Abstract
Mn(III) N-alkylpyridylporphyrins are among the most potent known SOD mimics and catalytic peroxynitrite scavengers, and modulators of redox-based cellular transcriptional activity. In addition to their intrinsic antioxidant capacity, bioavailability plays major role in their in vivo efficacy. While of identical antioxidant capacity, lipophilic MnTnHex-2-PyP is up to 120-fold more efficient in reducing oxidative stress injuries than hydrophilic MnTE-2-PyP. Due to limitations of analytical nature, porphyrin lipophilicity has been often estimated by thin-layer chromatographic Rf parameter, instead of the standard n-octanol/water partition coefficient, POW. Herein we used a new methodological approach to finally describe the MnP lipophilicity, by the conventional log POW means, for a series of biologically active ortho and meta isomers of Mn(III) N-alkylpyridylporphyrins. Three new porphyrins (MnTnBu-3-PyP, MnTnHex-3-PyP and MnTnHep-2-PyP) were synthesized to strengthen the conclusions. The log POW was linearly related to Rf and to the number of carbons in the alkyl chain (nC) for both isomer series; the meta isomers being 10-fold more lipophilic than the analogous ortho porphyrins. Increasing the length of the alkyl chain for 1 carbon atom increases the log POW value ~ 1 log unit with both isomers. Dramatic ~4 and ~5 orders of magnitude increase in lipophilicity of ortho isomers by extending pyridyl alkyl chains from 2 (MnTE-2-PyP, log POW = −6.25) to 6 (MnTnHex-2-PyP, log POW = −2.29) and 8 carbon atoms (MnTnOct-2-PyP, log POW = −0.77) parallels the increased efficacy in several oxidative-stress injury models, particularly those of the central nervous system where transport across the blood-brain barrier is critical. Although meta isomers are only slightly less potent SOD mimics and antioxidants than their ortho analogues, their higher lipophilicity and smaller bulkiness may lead to a higher cellular uptake and overall similar effectiveness in vivo.
Introduction
Oxidative stress is a common state in most pathological conditions such as cancer, diabetes, radiation injury, disorders of central nervous system (Alzheimer’s, Parkinson’s, multiple sclerosis, amyotrophic lateral sclerosis, stroke, spinal cord injury, chronic morphine tolerance), etc [1]. Thus synthetic antioxidants such as metal complexes (Mn salens [2], Mn cyclic polyamines [3], Mn porphyrins (MnP) [4]) and nitroxides [5] have been developed. Given the key role of mitochondria, compounds that accumulate there, such as MitoQ [6, 7] and Mn porphyrins [8] are of particular interest.
We have developed metalloporphyrin-based compounds that are potent mimics of superoxide dismutase based on structure-activity relationships (SAR) between the ability of MnPs to catalytically remove superoxide and the metal-centered redox potential for the MnIIIP/MnIIP redox couple, E1/2 [9, 10]. The ability of Mn porphyrins to scavenge superoxide parallels their ability to catalytically remove peroxynitrite, a more harmful and powerful oxidant in vivo [11]. Levels of O2·− and ONOO− and the other species that they generate (OH·, H2O2, CO3·−, NO2·) are increased under pathological conditions and damage biological molecules leading to their loss of function. Such species also upregulate cellular transcription, propagating oxidative stress and, thus, the related disease condition. Indeed we have found that Mn porphyrins inhibit activation of major transcription factors, AP-1 [12], NF-κB [13,14] and HIF-1α [15].
The Mn(III) ortho meso-tetrakis-N-ethylpyridylporphyrin, MnTE-2-PyP is the prototypical MnP-based SOD mimic/peroxynitrite scavenger most studied in vivo [16 – 25]. Its efficacy in vivo is at least in part due to its accumulation in mitochondria [8] and, despite its overall 5+ charge, its pharmacokinetics in the mouse shows that the drug distributes into major tissues and organs, including the brain [15].
The most efficacious compounds with high kcat, Mn(III) ortho meso-tetrakis-N-alkylpyridylporphyrins, have positively charged groups in ortho positions, in close vicinity to the metal site. Such design affords thermodynamic and electrostatic facilitation for the approach of negatively charged superoxide to the Mn center [10]. Recent studies have strongly supported the assumption that, in addition to high antioxidant potency, MnP bioavailability plays a major role, particularly in central nervous system disorders [26]. A MnTE-2-PyP analogue of equivalent antioxidant potency has been designed and synthesized with longer alkyl chains to yield an intuitively more lipophilic derivative, i.e. MnTnHex-2-PyP (Figure 1) [9]. Indeed, we have shown that MnTnHex-2-PyP is more effective in facilitating aerobic growth of SOD-deficient E. coli than its ethyl analogue (due at least in part to the fact that it accumulates in E. coli better [27]), thus offering protection at > 2 orders of magnitude lower concentration than MnTE-2-PyP [27]. The SOD-deficient E. coli that grew in the presence of MnTnHex-2-PyP has significantly higher ability to dismute O2·− than if E. coli grew in the presence of MnTE-2-PyP [27]. In animal models, MnTnHex-2-PyP was up to 120-fold more effective than MnTE-2-PyP (see Discussion also) at lowest therapeutically relevant doses (0.05 mg/kg) than any synthetic antioxidant thus far reported [1–4]. In rats, MnTnHex-2-PyP distributes between blood and brain at an 8:1 ratio 30 min after iv injection, while the corresponding ratio is 100:1 with MnTE-2-PyP [13, 15] [2]). In cerebral palsy model[3] preliminary data show that only MnTnHex-2-PyP, but not MnTE-2-PyP, was effective when given to pregnant rabbit dam twice (intravenously), before and after uterus ischemia at only 0.05 mg/kg (0.6 mg total dose per rabbit dam). The efficacy has been ascribed to the improved ability of MnTnHex-2-PyP to cross several lipid barriers before entering fetal brain [3]. While both MnTE-2-PyP and MnTnHex-2-PyP are promising for clinical development, the latter is advantageous for central nervous system (CNS) injuries due to its higher ability to cross the blood-brain barrier. To note, both MnTE-2-PyP and MnTnHex-2-PyP are extremely stable and do not lose redox-active Mn site in the presence of other ligands (EDTA). No loss of MnTnHex-2-PyP happens within 3 months in 12 M HCl and only 50% of MnTE-2-PyP was lost within a month [9].
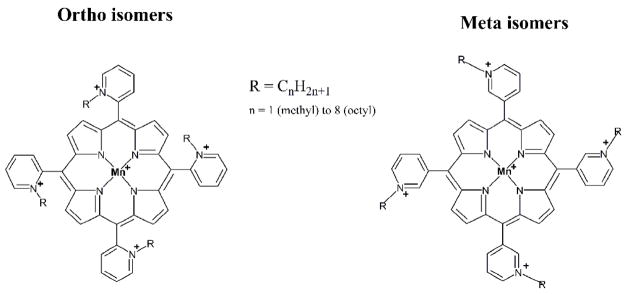
Figure 1.
Structures of ortho and meta isomers of Mn(III) N-alkylpyridylporphyrins.
Whereas the lipophilicity of MnPs seems to be a critical parameter for their action in vivo, it is often estimated in terms of the thin-layer chromatographic (TLC) retention factor values (Rf), which is not a common pharmaceutical parameter of drug lipophilicity and is meaningless for medical and pharmaceutical audiences, as it precludes the comparison with other drugs targeting similar diseases. A more familiar lipophilicity descriptor is the n-octanol/water partition coefficients (POW). However, MnPs distribute into n-octanol at extremely low levels, which imposes difficulties of analytical nature in determining log POW. Herein we have adapted a recent strategy [28] to determine the log POW of ortho and meta isomeric Mn porphyrins, and show that actual log POW, whose measurements are cumbersome, can be quickly and easily predicted based upon simple and straightforward Rf determinations.
Methods
General
Xanthine and equine ferricytochrome c (lot no. 7752) were from Sigma, whereas xanthine oxidase was prepared by R. Wiley [29] and was a gift from K.V. Rajagopalan. MnCl2·4H2O was purchased from J. T. Baker. Anhydrous N,N-dimethylformamide (DMF, Sigma-Aldrich Chemical Co) was kept over 4 Å molecular sieves. Ethyl ether (anhydrous), acetone, chloroform, and aluminum-backed silica gel 60 F254 TLC sheets were from EMD. NH4PF6 (>99.9999% purity) was from Advance Research Chemicals, Inc. KNO3 and methanol were from Mallinckrodt. Acetonitrile was from Fisher Scientific. Plastic-backed silica gel TLC plates (Z122777-25EA, Batch#3110), tetrabutylammonium chloride and ethyl p-toluenesulfonate were purchased from Sigma-Aldrich. The n-butyl, n-heptyl and n-hexyl esters of p-toluenesulfonic acid were purchased from TCI America. All other chemicals and solvents were of analytical grade and used without further purification.
Electrospray ionization mass spectrometric analyses (ESI-MS) were performed as described elsewhere [30] on an Applied Biosystems MDS Sciex 3200 Q Trap LC/MS/MS spectrometer at Duke Comprehensive Cancer Center, Shared Resource PK Labs; all samples of ~5 μM concentration were prepared in a H2O-acetonitrile (1:1, v/v; containing 0.1 % v/v HFBA) mixture and infused for 1 min at 10 μL/min into the spectrometer (curtain gas = 20 V; ion spray voltage = 3500 V; ion source = 30 V; T = 300 °C; declustering potential = 20 V; entrance potential = 1 V; collision energy = 5 V; gas = N2).
UV/vis spectra were recorded in H2O at room temperature on a Shimadzu UV-2501PC spectrophotometer with 0.5 nm resolution.
Elemental analyses were done by Atlantic MicroLab, Norcross, GA.
Porphyins
Caution needs to be exercised with all chemicals used in the synthesis including commercial porphyrins as their quality is variable [30]. The synthesis and characterization of all ortho isomers other than the n-heptyl analogue, which is given below, have been reported elsewhere [9]. The preparation of the other new compounds MnTnBu-3-PyP and MnTnHex-3-PyP are described below. N-alkylation . N-alkylation was performed as described previously [9]. Typically, to 100 mg of H2T-2(3)-PyP (Frontier Scientific) in 20 mL DMF at 100°C was added 4 mL of the corresponding p-toluenesulfonate. Reaction was followed by thin-layer chromatography on silica gel TLC plates using 8:1:1 acetonitrile:KNO3-saturated H2O:H2O as the mobile phase. Duration of N-alkylation is dependent upon the type of isomer and length of alkyl chains and ranged between few hours to few days. The course of N-alkylation was followed by TLC until no further change was observed. With meta isomers a single spot marked the completion of N-alkylation, while atropoisomers were observed with H2TnHep-2-PyP. Upon completion, the reaction mixture was poured in a separatory funnel containing 200 ml each of water and chloroform and shaken well. The chloroform layer was discarded and extraction with CHCl3 was repeated several times. The aqueous layer was filtered and the porphyrin was precipitated as PF6− salt by dropwise addition of a concentrated aqueous solution of NH4PF6, until no further precipitate formed. The precipitate was filtered and thoroughly washed with diethyl ether. After air drying the precipitate was dissolved in acetone, filtered and precipitated as the chloride salt by the addition of an acetone solution of tetrabutylammonium chloride. The precipitate was washed thoroughly with acetone and dried in vacuum at room temperature. Metallation. Metallation was achieved as previously described [9]. In brief, 20-fold excess MnCl2 is added to a pH 12.5 solution of porphyrins at 25 °C. Metallation occurs in a few hours at room temperature for butyl, but requires overnight heating at 100°C for the heptyl and hexyl porphyrins. The reaction progress was followed by uv/vis spectroscopy and TLC (8:1:1=acetonitrile:KNO3-saturated H2O:H2O) until the disappearance of both Soret band and fluorescence of the metal-free porphyrin. Upon completion, the suspension (excess of free Mn in the form of Mn oxo/hydroxo species) was filtered off, and the filtrate was precipitated with aqueous solution of NH4PF6 and washed with ether. The precipitate was dissolved in acetone, precipitated with an acetone solution of tetrabuthylammonium chloride and the resulting precipitate was washed with acetone. The PF6−(aq)/Cl−(acetone) precipitation procedure was repeated twice to assure removal of free manganese.
Metal-free porphyrins and their metal complexes were characterized by thin-layer chromatography, elemental analysis, UV/vis spectroscopy and electrospray ionization mass spectrometry (ESI-MS). UV/vis data: the molar absorption coefficients and corresponding λmax are summarized in Table 1, whereas ESI-MS data for the complexes are given in Table 2 and the peak assignments are consistent with those described for related compounds [30]. Elemental analysis: H2TnBu-3-PyPCl4·9H2O. (C56H80N8O9Cl4) Found: C, 58.99; H, 6.77; N, 9.88; Cl, 12.43 Calculated: C, 58.43; H, 7.00; N, 9.73; Cl, 12.31 %. H2TnHex-3-PyPCl4·13H2O (C64H104N8O13Cl4) Found: C, 57.49; H, 8.63; N, 8.49; Cl, 10.45 Calculated: C, 57.56; H, 7.85; N, 8.93; Cl, 10.45 %. H2TnHep-2-PyPCl4·12.5 H2O (C68H111N8O12.5 Cl4) Found: C, 58.93; H, 7.54; N, 8.12; Cl, 10.24 Calculated: C, 59.08; H, 8.08; N, 8.11; Cl, 10.26 %. MnTnBu-3-PyPCl5·14H2O (MnC56H88N8O14Cl5) Found: C, 50.59; H, 6.76; N, 8.45; Cl, 13.45 Calculated: C, 50.74; H, 5.85; N, 8.42; Cl, 13.33 %. MnTnHex-3-PyPCl5·13H2O Found: C, 53.80; H, 6.47; N, 7.88; Cl, 12.33 Calculated: C, 53.99; H, 7.22; N, 7.87; Cl, 12.33 %. MnTnHep-2-PyPCl5·20.5H2O (MnC68H125N8O20.5Cl5) Found: C, 50.43; H, 6.42; N, 7.24; Cl, 9.99 Calculated: C, 50.45; H, 8.02; N, 6.82; Cl, 10.85 %.
Table 1.
Molar absorption coefficients of chloride salts of metal-free porphyrins and their Mn(III) complexes synthesized in this work. The data for other compounds can be found elsewhere [9, 29][5] .
| Porphyrin | λmax ( log εa) |
|---|---|
| H2TnBu-3-PyP | 810.0(2.72); 638.5 (3.09); 582.0 (3.85); 514.5 (4.28); 417.5(5.53); 262.5 (4.41) |
| H2TnHex-3-PyP | 830.0 (3.08); 823.0(3.05); 582.0 (3.76); 525.0 (4.20); 417.5 (5.43); 330.0 (4.28); 317.0 (4.25); 288.0 (4.08); 263.5 (4.34) |
| H2TnHep-2-PyP | 639.0 (3.47); 584.0 (3.86); 543.0 (3.65); 510.5 (4.27); 416.5 (5.34); 263.0 (4.36) |
| MnTnBu-3-PyP | 843.0 (2.54); 830.0 (2.46); 786.0 (3.27); 675.0 (3.43); 558.0 (4.04); 502.5 (3.75); 460.0 (5.06); 396.0 (4.60); 373.5 (4.62); 262.0 (4.50) |
| MnTnHex-3-PyP | 565.5 (3.29); 675.5 (3.18); 557 (4.03); 501.5 (3.75); 460.0 (5.06); 395.5 (4.57); 372.0 (4.59); 330.5 (4.43); 262.0 (4.45) |
| MnTnHep-2-PyP | 779.5 (3.23); 559.5 (4.08); 502.5 (3.79); 454.0 (5.19); 414.5 (4.45); 364.5 (4.66); 261.5 (4.49) |
Molar absorption coefficient (M−1 cm−1) were determined in water at room temperature (± 5%). λmax (nm) were determined with errors within 0.5 nm.
Table 2.
Electrospray ionization mass spectrometry of Mn(III) N-alkylpyridylporphyrins. Samples at 5 μM concentration were prepared in a H2O-acetonitrile (1:1, v/v; containing 0.1 % v/v HFBA).
| Peak Assignment | m/z | |||||
|---|---|---|---|---|---|---|
| MnTnBu-3-PyPCl5 | MnTnHex-3-PyPCl5 | MnTnHep-2-PyPCl5 | ||||
| Found | Calculated | Found | Calculated | Found | Calculated | |
| [MnT(alkyl)-2,3-PyP5+ + 2HFBA−]3+/3 | 442.3, | 442.1 | 479.7 | 479.7 | 498.4 | 498.2 |
| [MnT(alkyl)-2,3-PyP5+ + HFBA—2H+]2+/2 | - | 600.6 | 612.5 | 612.2 | 639.5 | 640.2 |
| [MnT(alkyl)-2,3-PyP5+ + 2HFBA− - H+]2+/2 | 663.2, | 662.6 | - | 718.7 | 746.8 | 746.7 |
| [MnT(alkyl)-2,3-PyP5+ + 3HFBA−]2+/2 | 769.5 | 769.6 | 825.7 | 825.7 | 853.5 | 853.8 |
Thin-layer chromatography
Thin-layer chromatography of the purified compounds was performed on two types of silica gel TLC plates (plastic- or aluminum-backed) with 1:1:8 KNO3-saturated H2O: H2O: acetonitrile mixture as mobile phase. Typically, 1 μL of ~1 mM samples were applied at ~ 1cm of the strip border and the solvent front was allowed to run ~12 cm. The absolute Rf values and their ratio thereoff are sensitive to the degree of the saturation of vapor phase in the TLC chamber and may differ slightly from one to another experiment. Thus, the internal standardization of TLC is required for comparison purposes [30].
Partition coefficient
Partition between water and n-butanol was determined experimentally with more lipophilic ortho and meta isomeric Mn(III) meso-tetrakis-N-alkylpyridylporphyrins using a variation of the shake-flask method [28]. Briefly, 0.1 mL of a MnP solution in n-butanol-saturated water was shaken with 1 mL of water-saturated n-butanol for 3 min using Vortex-Genie in plastic tube. The biphasic mixture was centrifuged (3 min at 6000 rpm) and the layers were separated. The concentration of the MnP in each layer was measured spectrophotometrically. If dilution was needed for the UV/vis measurements, the aqueous layer was diluted with water, whereas for the organic layer, water-saturated n-butanol was used instead. Spectrum baseline was chosen accordingly. The log PBW was calculated using equation (1):
| (1) |
The log PBW values were converted to log POW using equation (2) as established recently [28] employing compounds that can be easily measured in both n-octanol and n-butanol (caffeine, pyridine, cyclohexanone, pyridazine, thiourea).
| (2) |
The POW values for the hydrophilic members of the porphyrin series were calculated from the log POW vs Rf correlation defined in this work (see below).
SOD-like activity
Determination of the catalytic rate constant for O2·− dismutation using cyt c assay was performed as previously described in details [9].
Results
SOD-activity
All MnP studied here are good-to-potent SOD mimics. The catalytic rate constants for the dismutation of O2·− are given in Table 3.
Table 3.
The characterization of the lipophilicity and SOD-like activity of chloride salts of ortho and meta isomers of Mn(III) N-alkylpyridylporphyrins. Rf values refer to those calculated using plastic-backed TLC plates (Rf(Plastic)).
| Porphyrin | Rf | log POW (determined)a | log POW (predicted) | log kcat |
|---|---|---|---|---|
| MnTM-2-PyP | 0.03 | −7.40c | 7.79 | |
| MnTE-2-PyP | 0.06 | −6.43c | 7.76 | |
| MnTnPr-2-PyP | 0.11 | −5.47c | 7.38 | |
| MnTnBut-2-PyP | 0.19 | −4.64 | 7.25 | |
| MnTnHex-2-PyP | 0.38 | −2.29 | 7.48 | |
| MnTnHep-2-PyP | 0.46 | −1.63 | 7.65 | |
| MnTnOct-2-PyP | 0.49 | −0.77 | 7.71 | |
| MnTM-3-PyP | 0.05 | −6.49d | 6.61 | |
| MnTE-3-PyP | 0.10 | −5.41d | 6.83 | |
| MnTnPr-3-PyP | 0.22 | −4.53 | 6.85 | |
| MnTnBut-3-PyP | 0.40 | −3.56 | 6.74 | |
| MnTnHex-3-PyP | 0.55 | −1.60 | 6.72 |
log PBW was determined experimentally using equation (1) and was converted to POW using equation (2);
conditions: 0.05 M phosphate buffer, 0.1 mM EDTA, pH 7.8, 40 μM xanthine, ~ 2nM xanthine oxidase, 10 μM cytochrome c. The rate of cytchrome c reduction by O2·−, kcyt c = 2.6 x 105 M−1 cm−1 is used for calculation of kcat [44]. The estimated errors are within ±10 %.
Calculated according to eq. (5).
Calculated according to eq. (6).
Thin-layer chromatography
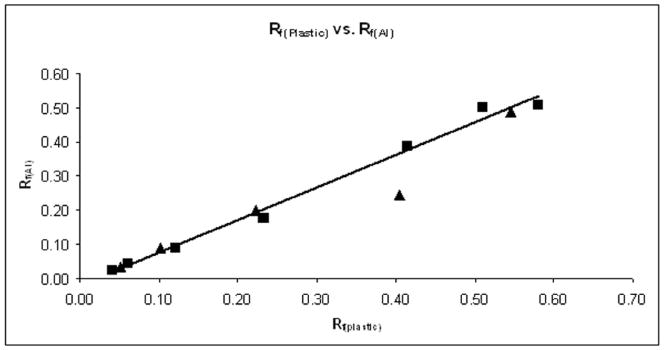
The Rf values measured for the compounds in silica gel plates (plastic-backed, Rf(Plastic)) are given in Table 3. A linear relationship between Rf(Plastic) values and the corresponding values obtained using aluminum-backed plates (Rf(Al)) was obtained and is depicted in Figure 2. Our data show that both plates exhibit equally well the effect of the lengthening of the alkyl chains. Because of such a linear relationship and for convenience, all Rf vales from hereon will refer to those calculated using plastic-backed TLC plates (Rf(Plastic)).
Figure 2.
The relationship between Rf obtained on TLC plastic-backed silica gel plates (Rf(Plastic)) and on TLC aluminum-backed silica gel plates (Rf(Al)) in acetonitrile: KNO3-saturated H2O:H2O = 8:1:1. The ortho porphyrins are presented as squares (from methyl to octyl) and meta as triangles (from methyl to hexyl). The relationship is described with equation Rf(plastic) = 0.91 x Rf(Al) + 0.01, R2 = 0.9609.
Partition coefficients
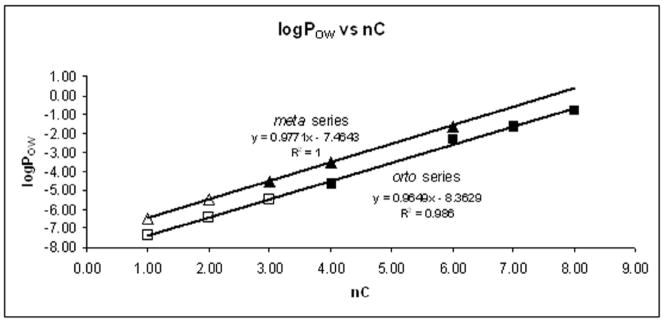
The log POW for the MnPs are given in Table 3. Linear relationships between log POW and the number of carbon atoms in the alkyl chain were observed in the series of both isomers (Fig. 3). Of note, all meta isomers (eq. 3) are more lipophilic than their respective ortho analogues (eq. 4).
Figure 3.
Relationship between the log POW and the number of carbon atoms (nC) for the series of ortho and meta Mn(III) N-alkylpyridylporphyrins. Full squares (ortho isomers) and triangles (meta isomers) present experimental values (determined in water/n-butanol system and converted to water/n-octanol system using equation 2) while empty squares and triangles present predicted values, respectively. The porphyrins are from bottom to top: MnTM-2,3-PyP, MnTE-2,3-PyP, MnTPr-2,3-PyP, MnTnBu-2,3-PyP, MnTnHex-2,3-PyP, MnTnHep-2-PyP, MnTnOct-2,3-PyP.
| (3) |
| (4) |
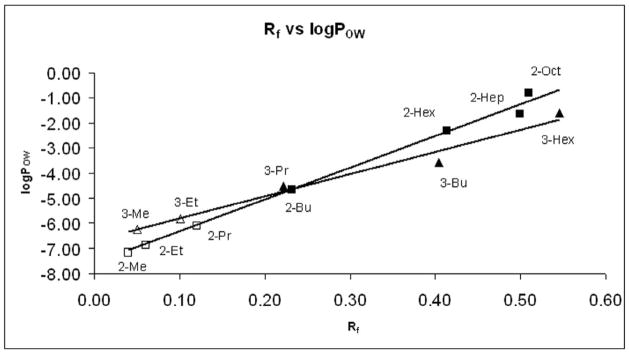
Linear correlations between the log POW and the Rf values were also observed (Figure 4, eqs. 5 and 6). This provides an easy tool for prediction of log POW values of hydrophilic Mn porphyrins, of which direct measurement of log POW values is experimentally inaccessible. Such an approach has been used for calculating the log POW of the lower members of the ortho and meta series, including MnTE-2-PyP, as presented in Table 3.
Figure 4.
Relationship between the thin-chromatographic parameter, Rf and log POW for the series of ortho and meta Mn(III) N-alkylpyridylporphyrins. Rf values refer to those calculated using plastic-backed TLC plates (Rf(Plastic)). Full squares (ortho isomers) and triangles (meta isomers) present experimental values (determined in n-butanol/water system and converted to n-octanol/water system using equation 2) while empty squares and triangles present predicted values, respectively. The porphyrins are from bottom to top: MnTM-2,3-PyP (2,3-Me), MnTE-2,3-PyP (2,3-Et), MnTPr-2,3-PyP (2,3-Pr), MnTnBu-2,3-PyP (2,3-Bu), MnTnHex-2,3-PyP (2,3-Hex), MnTnHep-2,3-PyP (2,3-Hep), MnTnOct-2,3-PyP (Rf value was determined on silica gel plates with plastic base in acetonitrile: KNO3-saturated H2O:H2O = 8:1:1.) Equations for ortho series is log POW = 12.69 x Rf - 7.59 (R2 = 0.9672) and for meta series is log POW = 8.89 x Rf - 6.70 (R2 = 0.9316).
| (5) |
| (6) |
Discussion
While development of MnP-based experimental therapeutics of tailored bioavailability have been intensively pursued [26, 27], limitations of analytical nature have often precluded appropriate quantification of the lipophilicity of the candidate drugs [31]. In the particular case of Mn(III) ortho N-alkylpyridylporphyrins, this issue has been alternatively worked around by describing the lipohilic character of the therapeutics by using their chromatographic Rf values. Such characterization of lipophilicity, while sound and relevant to chemists, is less descriptive and meaningful from a pharmaceutical/medical point of view. Partition coefficient between n-octanol and water is a common pharmaceutical measure of drug lipophilicity/bioavailability. Therefore, for a broader audience, log POW values would relate more convincingly the increase in the in vivo efficacy of a drug to its lipophilicity, and would also allow comparison of MnPs to other drugs of similar therapeutic use. A strategy to overcome the technical difficulties in determining POW values of compounds that do not distribute directly into the n-octanol phase at levels measurable by convenient methods (e.g., cationic MnP) may involve the use of an alternative solvent to n-octanol (such as n-butanol) and a corresponding calibration curve relating POW to the partition in the new solvent [32]. This approach was recently shown adaptable to porphyrin POW measurements [28] and was used here. The n-butanol/water distribution of the more lipophilic cationic Mn porphyrins was then measured and converted to n-octanol/water partition (eq. 2, Table 3). The POW for the hydrophilic members of the porphyrin series were calculated from the appropriate log POW vs Rf relationship (Table 3).
The log POW vs nC
Our data show that the log POW is linearly related to the number of carbon atoms in the alkyl side chain (nC) for both ortho and meta isomers. The slope of the curves is close to one in both systems, which means that each CH2 group in the side chain contributes to an ~10-fold increase in lipophilicity. Additionally, the parallel displacement of the meta isomer series by 1 log unit relative to the ortho series indicates that the meta isomers are ~10-fold more lipophilic than the corresponding ortho species (Figs. 3 and 4). Overall, this means that meta compounds share the same lipophificity of the ortho isomers that bear alkyl chains with one more CH2 group. Thus, the same lipophilicity of a shorter meta hexyl species (MnTnHex-2-PyP) was achieved with the longer ortho heptyl porphyrin, MnTnHep-2-PyP (Table 3).
The log POW vs Rf
We established that with both meta and ortho isomers the log POW values are linearly related to Rf values (Figure 3). It follows, thus, that both parameters are equally valid means for describing MnP lipophilicity. The linear correlation also indicates that significant differences in lipophilicity correspond with small differences in Rf values; e.g., Rf changes of 0.1 for the ortho isomers result in an ~17-fold lipophilicity increase (1.24 log POW change) (Table 3). The 0.32 difference in Rf values between ortho ethyl and hexyl porphyrins (MnTE-2-PyP and MnTnHex-2-PyP) yields 4 orders of magnitude difference in POW value.
As opposed to the log POW vs nC relationships, the log POW vs Rf plots of the ortho and meta series are not parallel to each other. The spatial (ortho vs meta) substitution pattern controls the overall exposure of the porphyrin core and, hence, the adsorption of the compounds onto silica gel [33]. As the core exposure in the meta isomers is considerably less affected by the length of the alkyl side-chain (nC) than the ortho analogues, the dependence of Rf values on nC are less pronounced in the meta isomers. Thus, the log POW are more responsive to Rf values in the ortho series than in the meta series, as indicated by the greater slope of eq. 5 compared to that of eq. 6).
Therapeutics that are widely used in clinics have log P values in a range from ca −3 to ca + 5 [34 – 36]. The members of the ortho series with higher lipophilicity (POW values ≥ ca −2) and in vivo efficacy, such as MnTnOct-2-PyP, MnTnHep-2-PyP, MnTnHex-2-PyP) (Table 2), may be the most promising Mn porphyrins for clinical development, particularly for CNS injuries. In five in vivo and in vitro models of oxidative stress injury MnTnHex-2-PyP proved up to 120-fold more effective than MnTE-2-PyP, offering protection at submicromolar concentrations in cell models and at only 0.05 mg/kg in animal models. In two studies (cerebral palsy and ataxia telangiectasia) the hydrophilic MnTE-2-PyP was ineffective. The models that employed MnTnHex-2-PyP are: E. coli [27], ataxia telangiectasia [37], kidney ischemia/reperfusion [38], chronic morphine tolerance [26], lung radioprotection 1 , stroke 2, cerebral palsy 3, amyotrophic lateral sclerosis G93A mouse model 4. In a hippocampus organotypic slice model the octyl analogue, MnTnOct-2-PyP, was 3000-fold more protective against oxygen and glucose deprivation than the hydrophilic MnTE-2-PyP [43].
Ortho vs meta isomeric Mn(III) N-alkylpyridylporphyrins
We have previously observed that with ortho isomers the SOD activity (kcat) drops from methyl to butyl and then increases again from butyl to octyl porphyrin [9] The data were discussed with respect to the interplay of electrostatic/steric and thermodynamic factors. However, there is essentially no difference in kcat among meta isomers (Table 3), which is consistent with the special charge distribution/eletrostatics experienced by the Mn center in the meta series [40]. Consequently, as the alkyl chain grows from methyl to butyl the difference in kcat between ortho and meta analogues drops from 15-fold for methyl to 3.2-fold for butyl porphyrin (Table 2), while the difference in lipophilicity remains the same (Figure 4). Further, meta isomers are less bulky. We have shown with methyl analogues that meta isomer, MnTM-3-PyP accumulates more in E. coli than bulkier ortho porphyrin MnTM-2-PyP [41]; the protection of aerobic growth of SOD-deficient E. coli with ortho and meta isomers was nearly identical. The unfavorable impact of bulkiness is much more pronounced with bigger molecules [27, 41, 42]. Although imidazolium derivatives (MnTDE-2-ImP and MnTDMOE-2-ImP) [27, 43] are equally potent SOD mimics, they accumulate less in E. coli and are therefore less protective than MnTE-2-PyP [27]. In summary, due to their higher lipophilicity and lesser bulkiness, meta porphyrins may accumulate to higher levels within a cell and that may compensate for somewhat lower kcat; in turn they may be in vivo of similar efficacy as are ortho isomers (work in progress) 5.
According to Hansch et al [34] the ideal compound would have log POW = ~ 2; ortho Mn(III) tetrakis(N-undecylpyridinium-2-yl)porphyrin with log POW = 1.98 or its meta decyl analogue would meet such criteria. Yet, although of appropriate lipophilicity Mn(III) tetrakis(N-undecylpyridinium-2-yl)porphyrin is likely too big to easily accumulate within cell. As discussed above to predict the drug antioxidant efficiency in vivo, other parameters should be considered, particularly stericity. The size and the shape of the molecule would determine the bulkiness of the molecule, but would also affect the shielding of the positive charges; combined these effects would dominate the transport and the interactions (likely unfavorable) with biomolecules which MnP would encounters on its way through the cell. In addition to steric factors, the redox property, i.e. the reducibility of Mn+3 porphyrin site with cellular reductants, must also be accounted for while designing or choosing the appropriate MnP, as experimental therapeutics in oxidative stress models.
Conclusions
Partition of isomeric Mn(III) N-alkylpyridylporphyrins between n-octanol and water, POW, was determined. The log POW is linearly related to thin-layer chromatographic parameter, Rf and to the number of carbon atoms with both ortho and meta isomers. Thus both parameters accurately describe drug lipophilicity. Yet, log POW may be more convenient and meaningful for the pharmaceutical and medical community particularly for the comparison of MnP to other drugs of similar therapeutic target that are in clinics or under development. The longer ortho N-alkylpyridylporphyrins, such MnTnHex-2-PyP and MnTnOct-2-PyP are significantly more lipophilic than MnTE-2-PyP and thus may be promising therapeutics, particularly in the cases of central nervous system injuries. Our data also show that meta isomers are 10-fold more lipophilic than ortho porphyrins and are in addition less bulky. Favorable lipophilicity and stericity would compensate for the lower antioxidant potency making meta isomers prospective antioxidants in vivo also.
Acknowledgments
IK, JSR, ZV and IBH acknowledge support from the National Institutes for Allergy and Infectious Diseases [U19AI067798], National Institute of Health [NIH R01 DA024074] and funding from The Wallace H. Coulter Translational Partners Grant Program; IS thanks NIH/NCI Duke Comprehensive Cancer Center Core Grant [5-P30-CA14236-29]. D.S. and I.B.H. thank for the support from NIH R01 DA024074. We are thankful to Irwin Fridovich for critically reading the manuscript.
Abbreviations Charges are omitted throughout text
- MnP
Mn porphyrin
- MnTalkyl-2
3-PyP5+ Mn(III) meso-tetrakis(N-alkylyridinium-2 or 3-yl)porphyrin, alkyl being methyl (M, AEOL10112), ethyl (E, AEOL10113), n-propyl (nPr), n-butyl (nBu), n-hexyl (nHex), n-heptyl (nHep), n-octyl (nOct)
- 2 and 3 relate to ortho and meta isomers
respectively
- MnTDE-2-ImP5+
Mn(III) tetrakis(N,N’-diethylimidazolium-2-yl)porphyrin, AEOL10150
- MnTDMOE-2-ImP5+
Mn(III) tetrakis[N,N’-di(2-methoxethyl)imidazolium-2-yl)porphyrin
- POW
partition coefficient between n-octanol and water
- PBW
partition coefficient between n-butanol and water
- TLC
thin-layer chromatography
- E1/2
half-wave reduction potential
- SOD
superoxide dismutase
- HIF-1α
hypoxia inducible factor-1, NF-κB, nuclear factor κB
- AP-1
activator protein-1
Footnotes
Gauter-Fleckenstein, B.; Fleckenstein, K.; Owzar, K.; Jiang, C.; Rebouças, J. S.; Batinic-Haberle, I.; Vujaskovic, Z. Early and late administration of antioxidant mimetic MnTE-2-PyP in mitigation and treatment of radiation-induced lung damage, unpublished.
Sheng, H.; Spasojevic, I.; Batinic-Haberle, I.; Warner, D, unpublished.
Tan, S.; Batinic-Haberle, I. The effect of MnTnHex-2-PyP in cerebral palsy rabbit model, unpublished.
row, J. P.; Batinic-Haberle, I. The effect of MnTnHex-2-PyP in delaying death in G93A ALS model, unpublished.
Kos, I.; Benov, L.; Spasojevic, I.; Batinic-Haberle, I. unpublished
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halliwell B, Gutteridge JMC. Free Radical Biology and Medicine. 4. Biosciences; Oxford: 2007. [Google Scholar]
- 2.Van Empel VPM, Bertrand AT, Van Oort RJ, Van der Nagel R, Engelen M, Van Rijen HV, Doevendans PA, Crijns HJ, Ackerman SL, Sluiter W, De Windt LJ. EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. J Am Coll Cardiol. 2006;48:8245–832. doi: 10.1016/j.jacc.2006.02.075. [DOI] [PubMed] [Google Scholar]
- 3.Salvemini D, Wang Z-Q, Zweier JL, Samouilov A, Macarthur H, Misko TP, Curie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity ion rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 4.Batinic-Haberle I, Benov L, Spasojevic I, Hambright P, Crumbliss AL, Fridovich I. The Relationship Between Redox Potentials, Proton Dissociation Constants of Pyrrolic Nitrogens, and in Vitro and in Vivo Superoxide Dismutase Activities of Manganese(III) and Iron(III) Cationic and Anionic Porphyrins. Inorg Chem. 1999;38:4011–4022. [Google Scholar]
- 5.Goldstein S, Samuni A, Hideg K, Merenyi G. Structure-activity relationship of cyclic nitroxides as SOD mimics and scavengers of nitrogen dioxide and carbonate radicals. J Phys Chem A. 2006;110:3679–3685. doi: 10.1021/jp056869r. [DOI] [PubMed] [Google Scholar]
- 6.James AM, Cocheme HM, Smith RAJ, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. J Biol Chem. 2005;28:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- 7.Smith RAJ, Porteous CM, Ganes AM, Murphy MP. Delivery of bioactive molecules to mitochondria. Proc Natl Acad Sci. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spasojevic I, Yumin C, Noel T, Yu I, Pole MP, Zhang L, Zhao Y, StClair DK, Batinic-Haberle I. Mn porphyrin-based SOD mimic, MnTE-2-PyP5+ targets mouse heart mitochondria. Free Radic Biol Med. 2007;42:1193–1200. doi: 10.1016/j.freeradbiomed.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batinic-Haberle I, Spasojevic I, Stevens RD, Hambright P, Fridovich I. Manganese(III) Meso Tetrakis Ortho N-alkylpyridylporphyrins. Synthesis, Characterization and Catalysis of O2·− Dismutation. J Chem Soc, Dalton Trans. 2002:2689–2696. [Google Scholar]
- 10.Rebouças JS, DeFreitas-Silva G, Idemori YM, Spasojevic I, Benov L, Batinic-Haberle I. The impact of electrostatics in redox modulation of oxidative stress by Mn porphyrins. Protection of SOD-deficient E. coli via alternative mechanism where Mn porphyrin acts as a Mn-carrier. Free Radic Biol Med. 2008;45:201–210. doi: 10.1016/j.freeradbiomed.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer-Sueta G, Vitturi D, Batinic-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. eactions of Manganese Porphyrins with Peroxynitrite and Carbonate Radical Anion. J Biol Chem. 2003;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Chaiswing L, Oberley TD, Batinic-Haberle I, StClair W, Epstein CJ, StClair D. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;65:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 13.Sheng H, Sakai H, Yang W, Fukuda S, Salahi M, Day BJ, Huang J, Paschen W, Batinic-Haberle I, Crapo JD, Pearlstein RD, Warner DS. Sustained treatment is required to produce long-term neuroprotective efficacy from a metalloporphyrin catalytic antioxidant in focal cerebral ischemia. Free Radic Biol Med. 2009 submitted . [Google Scholar]
- 14.Tse H, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: Implication for their use in targeting oxidation/reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–47. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Spasojevic I, Chen Y, Noel TJ, Fan P, Zhang L, Rebouças JS, StClair DK, Batinic-Haberle I. Pharmacokinetics of the potent redox modulating manganese porphyrin, MnTE-2-PyP5+ in plasma and major organs of B6C3F1 mice. Free Radic Biol Med. 2008;45:943–949. doi: 10.1016/j.freeradbiomed.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Tarpey MM, Patel RP, Batinic-Haberle I, White CR, Freeman BA. Oxygen Radical Inhibition of Nitric-Oxide Dependent Vascular Function in Sickle Cell Disease. Proc Natl Acad Sci USA. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Csont T, Viappiani S, Sawicka J, Slee S, Altarejos JY, Batinic-Haberle I, Schulz R. The Involvement of Superoxide and iNOS-Derived NO in Cardiac Dysfunction Induced by Pro-Inflammatory Cytokines. J Mol Cell Card. 2005;39:833–840. doi: 10.1016/j.yjmcc.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Cernanec JM, Weinberg BJ, Batinic-Haberle I, Guilak F, Fermor B. Oxygen tension modulates the effects of interleukin-1 on articular cartlidge matrix turnover via peroxynitrite. J Rheumatol. 2007;34:401–407. [PubMed] [Google Scholar]
- 19.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jian C, Batinic-Haberle I, Vujaskovic Z. omparison of two Mn porphyrin-based mimics of superoxide-dismutase (SOD) in pulmonary radioprotection. Free Radic Biol Med. 2008;44:982–989. doi: 10.1016/j.freeradbiomed.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moeller BJ, Batinic-Haberle I, Spasojevic I, Rabbani ZN, Anscher MS, Vujaskovic Z, Dewhirst MW. A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int J Rad Oncol Biol Phys. 2005;63:545–552. doi: 10.1016/j.ijrobp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of oxygenation, free radicals and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 22.Sheng H, Spasojevic I, Warner DS, Batinic-Haberle I. Mouse spinal cord compression injury is ameliorated by intrathecal manganese(III) porphyrin. Neurosci Lett. 2004;366:220–225. doi: 10.1016/j.neulet.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 23.Sompol P, Ittarat W, Tangpong J, Chen Y, Doubinskaia I, Mohammad Abdul H, Butterfield A, StClair DK. A neuronal model of Alzheimer’s disease: An insight into the mechanisms of oxidative stress-mediated mitochondrial injury. Neuroscience. 2008;153:120–130. doi: 10.1016/j.neuroscience.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piganelli JD, Flores SC, Cruz C, Koepp J, Young R, Bradley B, Kachadourian R, Batinic-Haberle I, Haskins K. A Metalloporphyrin Superoxide Dismutase Mimetic (SOD Mimetic) Inhibits Autoimune Diabetes. Diabetes. 2002;51:347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 25.Sheng H, Enghild J, Bowler R, Patel M, Calvi CL, Batinic-Haberle I, Day BJ, Pearlstein RD, Crapo JD, Warner DS. Effects of Metalloporphyrin Catalytic Antioxidants in Experimental Brain Ischemia. Free Radic Biol Med. 2002;33:947–961. doi: 10.1016/s0891-5849(02)00979-6. [DOI] [PubMed] [Google Scholar]
- 26.Batinic-Haberle I, Ndengele MM, Cuzzocrea S, Rebouças JS, Spasojevic I, Salvemini D. Lipophilicity is a critical parameter that dominates the efficacy of metalloporphyrins in blocking morphine tolerance through peroxynitrite-mediated pathways. Free Radic Biol Med. 2009;46:212–219. doi: 10.1016/j.freeradbiomed.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okado-Matsumoto A, Batinic-Haberle I, Fridovich I. Complementation of SOD-deficient Escheria Coli by manganese porphyrin mimics of superoxide dismutase activity. Free Radic Biol Med. 2004;37:401–10. doi: 10.1016/j.freeradbiomed.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 28.Engelmann FM, Rocha SVO, Toma HE, Araki K, Baptista MS. Determination of n-octanol/water partition and membrane binding of cationic porphyrins. Int J of Pharm. 2007;329:12–18. doi: 10.1016/j.ijpharm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Batinic-Haberle I, Benov L, Spasojevic I, Fridovich I. The Ortho Effect Makes Manganese (III) Meso-Tetrakis(N-methylpyridinium-2-yl)Porphyrin (MnTM-2-PyP) a Powerful and Potentially Useful Superoxide Dismutase Mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 30.Rebouças JS, Spasojevic I, Batinic-Haberle I. Quality of Mn-porphyrin-based SOD mimics and peroxynitrite scavengers for preclinical mechanistic/therapeutic purposes. J Pharm Biomed Anal. 2008;48:1046–1049. doi: 10.1016/j.jpba.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahaye D, Muthukumaran K, Hung C-H, Gryko D, Rebouças JS, Spasojevic I, Batinic-Haberle I, Lindsey JS. Design and synthesis of manganese porphyrins with tailored lipophilicity: Investigation of redox properties and Superoxide Dismutase activity. Bioorg Med Chem. 2007;15:7066–7086. doi: 10.1016/j.bmc.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas G. Medicinal Chemistry: An Introduction. 2. John Wiley & Sons; West Sussex: 2007. pp. 65–66. [Google Scholar]
- 33.Wagner RW, Johnson TE, Lindsey JS. Soluble Synthetic Multiporphyrin Arrays. 1. Modular Design and Synthesis. J Am Chem Soc. 1996;118:11166–11180. [Google Scholar]
- 34.Hansch C, Leo A, Mekapati SB, Karup A. QSAR & ADMS. Bioorg Med Chem. 3400;12:3391. doi: 10.1016/j.bmc.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 35.Proudfoot JR. The evolution of synthetic oral drug properties. Bioorg Med Chem Lett. 2005;15:1087 –1090. doi: 10.1016/j.bmcl.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Leo A, Hansch C, Elkins D. Partition coefficients and their uses. Chem Rev. 1971;71:525–616. [Google Scholar]
- 37.Pollard J, Rebouças JS, Durazo A, Kos I, Gralla EB, Valentine JS, Batinic-Haberle I, Gatti RA. Radioprotective effects of manganese-containing superoxide dismutase mimics on ataxia telangiectasia cells. Free Radic Biol Med. 2008 doi: 10.1016/j.freeradbiomed.2009.04.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saba H, Batinic-Haberle I, Munusamy S, Mitchell T, Lichti C, Megyesi J, MacMillan-Crow LA. anganese porphyrin reduces renal injury and mitochondrial damage during ischemia/reperfusion. Free Radic Biol Med. 2007;42:1571–1578. doi: 10.1016/j.freeradbiomed.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batinic-Haberle I, Spasojevic I, Stevens RD, Hambright P, Neta P, Okado-Matsumoto A, Fridovich I. New Class of Potent Catalysts of O2·− Dismutation. Mn(III) methoxyethylpyridyl- and methoxyethylimidazolylporphyrins. J Chem Soc Dalton Trans. 2004:1696–1702. doi: 10.1039/b400818a. [DOI] [PubMed] [Google Scholar]
- 40.Rebouças JS, Spasojevic I, Tjahjono DH, Richaud A, Méndez F, Benov L, Batinic-Haberle I. Redox modulation of oxidative stress by Mn porphyrin-based therapeutics: The effect of charge distribution. Dalton Trans. 2008:1233–1242. doi: 10.1039/b716517j. [DOI] [PubMed] [Google Scholar]
- 41.Batinic-Haberle I, Benov L, Spasojevic I, Fridovich I. The Ortho Effect Makes Manganese (III) Meso-Tetrakis(N-methylpyridinium-2-yl)Porphyrin (MnTM-2-PyP) a Powerful and Potentially Useful Superoxide Dismutase Mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 42.Wise-Faberowski L, Warner DS, Spasojevic I, Batinic-Haberle I. The effect of lipophilicity of Mn (III) ortho N-alkylpyridyl- and diortho N, N’-imidazolylporphyrins in two in-vitro models of oxygen and glucose deprivation -induced neuronal death. Free Radic Res. 2008 doi: 10.1080/10715760902736283. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batinic-Haberle I, Spasojevic I, Stevens RD, Bondurant B, Okado-Matsumoto A, Fridovich I, Vujaskovic Z, Dewhirst MW. New PEG-ylated Mn(III) porphyrins approaching catalytic activity of SOD enzyme. Dalton Trans. 2006:617–624. doi: 10.1039/b513761f. [DOI] [PubMed] [Google Scholar]
- 44.Spasojevic I, Batinic-Haberle I, Stevens RD, Hambright P, Thorpe AN, Grodkowski J, Neta P, Fridovich I. Manganese(III) biliverdin IX dimethyl ester: a powerful catalytic scavenger of superoxide employing the Mn(III)/Mn(IV) redox couple. Inorg Chem. 2001;40:726–739. doi: 10.1021/ic0004986. [DOI] [PubMed] [Google Scholar]