Abstract
Overall dietary energy intake, particularly the consumption of simple sugars such as fructose, has been increasing steadily in Western societies, but the effects of such diets on the brain are poorly understood. Here we used functional and structural assays to characterize the effects of excessive caloric intake on the hippocampus, a brain region important for learning and memory. Rats fed a high-fat, high-glucose diet supplemented with high-fructose corn syrup showed alterations in energy and lipid metabolism similar to clinical diabetes, with elevated fasting glucose and increased cholesterol and triglycerides. Rats maintained on this diet for eight months exhibited impaired spatial learning ability, reduced hippocampal dendritic spine density, and reduced LTP at Schaffer collateral – CA1 synapses. These changes occurred concurrently with reductions in levels of brain-derived neurotrophic factor in the hippocampus. We conclude that a high energy diet reduces hippocampal synaptic plasticity and impairs cognitive function, possibly through BDNF-mediated effects on dendritic spines.
Keywords: fructose, hippocampus, long-term potentiation, obesity, diabetes, BDNF, high-fat diet
Dietary energy intake has increased steadily in Western societies during the past 50 years resulting in increased obesity, diabetes and cardiovascular disease (Everitt et al., 2006). Simple sugars and saturated fats are believed to be major components of the Western diet that promote obesity and insulin resistance (Gross et al., 2004). Data from clinical, epidemiological and animal studies have suggested that excessive energy intake adversely affects the brain, particularly during aging. Studies suggest that individuals with a high energy intake are at increased risk of Alzheimer’s disease (Luschsinger et al., 2002). Animal studies have shown that high-calorie diets impair the structure and function of the hippocampus, a brain region critical for learning and memory (Farr et al., 2008; Greenwood and Winocur, 1990; Kanoski et al., 2007; Molteni et al., 2002; Winocur and Greenwood, 1999; Wu et al., 2004). The adverse effects of high calorie diets on learning and memory have been associated with impaired hippocampal synaptic plasticity and neurogenesis (Farr et al., 2008; Lindqvist et al., 2006), suggesting that the hippocampus may be particularly sensitive to changes in dietary energy intake.
In the present study we fed rats a diet high in saturated fats and simple sugars, and supplemented their water with high-fructose corn syrup. This diet increased fasting blood glucose levels and serum cholesterol and triglycerides. Additionally, we found that the diet impairs hippocampus-dependent learning, synaptic plasticity, and dendritic spine density. These adverse effects on brain function were associated with reduced levels of BDNF in the hippocampus and suggest that “Western” diets impair synaptic function and cognition by a mechanism involving reductions in BDNF and atrophy of dendritic spines. Detailed description of the methods and procedures used in these experiments are available as Supplementary Information.
Rats fed a high-fat, high-glucose food supplemented with 20% high-fructose corn syrup in the drinking water (HCD, high calorie diet) gained weight more rapidly than rats eating standard NIH chow and drinking plain water (Supplementary Fig. 1). Rats on the HCD had higher levels of serum cholesterol (cholesterol, t17=2.36, p=0.03; mg/dL, control=103.9 ± 8.62, HCD=164.3 ± 22.9). Changes were primarily due to increases in low-density lipoprotein levels (low-density lipoprotein, t12=2.75; p=0.02; mg/dL, control=59.99 ± 8.03, HCD= 88.31 ± 18.55; high-density lipoprotein, t13=1.41, p=0.18; mg/dL, control=40.88 ± 3.02, HCD=65.30 ± 8.35). Rats on the HCD had elevated fasting blood glucose levels (t10=2.92, p=0.02; mg/dL, control=121.8 ± 5.5, HCD=174.6 ± 7.8). There were no differences in serum insulin levels (t11=0.21, p=0.84; ng/ml, control=0.92 ± 0.29, HCD=0.86 ± 0.08). However, analysis of the relationship between glucose and insulin levels using a homeostatic model assessment (HOMA; Wallace et al., 2004) indicated that rats maintained on the HCD diet were insulin resistant (insulin sensitivity, t9=4.94, p=0.0008, control = 80.50 ± 11.95, HCD = 32.89 ± 3.14; β-cell function, t9=2.96, p=0.021, control = 79.57 ± 16.13, HCD = 32.82 ± 6.08).
Levels of corticosterone have also been shown to increase with high-fat feeding (Lindqvist et al., 2006). However, we did not observe any changes in circulating corticosterone in our animals (t17=0.51, p=0.62; ng/ml, control=129.0 ± 20.59, HCD=116.5 ± 14.91). Overall, the alterations in serum chemistry following long-term ingestion of the HCD are suggestive of insulin resistant diabetes and hyperlipidemia.
Rats on the HCD reduced the amount of food that they consumed each day (grams per day, control=29.81 ± 0.80, HCD=23.89 ± 0.76; t88=5.28, p<0.0001). When food weights are converted to caloric intake, the calories per day derived from food were nearly identical for rats on the HCD, and rats fed the standard NIH pellets (calories per day, control=123.10 ± 3.31, HCD=118.0 ± 3.77; t88=0.98, p=0.32). However, the rats did not derive their calories exclusively from food; rats on the HCD also received 20% high-fructose corn syrup via the drinking water. Rats drinking 20% high-fructose corn syrup drank more fluid per day than rats drinking plain water (ml/24hr, control=42.63 ± 1.22, HCD=66.94 ± 5.86; t27=3.43, p=0.002). When volumes were converted to additional calories per day derived from the 20% high-fructose corn syrup solution, rats on the HCD consumed more calories each day than rats eating standard chow with plain water (calories per day derived from chow and drinking; control=123.10 ± 3.31, HCD=200.50 ± 7.86; t27=5.92, p<0.0001).
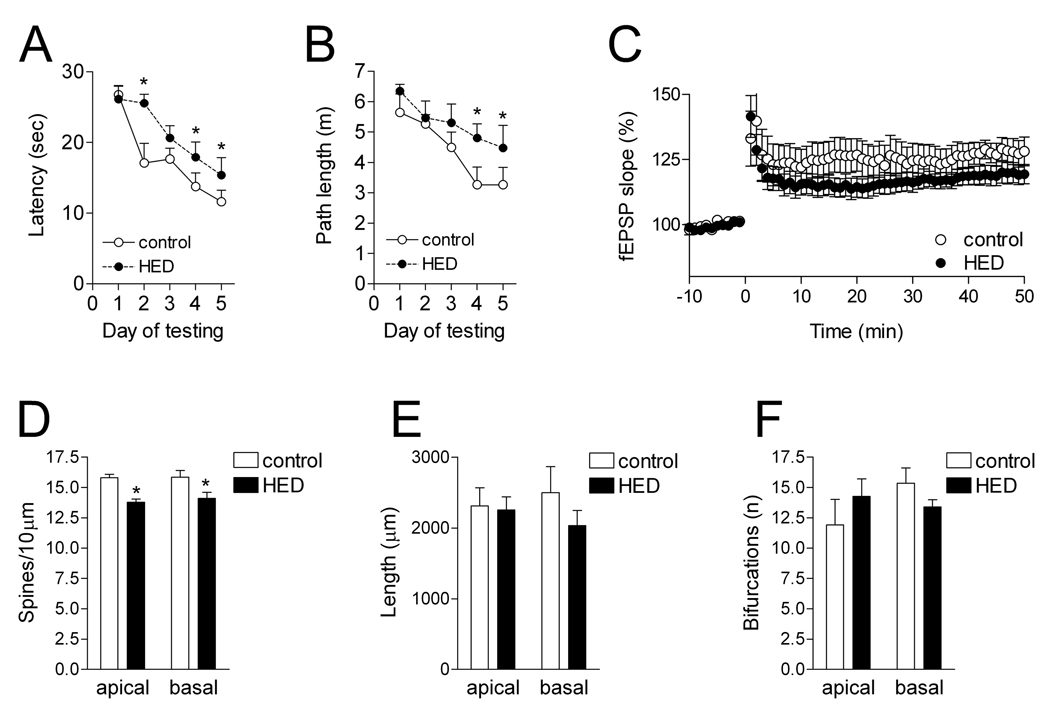
Following eight months on the HCD, rats performed poorly in the water maze compared to rats on the control diet. Rats maintained on the HCD took more time to locate the hidden platform, and also took a longer route to the platform (latency; F1,60=6.88, p=0.02; path length, F1,60=5.57, p=0.03; Fig. 1). These changes occurred in the absence of any changes in swim speed, the amount of time floating, or thigmotaxis (data not shown). There were no differences in performance on the visible-platform version of the water maze, which is not hippocampus dependent (latency to reach the visible platform, mean of 4 trials; control=12.15±2.12 sec, HCD=9.76±2.01 sec; t15=0.81, p=0.43). These results suggest that excessive energy intake impairs hippocampus-dependent memory.
Figure 1. A high calorie diet impairs hippocampus-dependent learning and synaptic plasticity, with correlated decreases in CA1 dendritic spine density.
A, Rats maintained on a high-calorie diet (HCD) took more time to find a hidden platform in the hippocampus-dependent water maze. B, Rats on the HCD also took a more circuitous route to the platform. C, Rats fed the HCD exhibited less LTP following stimulation of the Schaffer collateral pathway. D, The HCD reduced dendritic spine density on secondary and tertiary dendrites from both the apical and basal dendritic arbors of CA1 pyramidal neurons. E, There was no change in total dendritic length in rats fed the HCD compared to rats on the control diet. F, There were no significant differences in the complexity of the dendritic arbor, measured by counting the number of bifurcations. Asterisk (*) indicates significance at p<0.05 following repeated-measures ANOVA (A–C) or t-tests (D–F).
We measured LTP at Schaffer collateral synapses in hippocampal area CA1. Rats maintained on a standard diet exhibited robust LTP. In contrast, animals fed the HCD showed reduced LTP (F1,8=5.13, p=0.03). LTP deficits occurred without any changes in the input-output curve (fEPSP slope/fiber volley amplitude, control=1.03 ± 0.02, HCD=1.01 ± 0.01; t11=0.76, p=0.46). Moreover, there was no difference in the ability to elicit stable responses over a period of one hour in slices from rats on different diets, suggesting that overall slice health was not affected. These results are in support of our behavioral findings, and indicate that long-term caloric excess impairs hippocampal synaptic plasticity.
To test whether changes in dendritic spines might be occurring in the area where we observed impaired synaptic plasticity, we analyzed spine density and dendritic morphology on Golgi-impregnated neurons in hippocampal area CA1. We observed reduced dendritic spine density on secondary and tertiary dendrites in the apical dendritic arbor of rats on the HCD (t9=5.26, p=0.0005; Fig. 1–Fig. 2). We also analyzed dendritic spine density on the basal dendrites, and observed similar reductions in the density of spines (t9=2.35; p=0.04). Neither the basal nor the apical dendritic arbor showed any effect on the overall length of the dendritic tree (basal, t9=1.14, p=0.28; apical, t9=0.18, p=0.86). Dendritic complexity was also similar in the two diet groups (apical, t9=0.95, p=0.36; basal, t9=1.49, p=0.17). These morphological findings suggest that changes in spine density are associated with functional deficits in CA1 LTP and hippocampus-dependent learning.
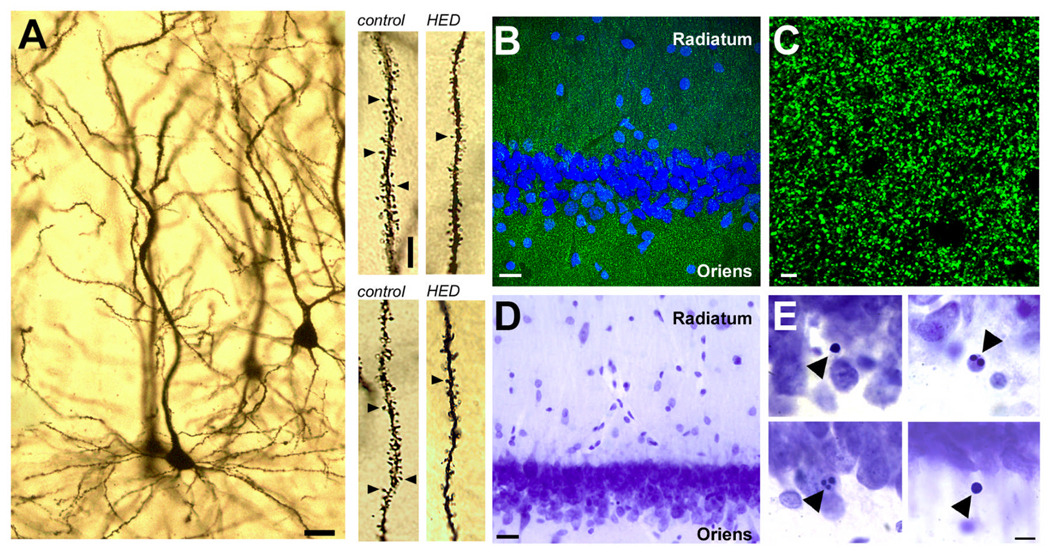
Figure 2. Excessive calorie intake reduces dendritic spine density and synaptic marker immunofluorescence, and increases the number of pyknotic nuclei in the CA1 region of the hippocampus.
A, Representative example of a CA1 neuron visualized with Golgi impregnation. Scale bar = 20 µm. Top right panel shows dendritic segments from the basal arbor of an animal on the control diet or the high-calorie diet (HCD), where indicated. Scale bar applies to all dendritic segments; length = 5 µm. Bottom right panel shows segments from apical oblique dendrites in animals fed the control diet or HCD, as indicated. B, Anatomical regions for analysis of synaptophysin labeling. Scale bar = 20 µm. C, Confocal micrograph showing synaptophysin labeling at the resolution and scale used for analysis. Scale bar = 2 µm. D, Low-magnification view of Nissl staining in hippocampal area CA1. Scale bar=20 µm. E, Examples of cells with condensed and/or fragmented nuclei. Scale bar=10 µm.
In order to further evaluate our observation of reduced dendritic spine density, we performed immunoblot analyses for the synaptic marker, synaptophysin. Band intensity for synaptophysin was reduced in whole-hippocampal homogenates from rats maintained on the HCD (t9=3.21, p=0.012; control=173.40 ± 25.75, HCD= 94.11 ± 7.69; Supplementary Fig. 2). To quantify synaptic marker expression in the specific anatomical region where we measured LTP and dendritic spine density, we performed immunofluorescence labeling for synaptophysin. The optical intensity of synaptophysin staining was reduced in both the CA1 stratum radiatum (optical intensity index, control=1.79×107 ± 4.05×106, HCD=1.06×107 ± 9.33×105; t9=1.92, p=0.04; Fig. 2), and in the CA1 stratum oriens (optical intensity index, control=1.45×107 ± 1.78×106, HCD=9.14×106 ± 6.61×105; t9=3.05, p=0.01).
Next, we measured levels of BDNF, a neurotrophic factor that is known to be regulated by dietary energy excess (Kanoski et al., 2007; Molteni et al., 2002; Wu et al., 2004). Rats on the HCD had reduced concentrations of BDNF in whole-hippocampal homogenates (pg/µg protein; control=9.41 ± 0.78, HCD=6.34 ± 0.76; t16=2.79, p=0.01). This suggests that synaptic loss was associated with reductions in hippocampal BDNF. Loss of synapses was probably not attributable to an overall loss of neurons in the hippocampus, because band intensity for the neuronal marker NeuN was not different across groups (t9=1.28, p=0.23; control=341.7 ± 84.92, HCD=585.1 ± 157.5; Supplementary Fig. 2). There was also no change in the volume of the CA1 cell body layer (mm3, control=0.87 ± 0.04, HCD=0.79 ± 0.07; t10=1.11, p=0.28). However, there was a nearly two-fold increase in the number of pyknotic nuclei in CA1 (t10=2.32, p=0.04; control = 236.0 ± 22.70, HCD= 420.0 ± 76.08; Fig. 2), suggesting that neurons in middle-aged rats on the HCD experience increased nuclear damage.
We observed impaired learning and LTP, as well as reduced dendritic spine density in response to excessive caloric intake. Impairment of dendritic spine density and synaptic plasticity was accompanied by reductions in hippocampal BDNF levels. Excessive caloric intake also increased fasting blood glucose levels and elevated serum lipids. Because alterations in hippocampal plasticity occurred in the context of a serum profile resembling diabetes, we conclude that the endocrine perturbations induced by excessive caloric intake may be detrimental for hippocampal structure and function.
Diets high in refined sugars and saturated fats increase the risk of type 2 diabetes (Everitt et al., 2006), and individuals with poorly controlled diabetes frequently exhibit impaired cognitive function (Gold et al., 2007). We recently demonstrated that some aspects of diabetes-induced hippocampal dysfunction are mediated by adrenal corticosteroids (Stranahan et al., 2008). However, rats in the present study did not exhibit elevated corticosterone levels, suggesting a different mechanism for the adverse effects of the HCD on hippocampal structure and function. The HCD did increase fasting plasma glucose concentrations, which may adversely affect neuronal function by promoting glucotoxicity (Tomlinson and Gardiner, 2008).
Deficits in hippocampal function may arise from peripheral insulin resistance and hyperlipidemia induced by a high-calorie diet. However, an alternative hypothesis would suggest that differences in the balance of proteins, carbohydrates, and fats between the two diets might have a direct effect on hippocampal plasticity, independently of peripheral metabolic alterations. In support of this idea, direct injection of triglycerides into the brain has detrimental consequences for learning and memory (Farr et al., 2008), while focal application of glucose enhances cognition (Dash et al., 2007). The extent to which different dietary components exert direct effects on central neuroplasticity remains to be determined.
Insulin resistance and obesity are associated with fluctuations in a number of different energy-regulating signaling peptides. For example, leptin enhances hippocampal synaptic plasticity and improves performance of rodents in learning and memory tasks (Harvey, 2007). As diabetes is associated with leptin resistance (Myers et al., 2008), it is possible that impaired leptin signaling contributes to the diet-induced deficits in hippocampal plasticity in the present study. Another such peptide, ghrelin, has previously been shown to promote spinogenesis and learning in the hippocampus (Diano et al., 2006). Levels of ghrelin are reduced in obesity (Tschop et al., 2001), opening the possibility that changes in neuronal structure and learning in our model may be mediated by reductions in ghrelin production. It is also possible to speculate that there may be some relationship between the actions of ghrelin and BDNF in the hippocampus.
Increases in the prevalence of obesity and diabetes are directly attributable to excessive caloric intake (Gross et al., 2004). The current findings suggest that elevated fasting glucose levels and hyperlipidemia are associated with neurological deficits. Although additional studies will be necessary to parse the relative contributions of diet-induced alterations in lipid and glucose metabolism to central neuroplasticity, it is clear that excessive caloric intake activates mechanisms that are likely to be detrimental for neuronal function.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Aging. We wish to thank Tamikia Lamb, Charlie Mae Lamb, Olga Carlson, and Charles Gordon for technical assistance.
References
- Everitt AV, Hilmer SN, Brand-Miller JC, Jamieson HA, Truswell AS, Sharma AP, Mason RS, Morris BJ, Le Couteur DG. Dietary approaches that delay age-related diseases. Clin Interv Aging. 2006;1:11–31. doi: 10.2147/ciia.2006.1.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J Neurosci. 2007;26:8048–8056. doi: 10.1523/JNEUROSCI.0671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008 doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Tsui W, Richardson S, Javier E, Convit A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav Neural Biol. 1990;53:74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774–779. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643–667. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007;182:57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE. The effects of high fat diets and environmental influences on cognitive performance in rats. Behav Brain Res. 1999;101:153–161. doi: 10.1016/s0166-4328(98)00147-8. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.