Abstract
While much is known about receptor affinity profiles of antipsychotic medications, less is known about their impact on functional brain systems in patients with schizophrenia. We conducted functional magnetic resonance imaging (fMRI) studies with first-episode schizophrenia patients as they made saccades to unpredictable visual targets before and after 4-6 weeks of antipsychotic treatment. Matched healthy individuals were scanned at similar time intervals. Pretreatment, patients had less activation in frontal and parietal eye fields and cerebellum. After treatment these disturbances were not present, suggesting improved function in attentional and sensorimotor systems. Other pretreatment abnormalities were noted in sensory and ventromedial prefrontal cortex, but after treatment these abnormalities were absent or less prominent, in line with improved function in attentional systems. In addition, although not abnormal at baseline, there was reduced activity after treatment in dorsal prefrontal cortex, dorsal striatum, and dorsomedial thalamus, suggesting a potential adverse effect of treatment on frontostriatal systems, perhaps related to dopamine blockade in the caudate. These findings provide evidence for a complex impact of antipsychotic medication on functional brain systems in schizophrenia and illustrate the potential of neuroimaging biomarkers for both adverse and beneficial drug effects on functional brain systems.
Keywords: saccadic eye movement, cognition, antipsychotic, frontal eye field, parietal eye field
1. Introduction
Studies of brain physiology after antipsychotic treatment in schizophrenia patients have primarily investigated effects on resting metabolism and blood flow (Miller et al., 2001; Ngan et al., 2002; Lahti et al., 2005). This work has been paralleled by short duration drug administration studies in healthy individuals (Honey et al., 2003; Lane et al., 2004). In contrast to studies of resting state physiology, fMRI provides an approach for examining drug effects on the functional brain systems that support cognitive and perceptual abilities in which change is the target for drug treatment. Two prior studies investigated treatment effects on task-related brain activation in initially medication-free first-episode schizophrenia patients and compared them with healthy participants. Each utilized behavioral flexibility tasks to target prefrontal function (Snitz et al., 2005; Brewer et al., 2007). While such work has helped characterize the impact of antipsychotics on prefrontally-mediated executive cognitive functions, more work is needed to improve understanding of medication effects throughout the brain and on more basic neurocognitive systems such as simple attention, sensory, and sensorimotor processing. Such work will help broaden understanding of the clinical effects of antipsychotic treatment, and provide more direct translational linkage to a wider range of animal model systems.
Examining automatic attention systems with simple saccadic eye movement tasks is one such approach. Visually-guided saccades are rapid shifts in gaze from one location to another in the visual field. Studies of nonhuman primates have mapped the neurophysiology and biochemistry of the oculomotor system, defining the unique contributions of different brain regions to sensorimotor and attentional aspects of eye movement control. The generation of visually-guided saccades is tightly linked with exogenous visual attention via processes mediated by neocortical areas that include and overlap with the frontal and parietal eye fields (Corbetta, 1998; Merriam et al., 2001). These cortical eye fields as well as striatum, thalamus, cerebellum, and brainstem contribute to sensorimotor aspects of eye movement control and their regulation by automatic attentional processes. Thus, oculomotor tasks provide a useful strategy for assessing the effects of drug treatment on attentional and sensorimotor brain systems. Of note, antipsychotic drugs have high affinity for dopamine receptors in the caudate nucleus of the striatum, and dopaminergic modulation in the caudate is important for saccade control (Hikosaka et al., 2000; Goldman-Rakic et al., 2004).
FMRI studies of medicated patients performing visually-guided saccades have found either slightly reduced activation in frontal, parietal, and supplementary eye fields, visual cortex, and anterior cingulate (Raemaekers et al., 2002) or no differences (McDowell et al., 2002) compared to healthy controls. In an fMRI study of saccadic eye movements in unmedicated first-episode patients in a subject cohort different from the one recruited for this study, significantly reduced activation was observed in frontal, supplemental, and parietal eye fields (Keedy et al., 2006). This is consistent with findings from a laboratory study of never medicated first-episode schizophrenia patients showing speeded saccade latencies prior to treatment (Reilly et al., 2005). Reduced attentional regulation from neocortical eye fields to brainstem oculomotor nuclei, suggested by the findings of Keedy et al. (2006), represent one potential cause of these speeded pretreatment saccade latencies (Everling and Munoz, 2000). Reilly et al. (2005) also found a slowing of the initially speeded responses after atypical antipsychotic treatment. This may be related to a normalization in saccade-related cortical eye field function after treatment, but this has not yet been directly examined.
To assess the effect of antipsychotic treatment on attentional and sensorimotor circuitry supporting visually-guided saccades, we performed fMRI studies with first-episode schizophrenia patients with no or limited prior antipsychotic treatment. They were unmedicated at the time of the first scan and were scanned again after 4-6 weeks of antipsychotic treatment. Matched healthy individuals were studied over a similar time interval. Our first aim was to characterize pretreatment group differences to assess illness-related abnormalities in oculomotor and attentional systems. For this aim, we anticipated reduced activation in frontal eye fields as reported previously by Keedy et al. (2006). Our second aim, which reflects the most novel aspect of the study, was to characterize brain function in oculomotor and attentional systems after treatment. Based on prior longitudinal laboratory studies, and previous fMRI studies where treated or untreated patients were studied separately, we predicted less abnormality in attentional and sensorimotor circuitry after treatment.
2. Method
2.1 Participants
The study was approved by the Institutional Review Board of the University of Illinois at Chicago, and all participants provided written informed consent. Six male and three female patients were recruited who met DSM-IV criteria for schizophrenia using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) and collateral clinical data reviewed at consensus diagnosis meetings. Nine healthy individuals who did not meet criteria for any present or past Axis I disorder according to SCID interviews were recruited from the surrounding community. They matched the patient group on age (t [df16] = 0.36, n.s.), gender (X2[df1] = 2.0, n.s.), and parental socioeconomic status (t [df14] = 2.1, n.s.; status could not be reliably ascertained for two patients). All participants met the following criteria: no known systemic or neurologic disease; no history of head trauma with loss of consciousness, no lifetime history of substance dependence or substance abuse within 3 months of study participation; and, no coffee, tea or cigarettes at least two hours prior to testing.
One schizophrenia patient was antipsychotic naïve. The remaining eight had brief prior second generation antipsychotic exposure (average of 2.8 [SD = 2.3] weeks). One had an additional 2 week exposure to a first generation antipsychotic before admission. Those taking antipsychotics at the time of consenting to the study were withdrawn from their medication under clinical supervision on an inpatient research unit. The minimum was 6 medication-free days prior to baseline fMRI studies (mean = 8.1 days). This was done to minimize acute treatment effects on fMRI data such as sedation, and to provide better comparative baseline data for examining medication effects to a degree that was clinically and ethically feasible.
Risperidone was the preferred treatment of choice, unless patients had a prior or emergent adverse reaction, or were inadequately responding to it per clinical judgment. At the time of follow-up scanning, six patients were still on risperidone (mean dose = 4.2 mg [2.1]). One was on ziprasidone (200mg) and two were on haloperidol (4 and 5 mg). Patients were rated on the Positive and Negative Symptom Scale (PANSS) (Kay et al., 1987) and Simpson-Angus Scale for extrapyramidal signs (SAS) (Simpson and Angus, 1970) at both time points. Mean PANSS scores at baseline were 73.5 (SD = 18.2) before treatment and 57.1 (SD = 20.9) after treatment. Although not statistically different, the reduction in PANSS scores show that patients were stabilizing during the interval between scans. Ratings of extrapyramidal side effects were low both before (0.44 [SD = .93]) and after (1.33 [SD = 2.07]) treatment. Healthy individuals were re-scanned after a time interval similar to that of the patients (t [df16] = 2.0, n.s.).
2.2 FMRI task
The behavioral task used in the scanner has been used in previous studies (Berman et al., 1999; Keedy et al., 2006) and was designed to contrast exogenously generated visual attention and visually guided saccades to unpredictable target displacements with central fixation in a block design paradigm. Six 30-second blocks of a visually-guided saccade task alternated with seven 30-second blocks of central fixation. During the saccade task, a circular target subtending 0.5° of visual angle moved in 4° steps between 5 possible locations (0, ± 4° and ± 8°) along the horizontal plane at a fixed interval of 810 msec. The direction of target movement was unpredictable except after the ± 8° locations, when the target always moved back toward center. Participants demonstrated the ability to understand and perform the tasks in a practice session prior to scanning. Task compliance during the scan was visually verified via an infrared video camera (30 Hz sampling, sufficient to verify performance but not to accurately measure saccade latencies or metrics).
2.3 Image acquisition and analysis
FMRI studies were conducted using a 3.0 Tesla whole body scanner (Signa VHi, General Electric Medical Systems, Waukesha, WI) with a gradient echo, echo-planar sequence (epiRT, 25 axial slices, 5mm thick, skip 1 mm, TR = 2500 ms, TE = 25ms, flip angle = 90, matrix = 64 × 64, FOV = 20 cm2, voxel size = 3.125 × 3.125 × 5 mm; 156 volumes acquired). Anatomic images were also acquired (three-dimensional spoiled gradient recalled, 1.5mm thick contiguous axial slices) for co-registration with the functional data. Images were reconstructed, autoscaled, and motion-corrected using Functional Image Analysis Software Computational Olio (Eddy et al., 1996). Individual volumes from the time series were excluded from analysis if head displacement was greater than 1.5mm or rotation was greater than 0.5 deg from the median volume location. The groups did not significantly differ on number of volumes excluded from analysis (mean [SD] number of excluded volumes: schizophrenia = 52.7 [24.2]; healthy = 55.3 [22.7]) or in head motion measurements in the remaining data used for analyses.
Effect size maps were generated to characterize BOLD signal differences between saccade and fixation blocks for each subject. These were expressed as Fisher z' statistics. The z' maps from each subject were warped into Talairach space and resampled to 3×3×3mm voxels (in-plane voxel resolution at acquisition). We then conducted between-group t tests to assess group differences at baseline and at follow-up, and applied a contiguity threshold to preserve a familywise Type 1 error rate of P < 0.05. The volume threshold was determined with AFNI's (Analysis of Functional Neuroimages) (Cox, 1996) AlphaSim Monte Carlo simulation program run with a template brain mask for restricting contiguity simulations to in-brain voxels.
Due to the limited sample size, we elected to focus primary hypothesis testing analyses on between group differences at each time point due to insufficient statistical power for evaluating group x time interaction effects. Within group analysis of change in the patient group was evaluated in regions where significant group differences were found, and the same contiguity threshold from between-groups analyses was applied. In reporting group differences, it is generally the case that both groups displayed activation in a region for the saccade vs. fixation contrast, but one group was significantly greater that the other. We note below if group differences resulted from any different patterns of activation in the saccade vs. fixation contrast.
3. Results
3.1 Task-related activation
Examination of healthy and schizophrenia groups' average activation maps showed that during the saccade task relative to fixation at both time points, both groups activated the well-known saccade/spatial attention system, including frontal, supplementary, and parietal eye fields, as well as visual cortex and cerebellum (Figure 1). Tables 1 and 2 provide details of activation differences between groups and of the within group change analysis of schizophrenia patients from pre- to posttreatment.
Fig 1.
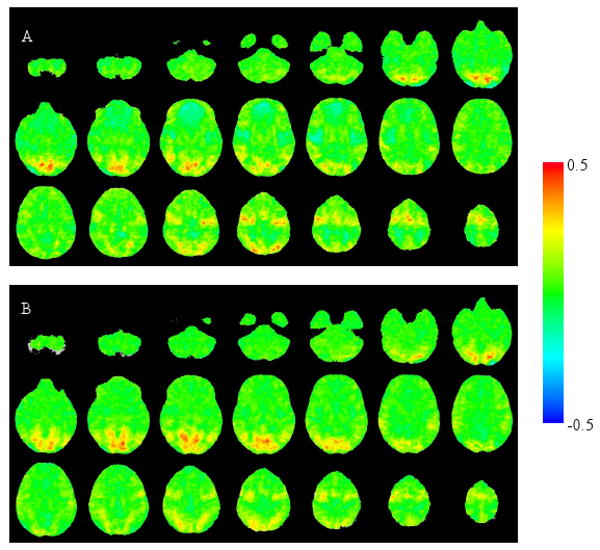
Unthresholded group average activation maps (expressed as Fisher z' effect sizes) in healthy controls (A) and schizophrenia patients (B) at baseline. These illustrate brain regions engaged by the saccade task relative to the fixation condition.
Table 1.
Significant group differences in activation in visual and oculomotor regions between schizophrenia patients and healthy individuals during a visually guided saccade task, and significant changes pre to post treatment for schizophrenia patients.
| Brain region | Volume (mm3) and peak difference (t-value and x,y,z coordinates) of baseline differences between groups a | Volume (mm3) and peak difference (t-value and x,y,z coordinates) of follow-up differences between groups a | Volume (mm3) and peak difference (t-value and x,y,z coordinates) of significant change pre to post treatment in patients b |
|---|---|---|---|
| L Frontal Eye Field | 1026 | 162 | |
| 4.01 | 0 | 3.09 | |
| -43, -13, 50 | -28, -7, 56 | ||
| R Supplementary Eye Field | 270 | 405 | 0 |
| 2.35 | 3.72 | ||
| 1, 1, 56 | 1, -4, 53 | ||
| L Supplementary Eye Field | 729 | 702 | 0 |
| 3.32 | 3.79 | ||
| -1, 4, 53 | -4, 4, 50 | ||
| R Cerebellum | 1026 | 513 | |
| 4.82 | 0 | 4.06 | |
| 7, -46, -33 | -25, -58, -48 | ||
| L Cerebellum | 189 | 54 | |
| 2.74 | 0 | 2.41 | |
| -10, -49, -36 | 22, -37, -27 | ||
| Vermis | 1188 | 783 | |
| 4.24 | 0 | 3.05 | |
| 1, -67, -45 | 1, -55, -33 | ||
| R Intraparietal Sulcus | 1512 | 4941 | |
| -3.43 | 0 | -6.54 | |
| 22, -46, 47 | 40, -43, 50 | ||
| L Intraparietal Sulcus | 1944 | 1620 | |
| -3.30 | 0 | -4.36 | |
| -19, -46, 53 | -28, -46, 44 | ||
| R Occipital/Occipito-temporal | 1053 | 3429 | 1377 |
| -3.00 | -4.14 | -4.38 | |
| 10, -91, -18 | 31, -40, -6 | 55, -61, 11 | |
| L Occipital/Occipito-temporal Gyrus | 1350 | 4860 | 378 |
| -3.18 | -3.76 | -2.99 | |
| -10, -55, 11 | -19, -79, 5 | -19, -85, 14 | |
| R Supramarginal Gyrus | 3186 | 756 | 1593 |
| -5.67 | 4.23 | -5.51 | |
| 55, -19, 26 | 58, -43, 35 | 55, -28, 26 | |
| L Supramarginal Gyrus | 3429 | 702 | 1647 |
| -4.54 | 3.94 | -5.59 | |
| -52, -34, 38 | -52, -46, 32 | -52, -28, 20 | |
| R Cingulate Motor | 1053 | 0 | |
| 0 | 3.56 | ||
| 10, 13, 41 | |||
| L Cingulate Motor | 1377 | 243 | |
| 0 | 4.53 | -3.903 | |
| -7, 4, 32 | -16, 4, 38 | ||
| R Caudate | 324 | 1890 | 0 |
| 2.97 | 3.93 | ||
| 10, 19, -3 | 13, 10, 8 | ||
| (ventral) | (dorsal) | ||
| L Caudate | 81 | 2376 | 81 |
| 2.64 | 4.48 | 2.29 | |
| -10, 10, 0 | -4, 10, 11 | -10, 4, 14 | |
| (ventral) | (dorsal) | ||
| R Lenticular Nucleus | 81 | 1917 | 135 |
| -2.19 | 3.70 | -2.94 | |
| 19, 16, -3 | 25, -22, 2 | 25, -13, 2 | |
| L Lenticular Nucleus | 108 | 2727 | 0 |
| -2.58 | 3.93 | ||
| -13, 4, -3 | -22, 1, -3 | ||
positive t indicates controls > patients ; negative t indicates patients > controls
positive t indicates increase pre to post treatment; negative t indicates decrease pre- to posttreatment.
L=left; R=right.
Table 2.
Significant group differences in activation in areas beyond the visual or oculomotor systems between schizophrenia patients and healthy individuals during a visually guided saccade task, and significant changes pre to post treatment for schizophrenia patients.
| Brain region | Volume (mm3) and peak difference (t-value and x,y,z coordinates) of baseline differences between groups a | Volume (mm3) and peak difference (t-value and x,y,z coordinates) of follow-up differences between groups a | Volume (mm3) and peak difference (t-value and x,y,z coordinates) of significant change pre to post treatment in patients b |
|---|---|---|---|
| R Superior Temporal Gyrus | 702 | 702 | 216 |
| -4.52 | -4.02 | -3.63 | |
| 43, 19, 8 | 49, -1, 5 | 34, -1, -15 | |
| L Superior Temporal Gyrus | 1377 | 459 | |
| -3.19 | 0 | -2.61 | |
| -61, -16, 14 | -46, 16, -9 | ||
| R Ventromedial Prefrontal Cortex | 1890 | 567 | |
| -4.21 | 0 | -5.42 | |
| 19, 37, -3 | 22, 49, -6 | ||
| L Ventromedial Prefrontal Cortex | 432 | 0 | 1836 |
| -2.96 | -6.71 | ||
| -16, 16, -9 | -22, 52, 2 | ||
| R Subgenual Cingulate | 1215 | 0 | 0 |
| -2.89 | |||
| 10, 43, -3 | |||
| L Subgenual Cingulate | 270 | 0 | 0 |
| -3.21 | |||
| -1, 19, -3 | |||
| R Insula | 1809 | 1350 | 486 |
| -4.08 | 4.01 | -3.22 | |
| 37, -16, 2 | 46, 19, 5 | 40, 28, 8 | |
| (posterior) | (anterior) | (anterior) | |
| L Insula | 1863 | 1161 | |
| 0 | 3.46 | -4.74 | |
| -25, 22, 2 | -43, -1, 11 | ||
| (anterior) | (anterior) | ||
| R Presupplementary Eye Field | 972 | 0 | |
| 0 | 5.43 | ||
| 7, 19, 47 | |||
| L Presupplementary Eye Field | 297 | 108 | |
| 0 | 3.24 | 3.78 | |
| -4, 7, 50 | -7, 16, 50 | ||
| R Dorsolateral Prefrontal Cortex | 0 | 4050 | 0 |
| 4.31 | |||
| 22, 49, 20 | |||
| L Dorsolateral Prefrontal Cortex | 1728 | 3213 | |
| 0 | 4.54 | -5.11 | |
| -25, 34, 32 | -31, 34, 32 | ||
| R Anterior Cingulate | 81 | ||
| -2.69 | |||
| 1755 | 10, 31, 20 | ||
| 0 | 6.40 | ---------------------- | |
| 10, 16, 35 | 189 | ||
| 2.85 | |||
| 13, 43, 17 | |||
| L Anterior Cingulate | 1188 | 0 | |
| 0 | 3.38 | ||
| -10, 19, 23 | |||
| R Dorsomedial Thalamus | 864 | 0 | |
| 0 | 2.97 | ||
| 7, -13, 0 | |||
| L Dorsomedial Thalamus | 324 | 0 | |
| 0 | 2.76 | ||
| 7, -4, 0 | |||
positive t indicates controls > patients ; negative t indicates patients > controls
positive t indicates increase pre to post treatment; negative t indicates decrease pre- to posttreatment.
L=left; R=right.
3.2 Group differences in visual and saccade systems
3.2.1 Baseline
Relative to healthy controls, schizophrenia patients had significantly less activation in bilateral supplementary eye fields (SEF) and the left frontal eye field (FEF, Fig. 2A) that are involved in attentional aspects of saccade control. They also had significantly less activation in the vermis and deep nuclei of the cerebellum, areas which are involved in motor aspects of saccade generation. In contrast, schizophrenia patients had significantly more activation than healthy individuals in three posterior cortical areas, including bilateral intraparietal sulci (IPS; Fig 2A), a region that contains parietal eye fields (PEF) involved in spatial orienting, occipital and occipitotemporal regions that support unimodal and heteromodal visual processing (Fig 2C and 2D), and the supramarginal gyri, involved in spatial processing (Fig 2B).
Figure 2.
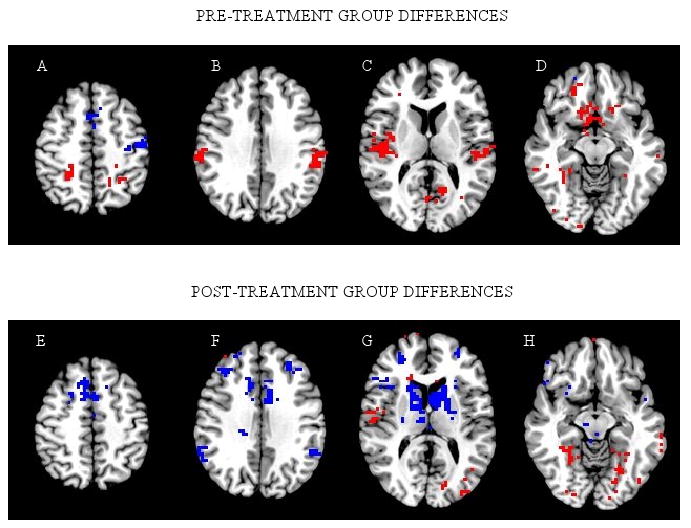
Significant group differences at each time point. Slices (left to right) are through z = 46, 31, 11, and -9. Blue voxels: Schizophrenia < Healthy; Red voxels: Schizophrenia > Healthy. Images are in radiological convention (right side of image is left hemisphere). PRE-TREATMENT GROUP DIFFERENCES (top row): A) Schizophrenia patients had reduced activation in bilateral supplementary and left frontal eye field, but greater activation in bilateral intraparietal sulcus. B) Schizophrenia patients had greater activation in bilateral supramarginal gyrus. C) Schizophrenia patients had greater activation in bilateral superior temporal gyrus, posterior insula, and visual cortex. D) Schizophrenia patients had greater activation in ventromedial prefrontal cortex, ventral striatum, and occipital and occipitotemporal cortex. POST-TREATMENT GROUP DIFFERENCES (bottom row): E) Schizophrenia patients had less activation in bilateral supplementary and presupplementary eye fields. F) Schizophrenia patients had less activation in dorsolateral prefrontal cortex, anterior cingulate, and supramarginal gyrus. G) Schizophrenia patients had less activation in dorsal striatum, particularly the head of the caudate, and greater activation in right superior temporal gyrus and visual cortex. H) Schizophrenia patients had greater activation in occipital and occitipotemporal cortex.
3.2.2 Follow-up
In contrast to pretreatment findings, no group differences were found in FEF, cerebellum, or IPS after treatment. For these regions, the patients' within group analysis confirmed that after treatment there were significant changes in the direction of normalization of function (higher activation in left FEF and bilateral cerebellum, less activation in bilateral IPS). Similar to pretreatment group differences, schizophrenia patients demonstrated less activation bilaterally in SEF relative to controls after treatment (Fig 2E) and consistent with this, no significant changes were noted in this region from pre- to posttreatment in patients.
Additional bilateral visual or oculomotor areas in which patients had less activation than controls at follow-up included the cingulate motor area, dorsal striatum, particularly the head of the caudate (Fig 2G), and supramarginal gyri (Fig 2F). Patients demonstrated significantly less activation after treatment in the left cingulate motor region, left caudate, and bilateral supramarginal gyri relative to pretreatment. The right cingulate motor region and caudate were also reduced but did not reach statistical threshold in these areas. Schizophrenia patients continued to show relatively greater activation than controls in occipital and occipitotemporal cortex (Fig 2G and 2H), although there were regions throughout visual cortex where there was significantly less activation after treatment within the patient group.
3.3 Observations beyond visual and oculomotor systems
3.3.1 Baseline
Schizophrenia patients showed greater activation than controls in bilateral superior temporal gyri which contain primary auditory cortex (Fig 2C). Also, schizophrenia patients had greater activation in ventromedial prefrontal cortex, subgenual cingulate, posterior right insula (Fig 2C), and ventral striatum (containing ventral caudate and nucleus accumbens; Fig 2D; see also Table 1 for baseline differences, which were ventral). In these regions generally linked to affective processing, the group differences were due to healthy individuals showing less activation during the saccade task relative to the fixation condition (see Fig 1A), although patients also displayed some activation in ventral striatum during the saccade task, contributing to group differences in that structure.
3.3.2 Follow-up
In the right superior temporal gyrus, as at baseline, schizophrenia patients had greater activation than controls (Fig 2G). However, in contrast to baseline, no group differences were found in left superior temporal gyrus, and within group analyses of the patient group revealed significantly decreased activity bilaterally in superior temporal gyri pre- to posttreatment. There were no group differences in ventromedial prefrontal cortex, subgenual anterior cingulate, or posterior right insula after treatment, as were seen pretreatment. Of these, there was significantly decreased activation from pre- to posttreatment in patients in ventromedial prefrontal cortex, consistent with a normalization of function. In anterior insula bilaterally (Fig 2G), schizophrenia patients had significantly less activation than controls after treatment, and in both right and left anterior insula patients had a significant decrease from pre-to posttreatment.
There was also less activation posttreatment in patients relative to controls in rostral frontal areas typically associated with higher cognitive aspects of eye movement control, including the presupplementary eye fields (Fig 2E), dorsolateral prefrontal cortex (Fig. 2F and 2G), dorsal anterior cingulate (Fig 2F), and dorsomedial thalamus (Fig 2G, right side only shown). In the within group analysis, patients had a small cluster of significantly increased activation in left presupplementary eye field, and on the right had generally decreased activation but this did not reach statistical threshold. Activation in left dorsolateral prefrontal cortex was significantly reduced after treatment relative to pretreatment in the patients; the right dorsolateral prefrontal cortex was also reduced but did not reach the statistical threshold. Finally, in right anterior cingulate there were small clusters of both increased and decreased activation relative to baseline data. Thus, in the left presupplementary eye field and the anterior cingulate, within group change was ambiguous in relation to the between group findings of relatively reduced activation compared with controls, but for right presupplementary eye field and dorsolateral prefrontal cortex, as in the caudate as previously reported, patients had changes after treatment consistent with disrupted normal function after treatment.
3.4 Caudate volume
Given the robust group differences in caudate activation after treatment and prior reports of caudate volumetric changes associated with some antipsychotic medications (Chakos et al., 1995; Corson et al., 1999; Lieberman et al., 2005), a supplemental analysis of caudate volume pre- and posttreatment was conducted. Right and left caudate nuclei were measured manually (test - retest reliability was 0.95) using BRAINS2 software (Magnotta et al., 2002), blind to group and time. Total intracranial volume (ICV) was measured by a semi-automated method. A multivariate analysis with ICV as a covariate showed no significant difference between or within groups in caudate volume change over time. Excluding patients on haloperidol did not change these results.
4. Discussion
4.1 Overview
In this fMRI study of antipsychotic treatment in first-episode schizophrenia, pretreatment abnormalities were observed in FEF and PEF, as expected, and in cerebellum, suggesting disturbance in visual attention and sensorimotor systems. After treatment, no deficits in these regions were found, suggesting antipsychotic treatment had an ameliorating effect in these systems. In addition, there were unanticipated but related findings in which patients demonstrated sensory and limbic system dysfunction that was less prominent after treatment. Similarly, patients also had pretreatment deficits in ventromedial prefrontal cortex that were not seen posttreatment, suggesting a possible normalization of “default mode” brain activity (Raichle et al., 2001; Greicius et al., 2003). These findings, noted in the context of a contrast between the attentionally-demanding saccade task vs. a passive fixation task, are in line with the notion of improved attentional control after antipsychotic treatment.
Findings of reduced function in some regions posttreatment where no abnormalities were detected at baseline are also noteworthy. This occurred in dorsal striatum, dorsomedial thalamus, dorsolateral prefrontal cortex, and anterior cingulate. These regions together comprise frontostriatal circuits known to support higher order attentional control over saccades (Sweeney et al., 1996) as well as other executive functions. Overall, findings from the present study are consistent with the predicted deficits in attentional and sensorimotor systems pretreatment, and a reduction in these deficits after treatment. However, some effects evident only after treatment suggest some possibly deleterious treatment-related outcomes in other aspects of attentional systems in areas that might be related to treatment-emergent or “secondary” negative symptoms.
4.2 Visual attention and oculomotor system
4.2.1 Cortical eye fields
Consistent with our previous fMRI study of visually guided saccades in first episode schizophrenia patients (Keedy et al., 2006), activation was reduced in FEF and SEF at baseline. However, in the present study, PEF had increased activation in patients before treatment. After treatment, FEF and PEF activation was no longer different from controls, but activation in SEF was still reduced.
SEF is reliably activated in healthy subjects during visually guided saccade tasks (Petit et al., 1997; Luna et al., 1998; Berman et al., 1999). However, its specific contribution to reflexive saccade generation is not yet well understood, as human lesion and animal studies have reported no impact on visually guided saccade generation in the context of compromised SEF function (Sommer and Tehovnik, 1997; Schiller and Chou, 1998). It appears to have an intermediate role between cognitive and sensorimotor functions, and as posttreatment changes for regions more specifically dedicated to these abilities were in opposite directions, interpretation of SEF effects after treatment are ambiguous.
Most noteworthy were the opposing abnormalities of the FEF and PEF at baseline (hypo-and hyperactive, respectively), and a subsequent normalization of both of these abnormalities after treatment. Normally activation in PEF and FEF is robust and highly correlated during saccade tasks (Sweeney et al., 1996; Luna et al., 1998). At baseline this cortico-cortical synchrony was disturbed in patients, but not after treatment. FEF primarily provides attentional regulation and sensorimotor transformation for saccade commands, whereas PEF plays a greater role in visuoperceptual analysis for guiding saccades. The pattern of disturbance in these areas at baseline suggests that patients may have reduced attentional regulation of sensorimotor control from FEF, and heightened levels of visual processing, as there was increased activity not only in PEF, but also in supramarginal gyrus, occipital, and occipitotemporal cortex.
Prior laboratory findings of visually guided saccades in first episode schizophrenia patients provides some insight into the possible functional relevance of the pretreatment abnormality found in FEF and PEF. Medication-naïve first episode schizophrenia patients had speeded saccade latencies in the absence of other abnormalities in saccade metrics or visual fixation (Reilly et al., 2005), a finding that suggested reduced frontal corticofugal input to midbrain structures controlling saccade execution. Thus, the present finding of reduced FEF activation at baseline is consistent with the laboratory study. Further, Reilly et al. (2005) reported that after 4-6 weeks of treatment with risperidone, patients' saccade latencies slowed to a normal rate, a finding consistent with the normal activation seen in FEF after treatment in the present study. Thus, the normalization of FEF activation after treatment may contribute to improved attentional control over sensorimotor processing. As PEF activation was also normal after treatment in the present study, treatment may have improved neural synchrony in this frontal-parietal cortical network subserving visual attention and visual orienting. On the other hand, supramarginal activation was abnormally reduced after treatment, and increased visual cortex activation was still present, which may relate to the persistent abnormalities in primary visual perceptual processes reported in psychophysical and visual perceptual research in schizophrenia (Chen et al., 1999; Kim et al., 2006; Silverstein et al., 2006).
4.2.2 Striatum, thalamus, and prefrontal cortex
Whereas no abnormalities were detected prior to treatment in dorsal striatum, patients demonstrated markedly reduced activation relative to controls after treatment, particularly in the head of the caudate, which is integral to saccade control (Hikosaka et al., 2000). The posttreatment changes may represent a functional consequence of the robust antagonism of D2 receptors in the striatum by antipsychotic medications (Buchsbaum et al., 1987; Liddle et al., 2000; Corson et al., 2002). We found no substantial increase in extrapyramidal ratings or alteration in caudate volumes at the end of the 4-6 week treatment period. The posttreatment reduction in striatal function during performance of a sensorimotor task may contribute to slower saccade latencies observed after treatment. The observation also suggests that fMRI may provide a more sensitive index of antipsychotic-induced changes in striatum than clinical ratings of extrapyramidal signs.
A pattern similar to that seen for the dorsal striatum was noted in dorsomedial thalamus, dorsolateral prefrontal cortex, and anterior cingulate. There were no pretreatment baseline abnormalities in these regions, but after treatment schizophrenia patients had significantly less activation than controls. To better understand these unexpected but provocative findings of reduced activation in dorsal frontal systems, it is instructive to note that activity in these regions has been reported in human imaging studies of simple saccadic eye movements tasks previously, so they are all known to play a role in simple attention/saccade control in addition to their role in higher cognitive functions (Corbetta, 1998; Berman et al., 1999; Simo et al., 2005). This is consistent with single-cell recordings in nonhuman primates' dorsolateral prefrontal cortex, showing populations of neurons firing in association with saccade execution independent of spatial coding (Funahashi et al., 1989; Wang et al., 2004). Clinically, the reduced activation in prefrontal systems may have relevance because adverse effects of antipsychotics on prefrontally-mediated cognitive tasks have been reported in animal models (Seamans et al., 1998; Skarsfeldt, 1996; Castner et al., 2000; Rinaldi et al., 2007). Clinical studies (Reilly et al., 2006a; Reilly et al., 2006b) of prefrontal function using tasks employed in animal studies (Goldman-Rakic et al., 2004) have found similar adverse treatment effects, and thus these changes may have relevance to the pattern of “secondary” negative symptoms observed in some patients after antipsychotic treatment (Buchanan, 2007).
4.3 Sensory/perceptual and limbic systems
In addition to the abnormalities found in schizophrenia patients in areas associated with attentional control and saccade execution, group differences also were found in sensory systems and in aspects of the limbic system. Specifically, greater pretreatment activation in patients was observed in both visual and auditory unimodal sensory cortex, heteromodal association cortex (occipitotemporal cortex involved in visual integration, and supramarginal gyrus involved in spatial processing), and limbic circuitry (ventral striatum and posterior insula). Increased activation in these regions during the acute phase of illness might be related to clinical problems such as reduced thalamocortical gating that might be associated with hallucinations, and to stress effects and emotional dysregulation (Dierks et al., 1999; van de Ven et al., 2005; Sumich et al., 2005; Sanjuan et al., 2007).
In interpreting these effects, it is important to recognize that these observations of greater sensory and limbic activation were made during the active visual attention task relative to the passive fixation condition, rather than as an alteration in resting state physiology. Thus, one possible explanation for over-activation in sensory systems is that the normal organizing and focusing influence of attention may be reduced during acute psychosis, resulting in increased processing of task-irrelevant (auditory and tactile) information from the fMRI environment. This interpretation is consistent with electrophysiology studies documenting deficits of sensory filtering/gating in schizophrenia. Well-replicated examples of this effect include reduced suppression of the P50 auditory evoked potential and reduced sensorimotor gating in prepulse inhibition paradigms (Oranje et al., 2006). Studies have demonstrated P50 deficits in schizophrenia patients at stimulus rates roughly equivalent to those of scanner pulse noise (Erwin et al., 1991), and P50 abnormality has been correlated with attention deficits (Yee et al., 1998; Erwin et al., 1998; Thoma et al., 2003). Increased sensory processing during a task that typically automatically elicits focused visual attention might represent increased throughput in thalamocortical systems secondary to either intrinsic thalamic disturbances (Lehrer et al., 2005) or reduced attentional modulation of either thalamocortical output or directly on heteromodal and unimodal cortex.
Hyperactivity in some sensory systems, such as primary auditory cortex, was less prominent after treatment. Attention modulates processing of task-irrelevant sensory information in healthy individuals (Rees et al., 1997), and so the observed reduction in patients of activation in sensory cortex after treatment may be due to an enhancement of the attentional modulation of sensory processing. However, task-relevant sensory input did not appear to as clearly benefit in the same way, as visual processing was still abnormally elevated after treatment in the between group comparison, although in the within group analysis, patients did evidence change in the direction of normalization of visual processing. Continued hyperactivity in sensory cortical areas even after treatment is consistent with persistent early sensory processing deficits seen in schizophrenia (Johnson et al., 2005; van der Stelt et al., 2006)
Finally, pretreatment group differences in ventromedial frontal cortex and ventral striatum were due to healthy individuals displaying deactivation in these areas during the active, attention-demanding task. This effect is consistent with numerous studies of healthy individuals showing deactivation in these regions during cognitively demanding tasks relative to less demanding ones or neutral states (Sweeney et al., 1996). This ventromedial deactivation has been termed the “default mode” of brain function (Raichle et al., 2001; Greicius et al., 2003). From this perspective, the failure of untreated schizophrenia patients to exhibit a default mode during active task engagement extends our observations of reduced attentional regulation of functional brain systems during task performance in unmedicated, acutely psychotic patients. That default mode deactivation was present in patients' ventromedial frontal cortex after treatment is consistent with the overall pattern of findings from this study of improved attentional regulation posttreatment.
4.4 Limitations and conclusions
Limitations of this study include the small sample size, which did not provide adequate power for a robust analysis of interaction effects to directly examine differential change over time in patients and healthy individuals. Second, eye movement monitoring during testing was sufficient to verify active performance but not to examine behavior-activation relationships. Third, most but not all patients were treated with risperidone, and thus medication specific effects cannot be evaluated given the small sample size. Fourth, patients changed in clinical state as well as medication status between pretreatment and follow-up testing. It therefore remains unspecified whether the changes we report reflect direct pharmacodynamic effects on specific neural systems or general changes in brain function associated with clinical recovery. Without an ethically unfeasible no-treatment condition, it is not possible to separate drug effects from changes related to clinical recovery in acutely ill first episode patients. First episode studies provide a strategy for examining treatment-related effects free from residual effects of prior long-term treatment and other course of illness effects, but generally they are not able to conclusively demonstrate direct drug effects for this reason. Finally, effects of experience with the paradigm (within a testing session and between sessions) may be significant, and may be different in patients and controls. However, because of the simple and reflexive nature of task performance in a simple saccade paradigm, such effects may be less likely than in studies of higher cognitive functions.
The present study is one of the few investigating functional brain systems in vivo before and after antipsychotic treatment in first-episode schizophrenia. By using fMRI with a systems neuroscience perspective, pretreatment illness-related disturbances in sensory, sensorimotor, and attentional systems were identified. Treatment-related improvement was seen in some aspects of these systems, but there were also suggestions of concurrent adverse treatment-related effects on prefrontal systems. Further research in this area is important in its own right, but may be especially useful as a biomarker strategy for assessing new treatments, as a complement to receptor studies to provide an integrated neurophysiological and biochemical understanding of drug effects, and to provide a mechanistic framework for understanding illness and treatment effects across multiple brain systems.
Acknowledgments
This research was supported by NIH grant MH62134 (PI - Sweeney).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, Sweeney JA. Cortical networks subserving pursuit and saccadic eye movements in humans: an fMRI study. Human Brain Mapping. 1999;8:209–225. doi: 10.1002/(SICI)1097-0193(1999)8:4<209::AID-HBM5>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer WJ, Yucel M, Harrison BJ, McGorry PD, Olver J, Egan GF, Velakoulis D, Pantelis C. Increased prefrontal cerebral blood flow in first-episode schizophrenia following treatment: Longitudinal positron emission tomography study. Australian and New Zealand Journal of Psychiatry. 2007;41:129–135. doi: 10.1080/00048670601109899. [DOI] [PubMed] [Google Scholar]
- Buchanan RW. Persistent negative symptoms in schizophrenia: An overview. Schizophrenia Bulletin. 2007;33:1013–1022. doi: 10.1093/schbul/sbl057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Wu JC, Delisi LE, Holcomb HH, Hazlett E, Cooper-Langston K, Kessler R. Positron emission tomography studies of basal ganglia and somatosensory cortex neuroleptic drug effects: Differences between normal controls and schizophrenic patients. Biological Psychiatry. 1987;22:479–494. doi: 10.1016/0006-3223(87)90170-3. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345:456–457. doi: 10.1016/s0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Nakayama K, Matthysse S, Palafox G, Holzman PS. Dependence of impaired eye tracking on deficient velocity discrimination in schizophrenia. Archives of General Psychiatry. 1999;56:155–161. doi: 10.1001/archpsyc.56.2.155. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proceedings of the National Academy of Sciences of the United States of America. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: Typical versus atypical neuroleptics. American Journal of Psychiatry. 1999;156:1200–1204. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- Corson PW, O'Leary DS, Miller DD, Andreasen NC. The effects of neuroleptic medications on basal ganglia blood flow in schizophreniform disorders: A comparison between the neuroleptic-naive and medicated states. Biological Psychiatry. 2002;52:855–862. doi: 10.1016/s0006-3223(02)01421-x. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dierks T, Linden DEJ, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W. Activation of Heschl's Gyrus during auditory hallucinations. Neuron. 1999;22:615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Eddy WF, Fitzgerald M, Genovese CR, Mockus A, Noll DC. Functional image analysis software - computational olio. In: Prat A, editor. Proceedings in Computational Statistics. Physica-Verlag; Heidelberg, Germany: 1996. pp. 39–49. [Google Scholar]
- Erwin RJ, Mawhinney-Hee M, Gur RC, Gur RE. Midlatency auditory evoked responses in schizophrenia. Biological Psychiatry. 1991;30:430–442. doi: 10.1016/0006-3223(91)90304-5. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Turetsky BI, Moberg P, Gur RC, Gur RE. P50 abnormalities in schizophrenia: Relationship to clinical and neuropsychological indices of attention. Schizophrenia Research. 1998;33:157–167. doi: 10.1016/s0920-9964(98)00075-9. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. Journal of Neuroscience. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. Journal of Neurophysiology. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological Reviews. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S, Ng V, Fletcher PC, Williams SCR, Brown J, Bullmore ET. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126:1767–1781. doi: 10.1093/brain/awg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Lowery N, Kohler C, Turetsky BI. Global-Local Visual Processing in Schizophrenia: Evidence for an Early Visual Processing Deficit. Biological Psychiatry. 2005;58:937–946. doi: 10.1016/j.biopsych.2005.04.053. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keedy SK, Ebens CL, Keshavan MS, Sweeney JA. Functional magnetic resonance imaging studies of eye movements in first episode schizophrenia: smooth pursuit, visually guided saccades and the oculomotor delayed response task. Psychiatry Research: Neuroimaging. 2006;146:199–211. doi: 10.1016/j.pscychresns.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophrenia Research. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Medoff DR, Tamminga CA, Holcomb HH. Functional effects of single dose first- and second-generation antipsychotic administration in subjects with schizophrenia. Psychiatry Research: Neuroimaging. 2005;139:19–30. doi: 10.1016/j.pscychresns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lane CJ, Ngan ETC, Yatham LN, Ruth TJ, Liddle PF. Immediate effects of risperidone on cerebral activity in healthy subjects: A comparison with subjects with first-episode schizophrenia. Journal of Psychiatry & Neuroscience. 2004;29:30–37. [PMC free article] [PubMed] [Google Scholar]
- Lehrer DS, Christian BT, Mantil J, Murray AC, Buchsbaum BR, Oakes TR, Byne W, Kemether EM, Buchsbaum MS. Thalamic and prefrontal FDG uptake in never medicated patients with schizophrenia. American Journal of Psychiatry. 2005;162:931–938. doi: 10.1176/appi.ajp.162.5.931. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Lane CJ, Ngan ET. Immediate effects of risperidone on cortico-striato-thalamic loops and the hippocampus. British Journal of Psychiatry. 2000;177:402–407. doi: 10.1192/bjp.177.5.402. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M. Antipsychotic drug effects on brain morphology in first-episode psychosis. Archives of General Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, Sweeney JA. Dorsal cortical regions subserving visually-guided saccades in humans: an fMRI study. Cerebral Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O'Leary DA, Yuh WTC, Heckel D. Structural MR image processing using the BRAIN2 toolbox. Computerized Medical Imaging and Graphics. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, Braff DL. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biological Psychiatry. 2002;51:216–223. doi: 10.1016/s0006-3223(01)01204-5. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Colby CL, Thulborn KR, Luna B, Olson CR, Sweeney JA. Stimulus-response incompatibility activates cortex proximate to three eye fields. Neuroimage. 2001;13:794–800. doi: 10.1006/nimg.2000.0742. [DOI] [PubMed] [Google Scholar]
- Miller DD, Andreasen NC, O'Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Comparison of the effects of risperidone and haloperidol on regional cerebral blood flow in schizophrenia. Biological Psychiatry. 2001;49:704–715. doi: 10.1016/s0006-3223(00)01001-5. [DOI] [PubMed] [Google Scholar]
- Ngan ETC, Lane CJ, Ruth TJ, Liddle PF. Immediate and delayed effects of risperidone on cerebral metabolism in neuroleptic naive schizophrenic patients: correlations with symptom change. Journal of Neurology, Neurosurgery and Psychiatry. 2002;72:106–110. doi: 10.1136/jnnp.72.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranje B, Geyer MA, Bocker KBE, Kenemans JL, Verbaten MN. Prepulse inhibition and P50 suppression: Commonalities and dissociations. Psychiatry Research. 2006;143:147–158. doi: 10.1016/j.psychres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Petit L, Clark VP, Ingeholm J, Haxby JV. Dissociation of saccade-related and pursuit-related activation in human frontal eye fields as revealed by fMRI. Journal of Neurophysiology. 1997;77:3386–3390. doi: 10.1152/jn.1997.77.6.3386. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Jansma JM, Cahn W, van der Geest JN, van der Linden JA, Kahn RS, Ramsey NF. Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3-dimensional event-related functional magnetic resonance imaging. Archives of General Psychiatry. 2002;59:313–320. doi: 10.1001/archpsyc.59.4.313. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Frith CD, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278:1616–1619. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Abnormalities in visually guided saccades suggest corticofugal dysregulation in never-treated schizophrenia. Biological Psychiatry. 2005;57:145–154. doi: 10.1016/j.biopsych.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MSH, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Archives of General Psychiatry. 2006a;63:1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MSH, Keshavan MS, Sweeney JA. Atypical antipsychotics exacerbate impairment on a translational spatial working memory task in first-episode schizophrenia. Biological Psychiatry. 2006b;59:153S–154S. doi: 10.1016/j.biopsych.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Rinaldi A, Mandillo S, Oliverio A, Mele A. D1 and D2 receptor antagonist injections in the prefrontal cortex selectively impair spatial learning in mice. Neuropsychopharmacology. 2007;32:309–319. doi: 10.1038/sj.npp.1301176. [DOI] [PubMed] [Google Scholar]
- Sanjuan J, Lull JJ, Aguilar EJ, Marti-Bonmati L, Moratal D, Gonzalez JC, Robles M, Keshavan MS. Emotional words induce enhanced brain activity in schizophrenic patients with auditory hallucinations. Psychiatry Research: Neuroimaging. 2007;154:21–29. doi: 10.1016/j.pscychresns.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Chou IH. The effects of frontal eye field and dorsomedial frontal cortex lesions on visually guided eye movements. Nature Neuroscience. 1998;1:248–253. doi: 10.1038/693. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D-1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. Journal of Neuroscience. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S, Uhlhaas PJ, Essex B, Halpin S, Schall U, Carr V. Perceptual organization in first episode schizophrenia and ultra-high-risk states. Schizophrenia Research. 2006;83:41–52. doi: 10.1016/j.schres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Simo LS, Krisky CM, Sweeney JA. Functional neuroanatomy of anticipatory behavior: Dissociation between sensory-driven and memory-driven systems. Cerebral Cortex. 2005;15:1982–1991. doi: 10.1093/cercor/bhi073. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica Supplement. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Skarsfeldt T. Differential effect of antipsychotics on place navigation of rats in the Morris water maze - A comparative study between novel and reference antipsychotics. Psychopharmacology. 1996;124:126–133. doi: 10.1007/BF02245612. [DOI] [PubMed] [Google Scholar]
- Snitz BE, MacDonald A, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: Functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. American Journal of Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Tehovnik EJ. Reversible inactivation of macaque frontal eye field. Experimental Brain Research. 1997;116:229–249. doi: 10.1007/pl00005752. [DOI] [PubMed] [Google Scholar]
- Sumich A, Chitnis XA, Fannon DG, O'Ceallaigh S, Doku VC, Faldrowicz A, Sharma T. Unreality symptoms and volumetric measures of Heschl's gyrus and planum temporal in first-episode psychosis. Biological Psychiatry. 2005;57:947–950. doi: 10.1016/j.biopsych.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Moses SN, Edgar JC, Huang MX, Weisend MP, Irwin J, Sherwood A, Paulson K, Bustillo J, Adler LE, Miller GA, Canive JM. Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. American Journal of Psychiatry. 2003;160:1595–1605. doi: 10.1176/appi.ajp.160.9.1595. [DOI] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Roder CH, Prvulovic D, Bittner RA, Dietz MG, Hubl D, Dierks T, Federspiel A, Esposito F, Di Salle F, Jansma B, Goebel R, Linden DEJ. The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. NeuroImage. 2005;27:644–655. doi: 10.1016/j.neuroimage.2005.04.041. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Lieberman JA, Belger A. Attentional modulation of early-stage visual processing in schizophrenia. Brain Research. 2006;1125:194–198. doi: 10.1016/j.brainres.2006.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Yee CM, Nuechterlein KH, Morris SE, White PM. P50 suppression in recent-onset schizophrenia: clinical correlates and risperidone effects. Journal of Abnormal Psychology. 1998;107:691–698. doi: 10.1037//0021-843x.107.4.691. [DOI] [PubMed] [Google Scholar]


