Abstract
Enhanced lipid biosynthesis is a characteristic feature of cancer. Deregulated lipogenesis plays an important role in tumour cell survival. These observations suggest that enzymes in the lipid synthesis pathway would be rational therapeutic targets for cancer. To this end, we review the enzymes in de novo fatty-acid synthesis and related pathways.
Keywords: lipid metabolism, fatty-acid synthase, acyl-CoA synthetase
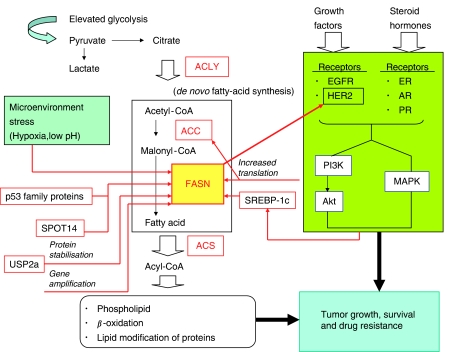
To establish an effective strategy in molecular cancer therapeutics, it is essential to identify characteristic features of the disease and to discover ways to selectively target them. Altered metabolism is one of the important features of cancer. Initially, Otto Warburg observed enhanced anaerobic glycolysis in cancer (Warburg, 1956). The elevated glucose catabolism produces an excess of the glycolytic end-product, pyruvate. Most of the pyruvate is converted to lactate, whereas some of it is converted to acetyl-CoA, which, in turn, is used in de novo fatty-acid synthesis (Figure 1). Highly proliferating cancer cells need to synthesise fatty acids de novo to continually provide lipids for membrane production. The synthesised fatty acids are also used for energy production through β-oxidation and lipid modification of proteins. De novo fatty-acid synthesis involves two key enzymes, acetyl-CoA carboxylase (ACC) and fatty-acid synthase (FASN). ACC carboxylates acetyl-CoA to form malonyl-CoA. The malonyl-CoA product is further converted by FASN to long-chain fatty acids. Most normal human tissues preferentially use dietary (exogenous) lipid for synthesis of new structural lipids, whereas de novo (endogenous) fatty-acid synthesis is usually suppressed, and FASN expression is maintained at low levels. By contrast, in cancer cells, de novo fatty-acid synthesis is commonly elevated and the supply of cellular fatty acid is highly dependent on the de novo synthesis. Therefore, deregulated de novo fatty-acid synthesis directly leads to cellular fatty-acid accumulation and affects fundamental cellular processes, including signal transduction and gene expression. Numerous studies have shown overexpression of FASN in various human epithelial cancers, including prostate, ovary, colon, lung, endometrium and stomach cancers (Kuhajda, 2000). Moreover, several reports have shown that FASN and related lipogenic enzymes play important roles in tumour cell survival at multiple levels.
Figure 1.
Coupling of elevated fatty-acid metabolism with growth factor signalling in cancer. Growth factors and hormone receptors play essential roles in tumour-related FASN overexpression. FASN and growth factor-dependent signalling are mutually regulated in cancer cells. Tumour microenvironment stress, as well as multiple other factors are involved in FASN overexpression and elevated lipogenesis in cancer. ACLY, ATP citrate lyase; ACC, acetyl-CoA carboxylase; FASN, fatty-acid synthase; ACS, acyl-CoA synthetase; EGFR, epidermal growth factor receptor; ER, oestrogen receptor; AR, androgen receptor; PR, progesterone receptor; PI3K, phosphatidylinositol-3-kinase; MAPK, mitogen-activated protein kinase; SREBP-1c, sterol regulatory element-binding protein 1c; USP2a, ubiquitin-specific protease-2a.
This minireview summarises recent advances in this field and provides a theoretical basis for the enzymes in lipogenic pathway as a new class of molecular targets to induce cancer selective cell death.
Regulation of FASN and de novo fatty-acid synthesis pathway in cancer
The increased de novo fatty-acid synthesis is caused by multiple mechanisms, including increased expression of lipogenic enzymes. Among them, FASN overexpression is observed in a wide variety of human cancers. In cancer cells, transcriptional regulation of FASN gene is one of the important mechanisms of FASN overexpression (Kuhajda, 2006; Menendez and Lupu, 2007). The FASN expression is regulated by growth factors – growth factor receptors, including epidermal growth factor receptor (EGFR) and HER2, and steroid hormone – steroid hormone receptors, such as oestrogen receptor (ER), androgen receptor (AR) and progesterone receptor (PR). Downstream of the receptors, the phosphatidylinositol-3-kinase (PI3K)-Akt and mitogen-activated protein kinase (MAPK) are candidate signalling pathways that mediate FASN expression through the sterol regulatory element-binding protein 1c (SREBP-1c). However, the regulations of FASN expression by the growth factor receptors are not simple. For instance, mutual regulation between FASN and the growth factor-dependent signalling is suggested. HER2 induces FASN expression through the downstream PI3K signalling (Kumar-Sinha et al, 2003). Conversely, FASN-dependent regulation of HER2 expression has also been reported (Menendez et al, 2004). Recent reports have further shown that the FASN expression is not only controlled by SREBP-1c, but also by other transcription factors, such as the p53 family proteins and the lipogenesis-related nuclear protein, SPOT14, which is overexpressed in breast tumours (D’Erchia et al, 2006; Martel et al, 2006).
As with these intracellular signalling molecules, extracellular tumour microenvironmental stresses play an important role in tumour-related FASN expression. Solid tumours are commonly characterised by a unique pathophysiological microenvironment, such as hypoxia, low pH, and nutrient starvation. This hostile microenvironment activates several intracellular signalling pathways that promote malignant progression. Hypoxia and low pH stress induce the FASN expression in cancer cells (Menendez et al, 2005; Furuta et al, 2008). Hypoxia upregulates SREBP-1, the major transcriptional regulator of the FASN gene, through phosphorylation of Akt.
Apart from the transcriptional regulation of FASN, it is also controlled at the posttranslational levels. Graner et al showed that the isopeptidase USP2a (ubiquitin-specific protease-2a) interacts with and stabilises FASN protein in prostate cancer (Graner et al, 2004). In breast cancer BT-474 cells that overexpress HER2, the expression of FASN and ACC are not mediated by SREBP-1, but by a mammalian target of rapamycin (mTOR)-dependent selective translational induction (Yoon et al, 2007). A significant copy number gain of FASN gene is also observed in prostate adenocarcinoma (Shah et al, 2006). These observations suggest that tumour-related FASN overexpression could be regulated at multiple levels (Figure 1).
FASN and de novo fatty-acid synthesis as promising targets of cancer
FASN is not only overexpressed in cancer, but it also plays an essential role in tumour growth and survival. Two well-known FASN inhibitors, the natural product cerulenin and the synthetic compound c75, have been studied. Treating tumour cells with pharmacological inhibitors of FASN effectively suppresses growth and induces apoptosis in breast cancer cells both in vitro and in vivo (Pizer et al, 2000). Similarly, FASN knockdown by specific siRNAs induces apoptosis in prostate cancer LNCaP cells (De Schrijver et al, 2003). By contrast, the RNAi-mediated inhibition of FASN did not affect growth rate or viability of nonmalignant cells. These data indicate that the elevated level of FASN observed in many cancers actually plays a predominant role in tumour growth and survival, suggesting that this enzyme could be a rational therapeutic target.
Although the precise mechanisms of FASN inhibition-induced cell death in cancer cells still remain unknown, several possibilities have been proposed. Initial studies indicate that FASN inhibition accumulates the toxic intermediary metabolite, malonyl-CoA, which induces apoptosis, whereas pharmacological inhibition of ACC by 5-(tetradecyloxy)-2-furoic acid does not (Pizer et al, 2000). These studies suggest that malonyl-CoA accumulation, but not end-product fatty-acid depletion, could be a critical factor for apoptosis induction. However, studies with siRNAs revealed that, as with FASN inhibition, ACC inhibition triggers apoptosis in prostate and breast cancer cells, but not in nonmalignant cells (Brusselmans et al, 2005; Chajes et al, 2006). Inhibiting ACC expression results in a major decrease in the cellular pool of palmitic acid and apoptosis induction. Moreover, supplementing the culture medium with palmitic acid completely rescues cells from both ACC and FASN knockdown-induced apoptosis (Chajes et al, 2006). These observations re-emphasise the importance of end-product fatty-acid starvation to cell death. At the same time, these data suggest that, apart from FASN inhibition, ACC inhibition could also be a rational strategy to induce selective death of tumour cells. As described above, FASN and tumour-related survival signals are mutually regulated, and FASN controls HER2-dependent signalling. Besides the role of FASN-dependent fatty-acid synthesis in fatty-acid supply for membrane production and energy production through β-oxidation, FASN overexpression is further associated with palmitoylation of signalling proteins (Fiorentino et al, 2008). Interference of these pathways could also be involved in the FASN inhibition-induced selective cancer cytotoxicity.
In addition to the essential role of FASN in cancer cell growth and survival, it is involved in other phases of cancer development. Browne et al showed that Orlistat, an antiobesity drug, inhibits FASN and suppresses endothelial cell proliferation and angiogenesis, suggesting a novel role of endothelial cell FASN in in vivo tumour growth (Browne et al, 2006). FASN overexpression also confers resistance to Adriamycin and mitoxantrone in breast cancer cells (Liu et al, 2008). These observations suggest that FASN inhibition could be a novel strategy to interfere with tumour survival through angiogenesis and reverse drug resistance of cancer. Recently, it was reported that FASN inhibition induces endoplasmic reticulum stress in cancer cells, and FASN inhibitors cooperate with the endoplasmic reticulum stress inducer to enhance tumour cell death (Little et al, 2007). Considering the fact that tumour microenvironment stresses are strong inducers of the endoplasmic reticulum stress (Murakami et al, 2007), the FASN inhibitors could show selective and enhanced cytotoxicity to cancer cells under the tumour microenvironment conditions.
The FASN-dependent de novo fatty-acid synthesis takes place in cytoplasm. Recently, however, another component of de novo fatty-acid synthesis in mitochondria was identified (Hiltunen et al, 2009). This suggests that the specificity of FASN inhibitors could be a critical key for successful molecular target therapy of cancer. In this aspect, recent determination of the crystal structure of FASN and molecular description of its active sites (Maier et al, 2008) would help in the development of improved inhibitors. Cancer cells also induce lipolysis in adipose tissue, a characteristic feature of cachexia, by producing lipid mobilisation factors (Tisdale, 2004). However, it is still not clear how the exogenous lipid mobilised from adipose tissue could affect the dependence of tumours on de novo fatty-acid synthesis. Polymorphisms of FASN gene are the other factors that could affect the effectiveness of FASN-targeting agents on cancer (Kovacs et al, 2004). Further studies are needed to clarify these points for effective application of the FASN inhibition strategy to cancer therapy.
Other enzymes in lipogenic pathway as potential cancer targets
ATP citrate lyase
ATP citrate lyase (ACLY) catalyses the conversion of citrate to cytosolic acetyl-CoA, thus linking the tumour-associated increase in glycolysis to enhanced lipogenesis (Figure 1). As with FASN and ACC, marked elevation of ACLY expression and activity has been reported in cancer cells (Bauer et al, 2005; Hatzivassiliou et al, 2005). In human lung adenocarcinoma, the expression of phosphorylated ACLY correlated with stage, differentiation grade, and a poorer prognosis (Migita et al, 2008). ACLY inhibition by RNAi leads to a significant impairment of glucose-dependent lipid synthesis (Bauer et al, 2005). Moreover, ACLY inhibition by siRNAs or the selective inhibitor SB-204990 suppresses the growth and survival of tumour cells in vitro and in vivo (Bauer et al, 2005; Hatzivassiliou et al, 2005; Migita et al, 2008). Although the molecular mechanisms of ACLY inhibition-dependent cell death are still not clear, these data suggest that this enzyme could also be a target for cancer therapy.
Acyl-CoA synthetase
Acyl-CoA synthetase (ACS) is an enzyme that acts downstream of FASN and converts long-chain fatty acids to acyl-CoA (Figure 1). This reaction is a critical step in several lipid metabolic pathways, including phospholipid and triacylglycerol biosynthesis, β-oxidation, and lipid modification of cellular proteins (reviewed in Coleman et al, 2002). In mammals, five ACS isoforms (ACSL1, 3, 4, 5, and 6) have been identified. All the five members catalyse saturated and unsaturated fatty acids of chain lengths 8–22 carbons, whereas ACSL4 prefers arachidonic acid (AA) as a substrate. AA metabolism plays a critical role in colon carcinogenesis. Cao et al have shown that intracellular-free AA is a mediator of apoptosis, and ACSL4 suppresses apoptosis by metabolically lowering the intracellular-free AA level (Cao et al, 2000). They further showed that ACSL4 is frequently upregulated in colon adenocarcinoma. These observations strongly suggest that ACSL4 could be involved in tumour cell survival. ACSL5, another ACS isoform, plays a role in the pathogenesis of cancer. It is frequently overexpressed in malignant gliomas and is involved in their growth and survival (Yamashita et al, 2000; Mashima et al, 2009). Studies with a specific inhibitor of ACS, Triacsin c, further revealed that ACS inhibition induces selective apoptosis in cancer cells through the mitochondria-mediated pathway (Mashima et al, 2005). These data indicate that ACS is involved in tumour cell survival and that ACS inhibition would be an effective strategy for cancer therapy. At present, it is not clear which ACS member's inhibition would best induce selective tumour cell death. Additional studies are required to clarify the role of each ACS isoform in normal and cancer cells.
Conclusion
Increased de novo fatty-acid synthesis has been recognised as a hallmark of cancer, whereas its significance in cancer pathogenesis has long been underestimated. However, the recent advances in this field have shown the importance of the lipogenic enzymes in tumour cell survival. From the viewpoint of cancer prevention, it would also be important to take into account the effects of dietary factors, such as conjugated linoleic acid, on lipid metabolism in carcinogenesis (Kelley et al, 2007). Further investigations on the regulation of these pathways will offer new strategies for tumour treatment, as well as for effective cancer prevention.
Acknowledgments
This work was funded by a Grant-in-Aid for Scientific Research on Priority Areas, Cancer, from the Ministry of Education, Culture, Sports, Science and Technology, Japan and by a Grant-in-Aid for Exploratory Research from Japan Society for the Promotion of Science.
References
- Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB (2005) ATP citrate lyase is an important component of cell growth and transformation. Oncogene 24: 6314–6322 [DOI] [PubMed] [Google Scholar]
- Browne CD, Hindmarsh EJ, Smith JW (2006) Inhibition of endothelial cell proliferation and angiogenesis by orlistat, a fatty acid synthase inhibitor. FASEB J 20: 2027–2035 [DOI] [PubMed] [Google Scholar]
- Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV (2005) RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res 65: 6719–6725 [DOI] [PubMed] [Google Scholar]
- Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM (2000) Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci USA 97: 11280–11285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chajes V, Cambot M, Moreau K, Lenoir GM, Joulin V (2006) Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res 66: 5287–5294 [DOI] [PubMed] [Google Scholar]
- Coleman RA, Lewin TM, Van Horn CG, Gonzalez-Baro MR (2002) Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J Nutr 132: 2123–2126 [DOI] [PubMed] [Google Scholar]
- De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV (2003) RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res 63: 3799–3804 [PubMed] [Google Scholar]
- D’Erchia AM, Tullo A, Lefkimmiatis K, Saccone C, Sbisa E (2006) The fatty acid synthase is a conserved p53 family target from worm to human. Cell Cycle 5: 750–758 [DOI] [PubMed] [Google Scholar]
- Fiorentino M, Zadra G, Palescandolo E, Fedele G, Bailey D, Fiore C, Nguyen PL, Migita T, Zamponi R, Di Vizio D, Priolo C, Sharma C, Xie W, Hemler ME, Mucci L, Giovannucci E, Finn S, Loda M (2008) Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Lab Invest 88: 1340–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, Kamada S, Saito K, Iiizumi M, Liu W, Ericsson J, Watabe K (2008) Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res 68: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Graner E, Tang D, Rossi S, Baron A, Migita T, Weinstein LJ, Lechpammer M, Huesken D, Zimmermann J, Signoretti S, Loda M (2004) The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell 5: 253–261 [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB (2005) ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8: 311–321 [DOI] [PubMed] [Google Scholar]
- Hiltunen JK, Schonauer M, Autio KJ, Mittelmeier TM, Kastaniotis AJ, Dieckmann CL (2009) Mitochondrial FAS type II – more than just fatty acids. J Biol Chem (in press) [DOI] [PMC free article] [PubMed]
- Kelley NS, Hubbard NE, Erickson KL (2007) Conjugated linoleic acid isomers and cancer. J Nutr 137: 2599–2607 [DOI] [PubMed] [Google Scholar]
- Kovacs P, Harper I, Hanson RL, Infante AM, Bogardus C, Tataranni PA, Baier LJ (2004) A novel missense substitution(Val1483Ile) in the fatty acid synthase gene(FAS) is associated with percentage of body fat and substrate oxidation rates in nondiabetic Pima Indians. Diabetes 53: 1915–1919 [DOI] [PubMed] [Google Scholar]
- Kuhajda FP (2000) Fatty acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition 16: 202–208 [DOI] [PubMed] [Google Scholar]
- Kuhajda FP (2006) Fatty acid synthase and cancer: new application of an old pathway. Cancer Res 66: 5977–5980 [DOI] [PubMed] [Google Scholar]
- Kumar-Sinha C, Ignatoski KW, Lippman ME, Ethier SP, Chinnaiyan AM (2003) Transcriptome analysis of HER2 reveals a molecular connection to fatty acid synthesis. Cancer Res 63: 132–139 [PubMed] [Google Scholar]
- Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ (2007) Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res 67: 1262–1269 [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Y, Zhang JT (2008) A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol Cancer Ther 7: 263–270 [DOI] [PubMed] [Google Scholar]
- Maier T, Leibundgut M, Ban N (2008) The crystal structure of a mammalian fatty acid synthase. Science 321: 1315–1322 [DOI] [PubMed] [Google Scholar]
- Martel PM, Binqham CM, Mcgraw CJ, Baker CL, Morganelli PM, Meng ML, Armstrong JM, Moncur JT, Kinlaw WB (2006) S14 protein in breast cancer cells: direct evidence of regulation by SREBP-1c, superinduction with progestin, and effects on cell growth. Exp Cell Res 312: 278–288 [DOI] [PubMed] [Google Scholar]
- Mashima T, Oh-hara T, Sato S, Mochizuki M, Sugimoto Y, Yamazaki K, Hamada J, Tada M, Moriuchi T, Ishikawa Y, Kato Y, Tomoda H, Yamori T, Tsuruo T (2005) p53-defective tumors with a functional apoptosome-mediated pathway: a new therapeutic target. J Natl Cancer Inst 97: 765–777 [DOI] [PubMed] [Google Scholar]
- Mashima T, Sato S, Sugimoto Y, Tsuruo T, Seimiya H (2009) Promotion of glioma cell survival by acyl-CoA synthetase 5 under extracellular acidosis conditions. Oncogene 28: 9–19 [DOI] [PubMed] [Google Scholar]
- Menendez JA, Decker JP, Lupu R (2005) In support of fatty acid synthase (FAS) as a metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J Cell Biochem 94: 1–4 [DOI] [PubMed] [Google Scholar]
- Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7: 763–777 [DOI] [PubMed] [Google Scholar]
- Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R, Lupu R (2004) Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad Sci USA 101: 10715–10720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, Ushijima M, Mashima T, Seimiya H, Satoh Y, Okumura S, Nakagawa K, Ishikawa Y (2008) ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res 68: 8547–8554 [DOI] [PubMed] [Google Scholar]
- Murakami S, Noguchi T, Takeda K, Ichijo H (2007) Stress signalling in cancer. Cancer Sci 98: 1521–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP (2000) Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res 60: 213–218 [PubMed] [Google Scholar]
- Shah US, Dhir R, Gollin SM, Chandran UR, Lewis D, Acquafondata M, Pflug BR (2006) Fatty acid synthase gene overexpression and copy number gain in prostate adenocarcinoma. Hum Pathol 37: 401–409 [DOI] [PubMed] [Google Scholar]
- Tisdale MJ (2004) Tumor-host interactions. J Cell Biochem 93: 871–877 [DOI] [PubMed] [Google Scholar]
- Warburg O (1956) On the origin of cancer cells. Science 123: 309–314 [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Kumabe T, Cho YY, Watanabe M, Kawagishi J, Yoshimoto T, Fujino T, Kang MJ, Yamamoto TT (2000) Fatty acid induced glioma cell growth is mediated by the acyl-CoA synthetase 5 gene located on chromosome 10q25.1-q25.2, a region frequently deleted in malignant gliomas. Oncogene 19: 5919–5925 [DOI] [PubMed] [Google Scholar]
- Yoon S, Lee MY, Park SW, Moon JS, Koh YK, Ahn YH, Park BW, Kim KS (2007) Up-regulation of acetyl-CoA carboxylase alpha and fatty acid synthase by human epidermal growth factor receptor 2 at the translational level in breast cancer cells. J Biol Chem 282: 26122–26131 [DOI] [PubMed] [Google Scholar]