MgCl2, 1 mM EGTA, 0.1 mM benzamidine, 5 mM β-mercaptoethanol, 0.5% CHAPS, 10% Glycerol) was added. Then a homogeneous solution was made by pipetting. This solution was kept in ice for 30 min before undergoing centrifusion. Approximately 80 µL of the top-layer solution was moved into a new tube, and the protein concentration was measured. The protein concentration was then diluted with a 1X CHAPS lysis buffer to make 1 µg/µL.
The TS primer (5'-AATCCGTCGAGCAGAGTT-3') to be used for the TRAP reaction was labeled with γ-32P-ATP (3,000 Ci/mM, 10 µCi/mL) and T4 polynucleotide kinase. The total amount of the TRAP reaction solution was 25 µL: 2.5 µL of the 10X TRAP buffer (200 mM Tris-HCl, pH 8.3, 15 mM MgCl2, 630 mM KCl, 0.5% Tween 20, 10 mM EGTA, 0.1% BSA), 0.5 µL of the 50X dNTPs mix (25 mM each of dATP, dTTP, dGTP and dCTP), 1 µL of the 32P-TS primer, 0.5 µL of the TRAP primer mix (RP primer, K1 primer, TSK1 template), 0.2 µL of Taq polymerase (5 units/µL, Takara Co., Shiga, Japan), 18.3 µL of distilled water, and 2 µL of the specimen. The solution was reacted at 30℃ for 30 min in a thermal cycler (Mastercycler 5330, Eppendorf Co., Hamburg, Germany), so that the telomerase in the specimen could react. Next, the reaction was terminated by heating for 30 sec at 94℃. The amplification of the product of reaction was repeated 30 times; one unit of amplification consisted of 30 sec at 94℃ and 30 sec at 60℃.
Loading dye (0.25% bromophenol blue, 0.25% xylene cyanol, 50% glycerol, 50 mM EDTA, pH 8.0) was put into each reaction tube, and electrophoresis was conducted in 12.5% polyacrylamide gel and 0.25X TBE buffer. The results were analyzed by using a phosphorimager (Molecular Dynamics Co., Sunnyvale, CA, U.S.A.).
Total RNA extraction
RNA was extracted using RNAzolB™ (WAK-Chemie Medical, Bad Homburg, Germany) according to the manufacture's instructions. The RNA yield was quantified by UV spectrophotometry. One microgram of total RNA was subjected to 1% agarose gel electrophoresis. Preservation of 28S and 18S rRNA species was used to assess the RNA integrity.
Quantitative detection of hTERT mRNA by real time RT-PCR
Quantitative detection of hTERT mRNA was performed with the commercially available LightCycler Telo TAGGG hTERT Quantification Kit (Roche Diagnostics GmbH, Mannheim, Germany) using the LightCycler instrument (Roche Molecular Systems, Alameda, CA, U.S.A.) for real-time PCR and all subsequent quantification steps according to the manufacturer's instructions. These experiments were repeated three times for each cell line.
hTERT mRNA was reverse transcribed, and a fragment of the cDNA were amplified with specific primers in a one-step RT-PCR reaction. The amplicon was detected by fluorescence using a specific pair of hybridization probes. The hybridization probes consisted of two different short oligonucleotides that hybridized to an internal sequence of the amplicon during the annealing phase of the amplification cycle. One probe was labeled at the 5'-end with LightCycler Red 640 and, to avoid extension, modified at the 3'-end by phosphorylation. The other probe was labeled at the 3'-end with fluorescein. The two probes were in close proximity only after hybridization to the template DNA, resulting in fluorescence resonance energy transfer (FRET) between the two fluorophores. During FRET, fluorescein, the donor fluorophore, was excited by the light source of the LightCycler instrument, and part of the excitation energy was transferred to LightCycler Red 640, the acceptor fluorophore. The emitted fluorescence of LightCycler Red 640 was then measured by the LightCycler instrument.
A typical 20 µL one-tube RT-PCR reaction contained 200 ng of total RNA (sample) or standard RNA templates provided with the kit. mRNA was reverse-transcribed for 10 min at 60℃. PCR amplifications were performed in separate tubes for 40 cycles (0.5 sec at 95℃; 10 sec at 60℃; 10 sec at 72℃) using manufacturer-supplied reaction mixtures specific for hTERT or the housekeeping gene PBGD, respectively. The PBGD reaction product served as a control for RT-PCR and as a reference for relative quantification of hTERT mRNA.
The number of PCR cycles to reach the fluorescence threshold was the cycle threshold (Ct). The Ct value for each sample was proportional to the log of the initial amount of input cDNA. By plotting the Ct value of an unknown sample on the standard curve, the amount of target sequences in the sample could be calculated. To normalize the hTERT mRNA expression for sample to sample differences in RNA input, RNA quality, and reverse transcriptase efficiency, we amplified the housekeeping gene PBGD. From each standard curve, we derived the copy numbers of PBGD and hTERT, respectively. The ratio between the copy numbers of hTERT and PBGD represented the normalized hTERT (NhTERT) for each sample and could be compared with that of other samples.
NhTERT=hTERT mRNA copies sample/PBGD mRNA copies sample
Quantitative detection of hTR by real-time RT-PCR
The quantitative detection of hTR was performed using the same procedures described for hTERT, in combination with the LightCycler Telo TAGGG hTR Quantification Kit (Roche Diagnostics GmbH). Because the hTR-encoding gene is intron-free, the PCR products could result from contamination by genomic DNA. These experiments were repeated three times for each cell line.
The number of PBGD and hTR transcripts in samples was calculated with the LightCycler software, using these standard curves. Each sample was normalized on the basis of its PBGD content (see above) according to the formula NhTR=hTR mRNA copies sample/PBGD mRNA copies sample.
RESULTS
Cell cytotoxicity test
After the treatment of cisplatin and 5-FU in PNUH-12 and SNU-899, the cell survival rate decreased in proportion to concentration. The IC50 difference between cisplatin and 5-FU in PNUH-12 and SNU-899 were 8-fold and 15-fold, respectively (Fig. 1).
Fig. 1.
Cytotoxic effects of cisplatin (A) and 5-fluorouracil (5-FU) (B) in PNUH-12, SNU-899, and HEp-2. As the concentration of the cisplatin and 5-FU increased, the survival decreased.
Telomerase activity by the TRAP assay
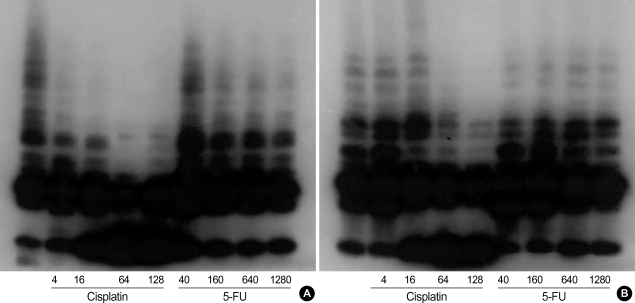
The trap assay was conducted in PNUH-12 and SNU 899 before drug treatment, and the activity of telomerase was displayed (Fig. 2). In both cell lines, after cisplatin exposure, telomerase activity decreased according to the drug concentration, but after 5-FU exposure, the change of telomerase activity was insignificant.
Fig. 2.
Photographs showing the telomerase activity of three head and neck cancer cell lines by cisplatin and 5-fluorouracil (5-FU) exposure. Telomerase activity was observed by telomerase assay in PNUH-12 (A) and SNU-899 (B) cell lines. The telomerase activity decreased in a concentration-dependent manner after cisplatin exposure, while the change in telomerase activity was insignificant after 5-FU exposure.
Real-time RT-PCR in PNUH-12
We used different concentrations of hTERT and hTR standard templates including 103, 104, 105, 106, and 107 copies/2 µL to perform quantitative PCR and calculate the standard curves, respectively. The correlation coefficients were both 1.00.
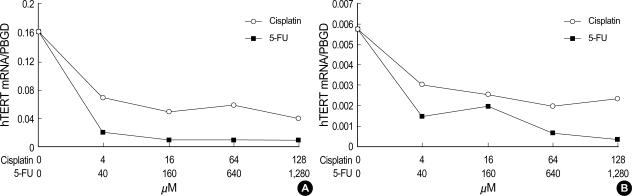
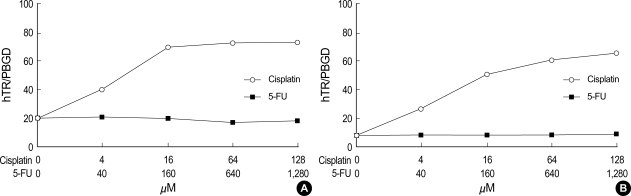
After cisplatin exposure in PNUH-12 and SNU-899, the expression of hTERT mRNA decreased, and the manifestation of hTERT mRNA also decreased after 5-FU exposure (Fig. 3). The expression of hTR increased after cisplatin exposure but did not show any significant change after 5-FU exposure in either cell lines (Fig. 4).
Fig. 3.
Detection of hTERT mRNA in PNUH-12 (A) and SNU-899 (B) samples by real-time RT-PCR analysis using the LightCycler. Concomitant detection of PBGD mRNA served as a reference for relative quantification. The normalized hTERT mRNA levels decreased after cisplatin and 5-fluorouracil (5-FU) exposure in both cell lines.
Fig. 4.
Detection of hTR in PNUH-12 (A) and SNU-899 (B) samples by real-time RT-PCR analysis using the LightCycler. Concomitant detection of PBGD mRNA served as a reference for relative quantification. In both cell lines, hTR levels were increased after cisplatin exposure, but, thy did not change significantly after 5-FU exposure.
DISCUSSION
A very strong correlation between telomerase expression and the presence of detectable hTERT mRNA has been accepted (4, 5). A high concentration of hTERT mRNA was detected in a cell with a high expression of telomerase activity. Though Akiyama et al. (12) reported that loss of telomerase activity was associated with a reduced catalytic domain (hTERT) mRNA level, the direct mechanism of hTERT mRNA related to telomerase regulation is unclear. Moreover, Cressey et al. (13) reported that reduced hTERT mRNA was produced by doxorubicin despite the lack of any effect on telomerase activity. Although hTR acts as a matrix to extend telomere, its expression is not significantly correlated with telomerase activity (7, 8).
This study examined the hTERT mRNA and hTR expression using real-time RT-PCR for the measurement of quantity and telomerase activity before and after the treatment of cisplatin and 5-FU in SNU-899 and PNUH-12. After cisplatin treatment, the telomerase activity and hTERT mRNA expression decreased, but hTR expression increased in both cell lines. Although telomerase activity did not show any significant change after 5-FU exposure, the expression of hTERT mRNA decreased and the expression of hTR did not show any significant change. The decrease in telomerase activity was not related to the decrease in numbers of cells as a percentage of viable cells.
Although Cressey et al. (13) reported that telomerase activity was not a reliable indicator of chemosensitivity in human testicular cancer cells, cisplatin may be more effective in decreasing telomerase activity than 5-FU, which did not cause any significant change. This result may also explain the fact that cisplatin is a more effective anticancer agent than 5-FU in the clinical treatment of head and neck cancers. The mechanism may be related to the findings in our previous study (14). In our previous study, when PNUH-12 cells were exposed to cisplatin and 5-FU, cisplatin induced p53-dependent apoptosis while 5-FU induced p53-dependent and p21WAF1/CIP1-dependent G1 phase cell-cycle arrest, not apoptosis (14). However, Ku et al. (15) reported that cisplatin and 5-FU did not inhibit telomerase activity in nasopharyngeal cancer cell lines. Although our results for 5-FU are in agreement with these reports, the results for cisplatin were different. There is a possibility that different results were achieved as a result of differences in cell lines and the concentration of anticancer drugs.
In PNUH-12 and SNU-899, the change of hTERT mRNA expression after cisplatin and 5-FU exposure was not significantly correlated with the alteration of telomerase activity. This means that other mechanisms may exist, such as the level of gene transcription, post-translational protein-protein interactions, and protein phosphorylation, for the regulation of telomerase activity, than hTERT mRNA expression that was thought as a limiting component of telomerase activity (16). Lin et al. (16) reported that telomerase activity was negatively correlated with the hTERT mRNA after treatment with anti-neoplastic agents in lymphoma cell lines and there was the potential relationship between p27/Kip 1 and telomerase activity. The results of our study correspond with those of Cressey et al. (13) who reported that hTERT was not related to a decrease in telomerase activity. In a study using real-time quantitative RT-PCR assay, Bieche et al. (17) reported that hTERT mRNA was detected in 100% of breast tumor RNAs but also in all normal breast RNAs. These studies suggested that hTERT mRNA itself was not indicative of telomerase activity.
In addition, in both cell lines, hTR expression increased when telomerase expression decreased after cisplatin exposure, but hTR expression remained unchanged when the telomerase expression did not change after 5-FU exposure. The telomerase activity as a result of cisplatin exposure is inversely correlated with the level of hTR expression. Our results differ from those of Cressey et al. (13) who reported that the expression of hTR was not related to the telomerase activity following drug-induced cell killing. However, the increase of hTR is in agreement with results of Rohde et al. (18) who showed that the expression levels of hTR were found to be markedly higher in normal renal tissue (no expression of telomerase activity) than in malignant renal tissue. There is a possibility that other mechanisms, such as the level of gene transcription, post-translational protein-protein interactions, and protein phosphorylation, are involved in the regulation of telomerase activity in PNUH-12 and SNU-899. These results also suggest the possible existence of a feedback mechanism, whereby a decrease in telomerase activity results in the induction of telomerase protein.
The rate limiting component that adjusts telomerase expression is known to be hTERT mRNA. The results of our study are contrary to the findings of previous research (6-8). The reason for this contradiction may be the fact that previous studies had conducted semi-quantitative evaluation of hTERT mRNA and hTR expression through RT-PCR (6-8). The authors used real-time RT-PCR, which could measure hTERT mRNA and hTR expression quantitatively. The real-time PCR method makes RNA quantitation much more precise and reproducible, rather than end point measurement of the amount of accumulated PCR product (19).
In conclusion, after cisplatin exposure, the telomerase activity and hTERT mRNA expression in PNUH-12 and SNU-899 decreased but hTR manifestation increased. After 5-FU exposure, the changes in telomerase and hTR activity in both cell lines were insignificant but hTERT mRNA manifestation decreased. These results suggest that there may be other important regulating components other than hTERT mRNA that regulate the telomerase activity in PNUH-12 and SNU-899.
References
- 1.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 2.Lee BJ, Wang SG, Choi JS, Lee JC, Goh EK, Kim MG. The prognostic value of telomerase expression in peripheral blood mononuclear cells of head and neck cancer patients. Am J Clin Oncol. 2006;29:163–167. doi: 10.1097/01.coc.0000207372.64733.b0. [DOI] [PubMed] [Google Scholar]
- 3.Liao CT, Tung-Chieh Chang J, Wang HM, Chen IH, Lin CY, Chen TM, Hsieh LL, Cheng AJ. Telomerase as an independent prognostic factor in head and neck squamous cell carcinoma. Head Neck. 2004;24:504–512. doi: 10.1002/hed.20007. [DOI] [PubMed] [Google Scholar]
- 4.Yu HP, Xu SQ, Lu WH, Li YY, Li F, Wang XL, Su YH. Telomerase activity and expression of telomerase genes in squamous dysplasia and squamous cell carcinoma of the esophagus. J Surg Oncol. 2004;86:99–104. doi: 10.1002/jso.20050. [DOI] [PubMed] [Google Scholar]
- 5.Shibuya K, Fujisawa T, Hoshino H, Baba M, Saitoh Y, Iizasa T, Sekine Y, Suzuki M, Hiroshima K, Ohwada H. Increased telomerase activity and elevated hTERT mRNA expression during multistage carcinogenesis of squamous cell carcinoma of the lung. Cancer. 2001;92:849–855. doi: 10.1002/1097-0142(20010815)92:4<849::aid-cncr1392>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Ducrest AL, Szutorisz H, Lingner J, Nabholz M. Regulation of the human telomerase reverse transcriptase gene. Oncogene. 2002;21:541–552. doi: 10.1038/sj.onc.1205081. [DOI] [PubMed] [Google Scholar]
- 7.Wisman GB, Knol AJ, Helder MN, Krans M, de Vries EG, Hollema H, de Jong S, van der Zee AG. Telomerase in relation to clinicopathologic prognostic factors and survival in cervical cancer. Int J Cancer. 2001;91:658–664. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1099>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Kanaya T, Kyo S, Takakura M, Ito H, Namiki M, Inoue M. hTERT is a critical determinant of telomerase activity in renal-cell carcinoma. Int J Cancer. 1998;78:539–543. doi: 10.1002/(sici)1097-0215(19981123)78:5<539::aid-ijc2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Majem M, Mesia R, Manos M, Gomez J, Galiana R, Cardenal F, Juan A, Montes A, Perez FJ, Nogues J, Llluch JR. Does induction chemotherapy still have a role in larynx preservation strategies? The experience of Institut Catala d'Oncologia in stage III larynx carcinoma. Laryngoscope. 2006;116:1651–1656. doi: 10.1097/01.mlg.0000231736.08477.47. [DOI] [PubMed] [Google Scholar]
- 10.Ku JL, Kim WH, Lee JH, Park HS, Kim KH, Sung MW, Park JG. Establishment and characterization of human laryngeal squamous cell carcinoma cell lines. Laryngoscope. 1999;109:976–982. doi: 10.1097/00005537-199906000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Roh HJ, Goh EK, Wang SG, Chon KM, Kim YS, Han JY. Establishment and Characterization of a Novel Cell Line (PNUH-12) Derived from a Human Squamous Cell Carcinoma of the Hypopharynx. Korean J Otolaryngol. 1999;42:72–81. [Google Scholar]
- 12.Akiyama M, Horiguchi-Yamada J, Saito S, Hoshi Y, Yamada O, Mizoguchi H, Yamada H. Cytostatic concentrations of anticancer agents do not affect telomerase activity of leukaemic cells in vitro. Eur J Cancer. 1999;35:309–315. doi: 10.1016/s0959-8049(98)00365-7. [DOI] [PubMed] [Google Scholar]
- 13.Cressey TR, Tilby MJ, Newell DR. Decreased telomerase activity is not a reliable indicatior of chemosensitivity in testicular cancer cell lines. Eur J Cancer. 2002;38:586–593. doi: 10.1016/s0959-8049(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 14.Lee BJ, Chon KM, Kim YS, An WG, Roh HJ, Goh EK, Wang SG. The effects of cisplatin, 5-fluorouracil, and radiation on cell cycle regulation and apoptosis in the hypopharyngeal carcinoma cell line. Chemotherapy. 2005;51:103–110. doi: 10.1159/000085769. [DOI] [PubMed] [Google Scholar]
- 15.Ku WC, Cheng AJ, Wang TC. Inhibition of telomerase activity by PKC inhibitors in human nasopharyngeal cancer cells in culture. Biochem Biophys Res Commun. 1997;241:730–736. doi: 10.1006/bbrc.1997.7874. [DOI] [PubMed] [Google Scholar]
- 16.Lin Z, Lim S, Viani MA, Sapp M, Lim MS. Down-regulation of telomerase activity in malignant lymphomas by radiation and chemotherapeutic agents. Am J Pathol. 2001;159:711–719. doi: 10.1016/S0002-9440(10)61742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bieche I, Nogues C, Paradis V, Olivi M, Bedossa P, Lidereau R, Vidaud M. Quantitation of hTERT gene expression in sporadic breast tumors with a real-time reverse transcription-polymerase chain reaction assay. Clin Cancer Res. 2000;6:452–459. [PubMed] [Google Scholar]
- 18.Rohde V, Sattler HP, Bund T, Bonkhoff H, Fixemer T, Bachmann C, Lensch R, Unteregger G, Stoeckle M, Wullich B. Expression of the human telomerase reverse transcriptase is not related to telomerase activity in normal and malignant renal tissue. Clin Cancer Res. 2000;6:4803–4809. [PubMed] [Google Scholar]
- 19.Bustin SA, Mueller R. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin Sci (Lond) 2005;109:365–379. doi: 10.1042/CS20050086. [DOI] [PubMed] [Google Scholar]