Abstract
The acquisition of metastatic ability by tumor cells is considered a late event in the evolution of malignant tumors. We report that untransformed mouse mammary cells that have been engineered to express the inducible oncogenic transgenes MYC and KrasD12, or polyoma middle T, and introduced into the systemic circulation of a mouse can bypass transformation at the primary site and develop into metastatic pulmonary lesions upon immediate or delayed oncogene induction. Therefore, previously untransformed mammary cells may establish residence in the lung once they have entered the bloodstream and may assume malignant growth upon oncogene activation. Mammary cells lacking oncogenic transgenes displayed a similar capacity for long-term residence in the lungs but did not form ectopic tumors.
Metastatic dissemination of cancer cells, the major cause of cancer mortality, is traditionally viewed as a late-stage event (1), although mammary epithelial cells have been shown to disseminate systemically from early neoplastic lesions in transgenic mice and from ductal carcinoma in situ in women (2). There is ample evidence that the ability of fully transformed tumor cells to metastasize depends on the regulation of developmental programs and external environmental cues (3–11), but to what extent the seeding or growth of tumor cells at the ectopic site is dependent on the initiating transforming event(s) is a subject of debate (12). We have developed a system that separates the process of seeding cells in the lung from the process of malignant growth at an ectopic site by using animals engineered to express potent oncogenes in a doxycycline-dependent mammary-specific manner. After intravenous (IV) injection of marked mammary cells that have different genetic potentials [no oncogenes, or oncogenes that will be expressed only after cells have taken up residence in an ectopic site (the lungs)], normal mammary cells can lodge in the lungs, grow slowly, and become frank metastatic malignancies once potent oncogenes are turned on.
We recently described tri-transgenic TetO-MYC;TetO-KrasD12;MMTV-rtTA (TOM;TOR; MTB) mice that coordinately express MYC and mutant Kras oncogenes in mammary epithelial cells when fed doxycycline (13). Doxycycline-naïve animals do not express the transgenic oncogenes and have morphologically and functionally normal mammary glands, but they develop diffuse autochthonous tumors within 3 to 4 weeks after doxycycline exposure. Tumors that form because of the expression of these oncogenes display malignant characteristics, such as transplantability and metastasis (fig. S1). Therefore, this model provides primary mammary cells that can be switched from a normal to a neoplastic state with simple experimental manipulation.
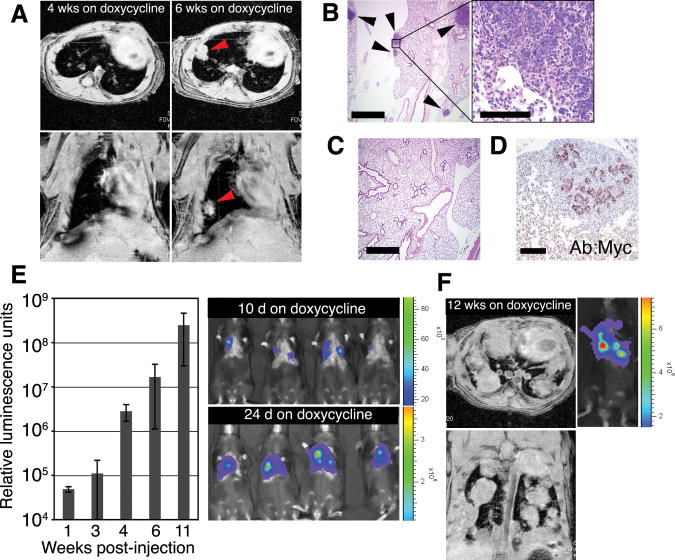
To investigate whether mammary cells from these mice can be induced to form metastasis in the absence of transformation at the primary site, we modified the traditional experimental metastasis assay (14). In the modified approach, instead of IV delivery of tumor cells from doxycycline-treated animals into new recipients, we injected dissociated morphologically normal mammary cells from mature TOM;TOR;MTB animals never exposed to doxycycline into the tail veins of Rag1−/− (15) females on a doxycycline diet. In this way, the injected cells can become transformed only in the bloodstream or tissues of the recipient mouse, an experimental situation that has not been previously examined. Magnetic resonance imaging (MRI) was used to survey Rag1−/− recipients for evidence of tumor foci in the lungs. Solitary nodules were observed in four out of four recipients 6 weeks after injection (Fig. 1A), and histological sections showed foci of mammary adenocarcinoma in the lung (Fig. 1B); in contrast, no tumors were found in control mice that did not receive doxycycline (Fig. 1C and table S1). The experimental metastases were histologically identical to the spontaneous metastases observed in tumor-bearing TOM;TOR;MTB mice (fig. S1) as well as to the primary mammary tumors arising in donor animals (13), and pulmonary nodules contained cells positive for MYC (Fig. 1D), keratin 8 (K8), smooth-muscle actin (SMA), and keratin 6 (K6) (fig. S2). These findings demonstrated that the tumorigenic capacity conferred on mouse mammary cells by coexpression of MYC and KrasD12 can be realized in the ectopic environment of the lung.
Fig. 1.
Untransformed mouse mammary cells form lung metastases after IV injection and induction of oncogenes. (A to D) Lung metastases develop from intravenously injected phenotypically normal mammary cells upon activation of MYC and KrasD12 transgenes. (A) Metastases were monitored by MRI in Rag1−/− mice after IV delivery of 1 × 106 dissociated primary mammary cells from doxycycline-naïve TOM;TOR;MTB mice. Recipient mice were fed doxycycline for 6 weeks starting 1 day before injection. Representative axial (top) and coronal (bottom) images obtained from the same animal 4 and 6 weeks after injection show development of a solid nodule (arrowheads) in the lung. (B) Foci of hematoxylin-eosin (H/E)–stained mammary adenocarcinoma (arrowheads) in paraffin-embedded lung sections of the same Rag1−/− mouse as in (A). Scale bars indicate 1 mm (left) and 0.1 mm (right). (C) No tumors were observed in lung sections of Rag1−/− mice that did not receive doxycycline after IV delivery of 1 × 106 primary mammary cells from doxycycline-naïve TOM;TOR;MTB mice. Scale bar, 1 mm. (D) Tumor cells from the same animal as in (A), but not the surrounding lung tissue, stained with anti-MYC antisera. Scale bar, 0.1 mm. (E and F) Lung metastases develop from intravenously injected phenotypically normal mammary cells upon activation of a PyMT transgene. (E) Donor cells expressing their transgene were detected by bioluminescence imaging after 5 × 105 primary mammary cells from doxycycline-naïve TOMT:IRES:Luc;MTB mice were injected intravenously into Rag1−/− mice that were placed on doxycycline 1 day before injection. Representative images at day 10 and day 24 after injection show the presence of signal-emitting cells in the thorax (right); temporal increases in bioluminescence (14) were quantified in relative luminescence units (left; n = 5 mice; error bars represent SD). (F) Axial (top) and coronal (bottom) MRI images of a mouse from (E) maintained on doxycycline for 12 weeks show solid nodules in the lung. A corresponding bioluminescence image is shown on the right.
We confirmed this observation in a different mouse model, TetO-PyMT:IRES:Luc;MMTV-rtTA (TOMT:IRES:Luc;MTB), recently generated in our lab (14). Expression of the polyoma middle T (PyMT) oncogene in this line is mammary gland–specific and doxycycline-dependent and can be monitored through the coordinate expression of the reporter gene (Luc) encoding firefly luciferase (fig. S3). When dissociated mammary cells from mature TOMT:IRES:Luc;MTB animals never exposed to doxycycline were injected intravenously in Rag1−/− females on a doxycycline diet, a bioluminescence signal was apparent over the thorax of recipient mice within 2 weeks (Fig. 1E). The development of mammary tumors in the lungs of injected mice was documented by MRI imaging (Fig. 1F) and by histology (Fig. 2B). Ectopic mammary tumor foci derived from either TOM;TOR;MTB or TOMT:IRES:Luc;MTB transgenic lines displayed characteristics associated with oncogene activation at the primary site, including robust proliferation (Fig. 2C) and dense vasculature (Fig. 2D). Therefore, upon activation of potent oncogenes, previously untransformed mouse mammary cells delivered to the systemic circulation produce metastatic-like disease in the pulmonary parenchyma without having undergone transformation at the primary site.
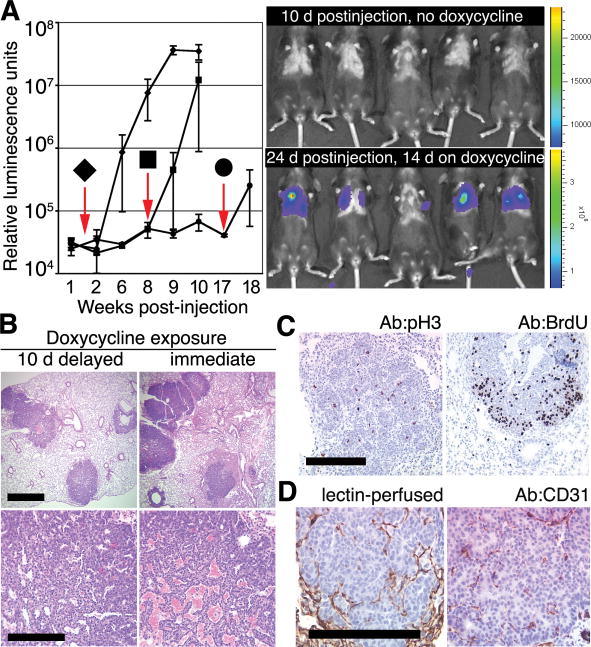
Fig. 2.
Delay in oncogene activation does not preclude the development of ectopic mammary tumors. (A) Bioluminescence in Rag1−/− mice after IV delivery of 1 × 105 mammary cells from doxycycline-naïve TOMT:IRES:Luc;MTB mice is undetectable before doxycycline exposure but can be induced at various times after placing mice on doxycycline 1.5 (◆, n = 7 mice), 8 (■, n = 3 mice), or 17 weeks (●, n = 2 mice) after IV injection. Downward arrows indicate times of addition of doxycycline to the diet. Error bars represent SD. Representative bioluminescence images (right) obtained 10 days after injection in the absence of doxycycline (top right) and after 2 additional weeks on doxycycline (bottom right). (B) Histologically similar metastatic tumors in lungs of Rag1−/− mice after IV delivery of 1 × 105 mammary cells from doxycycline-naïve TOMT:IRES:Luc;MTB mice exposed to doxycycline for 8 weeks starting 10 days after (left) or 1 day before (right) IV injection. Scale bars, 1 mm (top) and 0.1 mm (bottom). (C) Mitotic activity in tumor foci in lung sections from Rag1−/− recipient of TOM;TOR;MTB cells (left; stained with anti-pH3) or from Rag1−/− recipient of TOMT:IRES:Luc;MTB cells (right; stained with anti-BrdU serum after BrdU labeling) (14). Scale bar, 0.1 mm. (D) Angiogenic proficiency demonstrated by perfusion of the ectopic tumor with biotinylated lectin (left) (14) and by staining endothelial cells within tumor foci with anti-CD31 serum in a Rag−/− recipient of the TOMT:IRES:Luc; MTB cells (right). Scale bar, 0.1 mm.
To explore whether untransformed mammary cells can survive in the bloodstream and in the ectopic environment of the lung without oncogene expression yet still be induced to develop tumors at a later time, we injected dissociated mammary cells from TOMT:IRES:Luc;MTB animals never exposed to doxycycline into the lateral tail veins of Rag1−/− females on a doxycycline-free diet. No bioluminescence signal was observed in the lungs of recipient mice in up to 4 months of monitoring on this diet (Fig. 2A). When recipients were instead placed on doxycycline 1.5, 8, or 17 weeks after IV injection of transgenic mammary cells, we detected bioluminescence in the chest within 2 weeks of the start of doxycycline exposure (Fig. 2A). The histological appearance and the total number of foci in the lungs were similar in animals placed on a doxycycline diet 1 day before IV injection and 10 days after the injection [32 ± 6 (SD), n = 5 mice and 26 ± 6 (SD), n = 4 mice, respectively] (Fig. 2B). Conversion to malignancy at later times of induction was not measured because expression of the MMTV-rtTA transgene in the MTB line becomes non-uniform with age (16). These observations show that cells responsible for development of the ectopic mammary tumors can persist in the lung for up to 17 weeks in the absence of oncogene expression.
To rule out the possibility that low-level or transient expression of the transgenic oncogene(s) occurs in the absence of doxycycline, we carried out the modified experimental metastasis assay with mammary gland preparations from animals lacking any transgenic oncogenes (C57BL6/J mice) or from those expressing a gene encoding the “enhanced” green fluorescent protein (GFP) from the chicken β-actin promoter (β-actin-GFP) (17). Three weeks after the injection of mammary cells from β-actin-GFP mice, green foci were observed with fluorescent microscopy of the whole lungs in all recipients (Fig. 3A). These foci lacked the nodular appearance of metastatic tumors and were inconspicuous on routine histological inspection (Fig. 3B, top left). Staining with antibody to K8 facilitated the detection of the ectopic foci and confirmed their epithelial origin, whereas in the lungs of uninjected mice only the bronchial epithelium was stained (Fig. 3B, right).
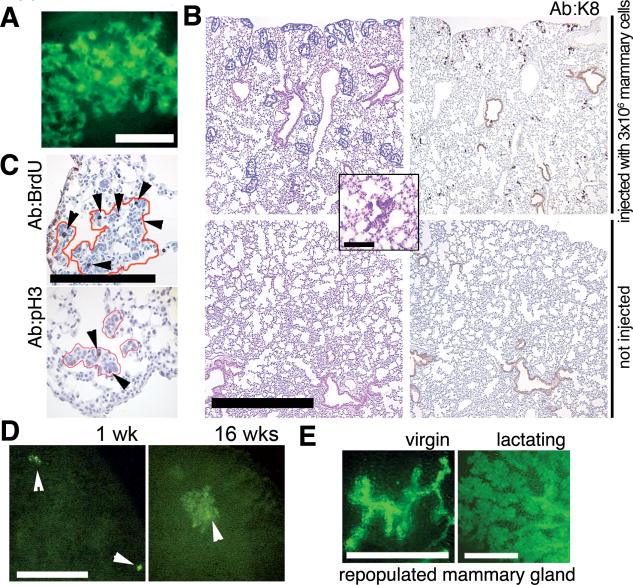
Fig. 3.
Mammary cells without an oncogenic transgene can persist in the lung. (A) Focus of green cells observed under excitation light (14) in a whole fresh lung of a Rag1−/− recipient 3 weeks after IV injection of 5 × 105 dissociated mammary cells from a β-actin-GFP mouse. Scale bar, 0.1 mm. (B) Representative size and distribution of the H/E–stained ectopic foci in lung sections from Rag1−/− mice injected intravenously with 3 × 106 mammary cells from a β-actin-GFP donor at 3 weeks after injection (top left). Inset shows a representative H/E–stained focus at high magnification. A consecutive section (top right) was stained with rat antiserum against K8. No foci were detected by H/E (bottom left) or K8 staining (bottom right) of lung sections from uninjected Rag1−/− mice. Scale bar, 1 mm. Inset scale bar, 0.2 mm. (C) Mitotic activity in ectopic epithelial outgrowths (outlined in red) demonstrated by BrdU-labeling detected with rat anti-BrdU serum (arrowheads, top) or by staining with anti-pH3 (arrowheads, left). Scale bar, 0.2 mm. (D) Larger foci of green fluorescent cells were observed by whole-lung imaging under excitation light (14) at 16 weeks after injection (right) as compared with 1 week after injection (left). Rag1−/− recipients were injected with 3 × 105 dissociated mammary cells from a β-actin-GFP mouse. Scale bar, 1 mm. (E) Mammary gland repopulation in secondary Rag1−/− recipients produces a green fluorescent mammary tree detectable under excitation light in the whole-mount preparations 4 or 7 weeks after transplantation (14). Glands were harvested from virgin recipients (left), or host animals were mated and the transplanted glands harvested 1 day postpartum (right). Scale bar, 1 mm.
To determine whether the ectopic foci of normal epithelial cells persist and grow in the foreign environment of the lung, we counted the total number of discrete foci in lung sections at different times after injection and looked at proliferation markers in these foci. The total number of foci found in lung sections from C57BL6/J recipients injected with 4 × 105 syngeneic mammary cells was similar in the animals surveyed at 3 weeks (n = 3 mice) and those surveyed at 10 weeks (n = 3 mice) after injection (42 ± 7 and 56 ± 22 in 10 paraffin lung sections, respectively). Moreover, the efficiency with which the wild-type cells were able to form these small epithelial clusters was similar to the efficiency with which we were able to induce ectopic tumors after injecting cells from doxycycline-naïve TOM;TOR;MTB donors [1.2 ± 0.4 (SD) versus 1.7 ± 1.4 (SD) per 10,000 cells injected, n = 6 and 8 mice, respectively; measured as described in (14)]. This result strongly argues that most or all of the mammary cells that are capable of surviving in the lung are able to respond to the initiating oncogene expression by forming an ectopic mammary tumor.
In both nontransgenic C57BL6/J– and β-actin-GFP–derived foci, occasional cells displayed mitotic activity (Fig. 3C). Consistent with this result, the green foci found under excitation light in the lungs of animals injected with mammary cells from β-actin-GFP mice 16 weeks after injection were larger in size than those found in recipients of the same preparation 1 week after injection (Fig. 3D). Ectopic epithelial outgrowths contained K8- and SMA-positive cells, such as observed in intact mammary glands (fig. S4A), and the outgrowths occasionally displayed a glandular appearance. Despite prolonged residence in the lung (up to 4 months), the green cells recovered from the recipients' lungs were competent to form hollow acinar structures in three-dimensional morphogenesis assays (fig. S4B) and secondary mammary outgrowths in cleared fat pads of Rag1−/− females (Fig. 3E). These findings establish that the ectopic cells residing in the lungs are indeed of mammary origin, that they are viable and mitotically active, and that at least some of them are multipotent and able to support full mammary development.
The experiments described here show that, in the absence of an active oncogene, dissociated cells from an untransformed mouse mammary gland can establish residence in the ectopic environment of the lung, grow slowly, and remain clinically undetectable after IV injection. The same cells can give rise to metastatic malignancies upon activation of oncogenes that can produce mammary tumors in an intact gland. It is widely acknowledged that multiple steps are required to establish metastases, including intravasion of cells from primary tumors into blood vessels or lymphatics; survival in the circulation, extravasation, and establishment of cells at ectopic sites; and malignant growth. Because we have injected mammary cells from transgenic mouse donors into tail veins of recipient mice, we have not examined the requirements for intravasation. We have, however, demonstrated that activated oncogenes and cellular transformation are not required for any of the subsequent steps, save for malignant growth at ectopic sites. These findings indicate that properties inherent in normal cells are sufficient for negotiating a substantial portion of the metastatic cascade. Considerable experimental and clinical evidence favors the idea that cells from small cancers may spread to distant sites early in tumorigenesis and account for dormancy and late relapse in human breast cancer (2, 18). Although we do not know whether premalignant cells can enter the systemic circulation during these early stages and become sources of later metastatic tumors, our observations argue that this hypothesis should be tested. The finding that metastatic disease can arise from untransformed mammary cells in the circulation refines our conception of cancer progression, and suggests that each step in the metastatic cascade should be examined to establish its functional requirements, including those performed by normal cells. Such functions might be susceptible to inhibitory strategies that can ablate disseminated pre-malignant or malignant cells and thereby diminish the mortality caused by cancer.
Supplementary Material
Acknowledgments
We thank M. A. Melnick, G. Sanchez, A. Giannakou, and J. Demers for expert handling of the mouse colony; L. Chodosh for providing MMTV-rtTA transgenic mice; D. Felsher and J. M. Bishop for providing TetO-MYC transgenic mice; A. Olshen for assistance with statistical analysis; and L. K. Tan for assistance with histological analysis. Supported in part by awards from NIH (K01 CA118731 to K.P., P01 CA94060 to H.V., and R24 CA83084 and P30-CA 08748, which provides partial support for core facilities used in conducting this investigation), the Martell Foundation (to H.V.), and the U.S. Department of Defense (W81XWH-05-1-0220 to M.J.).
Footnotes
Supporting Online Material: www.sciencemag.org/cgi/content/full/321/5897/1841/DC1, Materials and Methods, Figs. S1 to S4, Table S1, References
References and Notes
- 1.Hanahan D, Weinberg RA. Cell. 2000;100:57. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Husemann Y, et al. Cancer Cell. 2008;13:58. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Gupta GP, et al. Nature. 2007;446:765. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 4.Gupta PB, et al. Nat Genet. 2005;37:1047. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartwell KA, et al. Proc Natl Acad Sci USA. 2006;103:18969. doi: 10.1073/pnas.0608636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jechlinger M, et al. J Clin Invest. 2006;116:1561. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muraoka RS, et al. J Clin Invest. 2002;109:1551. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang Y, et al. Cancer Cell. 2003;3:537. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 9.Muller A, et al. Nature. 2001;410:50. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, et al. Cell. 2004;117:927. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Karnoub AE, et al. Nature. 2007;449:557. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 12.Bernards R, Weinberg RA. Nature. 2002;418:823. doi: 10.1038/418823a. [DOI] [PubMed] [Google Scholar]
- 13.Podsypanina K, Politi K, Beverly LJ, Varmus HE. Proc Natl Acad Sci USA. 2008;105:5242. doi: 10.1073/pnas.0801197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Materials and methods are available as supporting material on Science Online.
- 15.Mombaerts P, et al. Cell. 1992;68:869. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 16.Gunther EJ, et al. FASEB J. 2002;16:283. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- 17.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. FEBS Lett. 1997;407:313. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 18.Aguirre-Ghiso JA. Nat Rev Cancer. 2007;7:834. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.