Abstract
Abnormally high resistance to aqueous humor drainage via the trabecular meshwork and Schlemm’s canal is highly correlated with the development of primary open-angle glaucoma. Contractility of the actomyosin system in the trabecular cells or inner wall endothelium of Schlemm’s canal is an important factor in the regulation of outflow resistance. Cytoskeletal agents, affecting F-actin integrity or actomyosin contractility, or gene therapies, employing over-expression of caldesmon or Rho-A inhibition, can decrease outflow resistance in the drainage pathway. In this review, we discuss the mechanisms underlying these and similar effects on trabecular outflow resistance in living animals and/or in cultured ocular anterior segments from enucleated animal or human eyes.
Keywords: Actomyosin, cytoskeleton, glaucoma, intraocular pressure, outflow facility, trabecular meshwork
1. Introduction
The trabecular meshwork (TM) consists of arrays of collagen beams covered by endothelial-like cells, with loose extracellular matrix (ECM) occupying the spaces between the cells of the adjacent beams. The outermost juxtacanalicular (JXT) or cribriform region has no collagenous beams, but rather several cell layers immersed in a loose web of ECM fibrils. The adjacent Schlemm’s canal is a continuous endothelium-lined channel that drains aqueous humor to the general venous circulation (Lütjen-Drecoll et al., 1981; Rohen et al., 1981). TM structure and experimental flow studies suggest that flow resistance is maximal in the JXT region and/or the inner wall of Schlemm’s canal, although the exact location of the major resistance barrier is not clear (Johnson, 2005). Glaucoma is a progressive optic neuropathy commonly associated with elevated intraocular pressure (IOP) consequent to abnormally high resistance to aqueous humor drainage via the TM and Schlemm’s canal. Glaucomatous eyes exhibit fewer TM cells and abnormal appearing JXT ECM compared to eyes of age-matched normal individuals (Lütjen-Drecoll et al., 1981; Rohen et al., 1981), suggesting that cells and ECM in the JXT region may be critical in resistance regulation.
The actomyosin system, composed of actin microfilaments and associated proteins, is present in essentially all cells, and is highly organized in TM and Schlemm’s canal cells. There are numerous microfilament-based structures in cells along the trabecular outflow pathway. These structures primarily include focal contacts, adherens cell-cell junctions, and bundles of microfilaments (Geiger et al., 1995). Filamentous actin is the major component of microfilaments, but other actin-associated proteins modulate its organization. Additionally, a physiologically contracted state of the JXT- Schlemm’s canal region is required to maintain the microfilament-related structures in the outflow pathway. Microfilaments are involved in a variety of cellular processes from cell adhesion and motility to organelle traffic to adhesion-mediated signal transduction. Therefore, dynamics of the actomyosin system play important roles in changes in cell shape, volume, contractility, and adhesion to neighboring cells and to the ECM. These changes in TM and/or Schlemm’s canal cells, which could affect trabecular outflow resistance by altering the dimensions or direction of flow pathways and the amount and composition of the ECM, can be modulated directly by actin-disrupting agents or indirectly by inhibition of specific protein kinase(s) or cellular contractility through administration of protein kinase inhibitors or gene therapy. In this review, we discuss the effects of pharmacological and genetic perturbation of the actomyosin system in TM and Schlemm’s canal cells on trabecular outflow resistance.
2. Microfilament Disruptors
Latrunculins, marine macrolides that are specific and potent actin-disrupting agents, sequester monomeric G-actin, leading to massive disassembly of filamentous actin. Addition of latrunculin A or B (LAT-A, LAT-B) causes destruction of actin bundles and associated proteins in a wide variety of cell types, including human TM cells (Cai et al., 2000; Epstein et al., 1999). This effect is manifested by cell rounding and retraction of the lamellipodium, and accompanied by an apparent “arborization” of the cells. In living monkeys, LAT-A or -B administered intracamerally or topically induces major increases in outflow facility (up to 2- to 4-fold; Peterson et al., 1999; Peterson et al., 2000). Single or multiple topical treatments with LAT-A and/or –B also significantly decrease IOP in the monkey eye (Okka et al., 2004; Peterson et al., 1999, 2000). In organ cultures of enucleated porcine eyes or postmortem human eyes, LAT-B significantly increases outflow facility by 60 – 70% (Epstein et al., 1999; Ethier et al., 2006). Morphological studies indicate that the LAT-B-induced decrease in outflow resistance is associated with cytoskeleton disruption-related structural changes in the TM. Electron microscopy of live monkey eyes has revealed massive “ballooning” of the JXT region following LAT-B treatment, leading to a substantial expansion of the space between the inner wall of Schlemm’s canal and the trabecular collagen beams without observable separations between inner wall cells (see Figure 1; Sabanay et al., 2006). However, in postmortem human eyes, the facility increase is accompanied by increased openings between inner wall cells (more border or paracellular pores) with only very modest rarefaction of the JXT tissue and separation of the inner wall of Schlemm’s canal from JXT tissue (Ethier et al., 2006). This difference could be attributed to different models used in the two studies. Nevertheless, the facility increase and the extent of inner wall separation from the JXT region in the postmortem human eye are both qualitatively similar to that in the live monkey eye, although the magnitude of the facility increase and morphologic changes are much less in the former than in the latter (Sabanay et al., 2006; Ethier et al., 2006).
Figure 1.
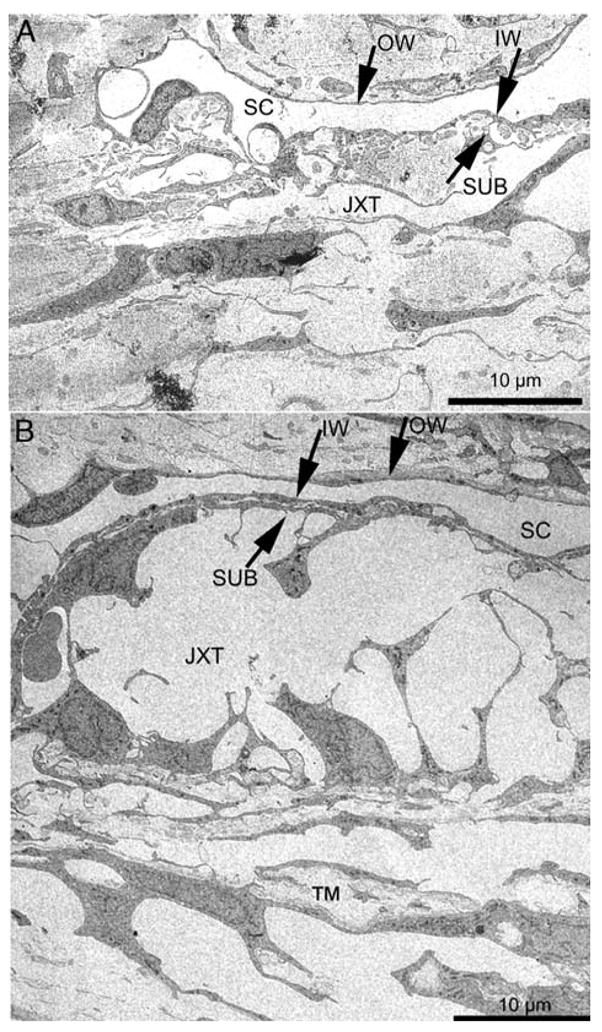
Transmission electron microscopy of the trabecular meshwork (TM) following LAT-B treatment. IW, inner wall; JXT, juxtacanalicular region; OW, outer wall; SC, Schlemm’s canal; SUB, sub-canalicular cells. Panel A shows normal JXT region and its circumjacent structures; Panel B indicates the massive “ballooning” of the JXT region and the retention of close contact between IW and SUB (compare to (A). The figure is modified from Sabanay, et al. (2006).
3. Protein Kinase inhibitors
The serine-threonine kinase inhibitor H-7, which affects a broad spectrum of protein kinases including myosin light chain kinase (MLCK), Rho kinase, and protein kinase C (PKC), inhibits actomyosin-driven contractility dramatically. This leads to cellular relaxation, deterioration of the actomyosin system and perturbation of its membrane anchorage, and loss of stress fibers and focal contacts in many types of cultured cells (Bershadsky et al., 1996; Epstein et al., 1999; Liu et al., 2001; Tian et al., 1998; Volberg et al., 1994;). H-7, administered intracamerally or topically to living monkey eyes, doubles outflow facility and decreases IOP (Tian et al., 1998, 2004) by a mechanism independent of the ciliary muscle (Tian et al., 1999a). In cultured anterior segments of porcine, human or monkey eyes, H-7 also significantly increases outflow facility (Bahler et al., 2004; Epstein et al., 1999; Hu et al., 2006). Morphological studies in the live monkey eye indicate that the H-7-induced increase in outflow facility is associated with cellular relaxation and drainage-surface expansion of the TM and Schlemm’s canal, accompanied by loss of ECM. The inner wall cells of Schlemm’s canal become highly extended, yet cell-cell junctions are maintained (Figure 2; Sabanay et al., 2000, 2004). The morphological changes in the TM of live monkey eyes are consistent with functional changes in isolated bovine TM strips, where the TM pre-contracted by carbachol was relaxed by H-7 (Thieme et al., 1999; Weiderholt et al., 2000). In postmortem cultured anterior segments of human eyes, H-7 causes a partial loss of the endothelial lining of Schlemm’s canal (Bahler et al., 2004).
Figure 2.
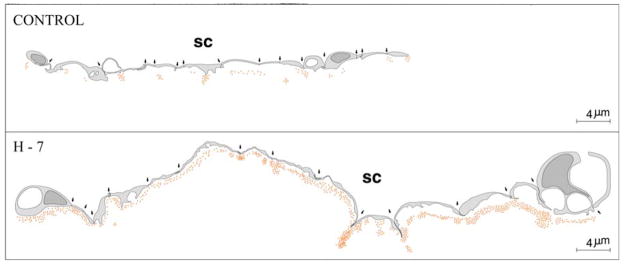
Schematic drawing depicting 15-cell stretches (cell-cell junctions marked by arrows) along Schlemm’s canal (SC) and distribution of perfused gold particles that crossed the juxtacanalicular area of control and H-7 treated eyes. Location of individual gold particles represented by red dots. The figure is modified from Sabanay, et al. (2000).
The specific target kinases affected by H-7 are not well defined, because the non-selective PKC inhibitor staurosporine, the specific PKC inhibitors chelerythrine and GF109203X, and the specific MLCK inhibitor ML-7 similarly increase outflow facility in living monkeys or cultured porcine anterior segments (Tian et al., 1999b, 2000; Khurana et al., 2003). More recently, evidence has shown that inhibition of Rho-associated coiled coil-forming protein kinase (ROCK; Rho kinase) may play a key role in regulating trabecular outflow. Rho kinase is a serine-threonine protein kinase that mainly promotes myosin II activity by inhibiting myosin light chain phosphatase as well as by phosphorylating the myosin regulatory light chain. This, in turn, induces the assembly of contractile acto-myosin bundles that generate strong tensile forces (Wettschureck and Offermanns, 2002). A specific Rho kinase inhibitor, Y-27632, induces reversible changes in cell shape and decreases in actin stress fibers, focal adhesions, and protein phosphotyrosine staining in human TM cells and Schlemm’s canal cells (Honjo et al., 2001a; Rao et al., 2001). In isolated bovine TM strips, Y-27632 completely blocks Ca2+ -independent phorbol myristate acetate or endothelin-1-induced contraction (Renieri et al., 2008; Rosenthal et al., 2005; Thieme et al., 2000). As expected, Rho kinase inhibitors, such as Y-27632, Y-39983, HA-1077, H-1152 and INS117548, increase outflow facility and/or decrease IOP in living rabbits, enucleated porcine eyes and/or living monkeys similar to H-7 (Honjo et al., 2001a, 2001b; Peterson et al, 2008; Rao et al., 2001, 2005b; Tian and Kaufman, 2005; Tokushige et al., 2007; Waki et al., 2001). A recent morphological study in bovine eyes indicates that, with Y-27632, the inner wall of Schlemm’s canal and the JXT connective tissue are significantly distended compared to control eyes, with discernible separation between the inner wall and JXT connective tissue. The average percent effective filtration length of the inner wall of Schlemm’s canal (filtration length/total length × 100) is 3-fold larger in Y-27632-treated eyes than in controls. A significant positive correlation is found between the average percent effective filtration length of the inner wall and the average percent separation length (separation length/total length × 100; separation length = length exhibiting separations between the JXT connective tissue and inner wall), suggesting that the structural correlate to the increase in outflow facility after Y-27632 is physical separation between the JXT connective tissue and inner wall of Schlemm’s canal (Lu et al., 2007).
These findings indicate that inhibition of myosin light chain phosphorylation and consequent cellular relaxation are likely the key mechanisms by which protein kinase inhibitors increase outflow facility. The target kinase(s) may include MLCK (Volberg et al., 1994), Rho kinase or ROCK (Thieme et al., 2000; Tian and Kaufman, 2005) and/or PKC (Khurana et al., 2003).
4. Exoenzyme C3 Transferase and Non-Muscle Caldesmon
TM relaxation can also be induced by modulating proteins, such as caldesmon, that negatively regulate actin–myosin interactions. When caldesmon is overexpressed, actin becomes uncoupled from myosin. Additionally, exoenzyme C3 transferase may also affect actin-myosin interactions by inhibiting Rho-GTP at the beginning of the Rho activation cascade, thereby blocking the whole Rho cascade. Within these pathways and among the end products are numerous targets not only for pharmacologic but also for gene therapy development.
Recently, adenovirus-delivered exoenzyme C3 transferase (C3-toxin) cDNA and non-muscle caldesmon cDNA have been successfully expressed in cultured human TM cells (Grosheva et al., 2006; Liu et al., 2005). Outflow facility in organ-cultured human or monkey eyes has been dramatically increased following overexpression of these genes (Gabelt et al., 2006; Liu et al., 2005). Specific inhibition of Rho-kinase activity in the TM by dominant negative Rho expression increases outflow facility in organ cultured human anterior segments (Rao et al., 2005a).
5. Microtubule Inhibitors
The microtubule system is composed of microtubules and associated proteins, and is one of the three major systems (the microfilament system, the microtubule system and the intermediate filament system) in the cytoskeleton network. Apparently, dynamics of the microtubule system may modulate outflow resistance since microtubule inhibitors increase outflow facility. Microtubules are not intrinsically contractile, but are important for directional cell motility and, driven by specific microtubule motor proteins for cytoplasmic trafficking of vesicles and organelles. Associated proteins that bind to microtubules can affect the latter’s stability and potentially attach the latter to other cytoskeletal filaments (e.g., microfilaments). Microtubule function could affect outflow pathway events through direct cellular mechanical effects (e.g., tensegrity) (Chen and Ingber, 1999), influences on ECM or cell membrane turnover (via vesicle movement), or through secondary signaling (e.g., leading to activation of the actin cytoskeleton). Ethacrynic acid (ECA), a sulfhydryl-reactive diuretic drug, inhibits microtubule assembly in vitro, and induces a rapid decrease in phosphotyrosine levels of focal adhesion kinase and a more subtle decrease in paxillin phosphorylation. Dephosphorylation of these proteins, which is detected before the onset of retraction, stress fiber disruption, or complete disruption of focal adhesions, disrupts signaling pathways that normally maintain the stability of the actin microfilaments and cellular adhesions, indicating a close relationship between the microtubule system and the actomyosin system. This action leads both to cell shape change in culture (Erickson-Lamy et al., 1992; O’Brien et al., 1997), and to facility changes in vivo (Epstein et al., 1987; Liang et al., 1992). Recent evidence has shown that several new derivatives of ECA significantly decrease IOP in cats and monkeys (Shimazaki et al., 2004, 2007). These ECA derivatives are more potent than ECA in terms of inducing cell shape alterations and decreasing actin stress fiber content in human TM cells (Rao et al., 2005c), suggesting that microtubule disruption may reduce outflow resistance at least partially through perturbation of the actomyosin system.
6. Clinical Relevance
At present, the only effective approach available to treat glaucoma is to reduce IOP. Hypotension medications used clinically include aqueous humor secretory inhibitors (e.g., beta-adrenergic receptor antagonists, alfa2-adrenergic agonists, and carbonic anhydrase inhibitors), uveoscleral-outflow enhancers (e.g., prostaglandin analogues), cholinergic drugs that affect trabecular outflow indirectly by contracting the ciliary muscle and deforming the TM, and epinephrine drugs that work on both the TM (inducing changes in cell shape through a beta-adrenergic receptor-cAMP/PKA-mediated cellular relaxation) and the uveoscleral (mediating endogenously synthesized prostaglandins) outflow routes. Secretory suppression may affect supplies of oxygen and nutrients to the non-vascularized cornea, lens and TM. Prostaglandin analogues do not substantially improve trabecular outflow. Cholinergic drug effects on the pupil and accommodation limit their clinical use. Epinephrine-like drugs are no longer used clinically because of their local and systemic side effects. Thus, there are no TM-selective outflow enhancers in current clinical use.
Numerous studies indicate that dynamics of the TM cytoskeleton may be involved in the regulation of aqueous humor outflow. Reduction of outflow resistance induced by cytoskeletal agents acting directly on the TM/Schlemm’s canal of glaucomatous eyes may mimic the normal physiological function. However, cytoskeletal drugs could, in principle, have detrimental effects on other anterior segment tissues, especially the cornea. Lower drug concentrations in larger volumes could minimize corneal toxicity without significantly sacrificing the drug’s effect on the TM following topical administration. This speculation is supported by several studies: intracameral doses of ECA reduce IOP in glaucoma patients without inducing corneal or anterior segment side effects (Melamed et al., 1992); a maximal resistance-reducing intracameral dose of H-7 produces general relaxation and expansion in the TM/Schlemm’s canal but no visible changes in the corneal endothelium or ciliary epithelium (Sabanay et al. 2000; Tian et al., 2001); and multiple doses of 0.01% topical LAT-B, which decreases IOP similar to a single dose of 0.02% LAT-B, did not increase corneal thickness as did the latter (Okka et al., 2004; Peterson et al., 2000). However, the potential cornea toxicity is still an obstacle to the use of higher concentrations of the drugs topically for a greater outflow facility increase.
To overcome this problem, novel methods of drug delivery need to be developed. Receptors might be different in different cell types or ECM, so understanding better the bio-molecular differences between cornea and TM, the different molecular targets or mechanisms for different actin-disrupting agents, and a pro-drug, gene therapy or other site-activated approach, could facilitate development of TM-selective “drugs” that reduce outflow resistance without affecting other ocular tissues.
7. Conclusions
This review has provided evidence supporting the crucial role of the actomyosin system in TM cells and endothelium of Schlemm’s canal in regulating trabecular fluid outflow. Relaxation of the TM and/or inner wall of Schlemm’s canal, which may be induced by pharmacological or genetic perturbation of the actin cytoskeleton, is the major structural change responsible for the increase in trabecular outflow facility. Cytoskeletal drugs and/or cytoskeleton-modulating gene therapies may have potential to open a new avenue in glaucoma treatments.
Acknowledgments
This study was supported by grants from the U.S. National Eye Institute (EY002698 and EY016665), Research to Prevent Blindness, the Wisconsin Alumni Research Foundation, and the Ocular Physiology Research and Education Foundation. BG is the incumbent of the E. Neter Chair in Cell and Tumor Biology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahler CK, Hann CR, Fautsch MP, Johnson DH. Pharmacologic disruption of Schlemm’s canal cells and outflow facility in anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2004;45:2246–2254. doi: 10.1167/iovs.03-0746. [DOI] [PubMed] [Google Scholar]
- Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- Cai S, Liu X, Glasser A, Volberg T, Filla M, Geiger B, Kaufman PL. Effect of latrunculin-A on morphology and actin-associated adhesions of cultured human trabecular meshwork cells. Mol Vision. 2000;6:132–143. http://www.molvis.org/molvis/v6/a18/ [PubMed]
- Chen CS, Ingber DE. Tensegrity and mechanoregulation: from skeleton to cytoskeleton. Osteoarthritis & Cartilage. 1999;7:81–94. doi: 10.1053/joca.1998.0164. [DOI] [PubMed] [Google Scholar]
- Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci. 1999;40:74–81. [PubMed] [Google Scholar]
- Epstein DL, Freddo TF, Bassett-Chu S, Chung M, Karageuzian L. Influence of ethacrynic acid on outflow facility in the monkey and calf eye. Invest Ophthalmol Vis Sci. 1987;28:2067–2075. [PubMed] [Google Scholar]
- Erickson-Lamy K, Schroeder A, Epstein DL. Ethacrynic acid induces reversible shape and cytoskeletal changes in cultured cells. Invest Ophthalmol Vis Sci. 1992;33:2631–2640. [PubMed] [Google Scholar]
- Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest Ophthalmol Vis Sci. 2006;47:1991–1998. doi: 10.1167/iovs.05-0327. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Hu Y, Vittitow JL, Rasmussen CR, Grosheva I, Bershadsky AD, Geiger B, Borrás T, Kaufman PL. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp Eye Res. 2006;82:935–944. doi: 10.1016/j.exer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Geiger B, Yehuda-Levenberg S, Bershadsky AD. Molecular interactions in the submembrane plaque of cell-cell and cell-matrix adhesions. Acta Anat (Basel) 1995;154:46–62. doi: 10.1159/000147751. [DOI] [PubMed] [Google Scholar]
- Grosheva I, Vittitow JL, Goichberg P, Gabelt BT, Kaufman PL, Borrás T, Geiger B, Bershadsky AD. Caldesmon effects on the actin cytoskeleton and cell adhesion in cultured HTM cells. Exp Eye Res. 2006;82:945–958. doi: 10.1016/j.exer.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BYJT, Shuh N, Honda Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001a;42:137–44. [PubMed] [Google Scholar]
- Honjo M, Inatani M, Kido N, Sawamura T, Yue BY, Honda Y, Tanihara H. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch Ophthalmol. 2001b;119:1171–1178. doi: 10.1001/archopht.119.8.1171. [DOI] [PubMed] [Google Scholar]
- Hu Y, Gabelt BT, Kaufman PL. Monkey organ-cultured anterior segments: Technique and response to H-7. Exp Eye Res. 2006;82:1100–1108. doi: 10.1016/j.exer.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Johnson M. What controls aqueous humour outflow resistance? Exp Eye Res. 2006;82:545–557. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana RN, Deng PF, Epstein DL, Rao PV. The role of protein kinase C in modulation of aqueous humor outflow facility. Exp Eye Res. 2003;76:39–47. doi: 10.1016/s0014-4835(02)00255-5. [DOI] [PubMed] [Google Scholar]
- Liang LL, Epstein DL, de Kater AW, Shahsafaei A, Erickson-Lamy KA. Ethacrynic acid increases facility of outflow in the human eye in vitro. Arch Ophthalmol. 1992;110:106–109. doi: 10.1001/archopht.1992.01080130108036. [DOI] [PubMed] [Google Scholar]
- Liu X, Cai S, Glasser A, Volberg T, Polansky JR, Fauss DJ, Brandt CR, Geiger B, Kaufman PL. Effects of H-7 on cultured human trabecular meshwork cells. Mol Vision. 2001;7:145–153. http://www.molvis.org/molvis/v7/a21/ [PubMed]
- Liu X, Hu Y, Filla MS, Gabelt BT, Peters DM, Brandt CR, Kaufman PL. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis. 2005;11:1112–1121. [PubMed] [Google Scholar]
- Lu Z, Overby DR, Scott PA, Freddo TF, Gong H. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008;86:271–281. doi: 10.1016/j.exer.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, Futa R, Rohen JW. Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1981;21:563–573. [PubMed] [Google Scholar]
- Melamed S, Kotas-Neumann R, Barak A, Epstein DL. The effect of intracamerally injected ethacrynic acid on intraocular pressure in patients with glaucoma. Am J Ophthalmol. 1992;113:508–512. doi: 10.1016/s0002-9394(14)74721-x. [DOI] [PubMed] [Google Scholar]
- O’Brien ET, Kinch M, Harding TW, Epstein DL. A mechanism for trabecular meshwork cell retraction: ethacrynic acid initiates the dephosphorylation of focal adhesion proteins. Exp Eye Res. 1997;65:471–483. doi: 10.1006/exer.1997.0357. [DOI] [PubMed] [Google Scholar]
- Okka M, Tian B, Kaufman PL. Effect of low-dose latrunculin B on anterior segment physiologic features in the monkey eye. Arch Ophthalmol. 2004;122:1482–1488. doi: 10.1001/archopht.122.10.1482. [DOI] [PubMed] [Google Scholar]
- Peterson JA, Tian B, Bershadsky AD, Volberg T, Gangnon RE, Spector I, Geiger B, Kaufman PL. Latrunculin-A increases outflow facility in the monkey. Invest Ophthalmol Vis Sci. 1999;40:931–941. [PubMed] [Google Scholar]
- Peterson JA, Tian B, Geiger B, Kaufman PL. Effect of latrunculin-B on outflow facility in monkeys. Exp Eye Res. 2000;70:307–313. doi: 10.1006/exer.1999.0797. [DOI] [PubMed] [Google Scholar]
- Peterson WM, Lampe J, Navratil T, Paran Y, Zvi K, Hennes EA, Gabelt BT, Geiger B, Kaufman PL, Vittitow JL. Topical administration of a novel and potent Rho kinase (ROK) inhibitor INS117548 alters the actin cytoskeleton, effectively lowers IOP, and is well tolerated on the ocular surface. Invest Ophthalmol Vis Sci. 2008;49 E-abstract 3816. http://abstracts.iovs.org/cgi/content/abstract/49/5/3816.
- Rao PV, Deng P, Maddala R, Epstein DL, Li CY, Shimokawa H. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol Vis. 2005a;11:288–297. [PubMed] [Google Scholar]
- Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res. 2005b;80:197–206. doi: 10.1016/j.exer.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Rao PV, Shimazaki A, Ichikawa M, Alvarado JA, Epstein DL. Effects of novel ethacrynic acid derivatives on human trabecular meshwork cell shape, actin cytoskeletal organization, and transcellular fluid flow. Biol Pharm Bull. 2005c;28:2189–2196. doi: 10.1248/bpb.28.2189. [DOI] [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–1037. Erratum in: Invest Ophthalmol Vis Sci 2001;42:1690. [PubMed] [Google Scholar]
- Renieri G, Choritz L, Rosenthal R, Meissner S, Pfeiffer N, Thieme H. Effects of endothelin-1 on calcium-independent contraction of bovine trabecular meshwork. Graefes Arch Clin Exp Ophthalmol. 2008 doi: 10.1007/s00417-008-0817-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Rohen JW, Futa R, Lütjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981;21:574–585. [PubMed] [Google Scholar]
- Rosenthal R, Choritz L, Schlott S, Bechrakis NE, Jaroszewski J, Wiederholt M, Thieme H. Effects of ML-7 and Y-27632 on carbachol- and endothelin-1-induced contraction of bovine trabecular meshwork. Exp Eye Res. 2005;80:837–845. doi: 10.1016/j.exer.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Sabanay I, Gabelt BT, Tian B, Kaufman PL, Geiger B. H-7 effects on the structure and fluid conductance of monkey trabecular meshwork. Arch Ophthalmol. 2000;118:955–962. [PubMed] [Google Scholar]
- Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Functional and structural reversibility of H-7 effects on the conventional aqueous outflow pathway in monkeys. Exp Eye Res. 2004;78:137–150. doi: 10.1016/j.exer.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp Eye Res. 2006;82:236–246. doi: 10.1016/j.exer.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Shimazaki A, Ichikawa M, Rao PV, Kirihara T, Konomi K, Epstein DL, Hara H. Effects of the new ethacrynic acid derivative SA9000 on intraocular pressure in cats and monkeys. Biol Pharm Bull. 2004;27:1019–1024. doi: 10.1248/bpb.27.1019. [DOI] [PubMed] [Google Scholar]
- Shimazaki A, Kirihara T, Rao PV, Tajima H, Matsugi T, Epstein DL. Effects of the new ethacrynic acid oxime derivative SA12590 on intraocular pressure in cats and monkeys. Biol Pharm Bull. 2007;30:1445–1449. doi: 10.1248/bpb.30.1445. [DOI] [PubMed] [Google Scholar]
- Thieme H, Nass JU, Nuskovski M, Bechrakis NE, Stumpff F, Strauss O, Wiederholt M. The effects of protein kinase C on trabecular meshwork and ciliary muscle contractility. Invest Ophthalmol Vis Sci. 1999;40:3254–3261. [PubMed] [Google Scholar]
- Thieme H, Nuskovski M, Nass JU, Pleyer U, Strauss O, Wiederholt M. Mediation of calcium-independent contraction in trabecular meshwork through protein kinase C and rho-A. Invest Ophthalmol Vis Sci. 2000;41:4240–6. [PubMed] [Google Scholar]
- Tian B, Sabanay I, Peterson JA, Hubbard WC, Geiger B, Kaufman PL. Acute effects of H-7 on ciliary epithelium and corneal endothelium in monkey eyes. Curr Eye Res. 2001;22:109–20. doi: 10.1076/ceyr.22.2.109.5529. [DOI] [PubMed] [Google Scholar]
- Tian B, Kaufman PL, Volberg T, Gabelt BT, Geiger B. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch Ophthalmol. 1998;116:633–43. doi: 10.1001/archopht.116.5.633. [DOI] [PubMed] [Google Scholar]
- Tian B, Wang R-F, Podos SM, Kaufman PL. Effects of topical H-7 on outflow facility, intraocular pressure and corneal thickness in monkeys. Arch Ophthalmol. 2004;122:1171–1177. doi: 10.1001/archopht.122.8.1171. [DOI] [PubMed] [Google Scholar]
- Tian B, Gabelt BT, Peterson JA, Kiland JA, Kaufman PL. H-7 increases trabecular facility and facility after ciliary muscle disinsertion in monkeys. Invest Ophthalmol Vis Sci. 1999a;67:293–5. [PubMed] [Google Scholar]
- Tian B, Brumback LC, Kaufman PL. ML-7, chelerythrine and phorbol ester increase outflow facility in the monkey eye. Exp Eye Res. 2000;71:551–66. doi: 10.1006/exer.2000.0919. [DOI] [PubMed] [Google Scholar]
- Tian B, Gabelt BT, Kaufman PL. Effect of staurosporine on outflow facility in monkeys. Invest Ophthalmol Vis Sci. 1999b;40:1009–1011. [PubMed] [Google Scholar]
- Tian B, Kaufman PL. Effects of the rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp Eye Res. 2005;80:215–225. doi: 10.1016/j.exer.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Tokushige H, Inatani M, Nemoto S, Sakaki H, Katayama K, Uehata M, Tanihara H. Effects of topical administration of y-39983, a selective rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest Ophthalmol Vis Sci. 2007;48:3216–3222. doi: 10.1167/iovs.05-1617. [DOI] [PubMed] [Google Scholar]
- Volberg T, Geiger B, Citi S, Bershadsky AD. Effect of protein kinase inhibitor H-7 on the contractility, integrity, and membrane anchorage of the microfilament system. Cell Motil Cytoskel. 1994;29:321–38. doi: 10.1002/cm.970290405. [DOI] [PubMed] [Google Scholar]
- Waki M, Yoshida Y, Oka T, Azuma M. Reduction of intraocular pressure by topical administration of an inhibitor of the rho-associated protein kinase. Curr Eye Res. 2001;22:470–4. doi: 10.1076/ceyr.22.6.470.5489. [DOI] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med. 2002;80:629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res. 2000;19:271–295. doi: 10.1016/s1350-9462(99)00015-4. [DOI] [PubMed] [Google Scholar]