Abstract
Intensification of glycemic control is associated with weight gain, however, less is known about weight change during the maintenance phase of glycemic management. On the basis of current models of energy homeostasis, we hypothesize that insulin use will result in less weight gain than oral antidiabetic agents in patients with well-controlled diabetes. This is a prospective cohort nested within a randomized control trial at an academic clinic, with enrollment from June 2002 to January 2005. A total of 163 patients with type 2 diabetes were enrolled after obtaining glycemic control. Insulin use was assessed by self-report at baseline. Participants were weighed at baseline and five follow-up visits over 24 months. The weight change was compared between insulin users and noninsulin users. The average (s.d.) age was 55 (11), 44% are female and 21% are black. The median duration of diabetes was 5 (0.5−10) years. At baseline, 88 participants (54%) reported insulin use with an average of 69 (6) units/day. Baseline BMI in the insulin users was 35 (6) and 33 (6) in noninsulin patients. Over 24 months, noninsulin patients gained 2.3 additional kilograms compared with insulin users (2.8 kg (6.8) vs. 0.5 kg (6.5), P = 0.065). After adjusting for age, race, sex, baseline weight, intervention status, and change in A1C, insulin users had 2.5 kg less weight gain than noninsulin users (P = 0.033). Less weight gain was observed over 24 months in insulin-treated patients. Whether this effect may be due to central catabolic effects of insulin merits additional confirmatory study and mechanistic investigation.
INTRODUCTION
Insulin therapy in the treatment of diabetes has been associated with weight gain in both clinical trials and clinical practice. Weight gain associated with insulin intensification of glycemic control was noted in the Diabetes Control and Complications Trial, the United Kingdom Prospective Diabetes Study, and other studies of either initiation and/or intensification of insulin therapy (1−4). One commentary from the United Kingdom Prospective Diabetes Study trial suggested that insulin should not be used as the initial medication in type 2 diabetes due to excessive weight gain (5). A recent retrospective study showed that patients treated with insulin gained more weight over 4 years than patients treated with oral agents, however, the study did not account for glycemic control (6). Patient anticipation of weight gain is a potent negative reinforcement and, from a medical perspective, weight gain is associated with other comorbidities such as hypertension and dyslipidemia (7). Weight gain is commonly attributed to reversal of negative energy balance by halting glycosuria and to intermittent hypoglycemia, a potent hyperphagic stimulus (8).
In a seeming contradiction, insulin has long been implicated as an important hormonal mediator of energy homeostasis (9−13). Insulin, together with the adipose-derived hormone leptin, functions as adiposity-related negative feedback signals to the central nervous system to regulate caloric intake and energy expenditure (14−16). Insulin, secreted in direct proportion to body adiposity, circulates to the brain and activates its receptors found on key homeostatic neurons in the mediobasal hypothalamus and elsewhere (17,18). In this way, insulin, together with other metabolic signals, functions to regulate the activity of neural circuits involved in the control of feeding behavior, energy expenditure, and other aspects of peripheral metabolism (17,18). Direct central nervous system administration of insulin leads to reduced feeding in several animal models and an array of molecular genetic studies confirm this role (12,19−25). Although the precise role of insulin action in the central nervous system in humans is less well described, insulin is believed to have similar catabolic actions in humans (13,18,26).
Weight change associated with long-term insulin use in well-controlled diabetes has not been described. According to the model just described, we hypothesize that in the setting of already well-controlled diabetes, eliminating the confounding factors of either initiation or intensification of insulin therapy, insulin use will result in less weight gain than the use of oral agents. Thus, we enrolled participants after they achieved stable glycemic control and followed them for 24 months. Weight change patterns were compared between insulin users and noninsulin users over this period in order to test the hypothesis that well-controlled patients with type 2 diabetes treated with insulin will have less weight gain compared with similar metabolically stable patients treated with other agents.
METHODS AND PROCEDURES
This study is a prospective cohort nested within a randomized controlled trial which comprised participants enrolled in the Glycemic Relapse Prevention Study previously described (27). This trial enrolled patients from June 2002 to January 2005 and evaluates the effectiveness of a telephonic intervention to prevent glycemic relapse over 24 months. Participants were recruited after the completion of a 12-week intensive outpatient diabetes improvement program at an academic medical center. To be eligible, participants must have type 2 diabetes, have been referred for uncontrolled diabetes, and achieved glycemic control during the intensive 12-week program. Patients were excluded if they were <18 or >75 years of age or if they were pregnant. A total of 165 participants were enrolled. One participant was inappropriately randomized and is excluded from the analysis. One participant received gastric bypass surgery during the follow-up period and is excluded from this analysis of weight change. Participant flow through the cohort study is depicted in Figure 1.
Figure 1.
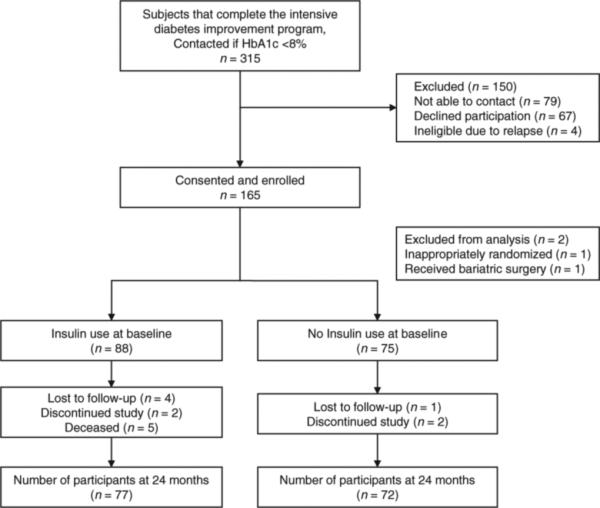
Cohort participation flowchart. HbA1c, glycated hemoglobin.
Primary exposure
Insulin used was assessed by self-report at enrollment. The average number of insulin units per day and shots per day was recorded at baseline and each follow-up period.
Primary outcome
Weight and height were obtained at enrollment, 3, 6, 12, 18, and 24 months. The majority of the weights were obtained on a Detecto balance scale (Detecto, Webb City, MO). However, on rare occasion (<5%), a weight from a clinic visit was substituted for the study visit.
Other measurements
Waist circumference was obtained at enrollment by measuring the horizontal circumference at the iliac crest. Hemoglobin A1c (A1C) was obtained at baseline and each follow-up time point in this study. The duration of diabetes was recorded at enrollment. The Center for Epidemiologic Studies Depression Scale was used to measure depression at baseline (28). Age, sex, and years of education were collected by self-report at baseline. Participants were asked to identify their race.
Statistical analysis
Demographic and clinical variables were described as mean and standard deviation or median and interquartiles ranges depending on the distribution of continuous variables. Continuous variables measured at baseline were compared using Wilcoxon rank sum test between insulin users and noninsulin users. The change in body weight was calculated by subtracting the baseline weight from the weight at 24 months. Among 24-month follow-up completers, Wilcoxon rank sum test was used to compare unadjusted distribution of change in body weight between insulin users and noninsulin users. In addition, we performed a multiple linear regression of the difference in weight at 24 months minus baseline and insulin as a factor adjusted for baseline weight, age, race, sex, intervention status of the randomized controlled trial, and change in A1C to assess the association of insulin use on the change in weight between baseline and weight at 24 months. Furthermore, using data of all subjects (N = 163), multivariable analyses were performed to explore the relationship between patient insulin use at baseline and rates of weight adjusted for age, sex, race, intervention status of the randomized controlled trial, and baseline weight. For weight outcome, a linear mixed effects regression models were used to compare the effect of the insulin use over time on the dependent variables (at baseline 3, 6, 12, 18, and 24 months) among all participants. Baseline weight, age, sex, race, and intervention status of the randomized controlled trial were included as covariates in the model. Change in A1C from baseline was included for each time point. The effect of the insulin was assessed by examining the interaction between insulin and follow-up time, which statistically tests for differences in rates of change between the regression slopes of the weight over time between the insulin users and noninsulin users (29). The covariance structures of the dependent variable was evaluated using Akaike Information Criteria and the structure that provided the best fit for the model was employed in the estimation of statistical significance (30,31). For all multivariable models, nonlinearity for follow-up time and age was assessed using restriction cubic splines (32). Regression residuals were examined graphically to assess normality of the residuals and satisfy regression requirements.
All calculations were performed using R 2.3.1. (R Foundation for Statistical Computing, Vienna, Austria.)
Ethics
This study was approved by the Vanderbilt University Institutional Review Board. Consent was obtained from each patient at enrollment.
RESULTS
The average (s.d.) age of participants was 55 (11) and 44% were female. Twenty-one percent of participants were black and the median (interquartile range) duration of diabetes was 5 (0.5−10) years. Eighty-eight participants (54%) reported insulin use at baseline, the average (s.d.) number of units was 69 (6) and the average (s.d.) number of units/kg was 0.68 (0.52). The mean (s.d.) number of shots per day at baseline was 2 (1.5). Baseline BMI (s.d.) in the insulin users was 35 (6) and in noninsulin users was 33 (6). Baseline characteristics of the study population are described in Table 1.
Table 1.
Baseline patient characteristics
| Insulin users (n = 88) | Nonusers (n = 75) | P valuea | |
|---|---|---|---|
| Age (years (±s.d.)) | 56 ± 10 | 54 ± 11 | 0.100 |
| Sex | |||
| Male (n (%)) | 44 (50) | 48 (64) | 0.072 |
| Female (n (%)) | 44 (50) | 27 (36) | |
| Race | |||
| White (n (%)) | 70 (80) | 54 (72) | 0.241 |
| Black (n (%)) | 17 (19) | 17 (23) | |
| Other (n (%)) | 1 (1) | 4 (5) | |
| Education | |||
| Less than 8th grade (n (%)) | 0 (0) | 1 (1) | <0.001 |
| 8th grade but less than high school (n (%)) | 15 (17) | 2 (3) | |
| High school or GED (n (%)) | 21 (24) | 19 (25) | |
| Some college (n (%)) | 32 (36) | 15 (20) | |
| College graduate (n (%)) | 16 (18) | 18 (24) | |
| Beyond college (n (%)) | 4 (5) | 20 (27) | |
| A1C (% (±s.d.)) | 6.99 ± 0.65 | 6.36 ± 0.56 | <0.001 |
| Duration of diabetes (years (IQR)) | 9 (5−15) | 0.6 (0.42−2.50) | <0.001 |
| BMI (kg/m2 (±s.d.)) | 34.6 ± 7.2 | 33.3 ± 6.5 | 0.196 |
| Weight (kg (±s.d.)) | 100 ± 21 | 100 ± 22 | 0.913 |
| Waist circumference (cm (±s.d.) (n = 159)) | 110 ± 15 | 107 ± 29 | 0.359 |
| Oral medications | |||
| Metformin (n (%)) | 42 (48) | 54 (72) | 0.002 |
| Sulfonylureas (n (%)) | 25 (28) | 40 (53) | 0.002 |
| Thiazolidines (n (%)) | 11 (12) | 9 (12) | 1.000 |
| CESD (mean (±s.d.) (n = 117)) | 11.6 ± 9.1 | 9.7 ± 7.2 | 0.401 |
CESD, Center for Epidemiologic Studies Depression Scale; GED, General Education Development Test; A1C, glycated hemoglobin. IQR, interquartile range.
χ2-Test for categorical variables and Wilcoxon test for continuous variables.
At baseline, a longer duration of diabetes was observed among insulin users. Because patients with diabetes often have disease progression that leads to insulin use, this finding was not unexpected. Due to collinearity with insulin use, duration of diabetes was not adjusted for in the statistical models of weight change. Furthermore, duration of diabetes was not associated with weight change in this study. Likewise, baseline A1C demonstrated collinearity with insulin use and was also not associated with weight change in this study.
The noninsulin users (oral-medication users) had more years of education. Metformin, sulfonylurea, and other secre tagogue use was more common in noninsulin users. Thiazolidinedione use was similar in both insulin and oral users. There was very little use of exenatide or pramilintide in this study and it did not vary between the groups. There were no significant differences in age, sex, race, depression scoring on the Center for Epidemiologic Studies Depression Scale (CESD), baseline waist circumferences, baseline weight, or baseline BMI between the two groups (Table 1).
The mean weight change from baseline at each follow-up visit by insulin status is shown in Figure 2 and described in Table 2. In the 144 participants who completed 24 months of follow-up, oral users gained an average (s.d.) of 2.9 (6.7) kg compared with insulin users who had an average (s.d.) weight gain of 0.5 (6.5) kg over 24 months (P = 0.064). After adjusting for age, race, sex, baseline weight, intervention status of the randomized controlled trial and change in A1C, insulin use was a significant predictor of less weight gain (insulin coefficient = −2.5 kg (95% confidence interval: −4.7 to −0.20); P = 0.033).
Figure 2.
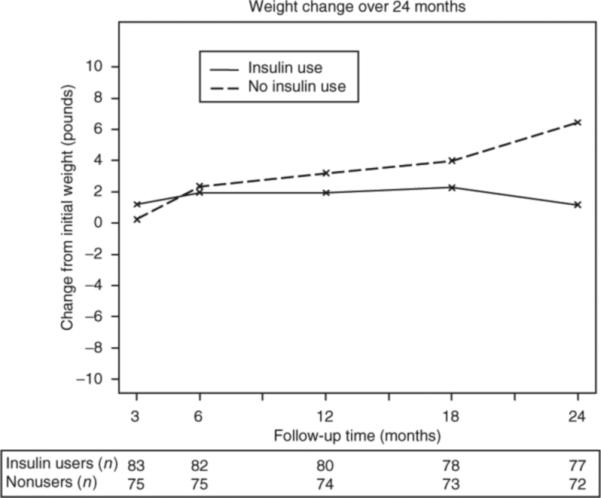
Weight change over 24 months. Weight change over 24 months by insulin status is shown. Participant follow-up at each time point is displayed below the x-axis.
Table 2.
Weight and A1C at 24 months
| Insulin users (n = 77) | Nonusers (n = 72) | P valuea | |
|---|---|---|---|
| Weight at 24 months (kg (±s.d.)) | 102 ± 20 | 102 ± 23 | 0.665 |
| Weight change over 24 months (kg (±s.d.)) | 0.5 ± 6.5 | 2.8 ± 6.8 | 0.064 |
| A1C at 24 months (% (±s.d.)) | 7.4 ± 1.2 | 7.0 ± 1.1 | 0.012 |
| Change in A1C over 24 months (% (±s.d.)) | 0.4 ± 1.2 | 0.6 ± 1.0 | 0.055 |
χ2-Test for categorical variables and Wilcoxon test for continuous variables.
The linear mixed effect adjusted model predicted weight change by insulin status and includes all weight data available for the 163 participants. The model was adjusted for age, sex, race, baseline weight, intervention status of the randomized controlled trial and change in A1C at each time point. Insulin use was associated with less weight gain over time (P value for insulin and follow-up time interaction = 0.045) (Figure 3).
Figure 3.
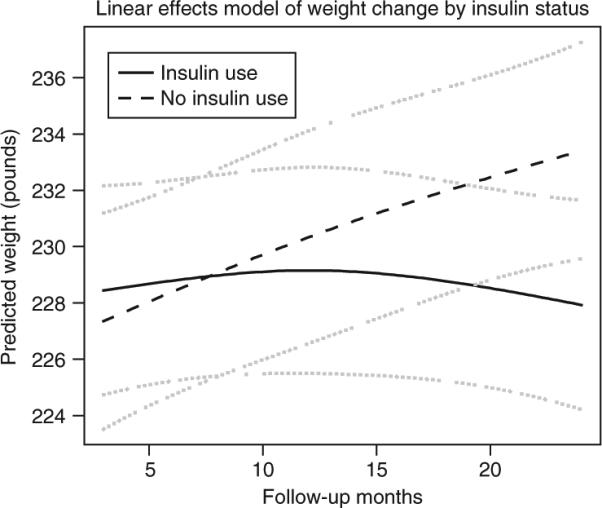
Linear mixed effects model of weight change by insulin status. An interaction between insulin use and follow-up time as nonlinear parameter was assessed (P = 0.045), model was adjusted for age, gender, intervention status, race, baseline weight and change in A1C at each time point. The dashed lines represent 95% confidence intervals.
DISCUSSION
Although insulin is a component of energy homeostatic systems, the clinical use of insulin has long been associated with weight gain. Many of the clinical studies that have described weight gain with insulin therapy have not considered the effect of glycemic control on weight gain. During initiation and intensification of insulin therapy, peripheral anabolic effects, hypoglycemic bouts or as yet unrecognized mechanisms may contribute to weight gain. Furthermore, the general population is, on average, gaining weight on a yearly basis and weight gain would not be unexpected in any cohort of diabetes patients. According to current models, once initiated and well controlled, the central catabolic effects of insulin should counter the peripheral anabolic effects in the form of a feedback loop in order to maintain weight stability. To our knowledge, our findings represent the first large scale demonstration of this effect in humans that supports these basic science and animal models.
The weight maintenance observed for insulin users in this study is contrary to the expectations of many providers and patients who fear that insulin use will lead to continued weight gain and may avoid insulin use for this reason. Although weight gain is a significant concern during the initial period of intensification, it is intriguing to consider that the adept long-term use of insulin may support weight maintenance. New strategies to control initial weight gain and to enhance weight stability or weight loss over time should be developed. Thus, these findings offer a new, biologically plausible perspective on the role of insulin and weight change during long-term management of diabetes.
There are several limitations to this study. This study was nested within a randomized controlled trial of maintenance diabetes care where some participants had telephonic contact with study personnel who discussed diet and exercise. We adjusted for intervention status in our analysis. In addition, there was no difference in weight change in the study arms. The participants in the study were on a variety of diabetic regimens and the regimens may have changed during the course of the study. Nonetheless, <5% of the participants changed insulin status, our primary exposure, over the course of 24 months. This study defines insulin use at baseline and does not allow for a change in status over the 24 months. The decision was made to treat insulin use as an intention-to-treat analysis a priori because it may aid clinicians to know what happens 24 months after achieving stable glycemic control. In addition to insulin use, there are many other factors that may have influenced weight change. No data were collected on many of these factors including, but not limited to, diet, physical activity, illness and other medications that affect weight. In addition, the weight change during the intensive diabetes improvement program prior to enrollment was not collected nor was the duration of insulin therapy. Residual confounding remains an important possible limitation of this prospective cohort study. These results should be confirmed with prospective, randomized studies.
There are several possible mechanisms that may lead well-controlled type 2 diabetes insulin users to gain less weight than noninsulin patients. The central catabolic actions of insulin may lead patients to have reduced caloric intake and to increase their energy expenditure over time. Alternatively, patients using insulin may have a heightened awareness of their caloric intake. Many diabetes improvement programs teach skills in calorie and carbohydrate counting, which are particularly important in meal-time insulin dosing. Patients who use insulin, especially those who match insulin to carbohydrate intake, may be more apt to monitor their caloric intake and hence gain less weight. Finally, the skill of the care provider in prescribing and monitoring insulin therapy with respect to hypoglycemia would also potentially significantly impact weight gain.
We have demonstrated that well-controlled type 2 individuals gain less weight during the maintenance phase of their care than type 2 individuals not on insulin independent of change in A1C. Future study directions include replication of this study in a different well-controlled type 2 diabetes population to ensure generalizability. In addition, investigation of the potential mechanisms of weight maintenance with insulin use in well-controlled type 2 diabetes should be pursued including monitoring of caloric intake and energy expenditure.
ACKNOWLEDGMENTS
The research was supported by the NIDDK R18 DK 062258 and the Robert Wood Johnson Foundation (T.A.E.), and NIDDK DK064857 and DK069927 (K.D.N.). The Vanderbilt Diabetes Research and Training Center is supported by the NIDDK P60 DK20593. M.M.H. was supported by the Vanderbilt Environmental Health Science Scholars Program (NIEHS 1K12 ES 015855).
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
reFerences
- 1.Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the Diabetes Control and Complications Trial. Diabetes Care. 2001;24:1711–1721. doi: 10.2337/diacare.24.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 3.Carver C. Insulin treatment and the problem of weight gain in type 2 diabetes. Diabetes Educ. 2006;32:910–917. doi: 10.1177/0145721706294259. [DOI] [PubMed] [Google Scholar]
- 4.Larger E. Weight gain and insulin treatment. Diabetes Metab. 2005;31:4S51–4S56. doi: 10.1016/s1262-3636(05)88268-0. [DOI] [PubMed] [Google Scholar]
- 5.United Kingdom Prospective Diabetes Study 24: a 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. United Kingdom Prospective Diabetes Study Group. Ann Intern Med. 1998;128:165–175. doi: 10.7326/0003-4819-128-3-199802010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhry ZW, Gannon MC, Nuttall FQ. Stability of body weight in type 2 diabetes. Diabetes Care. 2006;29:493–497. doi: 10.2337/diacare.29.03.06.dc05-1703. [DOI] [PubMed] [Google Scholar]
- 7.Brod M, Cobden D, Lammert M, Bushnell D, Raskin P. Examining correlates of treatment satisfaction for injectable insulin in type 2 diabetes: lessons learned from a clinical trial comparing biphasic and basal analogues. Health Qual Life Outcomes. 2007;5:8. doi: 10.1186/1477-7525-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Destefano MB, Stern JS, Castonguay TW. Effect of chronic insulin administration on food intake and body weight in rats. Physiol Behav. 1991;50:801–806. doi: 10.1016/0031-9384(91)90021-f. [DOI] [PubMed] [Google Scholar]
- 9.Woods SC, Porte D., Jr The role of insulin as a satiety factor in the central nervous system. Adv Metab Disord. 1983;10:457–468. doi: 10.1016/b978-0-12-027310-2.50024-4. [DOI] [PubMed] [Google Scholar]
- 10.Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity—A review. Neuropeptides. 2006;40:375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Niswender KD, Morrison CD, Clegg DJ, et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 12.Woods SC, Porte D, Jr, Bobbioni E, et al. Insulin: its relationship to the central nervous system and to the control of food intake and body weight. Am J Clin Nutr. 1985;42(Suppl 5):1063–1071. doi: 10.1093/ajcn/42.5.1063. [DOI] [PubMed] [Google Scholar]
- 13.Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niswender KD, Baskin DG, Schwartz MW. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab. 2004;15:362–369. doi: 10.1016/j.tem.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- 16.Steffens AB, Strubbe JH, Balkan B, Scheurink JW. Neuroendocrine mechanisms involved in regulation of body weight, food intake and metabolism. Neurosci Biobehav Rev. 1990;14:305–313. doi: 10.1016/s0149-7634(05)80040-5. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 18.Woods SC, Seeley RJ. Insulin as an adiposity signal. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S35–S38. doi: 10.1038/sj.ijo.0801909. [DOI] [PubMed] [Google Scholar]
- 19.Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143:2449–2452. doi: 10.1210/endo.143.6.8948. [DOI] [PubMed] [Google Scholar]
- 20.Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci. 1995;109:528–531. doi: 10.1037//0735-7044.109.3.528. [DOI] [PubMed] [Google Scholar]
- 21.Florant GL, Singer L, Scheurink AJ, et al. Intraventricular insulin reduces food intake and body weight of marmots during the summer feeding period. Physiol Behav. 1991;49:335–338. doi: 10.1016/0031-9384(91)90053-q. [DOI] [PubMed] [Google Scholar]
- 22.Lotter EC, Woods SC. Injections of insulin and changes of body weight. Physiol Behav. 1977;18:293–297. doi: 10.1016/0031-9384(77)90136-6. [DOI] [PubMed] [Google Scholar]
- 23.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 24.Woods SC, Stein LJ, McKay LD, Porte D., Jr Suppression of food intake by intravenous nutrients and insulin in the baboon. Am J Physiol. 1984;247:R393–R401. doi: 10.1152/ajpregu.1984.247.2.R393. [DOI] [PubMed] [Google Scholar]
- 25.Roehe R, Plastow GS, Knap PW. Quantitative and molecular genetic determination of protein and fat deposition. Homo. 2003;54:119–131. doi: 10.1078/0018-442x-00064. [DOI] [PubMed] [Google Scholar]
- 26.Porte D., Jr Central regulation of energy homeostasis: the key role of insulin. Diabetes. 2006;55(Suppl 2):S155–S160. [Google Scholar]
- 27.Huizinga MM, Shintani A, Michon S, et al. A randomized controlled trial to prevent glycemic relapse in longitudinal diabetes care: study protocol ( NCT00362193). Implement Sci. 2006;1:24. doi: 10.1186/1748-5908-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 29.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 30.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-Plus. Springer; New York, NY: 2000. [Google Scholar]
- 31.Littell R, Milliken G, Stroup W, Wolfinger R. SAS System for Mixed Models. SAS Institute; Cary, NC: 1996. [Google Scholar]
- 32.Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. Springer-Verlag; New York: 2001. [Google Scholar]


