Abstract
The incidence of second non-breast primary cancer following adjuvant treatment was evaluated using data from patients enrolled from 1978 to 1999 in four International Breast Cancer Study Group (IBCSG) trials. The occurrence of these tumours as sites of first failure was assessed separately for two treatment comparisons: toremifene versus tamoxifen for five years in 1035 patients in IBCSG Trials 12-93 and 14-93 with a median follow-up of eight years and endocrine therapy (toremifene or tamoxifen) versus chemoendocrine therapy (CMF or AC plus toremifene or tamoxifen) in 1731 patients from IBCSG Trials III, VII and 12-93, with a combined median follow-up of 14 years. No significant differences in second non-breast primary tumours were observed in either comparison. In particular the incidences of second primary uterine tumours with toremifene and tamoxifen were similar and no significant increase of secondary leukaemias was observed with chemoendocrine therapy compared with endocrine therapy.
Keywords: breast cancer, chemotherapy, endocrine therapy, second primary cancer
Introduction
Breast cancer mortality is decreasing as a result of earlier diagnosis and more effective adjuvant treatment [1]. As an increasing number of patients will die of other causes, side effects from adjuvant treatments become more relevant [2]. Late side effects of such treatments represent a unique challenge because they may take years or even decades to develop and as time passes it is less obvious whether adjuvant treatment is the cause. Together with cardiovascular and degenerative diseases, second non-breast primary cancers are probably the most relevant events other than breast cancer recurrence in the long-term follow-up of breast cancer patients.
Epidemiologic evidence suggests that the risk of second primary cancer is increased among women with early breast cancer [3,4]. Even if this risk is rather small (18% increase over the general population risk) [3], it should be addressed when adjuvant treatment and follow-up programs are considered for patients with early breast cancer. This increased risk may be the result of individual predisposition to specific types of tumours, but adjuvant treatments may also exert carcinogenic effects [4, 5].
The impact of therapy may be influenced by treatment doses and duration of exposure, the use of supportive therapies [6-8] and host factors such as age and individual predisposition [5]. This risk should be weighed against the hazard of recurrence and the expected benefit of adjuvant therapies. Early disclosure of results from adjuvant randomized clinical trials leads to a more rapid introduction in clinical practice of new therapies and new standards of treatment. Long-term results of clinical trials (both in terms of efficacy and safety) can provide valuable information on the risk of second primary cancer. The aim of this report is to evaluate the risk of second non-breast primary cancer in two cohorts of patients from four International Breast Cancer Study Group (IBCSG) adjuvant trials with long-term follow-up.
Patients and Methods
Patients were selected from four IBCSG adjuvant trials which enrolled patients from 1978 to 1999: Trials III, VII, 12-93 and 14-93 (Table 1). The first analysis evaluated whether there was a difference in the cumulative incidence of second non-breast primary cancer according to the type of selective oestrogen receptor modulator (SERM), toremifene or tamoxifen. This evaluation was based on the 1035 patients from IBCSG Trials 12-93 and 14-93 randomized to the SERM comparison. Since toremifene was not available in all countries, only 391 patients in Trial 12 and 644 in Trial 14 were evaluable for this question. Countries without access to toremifene only participated in the chemotherapy questions in these trials. The second analysis addressed the difference in the cumulative incidence of second non-breast primary cancer between endocrine therapy only (pooling results for toremifene and tamoxifen) and chemoendocrine adjuvant therapy in a population of patients from IBCSG Trials III, VII and the first 212 patients in 12-93 (Table 2). Patients in Trial III randomized to observation were excluded from this comparison.
Table 1. Characteristics of IBCSG Trials III, VII, 12-93, 14-93.
| Trial | Population | Years of accrual | Treatment arms | Enrolled pts | Median Follow-up (yrs) | References |
|---|---|---|---|---|---|---|
| III | Postmenopausal less than 65 yrs with positive nodes | 1978-1981 | Observation | 463 | 25.0 | 10 |
| p+Tam × 12 | ||||||
| CMFp+Tam × 12 | ||||||
| VII | Postmenopausal with positive nodes | 1986-1993 | Tam alone | 1212 | 14.7 | 11 |
| Tam + delayed CMF×3 | ||||||
| Tam + early CMF×3 | ||||||
| Tam + early CMF×3 + delayed CMF×3 | ||||||
| 12-93 | Postmenopausal with positive nodes | 1993-1997 | Six-arm randomization: | 212 | 8.0 | 12 |
| Concurrent AC×4 + Tor/Tam | (74) | |||||
| Sequential AC×4 + Tor/Tam | (68) | |||||
| Tor/Tam alone | (70) | |||||
| 1997-1999 | Endocrine randomization stratified by chemotherapy(Y/N) | 238 | ||||
| CT→ Tor/Tam | (143) | |||||
| Tor/Tam alone | (95) | |||||
| 14-93 | Postmenopausal with positive nodes | 1993-1997 | AC×4 + CMF×3 + Tam | 969 | 8.1 | 12 |
| AC×4 + Gap + CMF×3+ Tam | ||||||
| AC×4 + CMF×3 + Tor | ||||||
| AC×4 + Gap + CMF ×3 + Tor |
Abbreviations: CMF: C: cyclophosphamide 100 mg/m2 orally (po) days 1-14 of each cycle; M: methotrexate 40 mg/m2 intravenously (iv) days 1 and 8 of each cycle; F: 5-fluorouracil 600mg/m2 iv days 1 and 8 of each cycle; p: prednisone 7.5 mg/d PO. Delayed CMF: 3 cycles of CMF 9, 12, and 15 months after randomization. Early CMF: 3 cycles of CMF 1, 2, and 3 months after randomization. Tam: tamoxifen 20 mg po once daily. Tor: toremifene 60 mg daily. OFS: ovarian function suppression. AC: A: doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 iv day 1; C: cyclophosphamide 600 mg/m2 iv day 1 for every 21 days. Gap: 16 weeks without chemoendocrine therapy, beginning the week after the last AC dose.
Table 2. Characteristics of patient populations according to the study question.
| Study Questions | Trials | No. of Patients | Received Radiotherapy (%) | Median Follow-up (years) |
|---|---|---|---|---|
| Toremifene vs Tamoxifen | 12-93 | 391 | 208 (53) | 8.0 |
| 14-93 | 644 | 275 (43) | 8.1 | |
| Cumulative | 1035 | 483 (47) | 8.1 | |
| Endocrine vs chemoendocrine | III | 307 | 0 (0) | 25.0 |
| VII | 1212 | 268 (22) | 14.7 | |
| 12-93 | 212 | 91 (43) | 8.0 | |
| Cumulative | 1731 | 359 (21) | 14.4 |
Trial III enrolled patients between July 1978 and August 1981 and all patients received mastectomy, axillary dissection and no radiotherapy. In Trial III [9] postmenopausal patients aged 65 years or less, with axillary lymph node–positive disease, were randomly assigned to observation, tamoxifen plus low-dose prednisone for 1 year or to twelve 28-day courses of CMF plus low-dose prednisone and tamoxifen for 1 year.
Trial VII [10] enrolled postmenopausal patients with positive lymph nodes between July 1986 and April 1993. All patients had a mastectomy or a breast-conserving procedure and axillary node dissection. All patients received tamoxifen for 5 years and were randomly assigned to receive either three courses of classical CMF initially or no initial treatment. Irrespective of the first treatment, patients were also randomly assigned to receive no further treatment or three courses of classical CMF at months 9, 12, and 15 (delayed chemotherapy). Radiotherapy was mandatory in cases of breast-conserving surgery and had to be postponed until the end of the initial phase of chemotherapy. No radiotherapy was to be given after mastectomy.
Trials 12-93 and 14-93 enrolled patients between May 1993 and August 1999 [11]. IBCSG Trial 12-93 was a randomized three by two factorial phase III clinical trial comparing three adjuvant systemic regimens (chemotherapy with endocrine therapy starting concurrently versus chemotherapy with endocrine therapy starting sequentially versus endocrine therapy alone) and evaluating toremifene versus tamoxifen as the endocrine agent. The trial included both post- and perimenopausal women with node-positive, oestrogen receptor (ER)-positive breast cancer who were considered suitable for endocrine therapy alone. The chemotherapy regimen consisted of four courses of AC [anthracycline (doxorubicin 60 mg/m2 OR epirubicin 90 mg/m2 iv day 1) plus cyclophosphamide 600 mg/m2 iv day 1]. Toremifene and tamoxifen were given respectively at the dose of 20 mg and 60 mg daily for five years. Because the accrual rate was low, in 1997 the protocol was modified to discontinue the three-arm randomization for the chemotherapy-oriented question and continue exclusively with randomization to toremifene versus tamoxifen; the use and type of chemotherapy prior to initiation of toremifene or tamoxifen was left to the discretion of the investigators. Thus only the first 212 patients in Trial 12 were randomized to receive chemotherapy or not and were included in the chemoendocrine versus endocrine alone comparison.
IBCSG Trial 14-93 was a randomized two by two factorial phase III clinical trial comparing two ways of delivering adjuvant chemotherapy [with a 16-week gap between AC and CMF versus without the 16-week gap] and evaluating toremifene versus tamoxifen as the endocrine agent following the completion of chemotherapy. The trial included post- and perimenopausal women with node-positive disease who were considered not suitable for endocrine therapy alone. After mastectomy or breast conserving surgery all patients received four courses of AC followed, with or without a 16-week treatment-free interval, by three courses of classical CMF before starting endocrine therapy (toremifene 60 mg PO daily or tamoxifen 20 mg PO daily for five years from randomization).
In Trials 12-93 and 14-93, radiation therapy to the conserved breast was optional for patients having breast conservation surgery. No radiotherapy was to be given after mastectomy. In Trial 14-93 radiotherapy could be given either after all chemotherapy or integrated into CMF as agreed per institution.
In Trials III and VII follow-up visits were scheduled every three months for the first two years, six months for years three to five and yearly thereafter. The follow-up began similarly for Trials 12-93 and 14-93, but was reduced to every six months during year two and yearly thereafter.
The majority of cases of second primary tumours had pathological confirmation of diagnosis; all case report forms with notification of second-non breast cancer were medically reviewed by oncologists and queries were sent to investigators to clarify questionable cases. Disease-free survival (DFS) was defined as the time from the date of randomization to any relapse (including ipsilateral or contralateral breast recurrence), the appearance of a second primary malignancy, or death, whichever occurred first. Competing risk estimates with corresponding p-values controlling for breast cancer recurrences and deaths prior to breast cancer events were used to assess the cumulative incidences of second non-breast primaries [12]; competing risk hazard ratios and corresponding p-values were calculated using the method of Fine and Gray [13]. All probability values were obtained from two-sided tests.
Results
Tables 3 and 4 display second non-breast primaries as sites of first failure according to different treatments. Figures 1 and 2 display the Kaplan-Meier disease-free survival plots and cumulative incidence of second primary events for the two treatment comparisons.
Table 3. Toremifene vs tamoxifen: second non-breast primaries as sites of first failure.
| Toremifene | Tamoxifen | TOTAL | ||||
|---|---|---|---|---|---|---|
| Total patients | 525 | (100.0%) | 510 | (100.0%) | 1035 | (100.0%) |
| Total patients with second non-breast primary cancer | 20 | (3.8%) | 35 | (6.9%) | 55 | (5.3%) |
| 8-year cumulative incidence of second non-breast cancer* | 2.9% | 4.3% | 3.1% | |||
| (95% confidence interval) | (1.4%, 4.4%) | (2.4%, 6.2%) | (2.5%,3.6%) | |||
| Types of second non-breast cancer | ||||||
| Thyroid | . | 1 | (0.2%) | 1 | (<0.1%) | |
| Lung (non small cell) | 1 | (0.2%) | 1 | (0.2%) | 2 | (0.2%) |
| Lung (type not specified) | . | 1 | (0.2%) | 1 | (<0.1%) | |
| Gastric | . | 1 | (0.2%) | 1 | (<0.1%) | |
| Colon | 2 | (0.4%) | 6 | (1.2%) | 8 | (0.8%) |
| Rectal | . | 1 | (0.2%) | 1 | (<0.1%) | |
| Pancreatic | 1 | (0.2%) | 1 | (0.2%) | 2 | (0.2%) |
| Biliary | . | 1 | (0.2%) | 1 | (<0.1%) | |
| Renal | 2 | (0.4%) | 5 | (1.0%) | 7 | (0.7%) |
| Urothelial | 2 | (0.4%) | 3 | (0.6%) | 5 | (0.5%) |
| Ovarian | . | 2 | (0.4%) | 2 | (0.2%) | |
| Endometrial | 6 | (1.1%) | 5 | (1.0%) | 11 | (1.1%) |
| Cervical | . | 1 | (0.2%) | 1 | (<0.1%) | |
| Central nervous system: malignant meningioma | . | 1 | (0.2%) | 1 | (<0.1%) | |
| Melanoma | 2 | (0.4%) | 2 | (0.4%) | 4 | (0.4%) |
| Ocular melanoma | . | 1 | (0.2%) | 1 | (<0.1%) | |
| Skin (type not specified) | 1 | (0.2%) | . | 1 | (<0.1%) | |
| Leiomyosarcoma | 1 | (0.2%) | . | 1 | (<0.1%) | |
| Acute myeloid leukaemia | 1 | (0.2%) | 1 | (0.2%) | 2 | (0.2%) |
| Chronic lymphocytic leukaemia | 1 | (0.2%) | . | 1 | (<0.1%) | |
| Non-Hodgkin's lymphoma | . | 1 | (0.2%) | 1 | (<0.1%) | |
Competing risk analysis [13]
Table 4. Endocrine vs chemoendocrine: second non-breast primaries as sites of first failure (Trials III, VII, and the first 212 patients in Trial 12).
| Endocrine | Chemoendocrine | TOTAL | ||||
|---|---|---|---|---|---|---|
| Total patients | 529 | (100.0%) | 1202 | (100.0%) | 1731 | (100.0%) |
| Total patients with second non-breast primary cancer | 46 | (8.7%) | 93 | (8.0%) | 139 | (8.0%) |
| 14-year cumulative incidence of second non-breast cancer* | 6.5% | 7.2% | 7.0% | |||
| (95% confidence interval) | (4.3%, 8.6%) | (5.6%, 8.7%) | (5.7%,8.2%) | |||
| Types of second non-breast cancer | ||||||
| Head and Neck | 2 | (0.4%) | 1 | (<0.1%) | 3 | (0.2%) |
| Thyroid | 1 | (0.2%) | 3 | (0.2%) | 4 | (0.2%) |
| Lung (non small cell) | . | 3 | (0.2%) | 3 | (0.2%) | |
| Lung (type not specified) | . | 5 | (0.4%) | 5 | (0.3%) | |
| Lung carcinoid | 1 | (0.2%) | . | 1 | (<0.1%) | |
| Esophageal | . | 3 | (0.2%) | 3 | (0.2%) | |
| Gastric | 6 | (1.1%) | 5 | (0.4%) | 11 | (0.6%) |
| Small bowel | . | 1 | (<0.1%) | 1 | (<0.1%) | |
| Colon | 5 | (0.9%) | 7 | (0.6%) | 12 | (0.7%) |
| Rectal | 4 | (0.8%) | 3 | (0.2%) | 7 | (0.4%) |
| Pancreatic | 1 | (0.2%) | 5 | (0.4%) | 6 | (0.3%) |
| Hepatic | . | 1 | (<0.1%) | 1 | (<0.1%) | |
| Biliary | 1 | (0.2%) | 1 | (<0.1%) | 2 | (0.1%) |
| Colorectal (type not specified) | . | 1 | (<0.1%) | 1 | (<0.1%) | |
| Renal | 1 | (0.2%) | 3 | (0.2%) | 4 | (0.2%) |
| Urothelial | 2 | (0.4%) | 7 | (0.6%) | 9 | (0.5%) |
| Ovarian | 3 | (0.6%) | 6 | (0.5%) | 9 | (0.5%) |
| Endometrial | 5 | (0.9%) | 16 | (1.3%) | 21 | (1.2%) |
| Mixed Muellerian tumor | 1 | (0.2%) | 2 | (0.2%) | 3 | (0.2%) |
| Uterine sarcoma | . | 1 | (<0.1%) | 1 | (<0.1%) | |
| Uterine (type not specified) | . | 1 | (<0.1%) | 1 | (<0.1%) | |
| Cervical | 2 | (0.4%) | 2 | (0.2%) | 4 | (0.2%) |
| Vulvar | . | 1 | (<0.1%) | 1 | (<0.1%) | |
| Central nervous system (type not specified) | 1 | (0.2%) | . | 1 | (<0.1%) | |
| Melanoma | 4 | (0.8%) | 4 | (0.3%) | 8 | (0.5%) |
| Ocular melanoma | . | 1 | (<0.1%) | 1 | (<0.1%) | |
| Acute lymphocytic leukaemia | . | 1 | (<0.1%) | 1 | (<0.1%) | |
| Acute myeloid leukaemia | 1 | (0.2%) | 1 | (<0.1%) | 2 | (0.1%) |
| Chronic lymphocyticleukaemia | . | 2 | (0.2%) | 2 | (0.1%) | |
| Non-Hodgkin's lymphoma | 3 | (0.6%) | 4 | (0.3%) | 7 | (0.4%) |
| Lymphoma (type not specified) | 1 | (0.2%) | 1 | (<0.1%) | 2 | (0.1%) |
| Multiple myeloma | 1 | (0.2%) | . | 1 | (<0.1%) | |
| Primary peritoneal | . | 1 | (<0.1%) | 1 | (<0.1%) | |
Competing risk analysis [13]
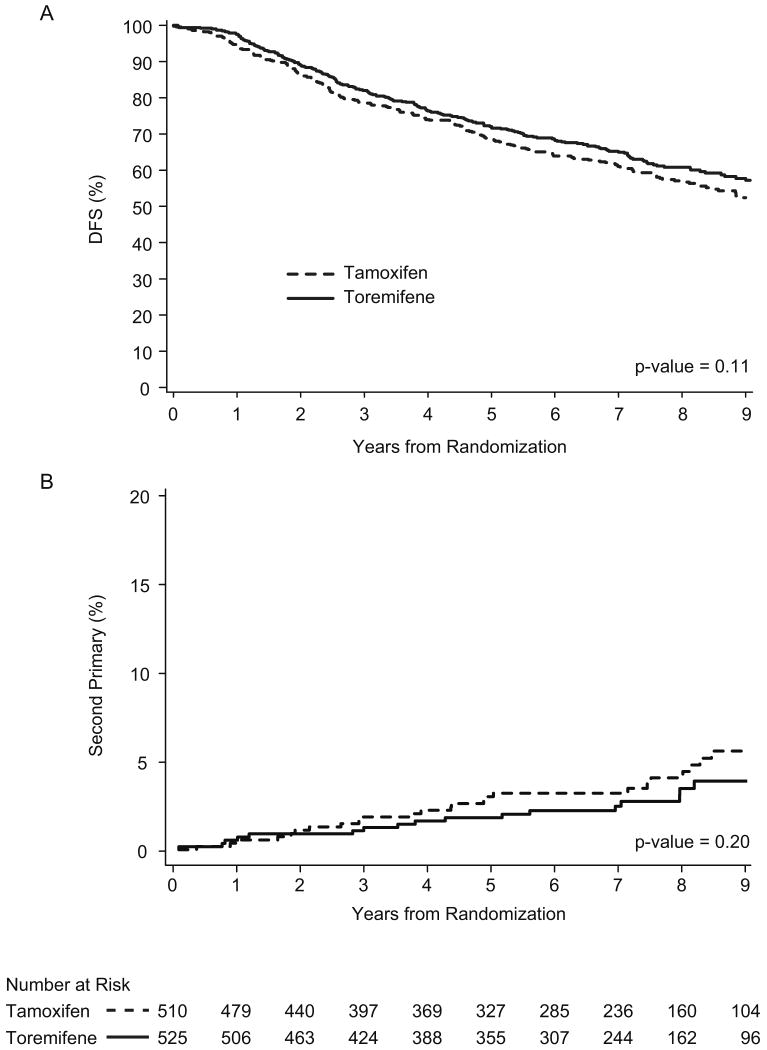
Figure 1.
Toremifene vs. tamoxifen according to disease-free survival (A) and competing risk of second non-breast primary cancer (B) among 1034 postmenopausal patients with node-positive early breast cancer enrolled in IBCSG Trials 12-93 and 14-93. P-value calculated according to Gray (12).
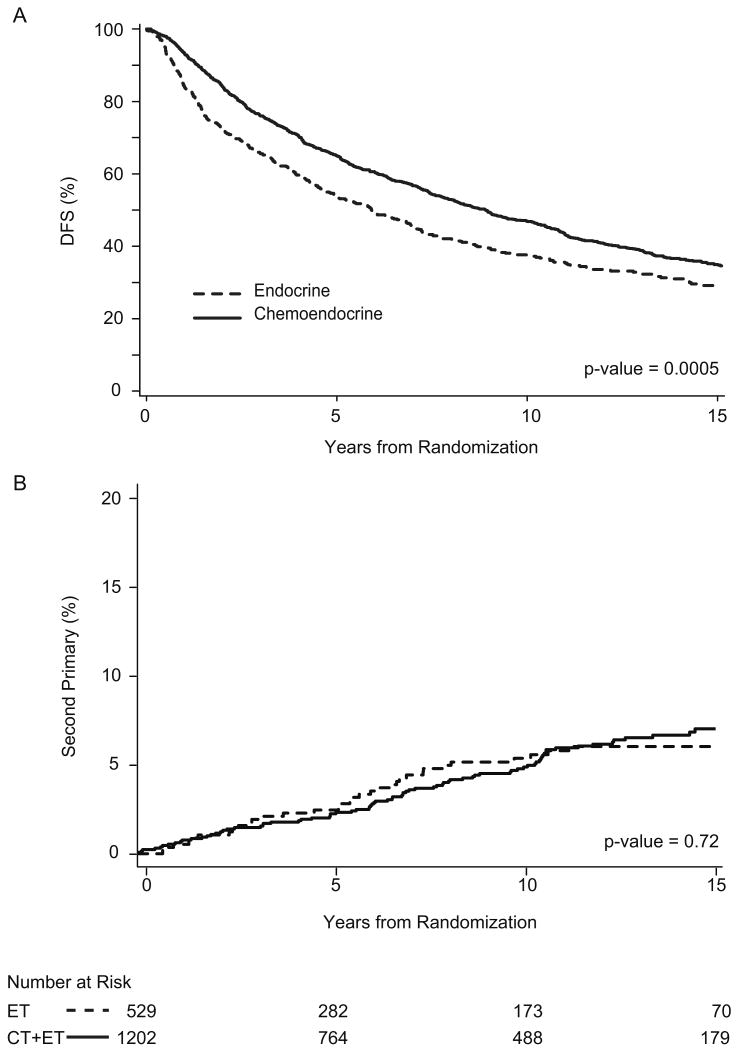
Figure 2.
Endocrine vs. chemoendocrine therapy according to disease-free survival (A) and competing risk of second non-breast primary cancer (B) among 1731 postmenopausal patients with node-positive early breast cancer enrolled in IBCSG Trials III, VII, and 12-93. P-value calculated according to Gray (12).
Toremifene versus Tamoxifen adjuvant therapy
A total of 1035 patients (391 from Trial 12-93 and 644 from Trial 14-93) were available for this comparison. After a median follow-up of 8.1 years, no significant differences were observed between toremifene and tamoxifen with regard to DFS (HR (Tor/Tam) = 0.86; 95% CI = 0.71, 1.04; p-value = 0.11) (Fig 1A). Fifty-five (5.3%) cases of second non-breast primary cancer were observed: 20 cases among the 525 (3.8%) toremifene-treated patients and 35 (6.9%) cases among the 510 tamoxifen-treated patients. The most frequent second primary cancers were endometrial cancer (1.1%), colon cancer (0.8%), renal cancer (0.7%), urothelial cancers (0.5%) (Table 3). No significant difference was evident for any sites of second primary cancer according to the different SERMs. No significant differences were observed between the two differently treated groups of patients with regard to the cumulative incidence of second primary cancers considering breast cancer relapses and deaths without recurrence as competing risks of second primary cancers (Fig 1B) (HR (Tor/Tam) = 0.67; 95% CI =0.37,1.95; p-value = 0.18).
Endocrine vs chemoendocrine adjuvant therapy
The second comparison was based on a total of 1731 patients, 529 treated with endocrine adjuvant therapy alone and 1202 treated with a combination of chemotherapy and endocrine treatments. Median follow-up was 14.4 years and varied among the three clinical trials: 25 years for Trial III, 14.7 years for Trial VII and 8.0 years for Trial 12-93. The incidence of second non-breast primary cancer was 8.0% (139 cases among 1731 patients). Again no significant difference was evident in the incidence of different types of second primary cancer (both solid tumours and acute leukaemia or other hematological malignancy) [endocrine 8.7% (46 events/ 529 patients), chemoendocrine 8.0% (93 events / 1202 patients)] between the two differently treated cohorts of patients (Table 4). No significant differences were observed between the two differently treated groups of patients with regard to the cumulative incidence of second primary cancers considering breast cancer relapses and deaths without recurrence as competing risks (Fig 2) (HR (Chemoendocrine/Endocrine) = 1.04; 95% CI = 0.70, 2.83; p-value = 0.84), although there was a statistically significant difference in DFS (HR Chemoendocrine/Endocrine = 0.80; 95% CI = 0.71, 0.91; p-value = 0.0005) favoring chemoendocrine therapy.
Discussion
Evidence from cancer registries and clinical studies suggests that patients with breast cancer have an increased risk of second primary cancer [3, 14-19]. New primary cancers of the breast account for nearly 40% of all subsequent malignancies, but the risk of other solid tumours and of hematologic malignancies [3,14] also seems higher than in the general population. A recent large population-based cohort study from the Netherlands found approximately one in every 20 breast cancer patients developed a second non-breast primary tumour within 10 years following a breast cancer diagnosis. Compared with the general female population, these breast cancer patients had a 22% increased relative risk in second non-breast primary cancers and an absolute excess risk of 13 cases per10,000 women-years, and occurrence of a second non-breast cancer was associated with a decrease in overall survival [20]. This increased risk of second primary malignancies may be related to common etiologic factors (hormonal and lifestyle), genetic predisposition [3, 4], or to the adjuvant treatment.
Tamoxifen is associated with a threefold increased risk of endometrial cancer and uterine sarcoma, and with a decrease of about a third in the incidence of contralateral breast cancer [5, 15]. The increased risk occurs predominantly among women who are 50 years or older [15,21] and treatment duration may affect the size of risk [4, 5]. An elevated risk of gastrointestinal cancer was also reported [14, 22, 23], but this association remains more controversial [5, 24-26]. Other SERMs such as raloxifene and toremifene may be safer for the uterus, and a lower risk of endometrial cancer has been reported [27-34]. Data from randomized adjuvant trials are conflicting. The Finnish adjuvant trial compared toremifene 40 mg/d with tamoxifen 20 mg/d given orally for 3 years in a population of postmenopausal women with lymph-node positive early breast cancer. At the first interim analysis on 899 out of 1489 accrued patients after a median follow-up of 3.4 years, two cases of endometrial cancer were observed in the tamoxifen arm and none in the toremifene group [33]. On the contrary, the first IBCSG report comparing toremifene and tamoxifen (IBCSG Trials 12-93 and 14-93) as adjuvant hormonal treatments for early breast cancer showed after a median follow-up of 5.5 years a similar incidence of second primary cancer with the two agents and no significant difference in endometrial cancer incidence [11]. The present report provides an update of the incidence of second non-breast primary cancer in patients treated with toremifene and tamoxifen in Trials 12-93 and 14-93 with longer follow-up. The results confirm our previous findings of the lack of a significant difference in endometrial cancer incidence with 6 (1.1%) and 5 (1.0%) patients in toremifene and tamoxifen treated patients, respectively. Overall second primary cancers were slightly less common among patients treated with toremifene, but the difference did not reach statistical significance. The issue of toremifene safety should be re-evaluated in light of updated reports regarding other clinical experiences with this SERM, even if the recent achievements of aromatase inhibitors in the adjuvant setting make this point less relevant.
The findings of the second comparison of this report, evaluating the impact of adding chemotherapy to endocrine treatment on the occurrence of second non-breast primary cancer, were similar to the first. The occurrence of second primary cancer, both solid tumours and secondary acute leukaemias, was not significantly increased in patients receiving chemoendocrine treatment compared with patients treated with endocrine therapy alone. Patients mainly received 3-12 cycles of classical CMF (or 4 cycles of AC in 212 patients) in addition to SERM endocrine therapy. This observation after a long follow-up period supports previous reports which found no increase in solid tumours and only a low excess risk of leukaemia with standard intensity CMF regimens [35,36]. The risk estimate of acute myeloid leukaemia (AML) varies among different studies, generally ranging from 0.2% to 1.7% [36]; differences are probably in part related to different drugs used, doses, schedules, length of follow-up and possibly some supportive therapies [6, 8, 37-46].
Even if secondary AML/Myelodisplastic syndrome occurs in a minority of patients who have received adjuvant chemotherapy and/or radiotherapy, and the expected gain in breast cancer mortality reduction widely exceeds risks even in the most favorable prognostic subgroup of patients [5], the principle of caution is always required; it should be recognized that the benefit of adding chemotherapy could be very low in patients with highly endocrine-responsive disease [48]. It would therefore be advisable to tailor the selection of adjuvant therapies according to biological characteristics of responsiveness and the risks of recurrence and toxicity [49,50]. The use of genetic testing to select patients at intermediate risk of recurrence who could benefit from adjuvant chemotherapy, as well as the identification of risk factors that may confer a higher susceptibility to the development of second non-breast primaries, such as polymorphisms in DNA repair and/or drug-metabolizing enzymes, may further improve the safety of modern breast cancer adjuvant treatment [51-54].
Radiotherapy probably accounts for the increased incidence of lung cancer reported in the literature in breast cancer patients (as well as cancers of esophagus, bone and soft tissue) [3]. In our study there were only fifteen cases of lung cancer (five in patients who had received radiation therapy), limiting the possibility of further analysis. Lung cancers appeared somewhat more frequently in patients treated with chemo-endocrine therapy (11 patients) than in those treated with endocrine therapy (4 patients). As all the trials selected for the present study were limited to postmenopausal patients, it is unlikely that age influenced this apparent difference.
This report, based on a substantial number of similarly-treated patients with eight to fourteen years of accurate follow-up reporting, seems reassuring that these agents used as adjuvant treatment of breast cancer in the comparisons studied do not appear to increase the incidence of second non-breast primary cancer in the comparisons studied. However a control group of untreated patients was lacking in our study and it is possible that the sample size was not large enough to detect a difference in the incidence of these tumours. Finally, experience from other studies shows that cancer survivors may be at higher risk of a second primary cancer even 25-30 years after treatments [19,54], and efforts to obtain long-term follow-up of our patients should continue.
Acknowledgments
We thank the patients, physicians, nurses, and data managers who participate in the International Breast Cancer Study Group trials.
Research Support: The International Breast Cancer Study Group is funded in part by: Swiss Group for Clinical Cancer Research, Frontier Science and Technology Research Foundation, The Cancer Council Australia, Australian New Zealand Breast Cancer Trials Group, National Cancer Institute (CA-75362), Swedish Cancer Society, Cancer Association of South Africa, Foundation for Clinical Research of Eastern Switzerland.
Role of Funding Source: No funding sources had a role in the preparation of this paper.
Appendix of Participating Centers that continue to follow patients on these trials
| Scientific Committee:; | A. Goldhirsch, A.S. Coates (Co-Chairs) |
| Foundation Council: | R. Stahel (President), M. Castiglione, A.S. Coates, J.P. Collins, M. de Stoppani, R.D. Gelber, A. Goldhirsch, M. Green, A. Hiltbrunner, S.B. Holmberg, D.K. Hossfeld, P. Karlsson, I. Láng, C.M. Rudenstam, B. Thürlimann, A. Veronesi |
| Coordinating Center Bern, Switzerland |
A. Hiltbrunner (Director), G. Egli, M. Rabaglio, R. Maibach, R. Studer, B. Ruepp, D. Bärtschi, M. Schärlig-Strausak, C. Hug. |
| Study Chairs | A. Goldhirsch, M. Castiglione, E. Simoncini, O. Pagani |
| Statistical Center Harvard School of Public Health and Dana-Farber Cancer Institute, Boston, MA, USA |
R. Gelber (Group Statistician), K. Price, B. Cole, S. Gelber, M. Regan, Z. Sun, A. Giobbie-Hurder, L. Nickerson |
| Data Management Center Frontier Science & Technology Research Foundation, Amherst, NY, USA |
L. Blacher (Director), R. Hinkle, J. Celano, T. Scolese |
| Pathology Office | B. Gusterson, G. Viale, E. Mallon |
| Centro di Riferimento Oncologico Aviano, Italy | A. Veronesi, D. Crivellari, S. Monfardini, E. Galligioni, M. D. Magri, A. Buonadonna, S. Massarut, C. Rossi, E. Candiani, A. Carbone, R. Volpe, M. Roncadin, M. Arcicasa, F. Coran, S. Morassut |
| Spedali Civili & Fondazione Beretta, Brescia, Italy | E. Simoncini (Study Chair), G. Marini, P. Marpicati, L. Lucini, P. Grigolato, L. Morassi, R. Farfaglia, A.M. Bianchi |
| General Hospital Gorizia, Italy | S. Foladore, L. Foghin, G. Pamich, C. Bianchi, B. Marino, A. Murgia, V. Milan |
| Groote Schuur Hospital and University of Cape Town, Rep. of South Africa | E. Murray, D.M. Dent, A. Gudgeon, I.D. Werner, P. Steynor, A. Hacking, J. Terblanche, A. Tiltman, E. Dowdle, R. Sealy, P. Palmer, P. Helman, E. McEvoy, J. Toop |
| Sandton Oncology Center, Johannesburg, Rep. of South Africa | D. Vorobiof, M. Chasen, G. C. Mohammed |
| West Swedish Breast Cancer Study Group, Göteborg, Sweden | C.M. Rudenstam, S. B. Holmberg, P. Karlsson, A. Wallgren, S. Ottosson-Lönn, R. Hultborn, G. Colldahl-Jädeström, E. Cahlin, J. Mattsson, O. Ruusvik, L.G. Niklasson, S. Dahlin, G. Karlsson, B. Lindberg, A. Sundbäck, S. BergegÂrdh, O. Groot, L.O. Dahlbäck, H. Salander, C. Andersson, M. Heideman, A. Nissborg, A. Wallin, G. Claes, T. Ramhult, J.H. Svensson, P. Liedberg, A. Nilsson, G. Oestberg, S. Persson, J. Matusik |
| European Institute of Oncology Milano, Italy | A. Goldhirsch, M. Colleoni, G. Peruzotti, G. Viale, G. Renne, G. Mazzarol, G. Martinelli, L. Orlando, F. Nolè, R. Torrisi, S. Dellapasqua, G. Curigliano, T. De Pas, F. de Braud, S. Cinieri, F. Peccatori, A. Luini, R. Orecchia, A. Costa, S. Zurrida, P. Veronesi, V. Sacchini, V. Galimberti, M. Intra, U. Veronesi |
| Ospedale S. Eugenio, Rome, Italy | M. Antimi, M. Minelli, V. Bellini, R. Porzio, E. Pernazza, G. Santeusanio, L.G. Spagnoli |
| The Institute of Oncology, Ljubljana, Slovenia | J. Lindtner, D. Erzen, T. Cufer, E. Majdic, B. Stabuc, R. Golouh, J. Lamovec, J. Jancar, I. Vrhovec, M. Kramberger, J. Novak, M. Naglas, M. Sencar, J. Cervek, S. Sebek, O. Cerar, A. Plesnicar, B. Zakotnik |
| Madrid Breast Cancer Group, Madrid, Spain | H. Cortès-Funes, C. Mendiola, C. Gravalos, Colomer, M. Mendez, F. Cruz Vigo, P. Miranda, A. Sierra, F. Martinez-Tello, A. Garzon, S. Alonso, A. Ferrero, C. Vargas |
| Australian New Zealand Breast Cancer Trials Group (ANZ BCTG) Operations Office, University of Newcastle | J.F. Forbes, D. Lindsay |
| Statistical Center, NHMRC CTC, University of Sydney | R.J. Simes, E. Beller, C. Stone, V. Gebski |
| The Cancer Council Victoria (formerly Anti-Cancer Council of Victoria), Melbourne, Australia | J. Collins, R. Snyder, E. Abdi, R. Basser, R. Bennett, P. Briggs, P. Brodie, W.I. Burns, M. Chipman, J. Chirgwin, R. Drummond, P. Ellims, D. Finkelde, P. Francis, J. Funder, T. Gale, M. Green, P. Gregory, J. Griffiths, G. Goss, L. Harrison, S. Hart, M. Henderson, V. Humenuik, P. Jeal, P. Kitchen, G. Lindeman, B. Mann, J. McKendrick, R. McLennan, R. Millar, C. Murphy, S. Neil, I. Olver, M. Pitcher, A. Read, D. Reading, R. Reed, G. Richardson, A. Rodger, I. Russell, M. Schwarz, L. Sisely, R. Stanley, M. Steele, J. Stewart, C. Underhill, J. Zalcberg, A. Zimet |
| Flinders Medical Centre, Bedford Park, South Australia | S. Birrell, M. Eaton, C. Hoffmann, B. Koczwara, C. Karapetis, T. Malden, W. McLeay, R. Seshadri |
| Calgary Mater Newcastle, Newcastle, Australia Gold Coast Hospital, Queensland, Australia | J.F. Forbes, J. Stewart, D. Jackson, R. Gourlay, J. Bishop, S. Cox, S. Ackland, A. Bonaventura, C. Hamilton, J. Denham, P. O'Brien, M. Back, S. Brae, A. Price, R. Muragasu, H. Foster, D. Clarke, R. Sillar, I. MacDonald, R. Hitchins |
| Royal Adelaide Hospital, Adelaide, Australia | I.N. Olver, D. Keefe, M. Brown, P.G. Gill, A. Taylor, A. Robertson, P.G. Gill, M.L. Carter, P. Malycha, E. Yeoh, G. Ward, A.S.Y. Leong, J. Lommax-Smith, D. Horsfall, R. D'Angelo, E Abdi, J. Cleary, F. Parnis |
| Royal Perth Hospital, Perth, Australia | E. Bayliss |
| Sir Charles Gairdner Hospital, Nedlands, Western Australia | M. Byrne, G. van Hazel, J. Dewar, M. Buck, D. Ingram, G. Sterrett, P.M. Reynolds, H.J. Sheiner, K.B. Shilkin, R. Hahnel, S. Levitt, D. Kermode, H. Hahnel, D. Hastrich, D. Joseph, F. Cameron |
| University of Sydney, Dubbo Base Hospital and Royal Prince Alfred Hospital, Sydney, Australia | M.H.N. Tattersall, A. Coates, F. Niesche, R. West, S. Renwick, J. Donovan, P. Duval, R.J. Simes, A. Ng, D. Glenn, R.A. North, J. Beith, R.G. O'Connor, M. Rice, G. Stevens, J. Grassby, S. Pendlebury, C. McLeod, M. Boyer, A. Sullivan, J. Hobbs, R. Fox, D. Hedley, D. Raghavan, D. Green, T. Foo, T.J. Nash, J. Grygiel, D. Lind |
| Prince of Wales, Randwick, NSW, Australia | C. Lewis, M. Friedlander |
| Auckland Breast Cancer Study Group, Auckland, New Zealand | R.G. Kay, I.M. Holdaway, V.J. Harvey, C.S. Benjamin, P. Thompson, A. Bierre, M. Miller, B. Hochstein, A. Lethaby, J. Webber, M.F. Jagusch, L. Neave, B. M. Mason, B. Evans, J.F. Carter, J.C. Gillman, D. Mack, D. Benson-Cooper, J. Probert, H. Wood, J. Anderson, L. Yee, G.C. Hitchcock, A. Lethaby, J. Webber, D. Porter |
| Waikato Hospital, Hamilton, New Zealand | I. Kennedy, G. Round, J. Long |
| SAKK (Swiss Group for Clinical CancerResearch) Inselspital, Bern | M.F. Fey, S. Aebi, M. Castiglione-Gertsch, E. Dreher, K. Buser, J. Ludin, G. Beck, J.M. Lüthi, H.J. Altermatt, M. Nandedkar |
| Kantonsspital, St. Gallen | H.J. Senn, B. Thürlimann, Ch. Oehlschlegel, G. Ries, M. Töpfer, U. Lorenz, A. Ehrsam, B. Späti, E. Vogel |
| Oncology Institute of Southern Switzerland Ospedale San Giovanni, Bellinzona | F. Cavalli, O. Pagani (Study Chair), H. Neuenschwander, C. Sessa, M. Ghielmini, E. Zucca, J. Bernier, E.S. Pedrinis, T. Rusca, E. Passega, L. Bronz, P. Rey, M. Galfetti, W. Sanzeni, T. Gyr, L. Leidi, G. Pastorelli, M. Varini, S. Longhi, C. Cafaro-Greco, R. Graffeo, A. Goldhirsch |
| Kantonsspital, Basel | R. Herrmann, C.F. Rochlitz, J.F. Harder, O. Köchli, U. Eppenberger, J. Torhorst |
| Hôpital des Cadolles, Neuchâtel | D. Piguet, P. Siegenthaler, V. Barrelet, R.P. Baumann |
| Kantonsspital, Zürich | B. Pestalozzi, C. Sauter, U. Haller, U. Metzger, P. Huguenin, R. Caduff |
| Centre Hospitalier Universitaire, Lausanne | L. Perey, K. Zaman, S. Leyvraz, P. De Grandi, W. Jeanneret-Sozzi, R. Mirimanoff, J.F. Delaloye |
| Hôpital Cantonal, Geneva | M. Nobahar, P. Alberto, H. Bonnefoi, P. Schäfer, F. Krauer, M. Forni, M. Aapro, R. Egeli, R. Megevand, E. Jacot-des-Combes, A. Schindler, B. Borisch, S. Diebold |
| Kantonsspital Graubünden, Chur | F. Egli, A. Willi, R. Steiner, J. Allemann, T. Rüedi, A. Leutenegger, U. Dalla Torre |
| Kantonsspital Aarau | A. Schönenberger, M. Wernli, M. Bargetzi, W. Mingrone, P. Schmid, E. Bärtschi, K. Beretta |
Footnotes
Conflicts of Interest Statement: None of the authors has any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lorenzo Gianni, Department of Oncology, Ospedale Infermi, Rimini and Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori, Meldola (FC), Italy. lgianni@auslrn.net.
Shari Gelber, IBCSG Statistical Center, Dana-Farber Cancer Institute and Frontier Science and Technology Research Foundation, Boston, MA, USA. shari@jimmy.harvard.edu.
Alberto Ravaioli, Department of Oncology, Ospedale Infermi, Rimini and Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori, Meldola (FC), Italy. aravaiol@auslrn.net.
Karen N. Price, IBCSG Statistical Center, Frontier Science and Technology Research Foundation, Boston, MA, USA. price@jimmy.harvard.edu
Ilaria Panzini, Department of Oncology, Ospedale Infermi, Rimini and Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori, Meldola (FC), Italy. ipanzini@auslrn.net.
Manuela Fantini, Department of Oncology, Ospedale Infermi, Rimini and Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori, Meldola (FC), Italy. mfantini@auslrn.net.
Monica Castiglione-Gertsch, IBCSG Coordinating Center, Bern, Switzerland. monica.castiglione@ibcsg.org.
Olivia Pagani, Oncology Institute of Southern Switzerland, Ospedale Italiano, Viganello, Lugano, Switzerland and Swiss Group for Clinical Cancer Research (SAKK), Bern, Switzerland. olivia.pagani@ibcsg.org.
Edda Simoncini, MD. Oncologia Medica-Spedali Civili, Brescia, Italy. bscivile@ns.numerica.it.
Richard D. Gelber, PhD. IBCSG Statistical Center, Dana-Farber Cancer Institute, Frontier Science and Technology Research Foundation, and Harvard School of Public Health, Boston, MA, USA. gelber@jimmy.harvard.edu
Alan S. Coates, MD. International Breast Cancer Study Group, Bern, Switzerland and University of Sydney, Australia. alan.coates@ibcsg.org
Aron Goldhirsch, MD. Oncology Institute of Southern Switzerland, Bellinzona, Switzerland and European Institute of Oncology, Milan, Italy. aron.goldhirsch@ibcsg.org.
References
- 1.Peto R, Boreham J, Clarke M, et al. UK and USA breast cancer deaths down 25% in year 2000 at ages 20-69 years. The Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 2.Hayes DF. Clinical practice. Follow-up of patients with early breast cancer. N Engl J Med. 2007;356:2505–13. doi: 10.1056/NEJMcp067260. [DOI] [PubMed] [Google Scholar]
- 3.Curtis RE, Ron E, Hankey BF, Hoover RN. New malignancies following breast cancer. In: Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF Jr, editors. New malignancies among cancer survivors: SEER Cancer Registries, 1973-2000 NIH Publ. No. 05-5302. Bethesda, MD: National Cancer Institute; 2006. pp. 181–205. [Google Scholar]
- 4.Van Leeuwen FE, Travis LB. Second Cancers. In: De Vita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 7th. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 2575–2602. [Google Scholar]
- 5.Matesich SM, Shapiro CL. Second cancers after breast cancer treatment. Semin Oncol. 2003;30:740–8. doi: 10.1053/j.seminoncol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukaemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 7.Shamsaldin A, Linassier C, Clisant S, de Vathaire F, Fenaux P, Hill C. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007;25:292–300. doi: 10.1200/JCO.2006.05.9048. [DOI] [PubMed] [Google Scholar]
- 8.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol. 2007;25:493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig Breast Cancer Study Group. Randomised trial of chemoendocrine therapy, endocrine therapy, and mastectomy alone in postmenopausal patients with operable breast cancer and axillary node metastasis. Lancet. 1984;i:1256–1260. [PubMed] [Google Scholar]
- 10.Crivellari D, Bonetti M, Castiglione-Gertsch M, et al. Burdens and benefits of adjuvant CMF and tamoxifen for elderly patients with breast cancer: The IBCSG Trial VII. J Clin Oncol. 2000;18:1412–1422. doi: 10.1200/JCO.2000.18.7.1412. [DOI] [PubMed] [Google Scholar]
- 11.International Breast Cancer Study Group. Toremifene and tamoxifen are equally effective for early-stage breast cancer: first results of International Breast Cancer Study Group Trials 12-93 and 14-93. Ann Oncol. 2004;15:1749–1759. doi: 10.1093/annonc/mdh463. [DOI] [PubMed] [Google Scholar]
- 12.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 13.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. JAMA. 1999;94:496–509. [Google Scholar]
- 14.Soerjomataram I, Louwman WJ, Lemmens VE, et al. Risks of second primary breast and urogenital cancer following female breast cancer in the south of The Netherlands, 1972-2001. Eur J Cancer. 2005;41:2331–7. doi: 10.1016/j.ejca.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Mellemkjaer L, Friis S, Olsen JH, et al. Risk of second cancer among women with breast cancer. Int J Cancer. 2006;118:2285–92. doi: 10.1002/ijc.21651. [DOI] [PubMed] [Google Scholar]
- 16.Soerjomataram I, Louwman WJ, de Vries E, Lemmens VE, Klokman WJ, Coebergh JW. Primary malignancy after primary female breast cancer in the South of the Netherlands, 1972-2001. Breast Cancer Res Treat. 2005;93:91–5. doi: 10.1007/s10549-005-4016-2. [DOI] [PubMed] [Google Scholar]
- 17.Levi F, Te VC, Randimbison L, La Vecchia C. Cancer risk in women with previous breast cancer. Ann Oncol. 2003;14:71–3. doi: 10.1093/annonc/mdg028. [DOI] [PubMed] [Google Scholar]
- 18.Evans HS, Lewis CM, Robinson D, Bell CM, Moller H, Hodgson SV. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer. 2001;84:435–40. doi: 10.1054/bjoc.2000.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown LM, Chen BE, Pfeiffer RM, et al. Risk of second non-hematological malignancies among 376,825 breast cancer survivors. Breast Cancer Res Treat. 2007;106:439–451. doi: 10.1007/s10549-007-9509-8. [DOI] [PubMed] [Google Scholar]
- 20.Schaapveld M, Visser O, Louwman MJ, et al. Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol. 2008;26:1239–46. doi: 10.1200/JCO.2007.11.9081. [DOI] [PubMed] [Google Scholar]
- 21.Hirsimäki P, Aaltonen A, Mäntylä E. Toxicity of antiestrogens. Breast J. 2002;8:92–6. doi: 10.1046/j.1524-4741.2002.08204.x. [DOI] [PubMed] [Google Scholar]
- 22.Rutqvist LE, Johansson H, Signomklao T, Johansson U, Fornander T, Wilking N. Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. Stockholm Breast Cancer Study Group. J Natl Cancer Inst. 1995;87:645–51. doi: 10.1093/jnci/87.9.645. [DOI] [PubMed] [Google Scholar]
- 23.Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18:937–47. doi: 10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook LS, Weiss NS, Pharris-Ciurej N, Schwartz SM, White E. Colorectal cancer following tamoxifen therapy for breast cancer (United States) Cancer Causes Control. 2001;12:405–10. doi: 10.1023/a:1011228501440. [DOI] [PubMed] [Google Scholar]
- 25.Curtis RE, Boice JD, Jr, Shriner DA, Hankey BF, Fraumeni JF., Jr Second cancers after adjuvant tamoxifen therapy for breast cancer. J Natl Cancer Inst. 1996;88:832–4. doi: 10.1093/jnci/88.12.832. [DOI] [PubMed] [Google Scholar]
- 26.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 27.Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–97. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 28.Vogel VG, Costantino JP, Wickerham DL, et al. National Surgical Adjuvant Breast and Bowel Project (NSABP) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 29.Harvey HA, Kimura M, Hajba A. Toremifene: an evaluation of its safety profile. Breast. 2006;15:142–57. doi: 10.1016/j.breast.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Pukkala E, Kyyrönen P, Sankila R, Holli K. Tamoxifen and Toremifene treatment of breast cancer and risk of subsequent endometrial cancer: a population-based case-control study. Int J Cancer. 2002;100:337–341. doi: 10.1002/ijc.10454. [DOI] [PubMed] [Google Scholar]
- 31.Pyrhonen S, Ellmen J, Vuorinen J, et al. Meta-analysis of trials comparing toremifene with tamoxifen and factors predicting outcome of antiestrogen therapy in postmenopausal women with breast cancer. Breast Cancer Res Treat. 1999;56:133–43. doi: 10.1023/a:1006250213357. [DOI] [PubMed] [Google Scholar]
- 32.Milla-Santos A, Milla L, Rallo L, Solano V. Phase III randomized trial of toremifene vs tamoxifen in hormonodependent advanced breast cancer. Breast Cancer Res Treat. 2001;65:119–24. doi: 10.1023/a:1006440802709. [DOI] [PubMed] [Google Scholar]
- 33.Holli K, Valavaara R, Blanco G, et al. Safety and efficacy results of a randomized trial comparing adjuvant toremifene and tamoxifen in postmenopausal patients with node-positive breast cancer. Finnish Breast Cancer Group. J Clin Oncol. 2000;18:3487–94. doi: 10.1200/JCO.2000.18.20.3487. [DOI] [PubMed] [Google Scholar]
- 34.Mäenpää J, Ellmén J, Pasanen T, Kaukonen M. Re: Effects of the antiestrogens Tamoxifen, Toremifene and ICI 182,780 on endometrial cancer growth. J Natl Cancer Inst. 1999;91:972. doi: 10.1093/jnci/91.11.972. [DOI] [PubMed] [Google Scholar]
- 35.Valagussa P, Moliterni A, Terenziani M, Zambetti M, Bonadonna G. Second malignancies following CMF-base adjuvant chemotherapy in resectable breast cancer. Ann Oncol. 1994;5:803–808. doi: 10.1093/oxfordjournals.annonc.a059008. [DOI] [PubMed] [Google Scholar]
- 36.Tallman MS, Gray R, Bennett JM, et al. Leukemogenic potential of adjuvant chemotherapy for early-stage breast cancer: the Eastern Cooperative Oncology Group experience. J Clin Oncol. 1995;13:1557–63. doi: 10.1200/JCO.1995.13.7.1557. [DOI] [PubMed] [Google Scholar]
- 37.Kroger N, Zander AR, Martinelli G, et al. European Group for Blood and Marrow Transplantation. Low incidence of secondary myelodysplasia and acute myeloid leukemia after high-dose chemotherapy as adjuvant therapy for breast cancer patients: a study by the Solid Tumors Working Party of the European Group for Blood and Marrow Transplantation. Ann Oncol. 2003;14:554–8. doi: 10.1093/annonc/mdg161. [DOI] [PubMed] [Google Scholar]
- 38.Le Deley MC, Suzan F, Cutuli B, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007;25:292–300. doi: 10.1200/JCO.2006.05.9048. [DOI] [PubMed] [Google Scholar]
- 39.Praga C, Bergh J, Bliss J, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol. 2005;23:4179–91. doi: 10.1200/JCO.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 40.Wilking N, Lidbrink E, Wiklund T, et al. Long-term follow-up of the SBG 9401 study comparing tailored FEC-based therapy versus marrow-supported high-dose therapy. Ann Oncol. 2007;18:694–700. doi: 10.1093/annonc/mdl488. [DOI] [PubMed] [Google Scholar]
- 41.Patt DA, Duan Z, Fang S, Hortobagyi GN, Giordano SH. Acute myeloid leukemia after adjuvant breast cancer therapy in older women: Understanding risk. J Clin Oncol. 2007;25:3871–6. doi: 10.1200/JCO.2007.12.0832. [DOI] [PubMed] [Google Scholar]
- 42.Martin M, Pienkowski T, Mackey J, et al. Breast Cancer International Research Group 001 Investigators. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–13. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 43.Roche H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24:5664–71. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 44.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–9. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 45.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–7. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 46.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–96. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 47.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–83. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 48.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–67. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldhirsch A, Coates AS, Gelber RD, Glick JH, Thürlimann B, Senn HJ, St Gallen Expert Panel Members First--select the target: better choice of adjuvant treatments for breast cancer patients. Ann Oncol. 2006;17:1772–6. doi: 10.1093/annonc/mdl398. [DOI] [PubMed] [Google Scholar]
- 50.Goldhirsch A, Wood W, Gelber R, Coates A, Thurlimann B, Senn HJ. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol; 10th St. Gallen Conference; 2007. pp. 1133–44. [DOI] [PubMed] [Google Scholar]
- 51.Seedhouse C, Russell N. Advances in the understanding of susceptibility to treatment-related acute myeloid leukaemia. Br J Haematol. 2007;137:513–29. doi: 10.1111/j.1365-2141.2007.06613.x. [DOI] [PubMed] [Google Scholar]
- 52.Haase D, Binder C, Bunger J, et al. Increased risk for therapy-associated hematologic malignancies in patients with carcinoma of the breast and combined homozygous gene deletions of glutathione transferases M1 and T1. Leuk Res. 2002;26:249–54. doi: 10.1016/s0145-2126(01)00124-2. [DOI] [PubMed] [Google Scholar]
- 53.Bolufer P, Collado M, Barragan E, et al. Profile of polymorphisms of drug-metabolising enzymes and the risk of therapy-related leukaemia. Br J Haematol. 2007;136:590–6. doi: 10.1111/j.1365-2141.2006.06469.x. [DOI] [PubMed] [Google Scholar]
- 54.van den Belt-Dusebout AW, de Wit R, Gietema JA, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25:4370–8. doi: 10.1200/JCO.2006.10.5296. [DOI] [PubMed] [Google Scholar]