Abstract
Ischemia and reperfusion (I/R)-induced liver injury occurs in several pathophysiological disorders including hemorrhagic shock and burn as well as resectional and transplantation surgery. One of the earliest events associated with reperfusion of ischemic liver is endothelial dysfunction characterized by the decreased production of endothelial cell-derived nitric oxide (NO). This rapid post-ischemic decrease in NO bioavailability appears to be due to decreased synthesis of NO, enhanced inactivation of NO by the overproduction of superoxide or both. This review presents the most current evidence supporting the concept that decreased bioavailability of NO concomitant with enhanced production of reactive oxygen species initiates hepatocellular injury and that endogenous NO or exogenous NO produced from nitrite play important roles in limiting post-ischemic tissue injury.
Keywords: liver ischemia, superoxide, peroxynitrite, NFkB, TNF, hemoglobin, free radicals, cytokines, superoxide
Introduction
Hepatic ischemia followed by reperfusion results in tissue injury that contributes substantially to the morbidity and mortality associated with shock, thermal injury, liver transplantation and resectional surgery. Indeed, liver ischemia and reperfusion (I/R)-induced hepatocellular damage is thought to be responsible for up to 10% of organ dysfunction in liver transplantation leading to acute and chronic rejection. Experimental and clinical evidence suggest that post-ischemic tissue damage occurs in an antigen-independent manner in which tissue injury initiates a cascade of innate immune responses that ultimately results in liver failure. For the warm I/R that occurs in shock or liver resectional surgery, it has been demonstrated that tissue damage occurs in two distinct phases termed the early (acute) phase and the late (subacute) phase [1–3]. The early phase of injury occurs in the absence of leukocyte infiltration and is thought to be initiated by a rapid change in the redox state of the tissue in favor of a more oxidative environment. The late phase of injury is dependent upon the production of several different cytokines and chemokines that promote the infiltration of large numbers of leukocytes into the tissue [1–4]. Extravasated polymorphonuclear leukocytes (PMNs) become metabolically activated and release large amounts of reactive oxygen species (ROS) together with extracellular matrix degrading enzymes such as collagenase and matrix metalloproteases[5]. The net result of this inflammatory infiltrate is an amplification of the acute injurious responses resulting in extensive inflammatory tissue injury.
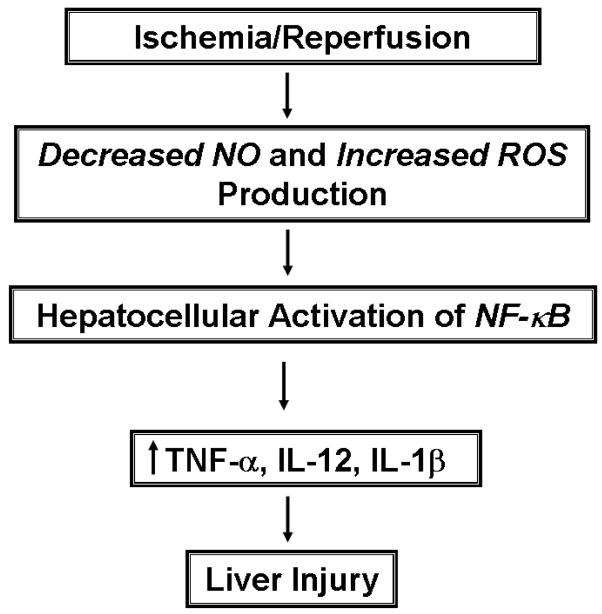
It is well-appreciated that endogenous nitric oxide (NO) may act to limit ROS- and PMN- mediated tissue injury as well as attenuate the subsequent inflammatory response in vivo [6–11]. Indeed, it has been demostrated that I/R-induced cellular injury is associated with a remarkable decrease in the bio-availability of NO which represents an important initiating event in the pathophysiology of post-ischemic injury in a variety of different tissues including the liver, heart, kidney and gut [6,12,13]. This review presents a brief overview of the evidence supporting the concept that a decrease in the bioavailability of NO coupled to increased production of superoxide initiate a pathophysiological cascade that ultimately leads to post-ischemic liver injury (Figure 1). The early innate immune responses occur in the absence of an inflammatory infiltrate but will ultimately promote a net oxidative stress within the Kupffer cells (KCs), sinusoidal endothelial cells (SECs) and hepatocytes thereby activating certain oxidant-sensitive transcription factors and enhancing the expression of injurious pro-inflammatory cytokines. The evidence implicating endogenous NO derived from endothelial cell nitric oxide synthase (eNOS) as well as exogenous NO generated from the metabolism of nitrite as important regulatory pathways molecule that limit I/R-induced liver injury are also discussed in this review.
Figure 1. Proposed pathophysiological mechanisms thought to be important in liver ischemia and reperfusion injury.
Ischemia and reperfusion of the liver induces a decrease in the bioavailability of NO concomitant with enhanced production of reactive oxygen species (ROS). This redox imbalance results in an alteration of the redox potential of the liver thereby producing a more oxidative environment. Enhanced “oxidative stress” within the Kupffer cells, sinusoidal endothelial cells and hepatocytes would promote the NFkB-dependent expression of certain pro-inflammatory cytokines that may injure the tissue.
Post-Ischemic Liver Injury: Divergent Roles of Superoxide and Nitric Oxide in the Acute Phase
The early phase of hepatocellular damage caused by I/R occurs within 1–6 hours following reperfusion and appears to be associated with KC and possibly lymphocyte activation [1,3,5,14]. This PMN-independent injury is thought to be initiated by an alteration in the redox state of the liver producing a more oxidative environment within the cells of the tissue. The mechanisms by which reperfusion of ischemic tissue induces this type of redox alteration is not known with certainty but has been the subject of active investigation for more than 10 years. One of the earliest events associated with I/R is dysfunction of the SECs which is characterized by a significant decrease in the steady state production of endothelial cell nitric oxide synthase (eNOS)-derived NO [6,8–10]. The decrease in NO production occurs within 5 min of reperfusion and is most probably due to decreased synthesis of NO, enhanced inactivation of NO by the overproduction of superoxide or both. Indeed, within minutes of reperfusion, there is enhanced production of ROS such as superoxide (O2−), hydrogen peroxide (H2O2) and/or hydroxyl radical (OH•) [1,3,15]. Cellular sources for these ROS that have been implicated are hepatocyte-derived xanthine oxidase and/or mitochondria as well as KC- and/or SEC-associated NADPH oxidase [1,3,15,16]. Although a great deal interest in hepatocyte-associated xanthine oxidase was generated early on in investigations of liver I/R, more recent work suggest that mitochondrial metabolism or KC-associated NADPH oxidase are probably more important sources of ROS in the post-ischemic liver [15,17]. A recent study using an isolated perfused liver preparation suggests that in the absence of Kupffer cells, hepatocytes exhibit substantial oxidative stress suggesting the presence of additional ROS generators in these cells as well [18]. Because certain oxygen-derived free radicals such as O2− will interact with and decompose NO, I/R-induced over-production of this free radical and H2O2 (coupled to decreased steady state levels of NO) will increase in redox potential of the liver from one of a reduced redox state to a more oxidized redox environment [8,9,19,20]. In fact numerous different studies, using pharmacologic interventions or genetic approaches have demonstrated significant protective effects of certain nonenzymatic or enzymatic antioxidants when administered prior to inducing liver ischemia [3,5,15,21]. Although these studies have identified a role for ROS in the pathophysiology of I/R-induced heptocellular injury, the cellular source(s) for these ROS-mediated events as well as the mechanisms by which these reactive species directly mediate hepatocyte damage have not been clearly delineated.
Superoxide and Post-Ischemic Liver Injury
Early studies into the role of ROS in the pathophysiology of liver I/R demonstrated significant protective effects of a variety of non-specific free radical scavengers. However, with the advent of new gene transfection technologies, more recent studies have shown that adenoviral transfection of the liver with manganese superoxide dismutase (Mn-SOD) significantly reduced post-ischemic liver injury implicating O2− as an important mediator of I/R injury [3]. While the use of adenoviral transfection of various SOD enzymes provides excellent mechanistic information, the clinical and therapeutic usefulness of this approach is limited by the known pro-inflammatory properties that these adenoviral vectors possess in the liver [22]. Exogenous administration of antioxidant enzymes such as SOD may provide a reasonable therapeutic approach, these proteins (Cu/ZnSOD or MnSOD) have extremely short half-lives (<7 minutes) in vivo making their use much less effective [23]. Chemical modifications of these proteins such as the covalent ligation of polyethylene glycol have extended the life span of these enzymes in the circulation [24–26]. Utilizing glycosylated forms of SOD thought to be taken up by hepatocytes and/or Kupffer cells, Fujita and colleagues as well as Yabe and associates were indeed able to significantly attenuate post-ischemic liver injury in the mouse [24–26]. SOD conjugated to either galactose or mannose resulted in a 50% reduction in serum ALT levels following 30 minutes of ischemia and 1 hour of reperfusion [24–26]. Further, these studies identified the mannosylated form of SOD to be taken up by non-parenchymal cells (i.e. endothelial cells, Kupffer cells, stellate cells, pit cells) resulting in substantial hepatocellular protection. These data suggest that Kupffer cell or SEC-generated superoxide may directly or indirectly mediate liver I/R injury.
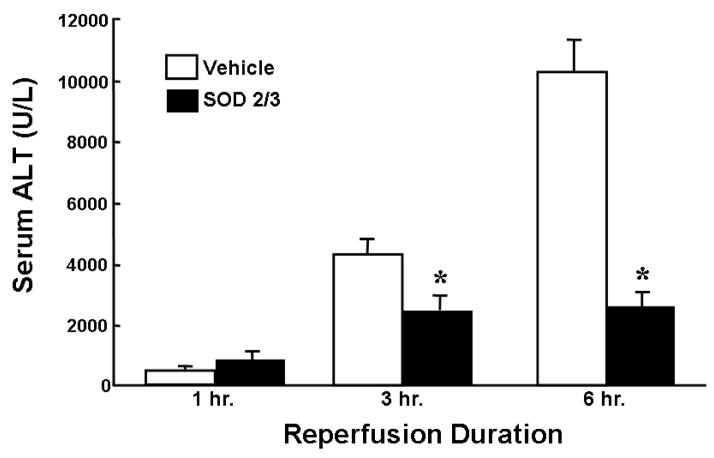
Recent work by Gao and colleagues have demonstrated that SOD may be genetically engineered to possess much longer half lives in vivo [23,27]. They have developed a fusion protein composed of the human polycationic extracellular SOD (SOD3) and the mitochondrial isoform MnSOD (SOD2). This fusion protein (SOD2/3) is capable of binding to the heparan sulfate moieties on the microvascular endothelium as well as the interstitial matrix and has been shown to have a greatly extended half-life (>30 hours) [15,18,23,27]. In contrast to the other modified forms of SOD described above, SOD2/3 remains on the extracellular surface of endothelial cells and hepatocytes allowing one to study the effects of extracellular O2−. We have shown that pretreatment of mice with this SOD2/3 significantly attenuates the post-ischemic liver injury incurred following 45 or 90 minutes of ischemia and 6 hours of reperfusion (Figure 2) [15]. The protection observed with this enzyme also correlated well with significant reductions in serum TNF-α, a known mediator of liver cell injury during I/R.
Figure 2. Effect of SOD2/3 on ischemia and reperfusion liver injury.
Mice were treated with the fusion protein SOD2/3 (1,000 U/kg, iv) or vehicle 15 minutes prior to being subjected to 90 minutes of ischemia and 6 hours of reperfusion. * p<0.05 vs. vehicle-treated mice controls. n ≥ 6 animals per group. Data derived from reference 15.
Taken together, these data clearly demonstrate that O2− mediates directly or indirectly much of the I/R-induced tissue injury. A new class of non-enzymatic, low molecular weight, membrane permeable, manganese-containing SOD mimetics have been created to circumvent some of the problems associated with native or modified enzyme administration. As discussed earlier, administration of native antioxidant enzymes is not therapeutically effective due to their extremely short half-life. Furthermore, administration of large amounts of protein, native or modified, in humans has been associated with immunological complications resulting in their withdrawal from patient use. Manganese-containing protoporphyrin compounds are devoid of these classical side effects. In experimental models of intestinal I/R, these compounds have proven extremely effective. Salvemini et al were able to significantly attenuate post-ischemic neutrophil infiltration in the rat small intestine following 45 minutes of superior mesenteric artery occlusion [15,28]. Further, these mimics have been associated with the reduction of TNF-α expression following splanchnic I/R injury. The use of these compounds in hepatic ischemia has also been examined. Treatment of mice with AEOL 10150 (Aeolus Pharmaceuticals), significantly reduced the post-ischemic liver injury [15]. These data, coupled to those obtained with the SOD2/3, suggest that both intracellular and extracellular O2− are important in the pathogenesis of liver I/R injury.
A number of different cellular sources of O2− have proposed to account for post-ischemic tissue injury. The most likely candidate is KC-associated NADPH oxidase although SEC NADPH oxidase may also play a role as well. KC-NADPH oxidase is multi-component O2−-producing protein complex that has been well-characterized in different phagocytic leukocytes and KCs. Recent data suggest that a similar O2− generating complex is found in endothelial cells and may be important for regulating vascular tone and blood flow [29]. In order to assess the importance of NADPH oxidase in a mouse model liver I/R, mice deficient in the gp91phox subunit of NADPH oxidase (gp91−/−) and lack the ability to produce O2- have been subjected to liver I/R. We found that gp91phox deficiency attenuated the post-ischemic liver injury during the acute phase [15]. Because the acute phase occurs in the absence of PMN infiltration, the most likely cellular sources of NADPH oxidase are the KC and possibly the sinusoidal and/or microvascular endothelial cells [16,30,31]. Indeed, inactivation of KC function by pretreatment of mice with gadolinium chloride 24 hours prior to the induction of ischemia dramatically reduce I/R-induced liver injury [15]. Exactly how NADPH oxidase-derived O2− directly or indirectly promotes liver injury is not known with certainty. It may be that generation of O2− early on during reperfusion may damage mitochondrial membrane lipids and proteins leading either directly or indirectly to the loss of inner membrane potential and ATP generating capacity [32]. In addition, O2− is known to inhibit certain enzymes within the mitochondria including aconitase which may lead to substantial reductions in ATP formation and ultimately cell death [33]. In addition to its direct effects, O2- may mediate hepatocellular damage indirectly via the up-regulation of certain injurious cytokines such as TNF-α [34,35]. This is supported by our observations that the protective effect of SOD2/3 and gp91 deficiency was associated with reductions in serum TNF-α as well [15,36]. The molecular and signaling pathways that are responsible for oxidant-induced cytokine production are discussed below.
Ischemia and Reperfusion Activates Transcription of Pro-Inflammatory Mediators
Over the years, enhanced production of ROS has been thought to injure cells and tissue via their ability to oxidize and/or degrade critical cellular constituents. However, more recent studies suggest that oxidant-induced tissue injury in the post-ischemic liver is not largely dependent upon ROS-mediated oxidative degradation of lipids or other biomolecules [37]. It is now thought that the pathophysiological effects of ROS may relate more to their ability to induce oxidative stress within cells thereby up-regulating (or down-regulating) the expression of certain redox-sensitive genes known to be important in cell proliferation, apoptosis and the inflammatory response. In fact, several lines of evidence suggest that I/R-induced ROS production may mediate hepatocellular injury and inflammation by activating redox-sensitive transcription factors such as NF-kB and activating protein-1 (AP-1) [1,3,34,38–40]. Several different studies demonstrate that liver I/R promotes nuclear translocation of NF-kB (and AP-1) prior to the onset of major liver damage suggesting one or both of these transcription factors may play critical roles in the pathogenesis of post-ischemic liver injury [1,3,34,39]. Although AP-1, as well as other transcription factors may be involved in different aspects of gene regulation in liver I/R, NF-kB is a critical regulatory element in initiating immune and inflammatory responses making this transcription factor a prime candidate for regulating the pathophysiology observed in liver I/R-induced injury. The mechanisms by which ROS-induced activation of NF-kB promotes post-ischemic liver injury has been the subject of active investigations over the past few years. NF-kB is a ubiquitous transcription factor and pleiotropic regulator of numerous inflammatory and immune response. Once activated, NF-kB translocates to the nucleus of the cell where it binds to its consensus sequence on the promoter-enhancer region of different genes thereby activating the transcription of genes known to be important in the immune and inflammatory responses. For example, NF-kB appears to regulate the transcription of several different cytokines (TNF-α, IL-1β, IL-2, IL-6, IL-8, IL-12,), certain endothelial cell adhesion molecules (ECAMs) such as ICAM-1, VCAM-1, E-selectin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) as well as NOS 2 (iNOS) and COX 2 [1,3,34,39].
As previously mentioned, a number of different oxidants, bacterial and viral products, cytokines and lipid mediators activate NF-kB. It is unlikely that each of these stimuli activates cytoplasmic NF-kB-IkB via completely different pathways. Indeed, there is a growing body of experimental data to suggest that many, if not all, of these stimuli activate multiple signaling pathways, which converge to enhance reactive oxygen signaling within the cell. The identity of the specific intracellular source(s) for this ROS signaling has (have) not been identified however prostaglandin synthase, xanthine oxidase, mitochondria, NADPH oxidase and cytochrome P-450 are likely candidates. NF-kB activation has also been shown to be inhibited by a wide variety of structurally diverse enzymatic or non-enzymatic antioxidants or free radical scavengers such as SOD, catalase, GSH peroxidase, N-acetylcysteine, vitamin E derivatives, α-lipoic acid and certain dithiocarbamates. The mechanisms by which small amounts of ROS activate NF-kB have not been defined. Intracellular oxidative stress is thought to activate, directly or indirectly, one or more of the IKKs. Recent work by Karin and coworkers suggest that ROS-mediated activation of NF-kB may be cell specific and may not occur in all tissue [41]. The mechanisms by which NFkB-dependent generation of pro-inflammatory cytokines mediate hepatocellular injury is not entirely clear. Investigations have suggested that much of the post-ischemic liver injury may be a result of massive liver apoptosis induced by TNF-α [42]. However, Jaeschke and coworkers found that when strict histopathological criteria were used to score the livers, virtually all liver injury occurred by “osmotic necrosis” and very little injury occurred by way of apoptosis [42]. However, it should be noted that the vast majority of dead and dying cells in the post-ischemic liver are hepatocytes. It is conceivable that SECs or KCs undergo significant apoptosis, which may not be apparent in whole liver preparations.
Another emerging aspect of liver biology that is receiving substantial attention is the concept that in addition to promoting tissue injury and inflammation, NF-kB activation may protect cells and tissues from a variety of different insults by virtue of its ability enhance the transcription of protective genes. Recent studies by Brenner and coworkers have confirmed that overexpression of liver IkB-α in mice inhibited NF-kB activation resulting in massive hepatocyte injury and apoptosis following partial hepatectomy in these animals [43]. The mechanisms by which NF-kB activation protects the liver are only now becoming understood. It is known for example that NF-kB activation induces several “anti-apoptotic” genes including MnSOD, a zinc finger protein termed A20, cellular inhibitors of apoptosis-2 (c-IAP-2), and Bcl-2 family members such as A1. Most of these genes (i.e. MnSOD, A20 and A1) are also known to inhibit NF-kB activation and thus may control the expression of certain pro-inflammatory genes. It may be that the balance between injurious vs protective genes upregulated during I/R as well as the cellular location of NFkB activation dictates whether NFkB activation will promote injury or protection.
Hepatocellular Protection by Nitric Oxide or Nitrite in Post-Ischemic Liver Injury
It is becoming increasingly appreciated that I/R results in rapid endothelial dysfunction in a number of different tissues which is characterized by the marked decrease in steady state production of endothelial cell-derived NO [6,8,10,20]. The decrease in NO bioavailability occurs within the first few minutes following reperfusion and appears to be due to decreased synthesis of NO, enhanced inactivation of NO by the overproduction of O2− or both. Because NO is known to interact with and rapidly decompose certain ROS such as O2−, OH., and ferryl hemoproteins, decreased steady state production of NO would in all likelihood increase the redox potential of the hepatocyte, KC and/or SEC in favor of a more oxidative environment [9,20,21]. Indeed, we have demonstrated that many of the pathophysiological charateristics induced by I/R (i.e. oxidative stress, leukocyte adhesion, enhanced vascular permeability) may be reproduced in normal tissue by administration of certain NOS inhibitors [8,10,20,44]. Taken together, these data suggest that eNOS-derived NO may act as an endogenous anti-inflammatory mediator in the microcirculation.
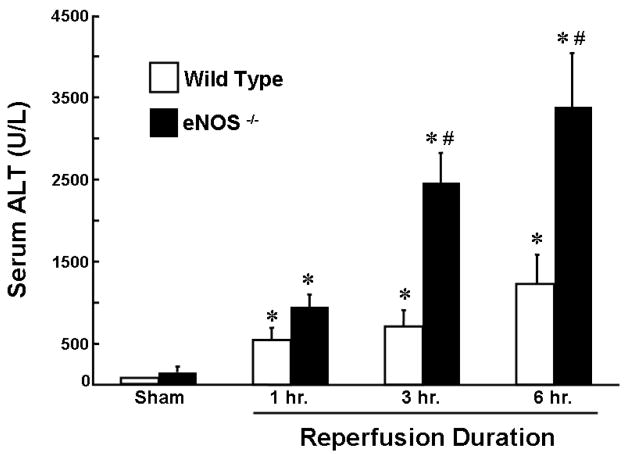
The use of certain NOS inhibitors to evaluate the role of NO in vivo has proven problematic. It is known for example that L-NAME and L-NMMA are nonspecific inhibitors of both eNOS and iNOS as well as potent vasoconstrictors in vivo [45]. Thus, the non-selective nature of these NOS inhibitors coupled to the fact that their exacerbatory effects may be due to their vasoconstrictor effects in a model of hypoperfusion makes their usefulness less than ideal to probe the role for NO in liver I/R. The potentially protective role of endogenous NO in liver I/R injury is supported by recently published studies demonstrating enhanced hepatocellular injury in post-ischemic animals rendered deficient in eNOS (Figure 3) [11,46–48]. In addition, investigators have demonstrated that NO donors or overexpression of liver eNOS protects mice from liver I/R-induced injury [49–51]. Although eNOS has been shown by several groups of investigators to play a critical protective role in liver I/R injury, the role of iNOS is less clear. Studies from our laboratory suggest that I/R-induced liver injury is enhanced in iNOS-deficient mice however interpretation of these data is complicated by the fact that we could not demonstrate upregulation of iNOS message in post-ischemic livers of wild type mice suggesting that the source of iNOS is not in the liver but in other tissue (ie intestine) or that a small population of cells such as KCs or SECs upregulate iNOS which we are unable to detect in whole liver preparations [52].
Figure 3. Effect of eNOS deficiency on ischemia and reperfusion liver injury.
eNOS deficient (eNOS−/−) or wild type mice were subjected to 45 minutes of ischemia and 6 hours of reperfusion. * p<0.05 vs. sham-operated mice; #p<0.05 vs. time matched wild type mouse. n ≥ 6 animals per group. Data derived from reference 15.
The mechanisms by which NO protects the liver against the injurious effects of I/R have not been clearly defined but several possibilities have been proposed. For example NO is known to inhibit NFkB activation. DeCaterina and coworkers provide evidence that NO enhances the denovo synthesis and/or stabilization of the natural inhibitor IkB-α [7]. In addition, NO-mediated S-nitrosation of a specific cysteine on the p50 and/or p65 subunits of NF-kB results in inhibition of binding of this heterodimer to it consensus sequences upstream of the different pro-inflammatory genes [53–55]. Furthermore, NO is known to interact with and decompose O2− or other reactive radicals or oxidants thereby limiting the formation of O2− derived H2O2 and preventing the downstream oxidant-induced pathways for NFkB activation [19]. Another possible mechanism may be that NO-dependent activation of soluble guanylyl cyclase (sGC) with the subsequent production of the vasorelaxant cGMP may protect against reperfusion injury by enhancing blood flow thereby limiting the degree of ischemia to the liver. It has been proposed that NO-mediated activation of protein kinase G via the sGC/cGMP pathway opens mitochondrial KATP channels which reduces calcium accumulation within the mitochondria and prevents the loss of cytochromc c from the mitochondrial intermembranal space (reviewed in [12])(Figure 4). Alternatively or in addition to, NO may reversibly inhibit mitochondrial respiration via interaction with complex I and/or cytochrome c oxidase. This would inhibit apoptosis, maintain small but significant amounts of O2 during ischemia and allow for a more controlled resumption of respiration following reperfusion [12] (Figure 4). Similar observations have been made with NO-dependent S-nitrosation of caspase-3 resulting in inactivation of this enzyme and inhibition of apoposis [12]. This would minimize free radical-mediated damage to the mitochondrial membrane and preserve cellular function. NO may attenuate the later stages of post-ischemic tissue damage by inhibiting platelet/leukocyte-endothelial cell interactions [8,10,11].
Figure 4. Proposed cytoprotective mechanisms for nitric oxide.

Nitric oxide (NO) derived from endothelial nitric oxide synthase or from the enzymatic (deoxyhemobin, dexoymyoglobin, cytochromes, xanthine oxidorectase) or nonenyzmatic (acid-dependent disproportionation) reduction of nitrite (NO2−) may protect tissue subjected to ischemia and reperfusion by: a) Inactivation of caspase-3 via the NO-dependent S-nitrosation of the protein; b) cGMP/protein kinase G (cGMP/PKG)-mediated opening of mitochondrial ATP-dependent potassium channels (KATP) which reduces the loss of cytochrome C, decreases calcium accumulation within the mitochondria, prevent the opening of the mitochondrial permeablility transition (MPT) pore and decrease apoptosis; c) inhibition of mitochondrial electron transport via the direct or indirect (S-nitrosation) inhibition of Complex I (and possible Complex IV) resulting in decreased reactive oxygen specie (ROS) generation, reduced cytochrome C release and decreased apoptosis.
Like any important signaling molecule, the generation and lifetime of NO is very short-lived in vivo. Indeed, NO may be inactivated rapidly by at least 3 different pathways. The first pathways involves the autoxidation of NO to nitrite (NO2−):
Although this pathway has been proposed to be a likely mechanism for NO decomposition in vivo, it is a 3rd order reaction with respect to NO interacting with O2 making this pathway too slow and unlikely to occur under physiological conditions. A more physiologically-relevant set of pathways for the inactivation of NO may be mediated by metal-catalyzed oxidation reactions. The copper-containing protein ceruloplasmin (P-Cu+2) has been shown to rapidly oxidize NO to NO2− under physiological conditions[56]:
In addition to ceruloplasmin, ferrous dioxygenated hemoglobin (Hb-Fe+2O2; oxyhemoglobin) rapidly rapidly decomposes NO to yield nitrate (NO3−):
These data suggest that circulating levels of nitrite and nitrate may be used to assess NOS activity in vivo. Indeed, eNOS deficient mice have plasma levels of nitrite that are only 30% of those found in wild type mice suggesting that eNOS-derived NO represents a major source of nitrite in the circulation [56]. In reality, nitrate is present in significantly greater concentrations that nitrite in the blood (25–35 uM vs 0.15–0.35 uM, respectively)[56]. In addition to NO-derived nitrite and nitrate, large amounts of nitrogen oxides are ingested in the diet from consumption of leafy vegetables (nitrate) and cured meats (nitrite) and contribute significantly to overall plasma levels of nitrate and nitrite. It has been estimated that one plate of leafy vegetables (lettuce, spinach) contains three times more nitrate (and nitrite) than can be produced by all 3 NOS isoforms in one day [56]. Historically, it has been assumed that blood and tissue nitrate and nitrite simply reflected inert endproducts of NO metabolism and food contaminants. However, it is now recognized that these oxidized metabolites of nitrogen can be metabolized in vivo to regenerate NO. This happens in two basic steps. The first step involves the reduction of nitrate to nitrite by the action nitrate reductase found in certain enteric and oral bacteria[56]:
Once formed, NO2− can be reduced further to yield NO via several different pathways. The first and best characterized is by the acid-catalzed disproportionation of nitrous acid (HNO2) via the intermediate formation of dinitrogen trioxide (N2O3):
This reaction is greatly enhanced by the presence of asorbic acid or different polyphenolic compounds [56]. In addition, nitrite can be reduced to NO by xanthine oxidoreductase (XO) or by ferrous deoxygenated hemoglobin or myoglobin (P-Fe+2):
Although much of the NO produced would escape the heme moiety making it available for further interactions, a portion of the NO would be trapped by the heme to form nitrosyl-hemoglobin (or myoglobin). The physiological significance of nitrosyl-heme formation is not clear at the present time. Taken together, these data demonstrate that endogeous or dietary nitrate and nitrite can be metabolized and recycled in vivo to generate biologically active NO. Because the rate of NO generation from nitrite is linearly dependent on reductions in tissue pO2 and pH, nitrite may be metabolized to NO in ischemic tissue (via deoxyHb or Mb) and exert NO-dependent protective effects site-specifically. Indeed, the tissue-specific reduction of nitrite to generate cytoprotective NO has important clinical significance in that treatment of different ischemic disorders with nitrite would reduce the untoward cardiovascular effects of systemic delivery of inhaled NO or intravenous administration of S-nitrosothiols. Indeed, a growing number of studies demonstrate that nitrite is cytoprotective in different models of tissue I/R including the liver, heart, kidney and brain [12]. The clinical usefulness of nitrite is currently being investigated as a potential therapeutic agent to treat ischemic disorders.
Concluding Remarks
There is now overwhelming evidence supporting a role for NO generated by eNOS or from nitrite as an important regulatory molecule that acts to limit post-ischemic tissue injury. The mechanisms by which this free radical protects the reperfused liver remain to be defined and is the subject of active investigation. Therapeutic uses of NO or nitrite are currently underway in several different laboratories to treat a variety of ischemic disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fan C, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver. J Mol Med. 1999;77:577–592. doi: 10.1007/s001099900029. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H. Mechanisms of reperfusion injury after warm ischemia of the liver. J Hepatobiliary Pancreat Surg. 1998;5:402–408. doi: 10.1007/s005340050064. [DOI] [PubMed] [Google Scholar]
- 3.Zwacka RM, Zhou W, Zhang Y, Darby CJ, Dudus L, Halldorson J, Oberley L, Engelhardt JF. Redox gene therapy for ischemia/reperfusion injury of the liver reduces AP1 and NF-kappaB activation. Nat Med. 1998;4:698–704. doi: 10.1038/nm0698-698. [DOI] [PubMed] [Google Scholar]
- 4.Husted TL, Lentsch AB. The role of cytokines in pharmacological modulation of hepatic ischemia/reperfusion injury. Curr Pharm Des. 2006;12:2867–2873. doi: 10.2174/138161206777947597. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia- reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 6.Lefer AM, Lefer DJ. Nitric oxideII. Nitric oxide protects in intestinal inflammation. Am J Physiol. 1999;276:G572–G575. doi: 10.1152/ajpgi.1999.276.3.G572. [DOI] [PubMed] [Google Scholar]
- 7.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granger DN, Kubes P. Nitric oxide as antiinflammatory agent. Methods Enzymol. 1996;269:434–442. doi: 10.1016/s0076-6879(96)69044-2. [DOI] [PubMed] [Google Scholar]
- 9.Gaboury J, Woodman RC, Granger DN, Reinhardt P, Kubes P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol. 1993;265:H862–H867. doi: 10.1152/ajpheart.1993.265.3.H862. [DOI] [PubMed] [Google Scholar]
- 10.Grisham MB, Granger DN, Lefer DJ. Modulation of leukocyte-endothelial interactions by reactive metabolites of oxygen and nitrogen: relevance to ischemic heart disease. Free Radic Biol Med. 1998;25:404–433. doi: 10.1016/s0891-5849(98)00094-x. [DOI] [PubMed] [Google Scholar]
- 11.Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, Huang PL, Scalia R. Leukocyte-endothelial cell interactions in nitric oxide synthase- deficient mice. Am J Physiol. 1999;276:H1943–H1950. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- 12.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: a brief review. Mol Med. 2008;14:337–345. doi: 10.2119/2007-00134.Vardanian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2005;289:G969–G976. doi: 10.1152/ajpgi.00223.2005. [DOI] [PubMed] [Google Scholar]
- 15.Hines IN, Hoffman JM, Scheerens H, Day BJ, Harada H, Pavlick KP, Bharwani S, Wolf R, Gao B, Flores S, McCord JM, Grisham MB. Regulation of postischemic liver injury following different durations of ischemia. Am J Physiol Gastrointest Liver Physiol. 2003;284:G536–G545. doi: 10.1152/ajpgi.00400.2002. [DOI] [PubMed] [Google Scholar]
- 16.Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79:115–136. doi: 10.1016/0009-2797(91)90077-k. [DOI] [PubMed] [Google Scholar]
- 17.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 18.Taniai H, Hines IN, Bharwani S, Maloney RE, Nimura Y, Gao B, Flores SC, McCord JM, Grisham MB, Aw TY. Susceptibility of murine periportal hepatocytes to hypoxia-reoxygenation: role for NO and Kupffer cell-derived oxidants. Hepatology. 2004;39:1544–1552. doi: 10.1002/hep.20217. [DOI] [PubMed] [Google Scholar]
- 19.Grisham MB, Jourd’heuil D, Wink DA. Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites:implications in inflammation. Am J Physiol. 1999;276:G315–G321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- 20.Kurose I, Wolf R, Grisham MB, Aw TY, Specian RD, Granger DN. Microvascular responses to inhibition of nitric oxide production. Role of active oxidants. Circ Res. 1995;76:30–39. doi: 10.1161/01.res.76.1.30. [DOI] [PubMed] [Google Scholar]
- 21.Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 23.Gao B, Flores SC, McCord JM. A site-directed mutant of Cu, Zn-superoxide dismutase modeled after native extracellular superoxide dismutase. Biol Trace Elem Res. 1995;47:95–100. doi: 10.1007/BF02790105. [DOI] [PubMed] [Google Scholar]
- 24.Yabe Y, Kobayashi N, Nishihashi T, Takahashi R, Nishikawa M, Takakura Y, Hashida M. Prevention of neutrophil-mediated hepatic ischemia/reperfusion injury by superoxide dismutase and catalase derivatives. J Pharmacol Exp Ther. 2001;298:894–899. [PubMed] [Google Scholar]
- 25.Yabe Y, Nishikawa M, Tamada A, Takakura Y, Hashida M. Targeted delivery and improved therapeutic potential of catalase by chemical modification: combination with superoxide dismutase derivatives. J Pharmacol Exp Ther. 1999;289:1176–1184. [PubMed] [Google Scholar]
- 26.Fujita T, Nishikawa M, Tamaki C, Takakura Y, Hashida M, Sezaki H. Targeted delivery of human recombinant superoxide dismutase by chemical modification with mono- and polysaccharide derivatives. J Pharmacol Exp Ther. 1992;263:971–978. [PubMed] [Google Scholar]
- 27.Gao B, Flores SC, Leff JA, Bose SK, McCord JM. Synthesis and anti-inflammatory activity of a chimeric recombinant superoxide dismutase: SOD2/3. Am J Physiol Lung Cell Mol Physiol. 2003;284:L917–L925. doi: 10.1152/ajplung.00374.2002. [DOI] [PubMed] [Google Scholar]
- 28.Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 29.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 30.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J Leukoc Biol. 1992;52:377–382. doi: 10.1002/jlb.52.4.377. [DOI] [PubMed] [Google Scholar]
- 31.De Minicis S, Bataller R, Brenner DA. NADPH oxidase in the liver: defensive, offensive, or fibrogenic? Gastroenterology. 2006;131:272–275. doi: 10.1053/j.gastro.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 32.Madesh M, Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hausladen A, Fridovich I. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J Biol Chem. 1994;269:29405–29408. [PubMed] [Google Scholar]
- 34.Shin T, Kuboki S, Lentsch AB. Roles of nuclear factor-kappaB in postischemic liver. Hepatol Res. 2007 doi: 10.1111/j.1872-034X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 35.Beutler BA. The role of tumor necrosis factor in health and disease. J Rheumatol. 1999;26(Suppl 57):16–21. [PubMed] [Google Scholar]
- 36.Harada H, Hines IN, Flores S, Gao B, McCord J, Scheerens H, Grisham MB. Role of NADPH oxidase-derived superoxide in reduced size liver ischemia and reperfusion injury. Arch Biochem Biophys. 2004;423:103–108. doi: 10.1016/j.abb.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 37.Jaeschke H. Kupffer cell-induced oxidant stress during hepatic ischemia-reperfusion: does the controversy continue? Hepatology. 1999;30:1527–1528. doi: 10.1002/hep.510300630. [DOI] [PubMed] [Google Scholar]
- 38.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 39.Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 40.Zwacka RM, Zhang Y, Zhou W, Halldorson J, Engelhardt JF. Ischemia/reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor kappaB independently of IkappaB degradation. Hepatology. 1998;28:1022–1030. doi: 10.1002/hep.510280417. [DOI] [PubMed] [Google Scholar]
- 41.Li N, Karin M. Is NF-kappaB the sensor of oxidative stress? FASEB J. 1999;13:1137–1143. [PubMed] [Google Scholar]
- 42.Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- 43.Iimuro Y, Nishiura T, Hellerbrand C, Behrns KE, Schoonhoven R, Grisham JW, Brenner DA. NFkappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest. 1998;101:802–811. doi: 10.1172/JCI483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conner EM, Aiko S, Fernandez M, Battarbee HD, Gray L, Grisham MB. Duration of the hemodynamic effects of N(G)-nitro-L-arginine methyl ester in vivo. Nitric Oxide. 2000;4:85–93. doi: 10.1006/niox.2000.0270. [DOI] [PubMed] [Google Scholar]
- 46.Hines IN, Kawachi S, Harada H, Pavlick KP, Hoffman JM, Bharwani S, Wolf RE, Grisham MB. Role of nitric oxide in liver ischemia and reperfusion injury. Mol Cell Biochem. 2002;234–235:229–237. [PubMed] [Google Scholar]
- 47.Hines IN, Harada H, Flores S, Gao B, McCord JM, Grisham MB. Endothelial nitric oxide synthase protects the post-ischemic liver: potential interactions with superoxide. Biomed Pharmacother. 2005;59:183–189. doi: 10.1016/j.biopha.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Kawachi S, Hines IN, Laroux FS, Hoffman J, Bharwani S, Gray L, Leffer D, Grisham MB. Nitric oxide synthase and postischemic liver injury. Biochem Biophys Res Commun. 2000;276:851–854. doi: 10.1006/bbrc.2000.3559. [DOI] [PubMed] [Google Scholar]
- 49.Duranski MR, Elrod JW, Calvert JW, Bryan NS, Feelisch M, Lefer DJ. Genetic overexpression of eNOS attenuates hepatic ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H2980–H2986. doi: 10.1152/ajpheart.01173.2005. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Billiar TR. Nitric Oxide. IV. Determinants of nitric oxide protection and toxicity in liver. Am J Physiol. 1999;276:G1069–G1073. doi: 10.1152/ajpgi.1999.276.5.G1069. [DOI] [PubMed] [Google Scholar]
- 51.Katsumi H, Nishikawa M, Yamashita F, Hashida M. Prevention of hepatic ischemia/reperfusion injury by prolonged delivery of nitric oxide to the circulating blood in mice. Transplantation. 2008;85:264–269. doi: 10.1097/TP.0b013e31815e902b. [DOI] [PubMed] [Google Scholar]
- 52.Hines IN, Harada H, Bharwani S, Pavlick KP, Hoffman JM, Grisham MB. Enhanced post-ischemic liver injury in iNOS-deficient mice: a cautionary note. Biochem Biophys Res Commun. 2001;284:972–976. doi: 10.1006/bbrc.2001.5069. [DOI] [PubMed] [Google Scholar]
- 53.Marshall HE, Hess DT, Stamler JS. S-nitrosylation: physiological regulation of NF-kappaB. Proc Natl Acad Sci U S A. 2004;101:8841–8842. doi: 10.1073/pnas.0403034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40:1688–1693. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 55.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: A state of nitric oxide resistance. Free Radic Biol Med. 2008;44:1506–1528. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]