Abstract
Background
Anabolic-androgenic steroid (AAS) dependence has been a recognized syndrome for some 20 years, but remains poorly understood.
Methods
We evaluated three groups of experienced male weightlifters: 1) men reporting no history of AAS use (N = 72); 2) nondependent AAS users reporting no history of AAS dependence (N = 42); and 3) men meeting adapted DSM-IV criteria for current or past AAS dependence (N = 20). We assessed demographic indices, lifetime history of psychiatric disorders by the Structured Clinical Interview for DSM-IV, variables related to AAS use, and results from drug tests of urine and hair.
Results
Nondependent AAS users showed no significant differences from AAS nonusers on any variable assessed. Dependent AAS users, however, differed substantially from both other groups on many measures. Notably, they reported a more frequent history of conduct disorder than nondependent AAS users (odds ratio [95% CI]: 8.0 [1.7, 38.0]) or AAS nonusers (13.1 [2.8, 60.4]) and a much higher lifetime prevalence of opioid abuse and dependence than either comparison group (odds ratios 6.3 [1.2, 34.5] and 18.6 [3.0, 116.8], respectively).
Conclusions
Men with AAS dependence, unlike nondependent AAS users or AAS nonusers, showed a distinctive pattern of comorbid psychopathology, overlapping with that of individuals with other forms of substance dependence. AAS dependence showed a particularly strong association with opioid dependence – an observation that recalls recent animal data suggesting similarities in AAS and opioid brain reward mechanisms. Individuals with AAS dependence and individuals with “classical” substance dependence may possibly harbor similar underlying biological and neuropsychological vulnerabilities.
Keywords: Anabolic-androgenic steroids, substance dependence, opioids, men
1. Introduction
The anabolic-androgenic steroids (AAS) are a group of drugs that includes testosterone, the natural male hormone, together with numerous synthetic testosterone derivatives created over the last 70 years (Pope and Brower, 2009). Since the 1950s, elite competitive athletes have used AAS for decades to improve performance. However, a majority of today's AAS users are not elite athletes at all, but simply individuals wanting to become more muscular (Buckley et al., 1988; Hildebrandt et al., 2006; Kanayama et al., 2001; Parkinson and Evans, 2006). As we have explained previously (Kanayama et al., 2008), this much larger but less visible population of ordinary AAS users started to emerge in the 1980s, as AAS spread out of the elite athletic community and into the general population. Now, illicit AAS use has grown into a widespread public health problem in the United States (Buckley et al., 1988; Johnston et al., 2006; McCabe et al., 2007; Yesalis et al., 1993) and many other Western countries (Galduroz et al., 2005; Handelsman and Gupta, 1997; Kokkevi et al., 2008; Melia et al., 1996; Nilsson et al., 2001; Pallesen et al., 2006; Rachon et al., 2006; Wanjek et al., 2007). AAS use is associated with a range of adverse medical effects, including dyslipidemia, cardiomyopathy, and prolonged suppression of the hypothalamic-pituitary-gonadal axis (Hartgens and Kuipers, 2004; Kanayama et al., 2008). In some individuals, AAS may also cause serious psychiatric effects, including especially major mood syndromes (Kanayama et al., 2008; Pagonis et al., 2006; Perry et al., 2003; Pope and Brower, 2009), which may sometimes be associated with aggression or violence (Klotz et al., 2006; Perry et al., 2003; Pope and Katz, 1990; Thiblin and Parlklo, 2002).
Over the last 20 years, a growing animal and human literature has demonstrated that AAS can produce a dependence syndrome. AAS dependence was first documented in human case reports and case series by the late 1980s (Brower et al., 1989; Brower et al., 1990; Hays et al., 1990; Tennant et al., 1988), and has subsequently been documented in about 30% of illicit AAS users in various larger field studies (see below). Animal studies have provided further evidence that AAS can induce dependence; for example, rats and mice will prefer to spend time in locations where they have previously received AAS (Alexander et al., 1994; Arnedo et al., 2000), and hamsters will self-administer testosterone by direct intracranial injection even to the point of death (Peters and Wood, 2005; Wood, 2006). Interestingly, animal observations suggest that AAS intoxication and withdrawal may involve opioid-like features. For example, symptoms of testosterone intoxication in hamsters are blocked by naltrexone, an opioid receptor antagonist (Peters and Wood, 2005). Although opioid antagonists do not precipitate full-scale opioid withdrawal in testosterone-treated hamsters (e.g., wetdog shakes, teeth chattering, yawning, or defecation), pretreatment with opioid antagonists will prevent testosterone self-administration (Peters and Wood, 2005). In other animal studies, AAS administration has been shown to influence opioidergic brain systems (Harlan et al., 2000; Johansson et al., 2000a; Johansson et al., 1997) and to modulate responses to opiate administration and withdrawal (Celerier et al., 2003; Wood, 2008). Thus, AAS may act as partial opioid agonists, although their dependence-inducing potential presumably involves other neural and endocrine mechanisms as well (Daly et al., 2001; Frye, 2007; Hochberg et al., 2003; Kindlundh et al., 2004; Lindqvist et al., 2002; Wood, 2008).
Human AAS dependence likely evolves in a more complex manner than in animals (Brower, 2002). At first, individuals typically begin AAS use in conjunction with weightlifting, as a pharmacological aid for gaining muscle and losing body fat. Individuals reporting prominent adolescent preoccupations with muscularity, and especially those with full-scale “muscle dysmorphia,” are particularly likely to initiate AAS use (Cafri et al., 2005; Hildebrandt et al., 2006; Kanayama et al., 2006; Kanayama et al., 2003b; Olivardia et al., 2000). Nevertheless, many of these individuals will not progress to AAS dependence, but will use only a few courses of AAS (colloquially called “cycles”) in a lifetime, for a total lifetime exposure of less than 12 months (Kanayama et al., 2006). Some, however, will go on to using AAS on an almost continuous basis despite adverse medical, psychiatric, social, and occupational effects; as this syndrome evolves, it increasingly seems to resemble “classical” drug dependence (Brower, 2002; Pope and Brower, 2009).
Over the last 20 years, seven studies, collectively evaluating 591 AAS users, have found that 177 (174 male, 3 female; a 30% overall rate) appeared to meet DSM-IV diagnostic criteria for AAS dependence; and Perry, P.J., personal communication, October 2008, and Malone, D.A., personal communication, January 2009). The low reported prevalence of AAS dependence in women seems understandable; women rarely wish to become highly muscular, and they are also subject to the masculinizing effects of AAS, such as beard growth, deepening of the voice, and masculinized sexual characteristics (Gruber and Pope, 2000; Kanayama et al., 2007). Aside from information on gender, age, and other demographic features, however, these seven studies have provided few direct comparisons of the attributes of AAS-dependent individuals versus the attributes of comparison groups, such as nondependent AAS users or AAS nonusers. One study (Copeland et al., 2000) compared 48 individuals with AAS dependence or AAS abuse (of whom 23 had AAS dependence) vs. 52 AAS users without dependence or abuse, and found no significant differences between groups on age at first use or mean weekly dose of AAS used. On the other hand, these investigators noted that the individuals with AAS dependence or abuse were significantly more likely than other AAS users to report having experienced “roid rage” (63.3% vs. 16.3%; odds ratio 3.9; 95% confidence interval 1.45 – 10.41). However, none of the studies systematically compared the prevalence of psychiatric disorders in AAS-dependent users vs. other groups.
To augment these limited data, we report the results of detailed psychiatric evaluations of dependent AAS users encountered in an ongoing study, and compare them against nondependent AAS users and AAS nonusers, recruited in the same study.
2.0 Methods
2.1 Participants
In the course of an ongoing study, we advertised in gymnasiums and sports supplement stores in Eastern Massachusetts and Southern Florida for men aged 18-40 who could bench-press 275 pounds for at least one repetition, currently or in the past, to participate in a psychiatric and medical evaluation for $150 compensation. The primary purpose of the study was to obtain retrospective assessments of childhood and adolescent risk factors for AAS use; participants over age 40 were excluded to reduce recall bias that might occur in older individuals. The requirement of a 275-pound bench press was simply a device to select a group of experienced weightlifters, and the study's focus on AAS use was not disclosed. Details of these recruitment methods are provided in previous publications describing a study of similar design (Kanayama et al., 2006; Kanayama et al., 2003b; Pope et al., 2004). All participants provided written informed consent for the study, which was approved by the McLean Hospital Institutional Review Board.
2.2 Study instruments
All participants were personally evaluated by two of the investigators (HGP and GK), using verbal interviews including 1) demographic questions; 2) the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2001); 3) modules derived from the Childhood Disorders Version of the SCID (the “KID-SCID”) (Brown-Séquard,; Matzner, 1994; Matzner et al., 1997), inquiring retrospectively about childhood attention deficit hyperactivity disorder (ADHD) and conduct disorder; and 4) the Body Dysmorphic Disorder Modification of the Yale-Brown Obsessive Compulsive Scale (BDD-YBOCS) (Phillips et al., 1997; Phillips et al., 1994; Phillips et al., 1993). We also 5) administered a computerized battery of psychological questionnaires regarding childhood and adolescent attributes, similar to questionnaires used previously (Kanayama et al., 2003b); 6) measured height, weight, body fat, and fat-free mass index (FFMI), using methods previously described (Kanayama et al., 2003b); and 7) collected urine and hair samples to be analyzed for drugs of abuse, including AAS.
The sequence of the evaluation was structured so that questions about substance use were placed at the end, with questions about AAS and other performance-enhancing drugs coming last, so that the investigators would be blinded to AAS history while eliciting all other information. The AAS history included questions about specific AAS used, doses, and durations. Participants reporting AAS use were also asked questions, patterned after those of the SCID, to assess history of AAS dependence and substance-induced mood disorders associated with AAS use.
2.3 Data analysis
For the present analysis, participants were classified into three groups: 1) nonusers reporting no history of AAS use; 2) nondependent AAS users reporting a history of AAS use but not meeting criteria for AAS dependence at any time; and 3) participants reporting a history of AAS dependence as diagnosed by DSM-IV criteria, interpreted and adapted for diagnosing AAS dependence as we have recently described (Kanayama et al., 2009). We initially compared the three groups on age, ethnicity, and study site (Florida versus Massachusetts); the groups differed on all of these three variables (see below). We then compared the three groups on a range of outcome variables, using linear and logistic regression with adjustment for age, ethnicity, and study site as discussed in more detail below. Alpha was set at 0.05, two-tailed, although the reader should note the possibility that, because of the multiple comparisons conducted, some might have reached this level of significance by chance alone.
3.0 Results
3.1 Sample characteristics
Of 146 men evaluated at the Florida and Massachusetts sites between July 2005 and October 2008, one was excluded for incomplete data and seven others were excluded for apparently untruthful answers on interview (AAS found in urine despite denial of AAS use by participant, N = 4; implausibly high muscularity and low body fat despite denial of AAS use, N = 2; and grossly inconsistent answers regarding history of conduct disorder, N = 1). Of the remaining 138 men, 72 reported no AAS use, 42 reported AAS use without evidence of dependence, 20 clearly met DSM-IV criteria for a lifetime history of AAS dependence, and 4 showed a history approaching criteria for AAS dependence, but could not be classified as such with certainty. These last four participants were excluded from subsequent comparisons, leaving 20 dependent AAS users.
The 72 nonusers were similar in age to the 42 nondependent AAS users (mean [SD]: 27.1 [6.0] years vs. 27.3 [5.7] years; p = 0.89 by t test, two-tailed) but the 20 dependent AAS users were significantly older than either of the other two groups (33.9 [4.7] years; p < 0.001 versus both other groups) – perhaps in part because older individuals had simply had more time to develop a dependence syndrome. The 93 men in the Florida subsample contained a smaller portion of AAS users (40 men, 8 of whom displayed a lifetime history of AAS dependence) than the 41 men in the Massachusetts subsample (22 AAS users, 12 with AAS dependence), probably because the Massachusetts participants were drawn from gymnasiums with a higher proportion of heavy AAS users. The 72 nonusers included 22 men of non-white ethnicity (11 African-American, 2 Asian, one Pacific Islander, 6 Hispanic-Caucasian, and 2 Hispanic-African-American) whereas the 62 AAS users included only three non-white individuals (one African-American and one Hispanic-African-American among the 42 nondependent users and one African-American among the 20 dependent users). Given these differences, all further comparisons among the three groups on the outcome variables were performed with adjustment for age (unordered categories based on quintiles of the age distribution), ethnicity (white versus non-white), and study site (Florida versus Massachusetts).
3.2 Demographic features
Dependent AAS users were more likely than nondependent AAS users to have had only a single parent (as a result of divorce, separation, or death) by the age of 13, and they were more likely than members of the other groups to report a first-degree relative with an alcohol or substance use disorder (Table 1). Despite their older mean age, dependent AAS users reported much lower educational attainment than the other groups; indeed, only one (5%) of these 20 individuals had graduated from college, as compared to 9 (21%) of the nondependent users and 27 (38%) of the nonusers.
Table 1. Demographic Features of Study Groups.
| Group | Between-group Comparisons | |||||
|---|---|---|---|---|---|---|
| Nonusers (N=72) | Nondependent AAS users (N = 42) | Dependent AAS users (N = 20) | Nondependent AAS users vs. nonusers | Dependent AAS users vs. nonusers non | Dependent vs. dependent AAS users | |
| N (%) | Odds Ratio (95% confidence interval)a | |||||
| Single parentb | 24 (33) | 10 (24) | 9 (45) | 0.5 (0.2, 1.4) | 2.2 (0.6, 7.2) | 3.9* (1.0, 14.8) |
| Family history of substance use disorderc | 22 (31) | 13 (33) | 10 (53) | 0.8 (0.3, 1.9) | 3.7* (1.0, 13.5) | 4.7* (1.2, 18.8) |
| Childhood sexual abuse | 7 (10) | 3 (7) | 2 (10) | 0.7 (0.2, 3.0) | 0.6 (0.1, 4.1) | 1.0 (0.1, 7.4) |
| Never married | 54 (75) | 35 (83) | 13 (65) | 1.9 (0.6, 6.2) | 2.4 (0.6, 9.3) | 1.3 (0.3, 5.5) |
| Any post-high-school education | 50 (69) | 32 (76) | 9 (45) | 1.5 (0.6, 3.9) | 0.2* (0.0, 0.7) | 0.1** (0.0, 0.5) |
| Mean (SD) | Mean Difference (95% confidence interval)a | |||||
| Age began regular weightlifting | 16.1 (3.4) | 16.2 (2.9) | 16.8 (3.6) | 0.1 (-1.2, 1.4) | 0.2 (-1.6, 2.1) | 0.1 (-1.8, 2.1) |
| Years of regular weightlifting | 7.4 (4.4) | 7.7 (4.6) | 12.6 (6.5) | 0.3 (-1.3, 1.8) | 0.9 (-1.3, 3.0) | 0.6 (-1.6, 2.8) |
| Fat-free mass index, kg/m2 | 22.6 (2.0) | 23.1 (2.5) | 25.8 (3.1) | 0.5 (-0.4, 1.5) | 3.2*** (1.8, 4.5) | 2.6*** (1.2, 4.0) |
Estimates adjusted for age, study site, and ethnicity (see text). * p < 0.05; ** p < 0.01; *** p < 0.001
The particpant reported that parents were separated or divorced, or that one parent died before he reached age 13; N = 41 for nondependent user group.
The participant reported at least one first-degree relative with apparent substance abuse or dependence; N = 71 non-users, 40 nondependent users, and 19 dependent users because of missing data (e.g., adopted or lacking information on biological relatives).
Dependent AAS users did not differ significantly from either of the other two groups in the mean age at which they started lifting weights or in their mean total years of lifting weights (Table 1). However, they were significantly more muscular than the other groups, as assessed by their fat-free mass index (Kouri et al., 1995), consistent with their extensive use of AAS.
3.3 Use of AAS and other performance-enhancing drugs
The dependent AAS users differed markedly from the nondependent AAS users on several variables related to AAS and other performance-enhancing drugs (Table 2). For example, dependent users reported a mean of almost six years of lifetime AAS use as compared with a mean of about six months in the nondependent AAS users; indeed, only three of the 42 nondependent AAS users reported more than one year of total lifetime AAS exposure. Interestingly, dependent AAS users and nondependent AAS users reported a similar mean age of onset of AAS use; however, dependent AAS users reported using significantly higher maximum doses of AAS, and they reported more frequent use of other performance-enhancing drugs. Among these other drugs were clenbuterol (reported by 14 [70%] of dependent users and 3 [7%] of nondependent users], growth hormone (13 [65%] and 3 [7%] of the groups, respectively), and several drugs reported exclusively by the dependent users: insulin (6 [30%] of dependent users), insulin-like growth factor 1 (IGF-1) (4 [20%]), and triiodothyronine (4 [20%]). Only one of the 72 AAS nonusers reported use of any performance-enhancing drug (clenbuterol). Only a small minority of AAS users in both groups reported using AAS specifically for performance in competitive sports.
Table 2. Features of AAS User Subgroups.
| Group | Between-group Comparisons | ||||
|---|---|---|---|---|---|
| Nondependent AAS users (N = 42) | Dependent AAS users(N = 20) | Dependent vs. nondependent AAS users | |||
| Mean (SD) | Mean Difference (95% confidence interval)a | ||||
| Age at first AAS use, years | 21.8 (4.7) | 23.6 (6.1) | -0.2 (-3.5, 3.0) | ||
| Lifetime weeks of AAS use | 25.7 (26.9) | 302.7 (245.4) | 238*** (142, 334) | ||
| Maximum weekly AAS dose, mg testosterone equivalent | 712 (631) | 1920 (966) | 993*** (531, 1456) | ||
| N (%) | Odds Ratio (95% confidence interval)a | ||||
| Used other performance-enhancing drugs | 5 (12) | 16 (80) | 28.5*** (4.3, 187.1) | ||
| Used AAS for competitive sports | 4 (10) | 3 (15) | 0.9 (0.1, 8.6) | ||
| AAS-associated mood disorder: | |||||
| - mania or hypomania | 3 (7) | 5 (25) | 4.3 (0.6, 20.4) | ||
| - major depression | 1 (2) | 2 (10) | 9.3 (0.4, 213.0) | ||
Estimates adjusted for age, study site, and ethnicity (see text). * p < 0.05; ** p < 0.01; *** p < 0.001
Five (25%) of the dependent AAS users reported a manic or hypomanic syndrome while taking AAS, and two of these same men displayed a major depressive episode during AAS withdrawal. None of these five men reported a manic or hypomanic episode at times when not using AAS. Among the remaining 15 dependent AAS users, six described increased irritability, aggressiveness, or violence while using AAS, but not to the point of meeting criteria for a hypomanic episode. The lifetime prevalence of AAS-associated major mood disorders among dependent AAS users was higher than in the nondependent AAS users, although these differences did not reach statistical significance, perhaps because of the small numbers involved.
3.4 Psychiatric and substance use disorders
The nonuser group and nondependent AAS user group showed no significant differences in lifetime prevalence of any of the various categories of psychiatric disorders assessed by the SCID and KID-SCID (Table 3), but dependent AAS users showed a strikingly higher lifetime prevalence of childhood conduct disorder. Among dependent AAS users, the relative odds [95% confidence interval] of a reported history of conduct disorder was 8.0 (1.7, 38.0) when compared to the nondependent users (p = 0.009) and 13.1 (2.8, 60.4) compared to the nonusers (p = 0.001). Dependent AAS users also reported a significantly higher lifetime prevalence of body dysmorphic disorder than nonusers. This latter difference was apparently attributable to a higher prevalence of muscle dysmorphia in the AAS-dependent group (see Table). Looking at substance use disorders, the three groups exhibited no significant differences in lifetime prevalence of alcohol dependence, but striking differences in their lifetime prevalence of non-alcohol substance dependence, particularly opioid dependence. Among dependent AAS users, the relative odds of opioid abuse or dependence was 6.7 (1.5, 31.3) when compared to nondependent users (p = 0.015), and 16.3 (3.4, 78.9) compared to the nonusers (p < 0.001). Dependent AAS users also frequently reported a history of cocaine dependence, but this diagnosis was also fairly common in the two comparison groups, so that differences between the dependent users and the comparison groups only barely reached the 0.05 level of significance (relative odds in dependent users vs. nonusers 4.5 [1.1, 17.5], p = 0.032; dependent users vs. nondependent users 5.0 [1.1, 22.1], p = 0.033).
Table 3. Lifetime Prevalence of Psychiatric and Substance Use Disorders in Study Groups.
| Group | Between-group Comparisons | |||||
|---|---|---|---|---|---|---|
| Nonusers (N=72) | Nondependent AAS users (N = 42) | Dependent AAS users (N = 20) | Nondependent AAS users vs. nonusers | Dependent AAS users vs. nonusers no | Dependent vs. ndependent AAS users | |
| N (%) | Odds Ratio (95% confidence interval)a | |||||
| ADHDb | 10 (14) | 8 (19) | 4 (20) | 1.2 (0.4, 3.6) | 1.3 (0.3, 5.7) | 1.0 (0.2, 5.1) |
| Conduct disorder | 15 (21) | 14 (33) | 11 (55) | 1.6 (0.6, 4.2) | 13.1*** (2.8, 60.4) | 8.0** (1.7, 38.0) |
| Major mood disorderc | 12 (17) | 8 (19) | 6 (30) | 1.1 (0.4, 3.2) | 2.7 (0.7, 10.5) | 2.4 (0.6, 10.2) |
| Anxiety disorderd | 7 (10) | 5 (12) | 4 (20) | 1.0 (0.3, 3.9) | 2.0 (0.4, 9.9) | 1.9 (0.3, 10.4) |
| Eating disorderse | 1 (1) | 3 (7) | 1 (5) | 6.9 (0.6, 80.6) | 7.1 (0.3, 167.0) | 1.0 (0.1, 13.9) |
| Body dysmorphic disorder | 2 (3) | 4 (10) | 5 (25) | 3.4 (0.5, 21.0) | 16.0** (1.9, 132.9) | 4.8 (0.8, 30.2) |
| -muscle dysmorphia only | - | 1 (2) | 4 (20) | 0.37f | 0.002f | 0.03f |
| Alcohol dependence | 12 (17) | 11 (26) | 2 (10) | 1.6 (0.6, 4.2) | 0.5 (0.1, 2.7) | 0.3 (0.1, 1.8) |
| Any non-alcohol substance dependence | 32 (44) | 24 (57) | 16 (80) | 1.5 (0.6, 3.5) | 12.5*** (2.7, 57.3) | 8.3** (1.7, 39.8) |
| Cannabis dependence | 30 (42) | 19 (45) | 6 (30) | 1.0 (0.4, 2.4) | 1.1 (0.3, 4.0) | 1.1 (0.3, 4.1) |
| Cocaine dependence | 12 (17) | 7 (17) | 9 (45) | 0.9 (0.3, 2.7) | 4.5* (1.1, 17.5) | 5.0* (1.1, 22.1) |
| Opioid dependence | 3 (4) | 6 (14) | 8 (40) | 2.9 (0.7, 13.3) | 18.6** (3.0, 116.8) | 6.3* (1.2, 34.5) |
| Opioid abuse or dependence | 5 (7) | 8 (19) | 10 (50) | 2.4 (0.7, 8.4) | 16.3*** (3.4, 78.9) | 6.7* (1.5, 231.3) |
Estimates adjusted for age, study site, and ethnicity (see text). * p < 0.05; ** p < 0.01; *** p < 0.001
Attention deficit hyperactivity disorder
Bipolar I (2 non-users, 0 nondependent users, 0 dependent users), bipolar II (1, 0, 0), and major depressive disorder (9, 8, 6); excludes AAS-induced mood disorders
Panic disorder without agoraphobia (0 non-users, 2 nondependent users, 1 dependent user), panic disorder with agoraphobia (1, 1, 1), agoraphobia without panic disorder (0, 1, 0), social phobia (2, 0, 0), obsessive-compulsive disorder (2, 1, 3), post traumatic stress disorder (3, 1, 1), or generalized anxiety disorder (1, 0, 0)
Anorexia nervosa (no cases), bulimia nervosa (1 non-user, 0 nondependent users, 0 dependent users), and binge-eating disorder (0, 3, 1)
p value by Fisher's exact test, two-tailed
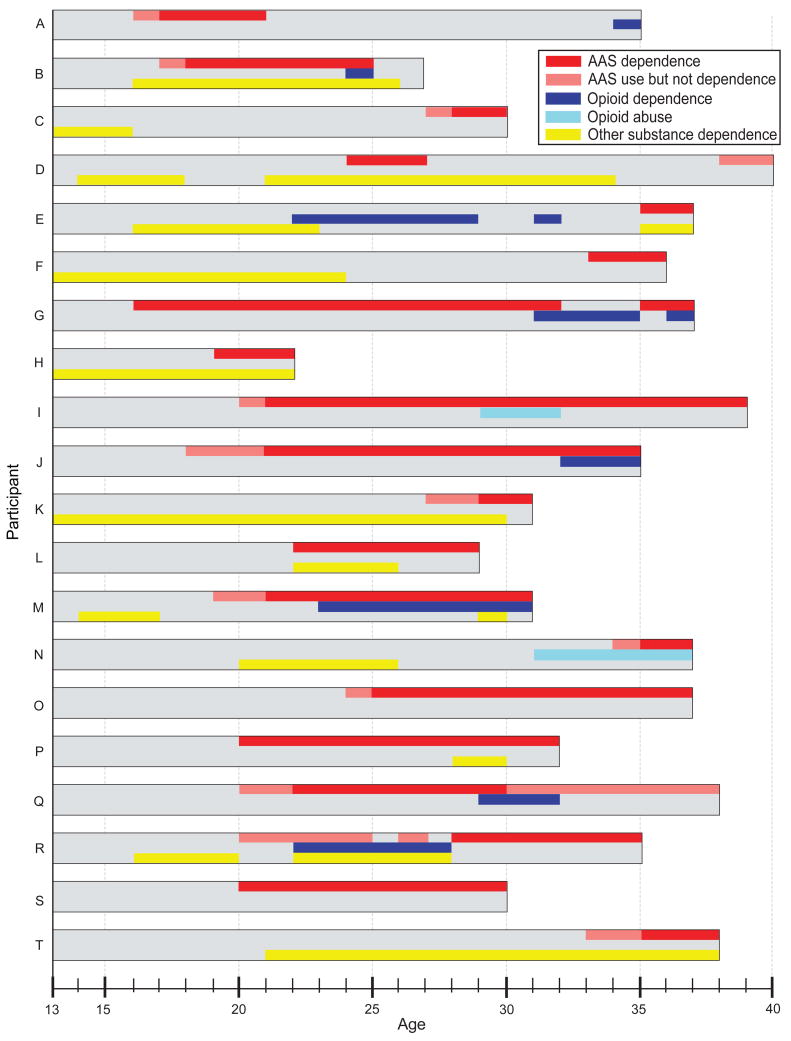
To explore further the nature of the possible relationship between AAS and other forms of substance abuse, we charted the chronology of AAS use and dependence, opioid abuse and dependence, and other substance dependence in the 20 AAS-dependent individuals (Figure 1). As will be seen, the onset of opioid abuse or dependence occurred both before and after the onset of AAS use and AAS dependence in different men; other forms of substance dependence also showed no consistent chronological relationship to AAS dependence. In some cases, the sequence of substance dependence disorders could be understood from the participant's history. For example, participant G developed a herniated intervertebral disk requiring surgery at age 31. He stopped lifting weights and discontinued AAS use, but became addicted to opioids prescribed by his physician, which he supplemented with opioids purchased illicitly. By age 35, he had resumed weightlifting, discontinued opioid use, and redeveloped AAS dependence. When evaluated at age 37, he had redeveloped opioid dependence as well. By contrast, participant R used AAS regularly, but below the threshold of AAS dependence, from age 20-24 and again at age 26. At age 23, he developed opioid and cocaine dependence requiring repeated detoxification admissions, and hence did not lift weights or use AAS regularly. At age 27, he successfully stopped all opioid and cocaine use, but then resumed regular weightlifting and quickly developed AAS dependence, which continued through the time of his evaluation at age 35.
Figure 1.
Lifetime course of substance use in 20 men with a history of anabolic-androgenic steroid dependence.
4.0 Discussion
In the course of an ongoing study of experienced weightlifters, we evaluated 72 men reporting no history of AAS use, 42 men reporting AAS use without evidence of AAS dependence, and 20 reporting a history of AAS dependence. Among all AAS users in our study, the proportion reporting AAS dependence (20 of 62 individuals, or 32%) was very similar to the 30% mean proportion found in the seven field studies cited earlier (Brower et al., 1991; Copeland et al., 1998b; Gridley and Hanrahan, 1994; Malone et al., 1995; Midgley et al., 1999; Perry et al., 2005; Pope and Katz, 1994). In our study, the 42 nondependent AAS users showed no significant differences from the 72 nonusers on any of the demographic, psychiatric, or substance-use variables that we assessed. However, the men with AAS dependence showed significant differences from the other two groups on a range of measures, including particularly a higher lifetime prevalence of childhood conduct disorder, lower levels of educational attainment, and a strikingly higher lifetime prevalence of other substance abuse disorders – especially opioid abuse or dependence. This last observation is consistent with previous reports suggesting a possible association between AAS use and opiate use (Arvary and Pope, 2000; Kanayama et al., 2003a; McBride et al., 1996; Wines et al., 1999), and a case study speculating that these classes of drugs might share similar mechanisms (Tennant et al., 1988). Looking more closely at the course of substance use disorders among the AAS-dependent men, we found no regular temporal sequence to suggest that AAS dependence tended to precede opioid dependence or vice versa – despite our own previously published speculations that AAS might serve as a “gateway” to opioid use (Arvary and Pope, 2000). Instead, the observations seem more suggestive of the hypothesis that these two forms of substance dependence might perhaps share a common diathesis.
As mentioned earlier, a substantial literature of animal studies also suggests that AAS and opioids may affect similar brain mechanisms (Celerier et al., 2003; Johansson et al., 2000a; Johansson et al., 2000b; Johansson et al., 1997; Peters and Wood, 2005; Wood, 2006, 2008). These data combine with our human observations to suggest that AAS dependence, opioid dependence, and perhaps other forms of substance dependence may share common underlying neuropsychological and neurobiological factors. At present, the nature of these factors remains speculative, but recent studies of individuals with classical substance dependence provide some possible leads. For example, substance-dependent individuals exhibit attributes such as increased impulsivity (Clark et al., 2006; Verdejo-Garcia et al., 2007), high levels of delay discounting (Kirby and Petry, 2004; Madden et al., 1999; Petry et al., 1998; Reynolds, 2006), and deficits in risk-taking and decision-making (Barry and Petry, 2008; Bechara and Martin, 2004; Ersche et al., 2006; Ersche et al., 2005; Fishbein et al., 2007; Passetti et al., 2008). Such attributes are also associated with antisocial or psychopathic traits (Petry, 2002; van Honk et al., 2002; Vassileva et al., 2007), including adolescent conduct disorder (Blair et al., 2001; Ernst et al., 2003; Kim et al., 2006) – the latter being a disorder strongly associated with AAS dependence in our present study. Testosterone and presumably other AAS may further shift the balance towards an increased sensitivity for reward and decreased sensitivity for threat or punishment, as suggested by animal (Bing et al., 1998; Boissy and Bouissou, 1994) and human studies (Hermans et al., 2007; van Honk et al., 2004). Thus it is tempting to speculate that this cluster of neuropsychological attributes – deficits in the ability to appreciate and balance future risks and rewards – might distinguish men who develop AAS dependence from men who display nondependent AAS use or men who do not use AAS at all.
It would seem important to explore further the psychological and neuropsychological features of men with AAS dependence, both to better understand the apparent comorbidity of AAS dependence and classical substance dependence, and to better identify individuals at risk for AAS dependence itself. This is particularly important because AAS-dependent individuals likely account for the majority of the public health problems posed by AAS use (Kanayama et al., 2008), whereas nondependent AAS users – at least in the view of some investigators (Cohen et al., 2007; Fost, 2005) – may not frequently experience major adverse effects.
Several limitations of this preliminary study should be considered. First, our sample was limited to male AAS users. However, as noted above, AAS use in women is rare (Gruber and Pope, 2000; Kanayama et al., 2007) and long-term AAS use leading to AAS dependence in women, although documented in 3 published cases (Copeland et al., 1998a, 2000), is likely extremely rare. Second, our sample of AAS-dependent men was small. Small samples, however, generally raise the possibility of a type II error (failing to find differences that actually exist) – whereas our study showed several highly significant differences despite the small sample size. Third, one must consider the possibility of selection bias, such as the possibility that individuals with multiple forms of substance dependence would be more likely to present for interview. However, participants for all three study groups were recruited in an identical manner, using an advertisement that focused on weightlifting and that did not discuss the investigators' interest in AAS or other drug use. Thus, any bias would likely have influenced selection similarly across all groups, mitigating the possibility of differential bias. Of course it could still be suggested that individuals with multiple substance dependence might refer friends with similar pathology to participate in the study, thus inflating apparent associations. However, it is our impression that cases observed in Massachusetts and Florida were similar, even though studied at different geographic locations.
One must also consider the possibility that we might have underestimated, rather than overestimated, levels of concomitant substance abuse and other pathology in the populations under study. First, individuals with active substance use disorders, or with psychiatric conditions such as major depression, might be less likely to visit a gymnasium for regular weightlifting, see our advertisement, and elect to participate in an interview study. Second, it should be recalled that our observations were derived from an ongoing study designed to look retrospectively at risk factors for AAS use, in which participants over age 40 were intentionally excluded to reduce recall bias. When it is considered that dependent AAS users are typically older than nondependent AAS users or nonusers (see Table 1), it follows that exclusion of individuals over age 40 might bias the sample against cases of AAS dependence, and might especially bias the sample against individuals with more prolonged and possibly more advanced cases.
In summary, our observations augment the growing evidence that AAS dependence is a valid entity, affecting a substantial portion of AAS users. Men with AAS dependence, unlike nondependent AAS users or AAS non-users, appear to show a distinctive pattern of comorbid psychopathology, overlapping with that of individuals with other forms of substance dependence, especially opioid dependence. These observations suggest that individuals with AAS dependence and individuals with classical substance dependence may perhaps share important underlying biological and neuropsychological vulnerabilities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GM, Packard MG, Hines M. Testosterone has rewarding affective properties in male rats: implications for the biological basis of sexual motivation. Behav Neurosci. 1994;108:424–428. doi: 10.1037//0735-7044.108.2.424. [DOI] [PubMed] [Google Scholar]
- Arnedo MT, Salvador A, Martinez-Sanchis S, Gonzalez-Bono E. Rewarding properties of testosterone in intact male mice: a pilot study. Pharmacol Biochem Behav. 2000;65:327–332. doi: 10.1016/s0091-3057(99)00189-6. [DOI] [PubMed] [Google Scholar]
- Arvary D, Pope HG., Jr Anabolic-androgenic steroids as a gateway to opioid dependence. N Engl J Med. 2000;342:1532. doi: 10.1056/NEJM200005183422018. [DOI] [PubMed] [Google Scholar]
- Barry D, Petry NM. Predictors of decision-making on the Iowa Gambling Task: independent effects of lifetime history of substance use disorders and performance on the Trail Making Test. Brain Cogn. 2008;66:243–252. doi: 10.1016/j.bandc.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychol. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bing O, Heilig M, Kakoulidis P, Sundblad C, Wiklund L, Eriksson E. High doses of testosterone increase anticonflict behaviour in rat. Eur Neuropsychopharmacol. 1998;8:321–323. doi: 10.1016/s0924-977x(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Mitchell DG. Somatic markers and response reversal: is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? J Abnorm Child Psychol. 2001;29:499–511. doi: 10.1023/a:1012277125119. [DOI] [PubMed] [Google Scholar]
- Boissy A, Bouissou MF. Effects of androgen treatment on behavioral and physiological responses of heifers to fear-eliciting situations. Horm Behav. 1994;28:66–83. doi: 10.1006/hbeh.1994.1006. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroid abuse and dependence. Current Psychiatry Reports. 2002;4:377–387. doi: 10.1007/s11920-002-0086-6. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Blow FC, Beresford TP, Fuelling C. Anabolic-androgenic steroid dependence. J Clin Psychiatry. 1989;50:31–33. [PubMed] [Google Scholar]
- Brower KJ, Blow FC, Young JP, Hill EM. Symptoms and correlates of anabolic-androgenic steroid dependence. Br J Addiction. 1991;86:759–768. doi: 10.1111/j.1360-0443.1991.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Eliopulos GA, Blow FC, Catlin DH, Beresford TP. Evidence for physical and psychological dependence on anabolic androgenic steroids in eight weight lifters. Am J Psychiatry. 1990;147:510–512. doi: 10.1176/ajp.147.4.510. [DOI] [PubMed] [Google Scholar]
- Brown-Séquard C. Des effets produits chez l'homme par des injections souscutanées d'un liquide retiré des testicules frais de cobaye et de chien. C R Séance Soc Biol. 1889:415–422. 429–431. [Google Scholar]
- Buckley WE, Yesalis CE, 3rd, Friedl KE, Anderson WA, Streit AL, Wright JE. Estimated prevalence of anabolic steroid use among male high school seniors. JAMA. 1988;260:3441–3445. [PubMed] [Google Scholar]
- Cafri G, Thompson JK, Ricciardelli L, McCabe M, Smolak L, Yesalis C. Pursuit of the muscular ideal: Physical and psychological consequences and putative risk factors. Clin Psychol Rev. 2005;25:215–239. doi: 10.1016/j.cpr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Celerier E, Yazdi MT, Castane A, Ghozland S, Nyberg F, Maldonado R. Effects of nandrolone on acute morphine responses, tolerance and dependence in mice. Eur J Pharmacol. 2003;465:69–81. doi: 10.1016/s0014-2999(03)01462-6. [DOI] [PubMed] [Google Scholar]
- Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006;60:515–522. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Cohen J, Collins R, Darkes J, Gwartney D. A league of their own: demographics, motivations and patterns of use of 1,955 male adult non-medical anabolic steroid users in the United States. J Int Soc Sports Nutr. 2007;4:12. doi: 10.1186/1550-2783-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Peters R, Dillon P. Anabolic-androgenic steroid dependence in a woman. Aust N Z J Psychiatry. 1998a;32:589. [PubMed] [Google Scholar]
- Copeland J, Peters R, Dillon P. A study of 100 anabolic-androgenic steroid users. The Med J Aust. 1998b;168:311–312. [PubMed] [Google Scholar]
- Copeland J, Peters R, Dillon P. Anabolic-androgenic steroid use disorders among a sample of Australian competitive and recreational users. Drug Alcohol Depend. 2000;60:91–96. doi: 10.1016/s0376-8716(99)00141-6. [DOI] [PubMed] [Google Scholar]
- Daly RC, Su TP, Schmidt PJ, Pickar D, Murphy DL, Rubinow DR. Cerebrospinal fluid and behavioral changes after methyltestosterone administration: preliminary findings. Arch Gen Psychiatry. 2001;58:172–177. doi: 10.1001/archpsyc.58.2.172. [DOI] [PubMed] [Google Scholar]
- Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am J Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology. 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders -- Patient Edition. Biometrics Research Department. New york state psychiatric institute; New york: 2001. [Google Scholar]
- Fishbein DH, Krupitsky E, Flannery BA, Langevin DJ, Bobashev G, Verbitskaya E, Augustine CB, Bolla KI, Zvartau E, Schech B, Egorova V, Bushara N, Tsoy M. Neurocognitive characterizations of Russian heroin addicts without a significant history of other drug use. Drug Alcohol Depend. 2007;90:25–38. doi: 10.1016/j.drugalcdep.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fost N. Steroid hysteria: Unpacking the claims. Virtual Mentor 7. [January 2002];2005 :2008. doi: 10.1001/virtualmentor.2005.7.11.oped2-0511. http://virtualmentor.ama-assn.org/2005/2011/oped2002-0511.html; [DOI] [PubMed]
- Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5alpha-reduced metabolite 3alpha-androstanediol. Pharmacol Biochem Behav. 2007;86:354–367. doi: 10.1016/j.pbb.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galduroz JC, Noto AR, Nappo SA, Carlini EA. Household survey on drug abuse in Brazil: study involving the 107 major cities of the country--2001. Addict Behav. 2005;30:545–556. doi: 10.1016/j.addbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Gridley DW, Hanrahan SJ. Anabolic-androgenic steroid use among male gymnasium participants: knowledge and motives. Sports Health. 1994;12:11–14. [Google Scholar]
- Gruber AJ, Pope HG., Jr Psychiatric and medical effects of anabolic-androgenic steroid use in women. Psychother Psychosom. 2000;69:19–26. doi: 10.1159/000012362. [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Gupta L. Prevalence and risk factors for anabolic-androgenic steroid abuse in Australian high school students. Int J Androl. 1997;20:159–164. doi: 10.1046/j.1365-2605.1997.d01-285.x. [DOI] [PubMed] [Google Scholar]
- Harlan RE, Brown HE, Lynch CS, D'Souza D, Garcia MM. Androgenic-anabolic steroids blunt morphine-induced c-fos expression in the rat striatum: possible role of beta-endorphin. Brain Res. 2000;853:99–104. doi: 10.1016/s0006-8993(99)02257-x. [DOI] [PubMed] [Google Scholar]
- Hartgens F, Kuipers H. Sports Med. Vol. 34. Auckland, NZ: 2004. Effects of androgenic-anabolic steroids in athletes; pp. 513–554. [DOI] [PubMed] [Google Scholar]
- Hays LR, Littleton S, Stillner V. Anabolic steroid dependence. Am J Psychiatry. 1990;147:122. doi: 10.1176/ajp.147.1.122a. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Gecks NM, Kenemans JL, van Honk J. Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology. 2007;32:1052–1061. doi: 10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Schlundt D, Langenbucher J, Chung T. Presence of muscle dysmorphia symptomology among male weightlifters. Compr Psychiatry. 2006;47:127–135. doi: 10.1016/j.comppsych.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr Rev. 2003;24:523–538. doi: 10.1210/er.2001-0014. [DOI] [PubMed] [Google Scholar]
- Johansson P, Hallberg M, Kindlundh A, Nyberg F. The effect on opioid peptides in the rat brain, after chronic treatment with the anabolic androgenic steroid, nandrolone decanoate. Brain Res Bull. 2000a;51:413–418. doi: 10.1016/s0361-9230(99)00263-4. [DOI] [PubMed] [Google Scholar]
- Johansson P, Lindqvist A, Nyberg F, Fahlke C. Anabolic androgenic steroids affects alcohol intake, defensive behaviors and brain opioid peptides in the rat. Pharmacol Biochem Behav. 2000b;67:271–279. doi: 10.1016/s0091-3057(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Johansson P, Ray A, Zhou Q, Huang W, Karlsson K, Nyberg F. Anabolic androgenic steroids increase beta-endorphin levels in the ventral tegmental area in the male rat brain. Neuroscience Res. 1997;27:185–189. doi: 10.1016/s0168-0102(96)01141-8. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2005 Volume II: College students and adults ages 19-45 (NIH Publication No 06-5884) National Institute on Drug Abuse; Bethesda, MD: 2006. [Google Scholar]
- Kanayama G, Barry S, Hudson JI, Pope HG., Jr Body image and attitudes toward male roles in anabolic-androgenic steroid users. Am J Psychiatry. 2006;163:697–703. doi: 10.1176/ajp.2006.163.4.697. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Boynes M, Hudson JI, Field AE, Pope HG., Jr Anabolic steroid abuse among teenage girls: An illusory problem? Drug Alcohol Depend. 2007;88:156–162. doi: 10.1016/j.drugalcdep.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG. Issues for DSM-V: Clarifying the diagnostic criteria for anabolic-androgenic steroid dependence. Am J Psychiatry. 2009 doi: 10.1176/appi.ajp.2009.08111699. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Cohane GH, Weiss RD, Pope HG. Past anabolic-androgenic steroid use among men admitted for substance abuse treatment: an underrecognized problem? J Clin Psychiatry. 2003a;64:156–160. doi: 10.4088/jcp.v64n0208. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: A looming public health concern? Drug Alcohol Depend. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Pope HG, Cohane G, Hudson JI. Risk factors for anabolic-androgenic steroid use among weightlifters: a case-control study. Drug Alcohol Depend. 2003b;71:77–86. doi: 10.1016/s0376-8716(03)00069-3. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Pope HG, Jr, Hudson JI. “Body image” drugs: a growing psychosomatic problem. Psychother Psychosom. 2001;70:61–65. doi: 10.1159/000056228. [DOI] [PubMed] [Google Scholar]
- Kim YT, Lee SJ, Kim SH. Effects of the history of conduct disorder on the Iowa Gambling Tasks. Alcohol Clin Exp Res. 2006;30:466–472. doi: 10.1111/j.1530-0277.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Kindlundh AM, Rahman S, Lindblom J, Nyberg F. Increased dopamine transporter density in the male rat brain following chronic nandrolone decanoate administration. Neurosci Lett. 2004;356:131–134. doi: 10.1016/j.neulet.2003.11.040. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Addiction. Vol. 99. Abingdon, England: 2004. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls; pp. 461–471. [DOI] [PubMed] [Google Scholar]
- Klotz F, Garle M, Granath F, Thiblin I. Criminality among individuals testing positive for the presence of anabolic androgenic steroids. Arch Gen Psychiatry. 2006;63:1274–1279. doi: 10.1001/archpsyc.63.11.1274. [DOI] [PubMed] [Google Scholar]
- Kokkevi A, Fotiou A, Chileva A, Nociar A, Miller P. Daily exercise and anabolic steroids use in adolescents: a cross-national European study. Subst Use Misuse. 2008;43:2053–2065. doi: 10.1080/10826080802279342. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Pope HG, Jr, Katz DL, Oliva P. Fat-free mass index in users and nonusers of anabolic-androgenic steroids. Clin J Sport Med. 1995;5:223–228. doi: 10.1097/00042752-199510000-00003. [DOI] [PubMed] [Google Scholar]
- Lindqvist AS, Johansson-Steensland P, Nyberg F, Fahlke C. Anabolic androgenic steroid affects competitive behaviour, behavioural response to ethanol and brain serotonin levels. Behav Brain Res. 2002;133:21–29. doi: 10.1016/s0166-4328(01)00408-9. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK, Jacobs EA. Discounting of delayed rewards in opioid-dependent outpatients: exponential or hyperbolic discounting functions? Exp Clin Psychopharmacol. 1999;7:284–293. doi: 10.1037//1064-1297.7.3.284. [DOI] [PubMed] [Google Scholar]
- Malone DA, Jr, Dimeff RJ, Lombardo JA, Sample RH. Psychiatric effects and psychoactive substance use in anabolic-androgenic steroid users. Clin J Sport Med. 1995;5:25–31. doi: 10.1097/00042752-199501000-00005. [DOI] [PubMed] [Google Scholar]
- Matzner F. Videotapes as training tools for the development of the KID-SCID. Scientific proceedings, American Academy of Childhood Adolescent Psychiatry, 42nd Annual Meeting.1994. [Google Scholar]
- Matzner F, Silva R, Silvan M, Chowdhury M, Nastasi L. Preliminary test-retest reliability of the KID-SCID. Scientific proceedings, American Psychiatric Association Annual Meeting.1997. [Google Scholar]
- McBride AJ, Williamson K, Petersen T. Three cases of nalbuphine hydrochloride dependence associated with anabolic steroid use. Br J Sports Med. 1996;30:69–70. doi: 10.1136/bjsm.30.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Brower KJ, West BT, Nelson TF, Wechsler H. Trends in non-medical use of anabolic steroids by U.S. college students: results from four national surveys. Drug Alcohol Depend. 2007;90:243–251. doi: 10.1016/j.drugalcdep.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia P, Pipe A, Greenberg L. The use of anabolic-androgenic steroids by Canadian students. Clin J Sport Med. 1996;6:9–14. doi: 10.1097/00042752-199601000-00004. [DOI] [PubMed] [Google Scholar]
- Midgley SJ, Heather N, Davies JB. Dependence-producing potential of anabolic-androgenic steroids. Addiction Res. 1999;7:539–550. [Google Scholar]
- Nilsson S, Baigi A, Marklund B, Fridlund B. The prevalence of the use of androgenic anabolic steroids by adolescents in a county of Sweden. Eur J Public Health. 2001;11:195–197. doi: 10.1093/eurpub/11.2.195. [DOI] [PubMed] [Google Scholar]
- Olivardia R, Pope HG, Jr, Hudson JI. Muscle dysmorphia in male weightlifters: a case-control study. Am J Psychiatry. 2000;157:1291–1296. doi: 10.1176/appi.ajp.157.8.1291. [DOI] [PubMed] [Google Scholar]
- Pagonis TA, Angelopoulos NV, Koukoulis GN, Hadjichristodoulou CS. Psychiatric side effects induced by supraphysiological doses of combinations of anabolic steroids correlate to the severity of abuse. Eur Psychiatry. 2006;21:551–562. doi: 10.1016/j.eurpsy.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Pallesen S, Josendal O, Johnsen BH, Larsen S, Molde H. Anabolic steroid use in high school students. Subst Use Misuse. 2006;41:1705–1717. doi: 10.1080/10826080601006367. [DOI] [PubMed] [Google Scholar]
- Parkinson AB, Evans NA. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc. 2006;38:644–651. doi: 10.1249/01.mss.0000210194.56834.5d. [DOI] [PubMed] [Google Scholar]
- Passetti F, Clark L, Mehta MA, Joyce E, King M. Neuropsychological predictors of clinical outcome in opiate addiction. Drug Alcohol Depend. 2008;94:82–91. doi: 10.1016/j.drugalcdep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Kutscher EC, Lund BC, Yates WR, Holman TL, Demers L. Measures of aggression and mood changes in male weightlifters with and without androgenic anabolic steroid use. J Forensic Sci. 2003;48:646–651. [PubMed] [Google Scholar]
- Perry PJ, Lund BC, Deninger MJ, Kutscher EC, Schneider J. Anabolic steroid use in weightlifters and bodybuilders: an internet survey of drug utilization. Clin J Sport Med. 2005;15:326–330. doi: 10.1097/01.jsm.0000180872.22426.bb. [DOI] [PubMed] [Google Scholar]
- Peters KD, Wood RI. Androgen dependence in hamsters: overdose, tolerance, and potential opioidergic mechanisms. Neuroscience. 2005;130:971–981. doi: 10.1016/j.neuroscience.2004.09.063. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of delayed rewards in substance abusers: relationship to antisocial personality disorder. Psychopharmacology. 2002;162:425–432. doi: 10.1007/s00213-002-1115-1. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Addiction. Vol. 93. Abingdon, England: 1998. Shortened time horizons and insensitivity to future consequences in heroin addicts; pp. 729–738. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacol Bull. 1997;33:17–22. [PubMed] [Google Scholar]
- Phillips KA, McElroy SL, Keck PE, Jr, Hudson JI, Pope HG., Jr A comparison of delusional and nondelusional body dysmorphic disorder in 100 cases. Psychopharmacol Bull. 1994;30:179–186. [PubMed] [Google Scholar]
- Phillips KA, McElroy SL, Keck PE, Jr, Pope HG, Jr, Hudson JI. Body dysmorphic disorder: 30 cases of imagined ugliness. Am J Psychiatry. 1993;150:302–308. doi: 10.1176/ajp.150.2.302. [DOI] [PubMed] [Google Scholar]
- Pope HG, Brower KJ. Anabolic-Androgenic Steroid-Related Disorders. In: Sadock B, Sadock V, editors. Comprehensive Textbook of Psychiatry. Ninth. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. pp. 1419–1431. [Google Scholar]
- Pope HG, Jr, Katz DL. Homicide and near-homicide by anabolic steroid users. J Clin Psychiatry. 1990;51:28–31. [PubMed] [Google Scholar]
- Pope HG, Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiatry. 1994;51:375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- Pope HG, Kanayama G, Ionescu-Pioggia M, Hudson JI. Addiction. Vol. 99. Abingdon, England: 2004. Anabolic steroid users' attitudes towards physicians; pp. 1189–1194. [DOI] [PubMed] [Google Scholar]
- Rachon D, Pokrywka L, Suchecka-Rachon K. Prevalence and risk factors of anabolic-androgenic steroids (AAS) abuse among adolescents and young adults in Poland. Sozial-und Praventivmedizin. 2006;51:392–398. doi: 10.1007/s00038-006-6018-1. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacology. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Tennant F, Black DL, Voy RO. Anabolic steroid dependence with opioid-type features. N Engl J Med. 1988;319:578. doi: 10.1056/NEJM198809013190910. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Parlklo T. Anabolic androgenic steroids and violence. Acta Psychiatr Scand. 2002;(Suppl):125–128. doi: 10.1034/j.1600-0447.106.s412.27.x. [DOI] [PubMed] [Google Scholar]
- van Honk J, Hermans EJ, Putman P, Montagne B, Schutter DJ. Defective somatic markers in sub-clinical psychopathy. Neuroreport. 2002;13:1025–1027. doi: 10.1097/00001756-200206120-00009. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Hermans EJ, Putman P, Tuiten A, Koppeschaar H. Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinology. 2004;29:937–943. doi: 10.1016/j.psyneuen.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Vassileva J, Petkova P, Georgiev S, Martin EM, Tersiyski R, Raycheva M, Velinov V, Marinov P. Impaired decision-making in psychopathic heroin addicts. Drug Alcohol Depend. 2007;86:287–289. doi: 10.1016/j.drugalcdep.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addictive Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Wanjek B, Rosendahl J, Strauss B, Gabriel HH. Doping, drugs and drug abuse among adolescents in the State of Thuringia (Germany): prevalence, knowledge and attitudes. Int J Sports Med. 2007;28:346–353. doi: 10.1055/s-2006-924353. [DOI] [PubMed] [Google Scholar]
- Wines JD, Jr, Gruber AJ, Pope HG, Jr, Lukas SE. Nalbuphine hydrochloride dependence in anabolic steroid users. Am J Addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 1999;8:161–164. doi: 10.1080/105504999305965. [DOI] [PubMed] [Google Scholar]
- Wood RI. Anabolic steroids: a fatal attraction? J Neuroendocrinol. 2006;18:227–228. doi: 10.1111/j.1365-2826.2006.01407.x. [DOI] [PubMed] [Google Scholar]
- Wood RI. Anabolic-androgenic steroid dependence? Insights from animals and humans. Front Neuroendocrinol. 2008;29:490–506. doi: 10.1016/j.yfrne.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesalis C, Kennedy N, Kopstein A, MS B. Anabolic-androgenic steroid use in the United States. JAMA. 1993;270:1217–1221. [PubMed] [Google Scholar]