Abstract
Fission yeast is an important model for epigenetic studies due to the ease with which genetic mutants can be isolated. However, it can be difficult to complement epigenetic phenotypes with genomic libraries in order to identify the genes responsible. This is because epigenetic phenotypes are typically unstable, and can prohibit complementation if silencing cannot be reestablished. Here we have resequenced the fission yeast genome following mutagenesis to readily identify a novel mutant involved in heterochromatic silencing. Candidate genes were identified as functional single base changes linked to the mutation, which were then reconstituted in a wild-type strain to recapitulate the mutant phenotype. By this procedure we identified a weak allele of ubc4, which encodes an essential E2 ubiquitin ligase, as responsible for the swi*603 mutant phenotype. In combination with a large collection of mutants and suppressor plasmids, next-generation genomic resequencing promises to dramatically enhance the power of yeast genetics, permitting the isolation of subtle alleles of essential genes, alleles with quantitative effects, and enhancers and suppressors of heterochromatic silencing.
The characterization of genes involved in epigenetic silencing in Schizosaccharomyces pombe has given valuable insight into the mechanisms regulating condensed, silent chromosome domains, such as those surrounding the centromere. Many of these genes were isolated in forward genetic screens (Ekwall et al. 1999). However, due to their variegated silencing defect, these genes can be extremely difficult to clone by complementation (Ekwall et al. 1999; Kato et al. 2005; Li et al. 2005). Further, because lab strains of S. pombe are all derived from the same progenitor, molecular markers are rare. Instead, insertions of reporter genes have been used to map point mutations for positional cloning (Kato et al. 2005), and in forward screens by insertional mutagenesis (Li et al. 2005). Recently, it has become possible to perform reverse genetic screens using S. pombe knockout libraries. However, many epigenetic silencing mutants are temperature sensitive (ts) lethal point mutations (Ekwall et al. 1999), so it is likely that screening knockout libraries will miss many essential genes. Next-generation whole-genome resequencing offers an alternative to cloning epigenetic silencing mutations generated by chemical mutagenesis (Hillier et al. 2008; Mardis 2008). When this study was instigated a single lane of an Illumina (Solexa) Genome Analyzer could generate 0.5–1.0 Gb of sequence and enough useful sequence to get >30-fold coverage of S. pombe's 14-Mb genome. We have used next-generation sequencing to identify a chemically induced point mutation that causes an epigenetic transcriptional silencing defect in S. pombe.
Results
Transcripts derived from pericentromeric repeats are silenced by two parallel mechanisms both involving histone H3K9 methylation: an RNAi-dependent mechanism and the Clr3 histone deacetylation pathway (Grewal and Jia 2007; Zaratiegui et al. 2007). In a genetic screen for transcriptional silencing defects, we assayed a subset of a large collection of mutagenized S. pombe strains that were temperature sensitive (ts) for growth. In each case, the growth defect could be suppressed by one or more plasmids recovered from a genomic library that had been systematically transformed into each strain (Matsumura et al. 2003; Hayashi et al. 2004; Yuasa et al. 2004). Twenty-seven ts strains were screened by reverse transcription polymerase chain reaction (RT-PCR) for repeat transcript accumulation at the non-permissive temperature of 36°C. One strain, designated ts603, accumulated centromeric repeat transcripts. Candidate genes from the most commonly recovered suppressor plasmids (Supplemental Table 1) were sequenced, and we identified a C-to-T base substitution within one of them, trz1(SPBC3D6.03c), that is predicted to cause a transition from an alanine to valine at amino acid 623 of the protein (A623V). When the trz1-A623V allele was regenerated in an otherwise wild-type (wt) background (strain DI13; Supplemental Table 2), the strain was ts for growth on minimal media, but centromeric transcripts did not accumulate (Supplemental Fig. 1A,B). Moreover, the silencing defect remained but the ts phenotype was lost when the trz1-A623V allele was corrected by targeted mutagenesis (strain DI36; Supplemental Table 2). Thus trz1-A623V was responsible for the growth, but not the silencing phenotype, which was instead caused by an unmapped mutation.
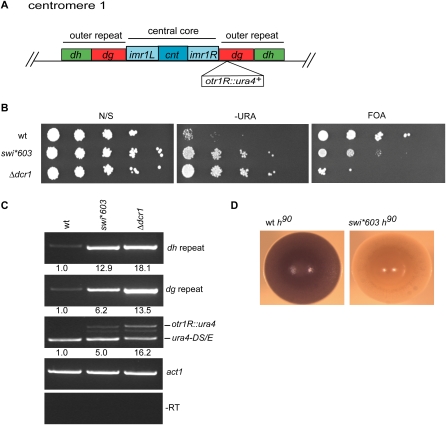
Mutants in RNAi can be distinguished from other silencing defects by their specific effects on reporter gene silencing at the centromere, rather than at the mating-type locus (Zaratiegui et al. 2007). Unlike mutants in RNAi, mutants in heterochromatic modification also affect mating-type silencing and switching in a homothallic (h90) strain. Efficient switching can be assayed by the production of spores, which stain dark brown with iodine vapors (Moreno et al. 1991). We found that silencing of the pericentromeric otr1R∷ura4+ reporter gene (Fig. 1A; Allshire et al. 1995) was relieved in the mutant strain, but to a lesser extent than in a Dicer deletion strain (Δdcr1) defective in RNAi-induced heterochromatin assembly (Fig. 1B,C). Further, while wt h90 colonies stained uniformly dark brown with iodine, mutant h90 colonies did not stain, remaining pale yellow (Fig. 1D). As heterothallic mutant strains of opposite mating-type (h− and h+) could mate and sporulate efficiently (data not shown), this indicated a defect in mating-type switching rather than RNAi. We therefore designated the unmapped mutation swi*603.
Figure 1.
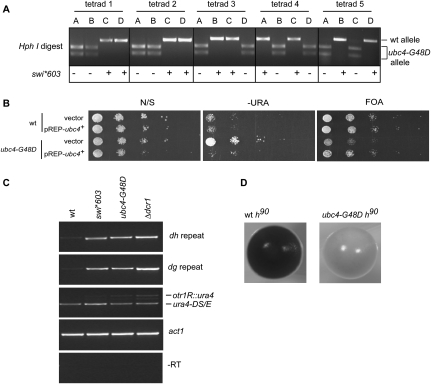
swi*603 is required for centromeric heterochromatin formation. (A) Cartoon of S. pombe centromere 1. The central core domain, where the kintochore forms, is flanked by outer repeat domains composed of dg and dh repeat sequences. The position of the centromeric otr1R∷ura4+ reporter gene used in this study is indicated. (B) swi*603 affects silencing of centromeric heterochromatin. Serial dilution (plating) assays were performed to measure the expression of the pericentromeric otr1R∷ura4+ reporter gene in wt, swi*603, and RNAi-defective Δdcr1 deletion cells. Cells were serially diluted 1/10 (starting with 1 × 104 cells) and spotted onto plates with no selection (N/S), plates lacking uracil (−URA), and counter-selective plates containing 5-fluoro-orotic acid (FOA). Yeast with an active ura4+ gene convert FOA to fluorodeoxyuridine, which is toxic to cells. (C) swi*603 results in the accumulation of transcripts derived from heterochromatic dg and dh centromere repeats. RT-PCR was performed using total RNA isolated from indicated strains to measure the amount of transcript derived from dg and dh centromeric repeats. In each sample, enrichment of each primer pair was measured relative to the control primer pair act1. Centromeric otr1R∷ura4+ expression was also analyzed and compared with the transcriptionally active but nonfunctional mini-gene ura4-DS/E at the ura4+ locus. –RT, minus reverse transcription. (D) swi*603 affects mating-type switching. Iodine staining phenotypes of homothallic (h90) wt strain SPG17 and swi*603 strain. Strains were streaked onto sporulation medium and allowed to grow at 26°C for 5 d before staining with iodine vapors.
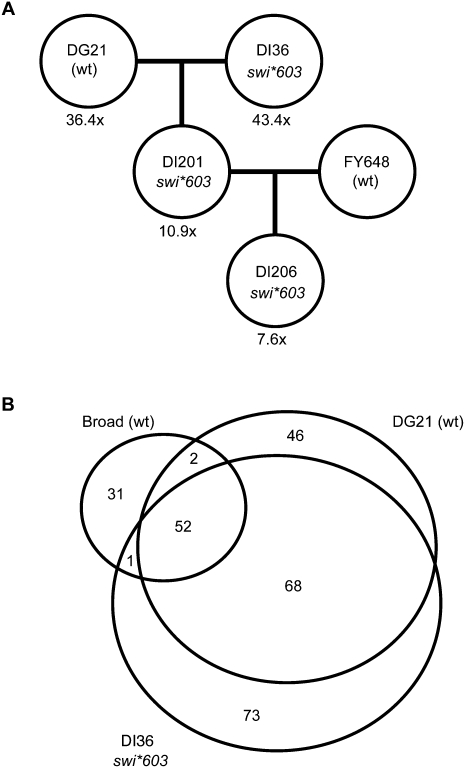
swi*603 was generated using the mutagen N-methyl-N′-nitro-N-nitrosoguanidine (MNG), which induces single nucleotide transitions (Lucchesi et al. 1986). Traditional mapping approaches in S. pombe are laborious and time consuming, so we undertook an alternative approach to genetic mapping based on whole-genome resequencing and mutation detection. The trz1-corrected swi*603 strain, DI36, was backcrossed twice to the same wt isolate (h− DG21 or h+ FY648) to generate swi*603 strains DI201 and DI206, respectively (Fig. 2A). Illumina genomic DNA libraries were generated from DNA isolated from wt DG21 and the three strains that carried the swi*603 allele. The libraries were sequenced on an Illumina Genome Analyzer to generate 43.3-fold sequence coverage of swi*603 strain DI36, 36.4-fold sequence coverage of wt DG21, 10.9-fold sequence coverage of swi*603 strain DI201, and 7.56-fold sequence coverage of swi*603 strain DI206 (Fig. 2A). Sequences were aligned to the published Sanger sequencing assembly of reference strain 972h− and analyzed for single base changes using MAQ (Mapping and Assembly with Qualities; Li et al. 2008). The Broad Institute has also recently resequenced wt strain 972h− to >200-fold sequence coverage and found 193 sequence discrepancies with the Sanger assembly, consistent with the very high accuracy of the 14-Mb reference sequence (Wood et al. 2002). Eighty-six of these discrepancies were single base changes (www.broad.mit.edu), which we included in our analysis.
Figure 2.
swi*603 strain pedigree and single base changes detected. (A) swi*603 strain DI36 was backcrossed twice. The DI36 strain, our wt (DG21/FY648), and strains derived from the two crosses were sequenced to various degrees of coverage. (B) Single base changes identified by 200-fold resequencing of 972h− by the Broad Institute, 36-fold sequencing of DG21 (wild type), and 43-fold sequencing of swi*603 strain DI36 were compared to identify 73 DI36-specific high-confidence single base changes potentially responsible for the observed phenotypes. In addition, divergences between wt DG21 and the recently sequenced 972h− were identified relative to the cosmid library-derived wt reference sequence (Wood et al. 2002).
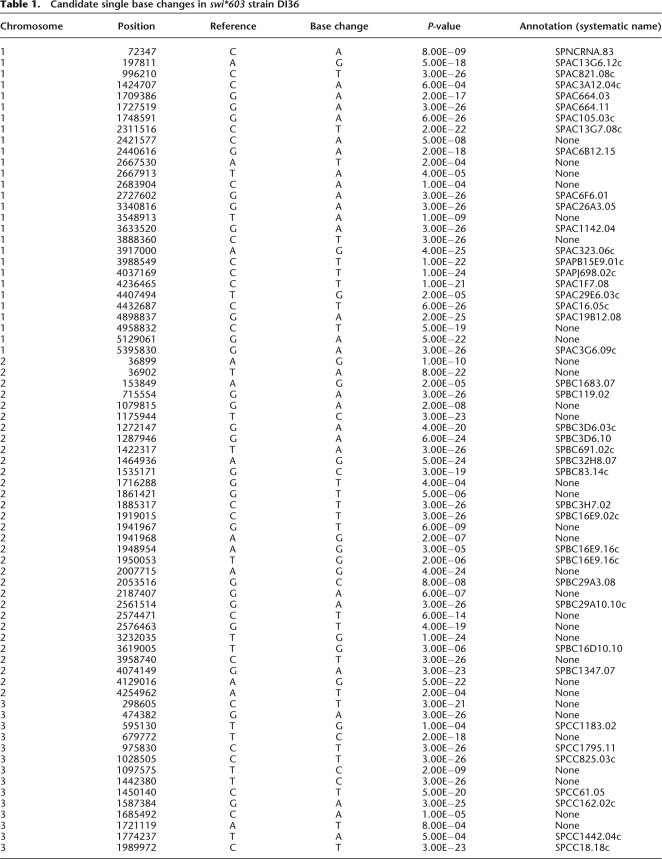
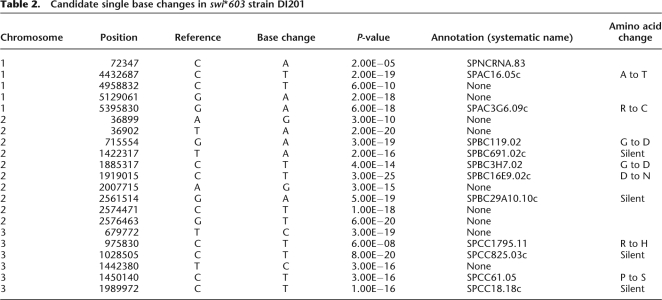
We identified 194 single base changes in swi*603 strain DI36 that were not in the Sanger reference. Seventy-three of these base changes were in DI36 alone, 52 shared with Broad 972h− and wt DG21, 68 shared with wt DG21 alone, and one with Broad 972h− alone (Fig. 2B). We expected the mutation causing swi*603 to be one of the 73 single base changes found in swi*603 strain DI36 alone (Table 1). Twenty-two of these single base changes were inherited in the backcross swi*603 strain DI201 (Fig. 2A), of which 11 were in genes, four of them silent substitutions (Table 2). Co-segregation analysis between single base changes and the silencing defect were performed using PCR and sequencing/digest, and a G-to-A base substitution at position 222 within the ubc4 gene (SPBC119.02) on chromosome II displayed 100% segregation with the centromeric silencing defect in five tetrads examined (Fig. 3A), indicating close linkage with swi*603. None of the other single base changes were closely linked to ubc4 and were eliminated from the analysis. The ubc4 gene encodes a ubiquitin-conjugating enzyme (Seino et al. 2003), and when expressed on a plasmid under the control of a medium-strength inducible promoter, nmt1, it complemented the loss of heterochromatic silencing in ubc4-G48D cells (Fig. 3B).
Table 1.
Candidate single base changes in swi*603 strain DI36
Table 2.
Candidate single base changes in swi*603 strain DI201
Figure 3.
Verification that the ubc4-G48D mutation is responsible for swi*603 strain phenotypes. (A) The ubc4-G48D mutant allele segregates 100% with the swi*603 centromeric otr1R∷ura4+ reporter gene loss of silencing phenotype. The ubc4-G48D allele is cut with HphI to yield two bands, while ubc4+ is not digested. Pericentromeric reporter gene otr1R∷ura4+ silencing is assayed by growth on plates lacking uracil (−URA). (−) Loss of ura4+ reporter gene silencing/growth on −URA plates/swi*603 allele; (+) silencing ura4+ reporter gene/no growth on −URA plates/wt. (B) The ubc4+ gene can complement the heterochromatic silencing defect in ubc4-G48D cells. Wild-type (wt) or ubc4-G48D cells were transformed with an empty G418-based plasmid or one expressing ubc4+ under the control of an inducible medium-strength nmt1 promoter. Serial dilution plating assays were performed (−thiamine) to measure the expression of the pericentromeric otr1R∷ura4+ reporter gene. Cells were serially diluted 1/10, starting with 1 × 104, and spotted onto nonselective plates (N/S), plates lacking uracil (−URA), and counter-selective plates containing 5-fluoro-orotic acid (FOA). (C) The ubc4-G48D allele when introduced into wt DG21 causes accumulation of centromeric transcripts and the pericentromeric otr1R∷ura4+ reporter gene. (D) The iodine staining phenotypes of homothallic (h90) wt SGP17 and a regenerated ubc4-G48D strain show that the ubc4-G48D allele affects mating-type switching.
The mutant allele is denoted as ubc4-G48D, as the base substitution causes an amino acid transition from a highly conserved glycine to aspartic acid at amino acid 48 of the protein (G48D) (Supplemental Fig. 2A). When the ubc4-G48D allele was regenerated in the heterothallic DG21 and homothallic SPG17 wt backgrounds (strains DI301 and DI304; Supplemental Table 2), the strains accumulated centromeric transcripts and failed to switch mating types, respectively (Fig. 3C,D). Further, when the ubc4-G48D allele was corrected in strain ts603 (strain DI302) the strain no longer accumulated centromeric transcripts (Supplemental Fig. 2B). Thus the ubc4-G48D allele was responsible for the centromeric silencing and the mating-type defects displayed by ts603. ubc4+ was recovered at a low frequency among the plasmids that suppressed the ts603 temperature sensitive phenotype, indicating it may also contribute to the ts603 growth phenotype on defined medium (Supplemental Table 1).
Discussion
Ubiquitin-conjugating enzymes, also known as E2 ligases, have diverse roles (Passmore and Barford 2004). Ubc4 is known to function with Ubc11 in the ubiquitination of mitotic cyclin Cdc13 by the APC/C (anaphase-promoting complex/cyclosome). Both ubiquitin-conjugating enzymes are directly required for the degradation of Cdc13 and the onset of the metaphase–anaphase transition (Seino et al. 2003), but this is unlikely to explain the silencing defect. Instead, the silencing defect in the ubc4-G48D strain resembles that of a cul4-1 mutant, which also exhibits loss of centromeric repeat silencing and mating-type switching defects (Jia et al. 2005). Cul4 is a cullin family protein, found in the Clr4 histone methyltransferase complex (Petroski and Deshaies 2005). It is possible that Ubc4 is the E2 ligase in the Cul4-pathway. In support of this idea, ubc4+ disrupts mitotic chromosome segregation when its cDNA is overexpressed (Javerzat et al. 1996), a phenotype shared with some silencing mutants.
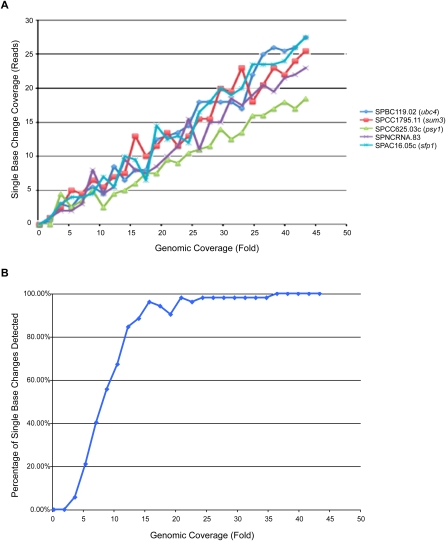
Next-generation sequencing provides a powerful technology for mapping mutations in forward screens, and for identification of subsequent revertants. Importantly, genome sequencing immediately distinguishes complementing and suppressing genes recovered by library transformation, even in cases where true complementation is rare, and multiple mutations are found in each strain (Srivatsan et al. 2008). This is particularly powerful for mutations that revert spontaneously, such as those in epigenetic silencing pathways. In order to determine the coverage required for this approach we performed regression analysis on reads randomly sampled from the swi*603 DI36 library (Fig. 4). We found that all single base changes analyzed including ubc4-G48D were efficiently recovered with 98% confidence at ∼22-fold coverage, using less than one Illumina flow cell (see Methods). The strategy can thus be summarized as follows: (1) sequence mutant strain to 20–25× coverage; (2) test candidate single base changes in backcross progeny until linked base change is found; (3) confirm by complementation. As the candidate single base changes include the mutation itself, no additional markers are required, allowing the use of monomorphic genomes such as S. pombe. Also because a candidate list is immediately accessible, new alleles in genes already known to result in the phenotype can be readily identified without further mapping.
Figure 4.
Relationship between library depth and single base change coverage. (A) The 17.04 million Solexa reads derived from swi*603 strain DI36 were sampled randomly to generate 25 libraries ranging from 0.25× to 43× coverage relative to the S. pombe reference genome. For each library, reads were aligned and single base changes detected using MAQ, and the number of sequences spanning five single base changes (including ubc4-G48D) was determined. The relationship between library depth and single base change coverage is generally linear but coverage can range from 1.5- to twofold at a given single base change, presumably due to localized differences in sequencing efficiency. (B) The relationship between DI36 library depth and the percentage confidence of finding all 52 single base changes shared between Broad 972h−, wt DG21, and swi*603 strain DI36. Approximately 22× coverage is required to get to 98% confidence of finding all 52 single base changes.
The accuracy of next-generation sequencing is key to this approach and in the course of this study we uncovered about one in 100,000 nucleotide (nt) differences between lab isolates derived from the same strain. Nothing in our analysis indicated that any of these differences were due to miscalled bases, indicating these are real polymorphisms that have arisen since these strains were derived from a common, recent isolate. This raises the possibility that natural variation and environmentally induced variation could be readily examined with this approach. For example, wild strains could be grouped phenotypically for sequence analysis in bulk (bulk segregation analysis) to identify single base changes common to one but not another population. These methods are not unique to yeast genetics. Organisms with small well-characterized genomes, such as Arabidopsis, Caenorhabditis elegans, and Drosophila, lend themselves to this approach and would still only require a single flow cell of data to detect mutations by this method (Sarin et al. 2008; Srivatsan et al. 2008). The range of genomes to which this approach can be applied continues to increase along with the output of next-generation sequencing instruments. In early 2009, resequencing a typical S. pombe mutant strain to 20–25× coverage costs less than $400. One lane of a flow cell with paired 76 nt reads and a high cluster density will yield 2.5 Gb of useful sequence, providing ∼180× coverage of the S. pombe 14-Mb genome. By multiplexing and running several strains per lane the cost of resequencing a mutant strain is a fraction of the cost of a lane ($2400–$2800). The minimal cost and the high likelihood of identifying the causative mutation with 20–25-fold coverage suggest this method as the method of choice for single nucleotide mutation discovery in S. pombe. The use of paired end sequencing coupled with more advanced processing of the resulting data will also enable the identification of chromosomal rearrangements and small indels. Sequencing could thus realistically be applied to reverse genetic approaches, such as TILLING, in which a population of chemically mutagenized plants (or yeast) is interrogated for mutations in known genes (McCallum et al. 2000).
On the basis of our results we propose a comprehensive screen to identify gene networks essential for growth in fission yeast. Using genome sequencing, we were able to determine which of the 10 or more genes that rescued a ts lethal strain was responsible for the mutation. By sequencing each of the 1000 ts lethal strains in this collection (Matsumura et al. 2003; Hayashi et al. 2004; Yuasa et al. 2004), the causative mutation underlying each strain will be revealed, identifying the other rescuing plasmids as allele-specific suppressors. This will rapidly lead to a genetic network of genes essential for growth, complementing more traditional and laborious approaches such as epistasis mapping (Roguev et al. 2008).
Methods
Strains and media
S. pombe strains used in this study are listed in Supplemental Table 2. Yeast cells were cultured in supplemented yeast extract (rich) adenine (YEA) medium. Minimal medium with ammonium chloride as a nitrogen source (EMM2) was used in complementation studies. Crosses and mating-type switching were performed with malt extract plus amino acids (ME+AA) medium. 250 μg/mL of each supplement was added to media to support full growth. 100 μg/mL of G418 was used in YEA to select KanMX6 positive clones. Serial dilution experiments were performed as described previously (Ekwall et al. 1999).
Genetic techniques
To mutagenize the trz1 gene a targeting vector containing the trz1-A623V allele was transformed into wt DG21. The targeting vector constructed in pBlueScript-KS+ consisted of a 2334-nt PCR fragment containing the coding region of trz1 plus some 3′ untranslated sequence. The QuickChange II site-directed mutagenesis kit (Stratagene) allowed the introduction of the alanine 623 to valine (A623V) mutation into the coding sequence of trz1. A KanMX resistance gene was cloned into an NheI site 3′ of the stop codon. To correct the trz1-A623V allele in ts603 the same targeting vector containing trz1+ was transformed into ts603. To mutagenize the ubc4+ gene a targeting vector containing the ubc4-G48D allele was transformed in wt strains DG21 and SPG17. The targeting vector constructed in pGEM-T (Promega) contained a 1563-nt PCR fragment containing the coding region of the ubc4-G48D allele amplified from ts603 and some 3′ untranslated sequence. The KanMX6 resistance gene was cloned into a SnaBI site 3′ of the stop codon. To correct the ubc4-G48D allele in ts603 a targeting vector containing ubc4+ was constructed as described above. All plasmid DNA was linearized and transformed as described in Krawchuk and Wahls (1999). Transformants were confirmed for site-specific recombination by PCR, the presence of the mutant or wt allele by a combined PCR/HphI digest, and the integrity of the remaining coding sequence by Sanger sequencing.
RNA analysis
Total RNA was extracted from cells growing at exponential phase in YEA using RiboPure Yeast (Ambion). RNA was treated with 8U of DNase I (DNA-free, Ambion) and analyzed by RT-PCR (One-Step RT-PCR Kit, Qiagen). Cycles of PCR for dg repeat, dh repeat, centromeric ura4+ reporter gene, and actin control were 28 cycles. Amplified DNA was separated in 2.0% agarose gels, stained with ethidium bromide, and visualized by Fluor-S Multimager (Bio-Rad).
Whole-genome resequencing and single base change discovery
Genomic DNA libraries for Illumina sequencing were prepared according to the manufacture's instructions. Libraries were sequenced on an Illumina Genome Analyzer. The resulting 32–36-bp sequence reads, including quality scores, were aligned to the S. pombe reference sequence using MAQ (Li et al. 2008) using the “map” function. A consensus genome was built using MAQ “assemble” using only sequence reads with a phred-scaled alignment quality of 30 or better. Single base changes were detected using the MAQ “cns2snp” function. Single base changes with phred-scaled quality scores <60 or with fewer than six reads covering them were discarded. Single base changes found in this study are listed in Supplemental Tables 3–6.
Acknowledgments
D.V.I. is a C.J. Martin postdoctoral fellow and D.B.G. was a U.S. Department of Energy-Biosciences postdoctoral fellow of the Life Sciences Research Foundation. Research in the authors' laboratory is supported by a grant from NIH to R.M. (RO1-GM076396).
Footnotes
[Supplemental material is available online at www.genome.org and at http://hispaniola.cshl.edu/irvine2009a/.]
Article published online before print. Article and publication date are at http://www.genome.org/cgi/doi/10.1101/gr.089318.108.
References
- Allshire R.C., Nimmo E.R., Ekwall K., Javerzat J.P., Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes & Dev. 1995;9:218–233. doi: 10.1101/gad.9.2.218. [DOI] [PubMed] [Google Scholar]
- Ekwall K., Cranston G., Allshire R.C. Fission yeast mutants that alleviate transcriptional silencing in centromeric flanking repeats and disrupt chromosome segregation. Genetics. 1999;153:1153–1169. doi: 10.1093/genetics/153.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal S.I., Jia S. Heterochromatin revisited. Nat. Rev. Genet. 2007;8:35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Fujita Y., Iwasaki O., Adachi Y., Takahashi K., Yanagida M. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. 2004;118:715–729. doi: 10.1016/j.cell.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hillier L.W., Marth G.T., Quinlan A.R., Dooling D., Fewell G., Barnett D., Fox P., Glasscock J.I., Hickenbotham M., Huang W., et al. Whole-genome sequencing and variant discovery in C. elegans. Nat. Methods. 2008;5:183–188. doi: 10.1038/nmeth.1179. [DOI] [PubMed] [Google Scholar]
- Javerzat J.P., Cranston G., Allshire R.C. Fission yeast genes which disrupt mitotic chromosome segregation when overexpressed. Nucleic Acids Res. 1996;24:4676–4683. doi: 10.1093/nar/24.23.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S., Kobayashi R., Grewal S.I. Ubiquitin ligase component Cul4 associates with Clr4 histone methyltransferase to assemble heterochromatin. Nat. Cell Biol. 2005;7:1007–1013. doi: 10.1038/ncb1300. [DOI] [PubMed] [Google Scholar]
- Kato H., Goto D.B., Martienssen R.A., Urano T., Furukawa K., Murakami Y. RNA polymerase II is required for RNAi-dependent heterochromatin assembly. Science. 2005;309:467–469. doi: 10.1126/science.1114955. [DOI] [PubMed] [Google Scholar]
- Krawchuk M.D., Wahls W.P. High-efficiency gene targeting in Schizosaccharomyces pombe using a modular, PCR-based approach with long tracts of flanking homology. Yeast. 1999;15:1419–1427. doi: 10.1002/(SICI)1097-0061(19990930)15:13<1419::AID-YEA466>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Goto D.B., Zaratiegui M., Tang X., Martienssen R., Cande W.Z. Two novel proteins, dos1 and dos2, interact with rik1 to regulate heterochromatic RNA interference and histone modification. Curr. Biol. 2005;15:1448–1457. doi: 10.1016/j.cub.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Li H., Ruan J., Durbin R. Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res. 2008;18:1851–1858. doi: 10.1101/gr.078212.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi P., Carraway M., Marinus M.G. Analysis of forward mutations induced by N-methyl-N′-nitro-N-nitrosoguanidine in the bacteriophage P22 mnt repressor gene. J. Bacteriol. 1986;166:34–37. doi: 10.1128/jb.166.1.34-37.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardis E.R. The impact of next-generation sequencing technology on genetics. Trends Genet. 2008;24:133–141. doi: 10.1016/j.tig.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Yuasa T., Hayashi T., Obara T., Kimata Y., Yanagida M. A brute force postgenome approach to identify temperature-sensitive mutations that negatively interact with separase and securin plasmids. Genes Cells. 2003;8:341–355. doi: 10.1046/j.1365-2443.2003.00637.x. [DOI] [PubMed] [Google Scholar]
- McCallum C.M., Comai L., Greene E.A., Henikoff S. Targeting induced local lesions IN genomes (TILLING) for plant functional genomics. Plant Physiol. 2000;123:439–442. doi: 10.1104/pp.123.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;1994:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Passmore L.A., Barford D. Getting into position: The catalytic mechanisms of protein ubiquitylation. Biochem. J. 2004;379:513–525. doi: 10.1042/BJ20040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski M.D., Deshaies R.J. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- Roguev A., Bandyopadhyay S., Zofall M., Zhang K., Fischer T., Collins S.R., Qu H., Shales M., Park H.O., Hayles J., et al. Conservation and rewiring of functional modules revealed by an epistasis map in fission yeast. Science. 2008;322:405–410. doi: 10.1126/science.1162609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin S., Prabhu S., O'Meara M.M., Pe'er I., Hobert O. Caenorhabditis elegans mutant allele identification by whole-genome sequencing. Nat. Methods. 2008;5:865–867. doi: 10.1038/nmeth.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino H., Kishi T., Nishitani H., Yamao F. Two ubiquitin-conjugating enzymes, UbcP1/Ubc4 and UbcP4/Ubc11, have distinct functions for ubiquitination of mitotic cyclin. Mol. Cell. Biol. 2003;23:3497–3505. doi: 10.1128/MCB.23.10.3497-3505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsan A., Han Y., Peng J., Tehranchi A.K., Gibbs R., Wang J.D., Chen R. High-precision, whole-genome sequencing of laboratory strains facilitates genetic studies. PLoS Genet. 2008;4:e1000139. doi: 10.1371/journal.pgen.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood V.R., Gwilliam M.A., Rajandream M., Lyne R., Lyne A., Stewart J., Sgouros N., Peat J., Hayles S., Baker D., et al. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871–880. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- Yuasa T., Hayashi T., Ikai N., Katayama T., Aoki K., Obara T., Toyoda Y., Maruyama T., Kitagawa D., Takahashi K., et al. An interactive gene network for securin-separase, condensin, cohesin, Dis1/Mtc1 and histones constructed by mass transformation. Genes Cells. 2004;9:1069–1082. doi: 10.1111/j.1365-2443.2004.00790.x. [DOI] [PubMed] [Google Scholar]
- Zaratiegui M., Irvine D.V., Martienssen R.A. Noncoding RNAs and gene silencing. Cell. 2007;128:763–776. doi: 10.1016/j.cell.2007.02.016. [DOI] [PubMed] [Google Scholar]