Abstract
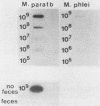
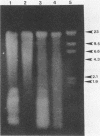
The lysis of mycobacterial cells typically has been difficult and time-consuming. We report a method for the physical rupture of Mycobacterium paratuberculosis and several other members of the family Mycobacteriaceae using a Mini-Beadbeater cell disrupter (Biospec Products; Bartlesville, Okla.) and zirconium beads, a process which yields DNA and RNA of high molecular weight and in greater quantity than that obtained by rupture in a Ribi pressure cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baess I., Bentzon M. W. Deoxyribonucleic acid hybridization between different species of mycobacteria. Acta Pathol Microbiol Scand B. 1978 Apr;86(2):71–76. doi: 10.1111/j.1699-0463.1978.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Bradley S. G. Relationships among mycobacteria and nocardiae based upon deoxyribonucleic acid reassociation. J Bacteriol. 1973 Feb;113(2):645–651. doi: 10.1128/jb.113.2.645-651.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Jacobs W. R., Docherty M. A., Ritchie L. R., Curtiss R., 3rd Molecular analysis of DNA and construction of genomic libraries of Mycobacterium leprae. J Bacteriol. 1985 Mar;161(3):1093–1102. doi: 10.1128/jb.161.3.1093-1102.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby G. K., Jones A. S., Kennedy J. F., Walker R. T. Isolation and analysis of the nucleic acids and polysaccharides from Clostridium welchii. J Bacteriol. 1970 Jul;103(1):159–165. doi: 10.1128/jb.103.1.159-165.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. J., Tabarés E. Separation of Mycobacterium gadium from other rapidly growing mycobacteria on the basis of DNA homology and restriction endonuclease analysis. J Gen Microbiol. 1986 Aug;132(8):2265–2269. doi: 10.1099/00221287-132-8-2265. [DOI] [PubMed] [Google Scholar]
- Gross W. M., Wayne L. G. Nucleic acid homology in the genus Mycobacterium. J Bacteriol. 1970 Nov;104(2):630–634. doi: 10.1128/jb.104.2.630-634.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E. B., Wayne L. G., Gross W. M. Purification of mycobacterial deoxyribonucleic acid. J Bacteriol. 1972 Dec;112(3):1033–1039. doi: 10.1128/jb.112.3.1033-1039.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda T., Kirchheimer W. F., Barksdale L. DNA isolated from Mycobacterium leprae: genome size, base ratio, and homology with other related bacteria as determined by optical DNA-DNA reassociation. J Bacteriol. 1982 Apr;150(1):414–417. doi: 10.1128/jb.150.1.414-417.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi Y., Tokunaga T. Method for isolation of deoxyribonucleic acid from mycobacteria. J Bacteriol. 1970 Nov;104(2):1020–1021. doi: 10.1128/jb.104.2.1020-1021.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Gross W. M. Isolation of deoxyribonucleic acid from mycobacteria. J Bacteriol. 1968 Apr;95(4):1481–1482. doi: 10.1128/jb.95.4.1481-1482.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]