Abstract
Opiates, such as morphine, decrease neurogenesis in the postnatal hippocampal subgranular zone (SGZ) by inhibiting progenitor proliferation and maturation. However, it is not known how morphine influences the growth factors and vasculature that encompass the neurogenic SGZ microenvironment. We examined morphine’s effect on pro- and anti-proliferative factors in the dentate gyrus (DG; Experiment 1) as well as the DG neurovasculature itself (Experiment 2). For Experiment 1, mice were implanted with sham or morphine pellets subcutaneously (s.c.; 0 and 48 hrs) and were decapitated 24 or 96 hrs later. One brain hemisphere was post fixed to examine proliferation by immunohistochemistry, and a DG-enriched sample was dissected from the other hemisphere to examine the neurogenic microenvironment via immunoblotting for known pro- and anti-proliferative factors. Consistent with previous results, morphine decreased the number of proliferating cells in the SGZ, as the number of Ki67-immunoreactive (IR) cells was decreased at 96 hrs. Morphine did not alter DG levels of the pro-proliferative factor BDNF, anti-proliferative factor IL1β, or their receptors TrkB and IL1R1 at either time point. However, morphine increased the pro-proliferative factor VEGF at 96 hrs. Given that VEGF is also a potent angiogenic factor, Experiment 2 examined whether the morphine-induced increase in VEGF correlated with altered DG neurovasculature. Mice were implanted with morphine pellets as in Experiment 1, and two hrs before perfusion (24 or 96 hrs) were administered bromodeoxyuridine intraperitoneally (BrdU, i.p.; 150 mg/kg). Tissue was co-stained for BrdU and the endothelial cell marker endoglin to enable examination of DG vessels and proximity of BrdU-IR cells to endoglin-IR vessels. At 96 hrs, endoglin-IR vessel area and perimeter were increased, but proximity of BrdU-IR cells to endoglin-IR vessels remained unchanged. These data suggest that following chronic morphine exposure, factors within the neurogenic microenvironment are maintained or upregulated to compensate for decreased SGZ proliferation.
Keywords: morphine, proliferation, VEGF, BDNF, neurovasculature, neurogenic niche
Introduction
Neurogenesis occurs in two primary brain areas in the postnatal mammalian brain: the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and the subventricular zone (SVZ) (Altman and Das, 1965; Ming and Song, 2005; Monje et al., 2002). Progenitor cells in both the SGZ and SVZ reside in neurogenic niches rich in vasculature and key factors, such as vascular endothelial growth factor (VEGF), interleukin-1β (IL1β), and brain-derived neurotrophic factor (BDNF) (Riquelme et al., 2008), and often proliferate in close proximity to vasculature elements (Heine et al., 2005; Mercier et al., 2002; Palmer et al., 2000). Many studies show a correlative relationship between manipulation of factors in the neurogenic niche and hippocampal neurogenesis. For example, exogenous application of growth factors and cytokines alters SGZ proliferation (Jin et al., 2002; Koo and Duman, 2008; Scharfman et al., 2005) and even influences hippocampal functions such as learning and memory (Cao et al., 2004; Mustafa et al., 2008; Warner-Schmidt et al., 2008). However, fewer studies have examined whether stimuli known to impact adult SGZ neurogenesis lead to alterations in hippocampal levels of factors such as BDNF, IL1β, or VEGF or changes in the vasculature.
Chronic opiate exposure negatively impacts SGZ proliferation and thus can be useful to elucidate the relationship between the neurogenic niche and decreased SGZ neurogenesis. Opiates decrease SGZ proliferation by inhibition of progenitor proliferation, maturation (Arguello et al., 2008; Eisch et al., 2000; Kahn et al., 2005) and alteration of the progenitor cell cycle (Arguello et al., 2008; Mandyam et al., 2004).
Notably, cognitive defects are evident in both laboratory animals chronically exposed to opiates (Spain and Newsom, 1991) as well as heroin abusers (Guerra et al., 1987). Heroin abusers also have altered levels of circulating growth factors (Angelucci et al., 2007) suggesting the possibility that neurotrophic factors may mediate the morphine-induced cognitive deficit in both humans and rodents (Spain and Newsom, 1991). However morphine’s influence on the neurogenic niche in vivo, including levels of BDNF, IL1β, VEGF, and vasculature elements, has not been examined. Therefore, the present study addressed whether chronic morphine altered the neurogenic microenvironment of the DG SGZ. We hypothesized that chronic morphine would decrease the pro-proliferative factors VEGF and BDNF but increase the anti-proliferative factor IL1β, correlating with decreased SGZ proliferation.
To gain insight into whether the chronic morphine-induced decrease in SGZ proliferation correlates with changes in factors in the neurogenic niche, a subcutaneous (s.c.) morphine pellet administration paradigm was utilized where C57BL/6J mice were exposed to morphine for 24 or 96 hrs (Arguello et al., 2008; Fischer et al., 2008). To correlate changes in proliferation with changes in the microenvironment, mice were killed via decapitation, one hemisphere was post fixed to observe changes in proliferation via immunohistochemistry (IHC), and the DG/CA1 region was dissected from the other hemisphere to observe changes in the neurogenic niche by immunoblotting.
Materials and Methods
Animals
For all experiments, C57BL/6J mice from Jackson Laboratories (stock #000664) were used. Mice were housed four to a cage with free access to food and water in a UT Southwestern Medical Center facility on a 12 hr light/dark cycle. Mice were allowed to habituate for one week prior to start of experiments. All experimental procedures were approved by The Institutional Animal Care and Use Committee and were in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drug treatment and Bromodeoxyuridine (BrdU) injections
For Experiment 1, mice were implanted with one 25 mg morphine pellet (generously provided by the National Institute on Drug Abuse, Bethesda, MD) or sham pellet s.c. under isoflurane anesthesia (2% in compressed oxygen for 1–2 minutes) at 0 and 48 hrs and were killed via decapitation after 24 or 96 hrs of morphine exposure. One hemisphere of the brain was post fixed in 4% paraformaldehyde in 0.1M phosphate buffered saline (PBS; pH 7.4) for three days, followed by cryoprotection in 30% sucrose as previously described (Lagace et al., 2007). The other hemisphere was regionally dissected (DG/CA1 and CA3) and snap frozen for future immunoblotting use. For Experiment 2, mice were also implanted with s.c. morphine or sham pellets (0 and 48 hrs). Two hours before perfusion (at 24 or 96 hrs) mice were given a single 150 mg/kg injection of BrdU intraperitoneally (i.p., Roche Diagnostics, dissolved in 0.9% saline, 0.007N NaOH at 10 mg/mL) and anesthetized with chloral hydrate (120 mg/kg dissolved in 0.9% saline). Mice were then transcardially exsanguinated with 0.1M PBS followed by perfusion of the fixative (4% paraformaldehyde in 0.1M PBS, pH 7.4), each provided in a 7 ml/min flow rate as previously described (Harburg et al., 2007).
Tissue sectioning
Serial sets of 30μm coronal sections from brains were collected on a freezing microtome (Leica, SM2000R). Every ninth section through the entire mouse hippocampus was slide mounted prior to IHC (−0.82 to −4.24 from bregma) (Franklin and Paxinos, 1997). Slides were coded before IHC and the code was not broken until data analysis was complete (Mandyam et al., 2004).
IHC
Antibodies
The following antibodies were used for IHC: rat anti-BrdU (Accurate, Westbury, NY, Cat #: OBT0030, Lot #: H8365, 1:300); rabbit anti-Ki67 (SP6, LabVision Corporation NeoMarkers, Fremont, CA, Cat#: RM-9128-R1, Lot#: 9106S603C, 1:400); rat anti-mouse endoglin (MJ7/18, Developmental Studies Hybridoma Bank, University of Iowa, Illinois, 1:500). For each antibody, the specificity of staining was determined by lack of signal after omission and/or dilution of the primary antibody, as previously reported: endoglin (Ge and Butcher, 1994; Korpanty et al., 2007) present study, BrdU (Mandyam et al., 2004), Ki67 (Dayer et al., 2003; Mandyam et al., 2007).
Single label IHC
Tissue from Experiment 1 was used for Ki67 single label IHC. The following pretreatment steps were used: antigen unmasking (0.01M citric acid, pH 6.0, 100°C, 10 min) and quenching of endogenous peroxidases (0.3% H2O2, 30 min). Nonspecific binding was blocked (3% serum, 0.3% Triton-X, 30–60 min) followed by primary antibody incubation overnight (3% serum, 0.3% Tween-20). Sections were then incubated with biotin-tagged secondary antibody (1:200, 60 min) and IHC was completed with the avidin-biotin/CY3 tyramide signal amplification method (ABC Elite, Vector Laboratories, Burlingame, CA and CY3-TSA, Perkin Elmer Life Sciences, Inc., Boston, MA, Cat#: SAT704A001EA, 1:50) as previously described (Lagace et al., 2007) followed by counterstain with DAPI and quenching of autofluorescence with 1X cupric sulfate (Fischer et al., 2008).
Double label IHC
Tissue from Experiment 2 was used for endoglin and BrdU double label IHC. Staining for endoglin was completed followed by staining for BrdU. Endogenous peroxidase quenching and blocking steps were performed for endoglin followed by incubation with endoglin primary antibody overnight. IHC for endoglin was completed via avidin-biotin/CY3 tyramide signal amplification as for Ki67. Sections then underwent the following pretreatment steps for BrdU: antigen unmasking, membrane permeabilization (trypsin in 0.1M Tris, 0.1% CaCl2, 10 min) and DNA denaturation (2M HCl in 1X PBS, 30 min), as previously described (Donovan et al., 2006). Sections were then incubated in blocking serum followed by BrdU primary antibody incubation overnight. BrdU was visualized with a CY2-conjugated fluorophore (1:200, 4 hrs) and counterstained as described above.
Immunoblotting
Snap frozen DG/CA1 regionally dissected tissues from Experiment 1 were lysed with 50 mM NaF, 1% SDS buffer (for BDNF, TrkB, IL1β, IL1R1) or 50mM Tris, 50 mM NaF, 32 mM sucrose, 1% SDS (with 1X protease inhibitor cocktail (Roche Diagnostics, Cat# 11697498001) 1X phosphatase inhibitor 1 and 1X phosphatase inhibitor 2 (Sigma, Cat# P2850 and P5726, respectively)). Samples were sonicated for 10 seconds with 100°C lysis buffer, followed by 10 minutes of boiling. Following lysis, protein concentration was determined via BCA Protein Assay Kit (Pierce, Rockford, IL, Cat#: 23225). Forty μg of protein was loaded on a 4–20% gradient gel. Protein was transferred to nitrocellulose membranes for 1 hour at 100 V. Membranes were blocked in 5% milk for one hour, followed by primary antibody overnight at 4°C. The following primary antibodies from Santa Cruz Biotechnology (Santa Cruz, CA) were used: rabbit anti-BDNF(N-20, Cat#: sc-546, Lot#: C1804, 1:500); rabbit anti-IL1β (H-153, Cat#: sc-7884, Lot#: C1904, 1:250); rabbit anti- IL1R1(M-20, Cat#: sc-680, Lot#: E102, 1:1000). Additional antibodies used were: rabbit anti-TrkB (Millipore, Billerica, MA 01821, Cat#: 07–225, Lot#: 28846, 1:5000); rabbit anti-VEGF (SP28, LabVision Corporation NeoMarkers, Fremont, CA, Cat#: RM-9128-R1, Lot#: 9128R609E, 1:300); rabbit anti-VEGFR2 (T014, (Feng et al., 2000), 1:300); mouse anti-GAPDH (Research Diagnostic Inc., Flander, NJ, 07836, Cat#: RDI-TRK5G4-6C5, 1:200,000). Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (1:10,000 for BDNF, IL1β, IL1R1, TrkB, VEGF, VEGFR2; 1:100K for GAPDH) and developed with ECL Plus Detection Reagent (GE Healthcare, Buckinghamshire, UK). Membranes were washed with stripping buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 100 mM βME in 1X Tris-Buffered Saline Tween-20) before being reprobed with GAPDH. Proteins of interest were normalized to GAPDH and bands and were quantified with NIH Image J 1.63. Data are expressed as percent of sham (mean ± SEM).
Microscopic analysis and quantification
Ki67-IR cell quantification
Ki67-IR cell number was quantified at 400X magnification on an Olympus BX-51 microscope (Tokyo, Japan). Cell numbers were quantified with modified stereology via the optical fractionator method throughout the rostral-caudal axis of the hippocampus (−0.82 to −4.24 from bregma) (Franklin and Paxinos, 1997). Cell counts were collected from the SGZ (defined as the region bordering the granule cell layer and hilus: three cell widths into the hilus and half of the granule cell layer), with continuous adjustment of the focal plane, as described previously (Arguello et al., 2008; Donovan et al., 2006).
Vessel and BrdU proximity analysis
Tissue from Experiment 2 was used for vessel analysis. We used a modified protocol to quantify endoglin staining: number of vessels, vessel area, and vessel perimeter (Heine et al., 2005). Sections of the DG were examined on a Nikon Eclipse E600 microscope at 100× (Nikon, Lewisville, TX) and images were captured with a Photometric Coolsnap HQ camera (Nikon) and imported into Metamorph software (Universal Imagining Corporation) for analysis. Photos of DG sections (−1.3 to −3.1μm from bregma) were captured for each stain (endoglin or BrdU) under identical conditions (exposure time, high and low limits, and scaling), and background levels were thresholded from control sections stained with secondary antibody alone. Integrated optical density and optical intensity were measured using MetaMorph’s Integrated Morphometry Analysis for number of endoglin-IR vessels, vessel area and vessel perimeter, as previously described (Arnold et al., 2008). Additionally, proximity of BrdU-IR cells to endoglin-IR vessels was categorized by a blinded observer. Similar to previous studies (Heine et al., 2005), BrdU-IR cells that were vascular-associated were categorized as: “touch” if touching or overlapping a vessel. Non vascular-associated BrdU-IR cells were further distinguished as either “near”, within 10 μm of vessel, or “far”, beyond 10 μm of vessel (Heine et al., 2005).
Statistical analyses
Data are reported as mean ± SEM. Statistical analyses were performed using student’s t-test for comparison of two groups. All statistical analyses were performed using Prism (version 5.0) software. Statistical significance was defined as p≤ 0.05 with *p≤ 0.05, **p<0.01.
Results
Chronic morphine inhibits the total population of proliferating cells in the SGZ
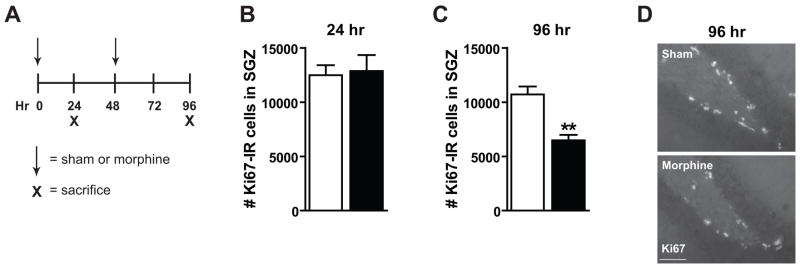
Previous work has determined that chronic morphine decreases SGZ progenitor cell proliferation (Fischer et al., 2008). To correlate changes in the neurogenic SGZ microenvironment with a change in proliferation, C57BL/6J mice were implanted with morphine or sham pellets s.c. at 0 and 48 hrs and were killed at 24 or 96 hrs (Figure 1A). Progenitor cell proliferation was assessed with the endogenous cell cycle marker Ki67 in the SGZ as previously described (Arguello et al., 2008). The number of Ki67-IR cells, or total population of proliferating cells, was not decreased after 24 hrs of morphine exposure (Figure 1B) as previously shown (Fischer et al., 2008). However, after 96 hrs of morphine exposure, the number of Ki67-IR cells was significantly decreased from sham mice (Figure 1C; t6=4.74, p=0.0032). A representative IHC image depicts this decrease qualitatively (Figure 1D). This confirms earlier work that the total population of proliferating cells is decreased after 96 hrs but not 24 hrs of morphine exposure.
Figure 1. Chronic morphine inhibits the total population of SGZ proliferating cells.
(A) C57BL/6J mice were implanted with s.c. sham or morphine pellets at 0 and 48 hrs and killed at 24 or 96 hrs via decapitation. (B) The number of Ki67-IR cells in the SGZ was not changed after 24 hrs. (C) The number of Ki67-IR cells was significantly decreased after 96 hrs of morphine exposure. (D) Representative IHC demonstrated decreased numbers of Ki67-IR cells in the SGZ. At all time points sham: n=4, morphine: n=4. Scale bar=100 μm, **p<0.01.
Chronic morphine does not alter certain pro- or anti-proliferative factors in the DG
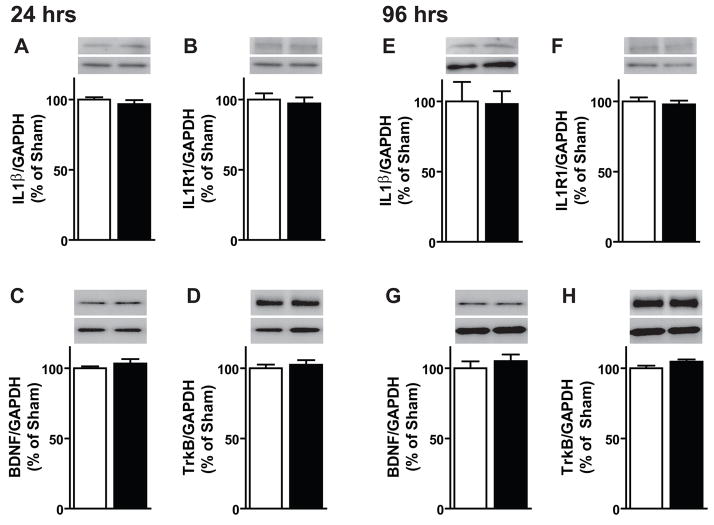
To assess changes in pro- or anti-proliferative factors in the DG after chronic morphine, DG-enriched protein samples were immunoblotted for BDNF and IL1β and their corresponding receptors TrkB and IL1R1. DG levels of IL1β and IL1R1 were unchanged after 24 hrs of morphine exposure (Figure 2A, B). BDNF and TrkB levels also remained unchanged after 24 hrs of morphine exposure (Figure 2C, D). The same result was observed for IL1β, IL1R1, BDNF and TrkB at the 96 hr time point (Figure 2E-H). Therefore at a time point in which proliferation was decreased by morphine (96 hrs), DG levels of the pro- and anti-proliferative factors BDNF and IL1β remained unchanged.
Figure 2. Chronic morphine does not alter DG levels of BDNF or IL1β.
To determine if the microenvironment of the hippocampus was altered by chronic morphine, DG-enriched extracts were used to look for changes in protein levels via immunoblotting. (A–D) After 24 hrs of morphine exposure, dentate levels of IL1β, BDNF and their respective receptors, IL1R1 and TrkB remained unchanged. (E–H) After 96 hrs of morphine exposure, dentate levels of IL1β and BDNF and their respective receptors, IL1R1 and TrkB remained unchanged. At all time points sham: n=4–8, morphine: n=5–8.
Chronic morphine dynamically alters DG levels of VEGF
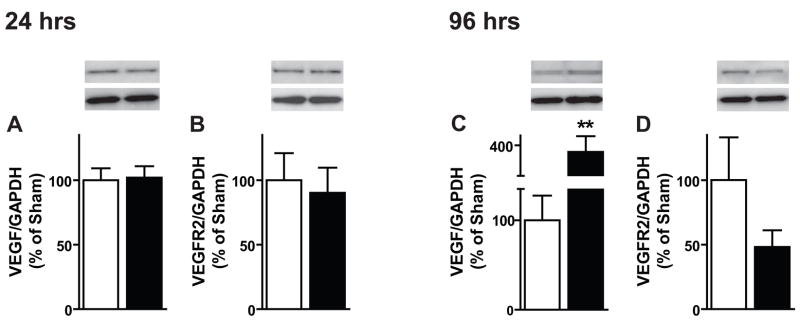
Given previous work linking VEGF to regulation of SGZ neurogenesis (Jin et al., 2002), protein levels of VEGF and VEGFR2 were also assessed after chronic morphine. After 24 hrs of morphine exposure, DG levels of VEGF and its primary receptor VEGFR2 were unchanged (Figure 3A,B). Interestingly, after 96 hrs of exposure (a time point in which numbers of Ki67-IR cells were decreased) levels of VEGF were significantly increased (Figure 3C; t8=3.375, p=0.0097). Levels of VEGFR2 remained unchanged at 96 hrs (Figure 3D). These data suggest that rather than a parallel change, VEGF levels negatively correlate with the change in SGZ proliferation.
Figure 3. Chronic morphine dynamically alters levels of VEGF in the DG.
(A, B) After 24 hrs of morphine, DG protein levels of VEGF and VEGFR2 are unchanged as measured via immunoblotting. (C, D) After 96 hrs, VEGF protein levels are increased whereas receptor levels remain unchanged. At all time points sham: n=5–6, morphine: n=4–8, **p<0.01.
Endoglin expression in the hippocampal DG
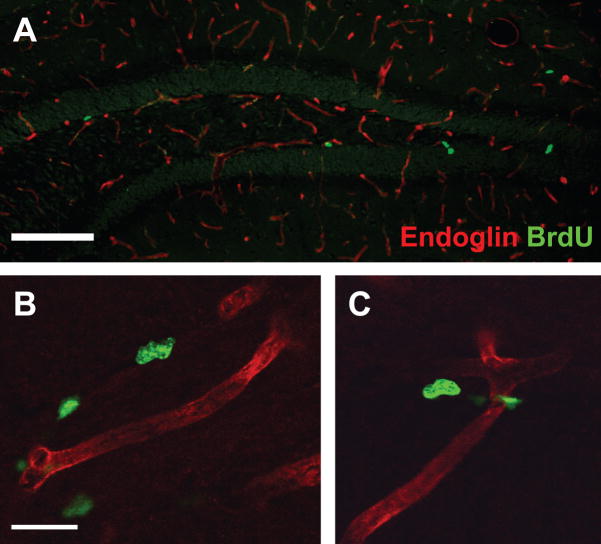
VEGF is a unique pro-proliferative factor in that it also can function as an angiogenic factor (Palmer et al., 2000). To determine if the morphine-induced increase of VEGF levels correlated with altered neurovasculature, it was first necessary to stain for an appropriate vasculature marker in the SGZ. C57BL/6J mice were given morphine or sham pellets s.c. at 0 and 48 hrs and two hrs before perfusion (24 or 96 hrs) received a single 150mg/kg i.p. injection of BrdU. Co-labeling of endoglin, an endothelial cell marker, and BrdU demonstrated that endoglin-IR vessels are present throughout the DG (Figure 4A). Confocal images of endoglin-IR vessels revealed that BrdU-IR cells were closely associated with vasculature (Figure 4B,C). This allowed examination of properties of the vessel themselves, as well as association of proliferating cells with the neurovasculature.
Figure 4. Endoglin expression in the hippocampal DG.
(A) Low-power image shows that endoglin staining is present throughout the hippocampus and in the SGZ where the majority of proliferating BrdU-IR cells reside. Scale bar=100 um. (B–C) Confocal images show close proximity of BrdU-IR cells to endoglin-IR vessels. Scale bar=10 um.
Chronic morphine-induced increase in VEGF correlates with altered vasculature in dentate SGZ
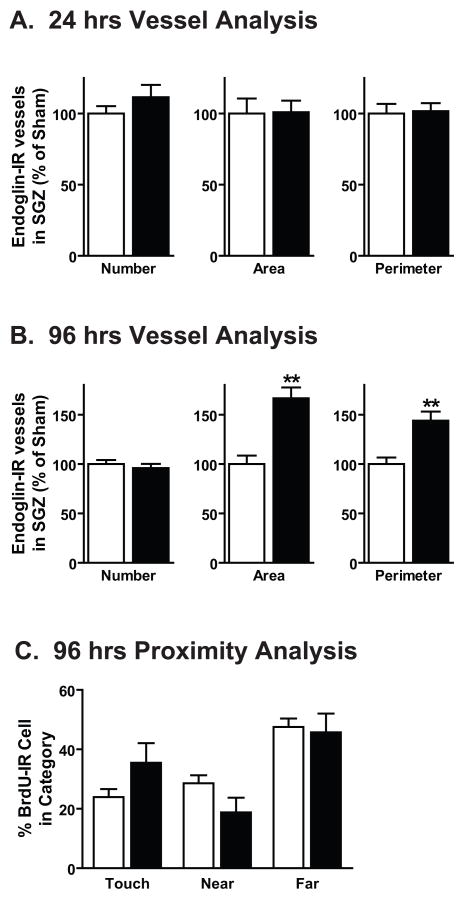
To determine if the morphine-induced increase in VEGF correlated with vasculature changes in the DG, several measures of vasculature remodeling were examined: endoglin-IR vessel number, area and perimeter (Heine et al., 2005). First, to assess if increased VEGF correlated with increased angiogenesis, the number of endoglin-IR vessels was compared between sham and morphine exposed mice. The numbers of endoglin-IR vessels, vessel area and perimeter in the DG SGZ were unchanged after 24 hrs of morphine exposure (Figure 5A), a time point in which proliferation and VEGF levels were unchanged. This suggests that there was no change in angiogenesis or the neurovasculature after 24 hrs of morphine exposure. Similarly, after 96 hrs of morphine exposure the number of endoglin-IR vessels was the same between sham and morphine groups. However, the area and perimeter of endoglin-IR vessels was significantly increased (Figure 5B; area: t8=4.803, p=0.0014; perimeter: t8=3.890, p=0.0046). This suggests that although angiogenesis was unchanged, the neurovasculature was altered after 96 hrs of morphine exposure. To determine if morphine exposed mice had diminished association between proliferating cells and the neurovasculature, the proximity of BrdU-IR cells to endoglin-IR vessels was examined (Heine et al., 2005). No changes in progenitor cell proximity to endoglin-IR vessels was observed after 96 hrs of morphine exposure (Figure 5C).
Figure 5. Chronic morphine-induced increase in VEGF correlates with altered DG neurovasculature.
(A)After 24 hrs of morphine exposure, various properties of endoglin-IR cells including vessel number, area and perimeter remain unchanged. (B) After 96 hrs of morphine exposure, the number of endoglin-IR vessels between sham and morphine groups did not differ. However the area and perimeter of endoglin-IR vessels are increased. (C) The proximity of BrdU-IR cells to endoglin-IR vessels, does not differ between sham and morphine treated groups. Proximity was measured as “touch”, touching or overlapping the vessel; “near”, within 10 μm of vessel, or “far”, beyond 10μm of vessel. For all time points sham: n=4–5, morphine: n=4–5, **p<0.01.
Discussion
The current results suggest that following chronic morphine exposure, DG levels of factors within the neurogenic microenvironment are either maintained or up-regulated. At a time point in which progenitor cell proliferation was decreased, levels of both pro and anti-proliferative factors (BDNF and IL1β, respectively) were maintained. Interestingly, VEGF, known as both a pro-proliferative and angiogenic factor, was increased when proliferation was decreased. Furthermore, although the morphine-induced increase in VEGF did not correlate with altered angiogenesis of endoglin-IR vessels within the DG, properties of the vessels themselves such as area and perimeter were also increased.
Chronic morphine inhibits proliferating cells in the hippocampal SGZ
The effect of chronic morphine on SGZ progenitor cells has been well characterized (Arguello et al., 2008; Fischer et al., 2008). However it was necessary to confirm changes in proliferation so that they could be correlated with possible alterations in the neurogenic microenvironment within a single subject. As expected from previous data, the current experiments demonstrate that the total population of proliferating cells was only decreased after 96 hrs of morphine exposure.
DG levels of BDNF and IL1β are not altered by chronic morphine
An interesting and unexpected finding from the present study was that DG levels of BDNF and IL1β, as well as their respective receptors TrkB and IL1R1, were not decreased after 24 or 96 hrs of morphine exposure. This is of interest as other studies show that exogenously applied BDNF or IL1β are pro-proliferative or anti-proliferative, respectively (Koo and Duman, 2008; Scharfman et al., 2005). However the present findings are not unprecedented, as another report also observed a disconnect between levels of proliferation and growth factor mRNA levels (Warner-Schmidt et al., 2008). Perhaps these differences arise from different experimental designs: examination of the effect of exogenously applied growth factors versus examination of the effect of drug administration on endogenous growth factors. It is also possible that immunoblotting from a DG-enriched sample is not sensitive enough to detect changes in protein levels that may occur in discrete cellular populations. For example, levels of BDNF are increased in one cell population and decreased in another, resulting in the lack of change reported here. However, several studies have detected changes in cell death proteins in the hippocampus via immunoblotting following morphine exposure (Emeterio et al., 2006), as well as proBDNF after prenatal exposure to opiates (Schrott et al., 2008). Given that in the present work we can detect a robust change in VEGF levels from DG-enriched samples, we can minimally say that the immunoblotting approach used here is sensitive enough to detect large changes in hippocampal levels of growth factors. A third explanation for our results could center on temporal specificity. A previous study determined that an increase in VEGF precedes an increase in BDNF levels (Louissaint et al., 2002). Thus it is possible that BDNF protein levels would not be altered at 24 and 96 hr time points, but would only be revealed at later time points. However, examination of factors within the neurogenic niche would be challenging with the present morphine paradigm since mice may experience somatic withdrawal due to the decline of morphine blood levels when time points beyond 96 hrs are examined (Fischer et al., 2008; unpublished observations). Since i.p. injections of morphine decrease SGZ proliferation in the rat (Kahn et al., 2005), a reasonable alternative to s.c. pellet administration is a paradigm using i.p. injections of escalating morphine doses (Shaw-Lutchman et al., 2002). However, we have recently showed that escalating injection paradigms in the mouse do not decrease SGZ proliferation (Fischer et al., 2008). Thus, examination of longer time points awaits development of alternative opiate administration paradigms that result in decreased SGZ proliferation in the mouse without somatic withdrawal symptoms (e.g. self-administration or alternative injection paradigms). Taken together the present data suggest that factors in the neurogenic niche are differentially sensitive to exogenous stimuli.
DG levels of VEGF are dynamically regulated
Another significant finding of the present study was that at a time point in which progenitor proliferation was decreased, DG levels of VEGF were increased. This was unexpected as levels of VEGF are usually positively correlated with changes in proliferation (Cao et al., 2004; Jin et al., 2002; van Praag et al., 2005; Warner-Schmidt et al., 2008). Furthermore, VEGF levels increase after myocardial infarction and morphine treatment prevents this increase (Balasubramanian et al., 2001; Roy et al., 2003). One potential hypothesis in the case of chronic morphine administration is that an increase in VEGF in the DG may reflect a compensatory response of the DG to prevent a further decrease in proliferation. This is very interesting in that although 30% of proliferating cells are inhibited by morphine, 70% of proliferating cells continue to divide, which suggests that morphine alters a subpopulation of proliferating cells that are not be able to respond to the factors that are maintained in the neurogenic niche.
Chronic morphine-induced increase in VEGF correlates with altered SGZ neurovasculature
Although increased DG levels of VEGF were observed after 96 hr of morphine exposure, it was unclear if this increase was functional. Therefore we examined whether the increased VEGF levels correlated with a measureable change in the vasculature. Several studies have observed that increased VEGF levels are associated with altered properties of neurovasculature such as increased area and perimeter (Louissaint et al., 2002; van Praag et al., 2005) as well as number of vessels (Cao et al., 2004). In the present study, increased VEGF levels did not correlate with the formation of new vessels, but did correlate with increased area and perimeter of endoglin-IR vessels, suggesting potential remodeling of the neurovasculature. It is possible that the morphine-induced increase in VEGF levels was not robust enough to lead to angiogenesis, as observed with previous overexpression studies (Cao et al., 2004). Given the discovery that progenitor cells are closely associated with the vasculature, (Palmer et al., 2000) the increase in vessel size after morphine treatment supports the hypothesis that the neurogenic niche may be providing more vascular support to compensate for decreased SGZ proliferation. To further explore the relationship between the morphine-induced alterations in VEGF and vasculature, future experiments should take advantage of the documented lack of morphine’s effect on SVZ proliferation (Eisch et al., 2000). Comparison between the potential changes in morphine-induced alterations in neurovasculature between the SVZ and the SGZ would shed light on the similarities and differences between these two neurogenic niches.
While this correlative study highlights several interesting findings, key questions remain to be answered. What cell populations are secreting VEGF? More importantly, which progenitor cell populations express VEGFR2 and might therefore be able to respond to increased VEGF levels? Several studies have shown that progenitor cells and the surrounding granule cells express receptors to respond to growth factors, such as TrkB (Donovan et al., 2008; Li et al., 2008) and VEGFR2 (Cao et al., 2004; Jin et al., 2002), suggesting that the mature neurons themselves might provide trophic support for dividing cells. Similar to TrkB and VEGFR2 expression, IL1R1 is highly expressed in the DG and has been observed on proliferating progenitor cells (Koo and Duman, 2008). Immunohistochemical staining has shown expression of the primary VEGF receptor VEGFR2 (Flk1) on progenitor cells in the DG. Specifically, VEGFR2 expression is present on immature doublecortin (DCX)-IR cells as well as mature BrdU-IR cells (Jin et al., 2002; Palmer et al., 2000). A previous study by our lab determined that the total population of DCX-IR cells was not altered after chronic morphine (Arguello et al., 2008), suggesting that a subpopulation of DCX-IR cells that are also VEGFR2-IR are responsive to the morphine-induced increase in VEGF. However, VEGFR2 presence is not restricted to progenitor cells, as colocalization of VEGFR2 has also been reported on both astrocytes and neurons in the hilus, and granule neurons of the DG (Heine et al., 2005; Palmer et al., 2000). Future studies should focus on determining if the expression pattern of VEGFR2 on specific progenitor cell types is altered after chronic morphine and whether over-expression of VEGF could attenuate the morphine-induced decrease in proliferation.
Conclusion
In sum, we show that chronic morphine results in decreased SGZ proliferation, no change in DG levels of the cytokine IL1β, growth factor BDNF, or their respective receptors, but does enhance DG levels of VEGF and certain characteristics of the vasculature. The present results underscore the complex relationship between adult hippocampal neurogenesis and pro-and anti-proliferative factors within the neurogenic niche. Future work is warranted to more fully explore the functional consequence of treatments that enhance proliferation on neurovasculature (Newton and Duman, 2004). Interestingly, VEGF is important for spatial learning and memory formation (Cao et al., 2004) and human heroin abusers and rodents exposed to morphine have altered neurogenesis and impaired memory (Eisch et al., 2000; Guerra et al., 1987; Spain and Newsom, 1991; Weber et al., 2006). Therefore, future use of an opiate-self administration paradigm in rodents would be extremely useful in testing whether knockdown or overexpression of certain factors in the neurogenic niche, such as VEGF, would impair or enhance learning of drug-context memories, indicating novel treatment avenues for addiction.
Acknowledgments
This work was supported by grants from NIDA (R01 DA016765 and K02 DA023555 to AJE, minority supplement to DA016765 to AJE/AAA). JRS and RWM were supported by an education grant (R25 DA18843). The authors thank Shanna Arnold for excellent technical assistance with vessel analysis.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- BrdU
bromodeoxyuridine
- DCX
doublecortin
- DG
dentate gyrus
- IHC
immunohistochemistry
- IL1β
interleukin-1β
- i.p
intraperitoneal
- IR
immunoreactive
- s.c
subcutaneous
- SGZ
subgranular zone
- SVZ
subventricular zone
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Ricci V, Pomponi M, Conte G, Mathe AA, Attilio Tonali P, Bria P. Chronic heroin and cocaine abuse is associated with decreased serum concentrations of the nerve growth factor and brain-derived neurotrophic factor. J Psychopharmacol. 2007;21(8):820–825. doi: 10.1177/0269881107078491. [DOI] [PubMed] [Google Scholar]
- Arguello AA, Harburg GC, Schonborn JR, Mandyam CD, Yamaguchi M, Eisch AJ. Time course of morphine’s effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience. 2008;157(1):70–79. doi: 10.1016/j.neuroscience.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S, Mira E, Muneer S, Korpanty G, Beck AW, Holloway SE, Manes S, Brekken RA. Forced expression of MMP9 rescues the loss of angiogenesis and abrogates metastasis of pancreatic tumors triggered by the absence of host SPARC. Exp Biol Med (Maywood) 2008;233(7):860–873. doi: 10.3181/0801-RM-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Ramakrishnan S, Charboneau R, Wang J, Barke RA, Roy S. Morphine sulfate inhibits hypoxia-induced vascular endothelial growth factor expression in endothelial cells and cardiac myocytes. J Mol Cell Cardiol. 2001;33(12):2179–2187. doi: 10.1006/jmcc.2001.1480. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36(8):827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460(4):563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Donovan MH, Yamaguchi M, Eisch AJ. Dynamic expression of TrkB receptor protein on proliferating and maturing cells in the adult mouse dentate gyrus. Hippocampus. 2008 doi: 10.1002/hipo.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495(1):70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97(13):7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeterio EP, Tramullas M, Hurle MA. Modulation of apoptosis in the mouse brain after morphine treatments and morphine withdrawal. J Neurosci Res. 2006;83(7):1352–1361. doi: 10.1002/jnr.20812. [DOI] [PubMed] [Google Scholar]
- Feng D, Nagy JA, Brekken RA, Pettersson A, Manseau EJ, Pyne K, Mulligan R, Thorpe PE, Dvorak HF, Dvorak AM. Ultrastructural localization of the vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) receptor-2 (FLK-1, KDR) in normal mouse kidney and in the hyperpermeable vessels induced by VPF/VEGF-expressing tumors and adenoviral vectors. J Histochem Cytochem. 2000;48(4):545–556. doi: 10.1177/002215540004800412. [DOI] [PubMed] [Google Scholar]
- Fischer SJ, Arguello AA, Charlton JJ, Fuller DC, Zachariou V, Eisch AJ. Morphine blood levels, dependence, and regulation of hippocampal subgranular zone proliferation rely on administration paradigm. Neuroscience. 2008;151(4):1217–1224. doi: 10.1016/j.neuroscience.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. p. 216. [Google Scholar]
- Ge AZ, Butcher EC. Cloning and expression of a cDNA encoding mouse endoglin, an endothelial cell TGF-beta ligand. Gene. 1994;138(1–2):201–206. doi: 10.1016/0378-1119(94)90808-7. [DOI] [PubMed] [Google Scholar]
- Guerra D, Sole A, Cami J, Tobena A. Neuropsychological performance in opiate addicts after rapid detoxification. Drug Alcohol Depend. 1987;20(3):261–270. doi: 10.1016/0376-8716(87)90036-6. [DOI] [PubMed] [Google Scholar]
- Harburg GC, Hall FS, Harrist AV, Sora I, Uhl GR, Eisch AJ. Knockout of the mu opioid receptor enhances the survival of adult-generated hippocampal granule cell neurons. Neuroscience. 2007;144(1):77–87. doi: 10.1016/j.neuroscience.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21(5):1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn L, Alonso G, Normand E, Manzoni OJ. Repeated morphine treatment alters polysialylated neural cell adhesion molecule, glutamate decarboxylase-67 expression and cell proliferation in the adult rat hippocampus. Eur J Neurosci. 2005;21(2):493–500. doi: 10.1111/j.1460-9568.2005.03883.x. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007;13(1):323–330. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, Decarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, Dileone RJ, Greer CA, Mandyam CD, Eisch AJ. Dynamic Contribution of Nestin-Expressing Stem Cells to Adult Neurogenesis. J Neurosci. 2007;27(46):12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34(6):945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007;146(1):108–122. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J Neurosci Res. 2004;76(6):783–794. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451(2):170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Mustafa S, Walker A, Bennett G, Wigmore PM. 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. 2008;28(2):323–330. doi: 10.1111/j.1460-9568.2008.06325.x. [DOI] [PubMed] [Google Scholar]
- Newton SS, Duman RS. Regulation of neurogenesis and angiogenesis in depression. Curr Neurovasc Res. 2004;1(3):261–267. doi: 10.2174/1567202043362388. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos Trans R Soc Lond B Biol Sci. 2008;363(1489):123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Balasubramanian S, Wang J, Chandrashekhar Y, Charboneau R, Barke R. Morphine inhibits VEGF expression in myocardial ischemia. Surgery. 2003;134(2):336–344. doi: 10.1067/msy.2003.247. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schrott LM, Franklin LM, Serrano PA. Prenatal opiate exposure impairs radial arm maze performance and reduces levels of BDNF precursor following training. Brain Res. 2008;1198:132–140. doi: 10.1016/j.brainres.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, Duman RS, Storm D, Nestler EJ. Regional and cellular mapping of cAMP response element-mediated transcription during naltrexone-precipitated morphine withdrawal. J Neurosci. 2002;22(9):3663–3672. doi: 10.1523/JNEUROSCI.22-09-03663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain JW, Newsom GC. Chronic opioids impair acquisition of both radial maze and Y-maze choice escape. Psychopharmacology (Berl) 1991;105(1):101–106. doi: 10.1007/BF02316870. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Madsen TM, Duman RS. Electroconvulsive seizure restores neurogenesis and hippocampus-dependent fear memory after disruption by irradiation. Eur J Neurosci. 2008;27(6):1485–1493. doi: 10.1111/j.1460-9568.2008.06118.x. [DOI] [PubMed] [Google Scholar]
- Weber M, Modemann S, Schipper P, Trauer H, Franke H, Illes P, Geiger KD, Hengstler JG, Kleemann WJ. Increased polysialic acid neural cell adhesion molecule expression in human hippocampus of heroin addicts. Neuroscience. 2006;138(4):1215–1223. doi: 10.1016/j.neuroscience.2005.11.059. [DOI] [PubMed] [Google Scholar]