Abstract
Epileptiform activity (EA) in vivo and in vitro induces a loss of dendritic spines and synapses. Because CaMKII has been implicated in synaptogenesis and synaptic plasticity, we investigated the role of CaMKII in the effects of EA on spines, using rat hippocampal slice cultures. To visualize dendrites and postsynaptic densities (PSDs) in pyramidal neurons in the slices, we used biolistic transfection to express either free GFP or a PSD-95-YFP construct that specifically labels PSDs. This allowed us to distinguish two classes of dendritic protrusions: spines, that contain PSDs, and filopodia, that lack PSDs, and which are, on average, longer than spines. By these criteria, 48 hour of EA caused a decrease specifically in the number of spines. Immunoblots showed that EA increased CaMKII activity in the slices. Inhibition of CaMKII by expression of AIP, a specific peptide inhibitor of CaMKII, reduced spine number under basal conditions and failed to prevent EA-induced spine loss. However, in EA conditions, AIP increased the number of filopodia and the number of PSDs on the dendritic shaft. These data show at least two roles for CaMKII activity in maintenance and remodeling of dendritic spines under basal or EA conditions: First, CaMKII activity promotes the maintenance of spines and spine PSDs. Second, CaMKII activity suppresses EA-induced formation of filopodia and suppresses an increase in shaft PSDs, apparently by promoting translocation of PSDs from dendritic shafts to spines and/or selectively stabilizing spine rather than shaft PSDs.
Keywords: Epileptiform Activity, CaMKII, Hippocampal Slice Culture, Postsynaptic Density, Synaptogenesis
INTRODUCTION
Dendritic spines undergo dynamic remodeling during synaptogenesis (Dailey and Smith 1996; Dunaevsky et al. 2001; Dunaevsky et al. 1999; Ziv and Smith 1996) and in response to neuronal activity (Engert and Bonhoeffer 1999; Maletic-Savatic et al. 1999; Toni et al. 1999). While many studies have examined acute activity-induced changes (Reviewed by (Segal 2005), the effect of protracted changes in neuronal activity on spine remodeling has not been well studied. We used an “epileptiform activity” (EA) paradigm to cause bursts of elevated neural activity in cultured rat hippocampal slices. While distinct from epilepsy in vivo, which involves periodic episodes of increased activity over an extended period, EA in vitro similarly involves a pattern of neuronal activity characterized by synchronized firing of neighboring neurons. Previous studies show that prolonged EA induces a loss of spines and postsynaptic densities (PSDs) in hippocampal and cortical neurons in vitro (Muller et al. 1993; Drakew et al. 1996; Zha et al. 2005), similar to what has been observed in epilepsy in vivo in humans (Bothwell et al. 2001; Swann et al. 2000) and in animal models (Jiang et al. 1998; Swann et al. 2000). We also previously noted a preferential loss of spine, as opposed to dendritic shaft synapses (Zha et al. 2005). Here, we investigate the underlying molecular mechanisms of this spine and synapse loss.
Ca2+/calmodulin-dependent protein kinase II (CaMKII), a prominent component of the postsynaptic density in spines, has been implicated in synaptogenesis and plasticity (Hudmon and Schulman 2002; Lisman et al. 2002). Synaptic activity induces CaMKII activation (Fink and Meyer 2002; Lisman et al. 2002) and regulates its localization at postsynaptic sites (Ahmed et al. 2006; Gleason et al. 2003; Shen and Meyer 1999). In turn, activated CaMKII at postsynaptic sites facilitates synaptic transmission by phosphorylating and promoting synaptic insertion of AMPA receptors (Bayer et al. 2001; Wu et al. 1996). In addition to its acute effect on synaptic function, CaMKII regulates the formation, growth and/or branching of axons and dendrites (Hansen et al. 2003; Redmond et al. 2002; Vaillant et al. 2002; Zou and Cline 1996; Zou and Cline 1999). CaMKII may also play important roles in regulating the number of synapses. In dissociated cortical neurons, CaMKII activation decreases while CaMKII inhibition increases the number of synapses (Pratt et al. 2003).
Given the abundance of CaMKII at postsynaptic sites, its regulation by synaptic activity, and its effects on neuronal architecture, we hypothesize a role for CaMKII in the control of spine and synapse number by prolonged EA. We show here that EA induces CaMKII activation. By expressing a fluorescent genetically-encoded CaMKII inhibitor (Bok et al. 2007; Zha et al. 2006) in isolated cells in hippocampal slice cultures, we examined the role of postsynaptic CaMKII in EA-induced spine remodeling. Although CaMKII inhibition appeared to partially reverse EA-induced loss of dendritic protrusions, by distinguishing spines (protrusions that contain PSDs) from filopodia, we determined that CaMKII inhibition did not block EA-induced loss of mature spines but rather increased the number of filopodia. Also, CaMKII inhibition during EA increased the number of shaft PSDs. These results indicate that synaptic remodeling in EA, while favoring a net loss of spines, does include some conversion of filopodia to new spines, at least in part by translocation of PSDs from shaft to filopodium. We suggest that this latter process is CaMKII-dependent because blockade of postsynaptic CaMKII activity leads to accumulation of “empty” filopodia and shaft PSDs.
METHODS AND MATERIALS
Constructs and reagents
The PSD95-YFP constructs were kindly provided by the late Dr. Alaa El-Husseini (Univ. of British Columbia) and by Dr. Bonnie Firestein (Rutgers Univ.). EGFP-C1 and ECFP-C1 expression vectors were purchased from Clontech.
GFP-AIP was constructed by inserting oligonucleotides encoding autocamtide-2 related inhibitory peptide (AIP; KKALRRQEAVDAL) into the EGFP-C1 vector between BamHI and XbaI and has been previously described in detail (Bok et al. 2007). CFP-AIP was constructed similarly by subcloning the AIP sequence into ECFP-C1 (Zha et al. 2006). Efficacy and specificity of GFP-AIP were shown by its ability to inhibit activation of a CRE-reporter construct by cotransfected constitutively-active CaMKII while not inhibiting activation by CaMKIV or by cAMP-dependent protein kinase (Bok et al. 2007).
The monoclonal anti-CaMKIIα antibody (Leonard et al. 1999) was a gift of Dr. Johannes Hell. Other antibodies used are: monoclonal phospho-Thr 286 specific CaMKIIα antibody from Affinity Bioreagents or Promega, anti-actin IgM from the University of Iowa Developmental Studies Hybridoma Bank, and HRP-conjugated secondary antibodies from Sigma and Santa Cruz. Other reagents used: SR-75531 (gabazine, Gbz) from Sigma and RBI, tetrodotoxin (TTX) from RBI.
Hippocampal slice culture and transfection
Rat hippocampal organotypic slice cultures were prepared as previously described (Zha et al. 2005), using methods modified from Stoppini et al (Stoppini et al. 1991). Briefly, hippocampi from postnatal 6 to 7 day old rats were dissected and cut into 300 µm thick transverse slices using a manual tissue chopper. Slices were placed in Falcon polyethylene terephthalate-etched membrane culture inserts containing 1 µm pores (Fisher). Slices were maintained in filter culture medium (FCM) (25% horse serum, 25% Hanks Balanced Salt Solution, 50% MEM, 2 mM glutamine, 44 µg/ml NaHCO3, and 10 U/ml penicillin-streptomycin) without addition of HEPES or glucose. Slices were cultured at 37°C in a 5% CO2 humidified incubator. The medium was changed every 2–3 days.
Biolistic transfection of slice cultures was done using a Helios Gene Gun (Bio-Rad), as previously described (Marrs et al. 2001). For spine analysis, P6 or P7 rats were cultured for 4 days before transfection. For PSD analysis, P6 rats were cultured for 5 days, or P7 rats were cultured for 4 to 5 days before transfection and slices were allowed to express transfected genes for 10–18 hr before transition to experimental conditions. Typically, <6 neurons/slice express the transgene(s), allowing observation of the effect on individual neurons.
Experimental culture conditions used in this study include: control FCM, FCM with 10 µM Gbz, FCM with 10 µM Gbz plus 0.2 µM TTX, or FCM with 0.2 µM TTX. Using Ca2+ imaging, we showed previously that Gbz treatment induced periodic waves of synchronous activity (epileptiform activity, EA) in hippocampal slices within 20 m of treatment and that the frequency of the EA did not change significantly after 24 hr of treatment (Zha et al. 2005).
Comparisons among culture conditions were performed on slices dissected and cultured in parallel at the same time. For counting spines and PSDs, slices were fixed with 4% paraformaldehyde in 25% horse serum, 25% Hanks Balanced Salt Solution, 50% MEM, 25 mM HEPES, pH 7.2, 48 hrs after transition to experimental conditions.
Confocal Microscopy
Confocal imaging was done as described previously (Dailey 2002). Briefly, images were captured using scanning laser confocal microscopes (either a Leica TCS NT or a Leica SP2 AOBS; Leica, Inc.). For Leica TCS NT, illumination was provided by two separate lasers, argon (Ar) and krypton (Kr). For Leica SP2 AOBS, illumination was provided by Ar and green He-Ne lasers. For analysis of spines and synapses in fixed tissues, a series of high-resolution images (1024 × 1024 pixel array) was captured at a z-step of 0.8 µm using a 63×/1.2 PL APO water lens with an additional electronic zoom of 1.5 or 2 for Leica TCS NT or 2.8 for Leica SP2 AOBS. Each captured image was an average of 3–4 scans in a single plane. For each transfected neuron, one segment of an apical dendrite (about 50–150 µm away from the cell body layer) was imaged and used for spine and synapse analysis. For CFP and YFP cotransfected cells, CFP was excited with the 458 nm laser line and YFP with the 514 nm line.
Data analysis and statistics
Dendritic segments and spines were analyzed quantitatively using Scion Image (Scion Corp.) or ImageJ (NIH) software as described earlier (Zha et al. 2005). Briefly, spine length was measured as the distance from the tip of the spine to the intersection between spine and dendrite shaft. In the case where spines branched, the length was measured along the spine from the farthest tip to the base. 3-D image stacks were used to determine the length of each protrusion independent of its orientation.
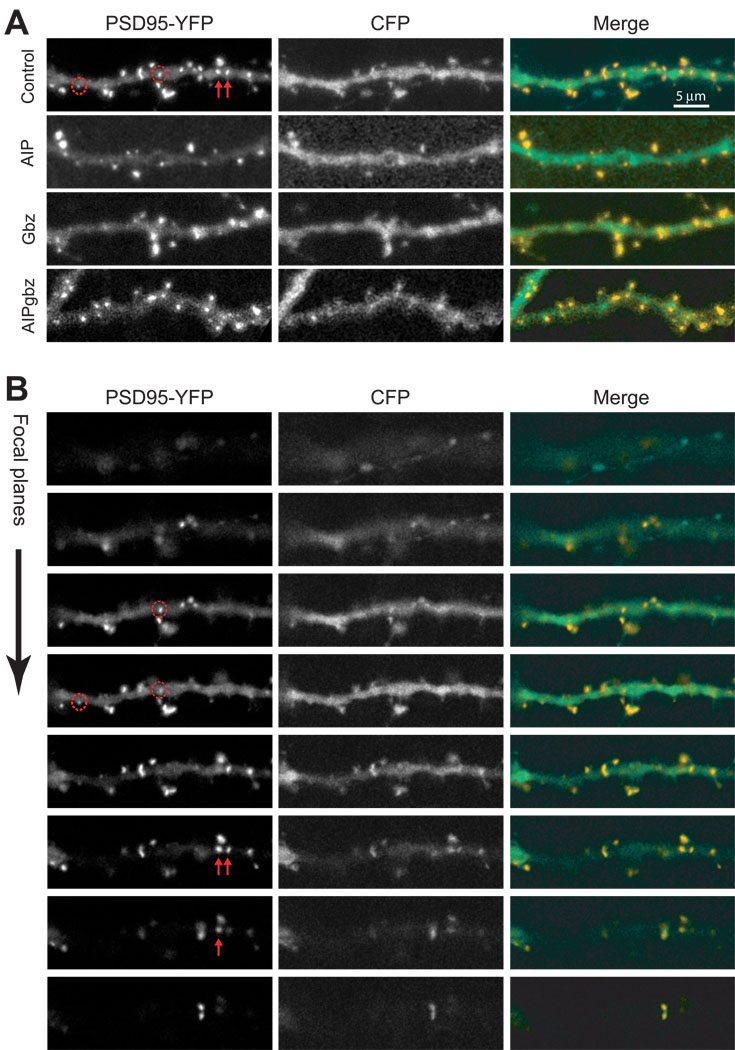
PSDs were scored using 3-D image stacks such that if the best-focus image of a PSD was located one or more focal planes above or below the surface of the dendrite shaft, it was counted as a spine PSD rather than shaft PSD (see Figure 4 for detail). Thus, we could distinguish between most spine and shaft PSDs regardless of the orientation of the spine. It is possible that a small number of PSDs on spines that protrude slightly (<0.2 µm) from the shaft were scored as shaft PSDs. While this technical limitation could cause a slight overestimate of the number of shaft PSDs across experimental conditions, it should not affect our major conclusions.
Figure 4. Representative images of PSD-95 clusters under different conditions.
Pyramidal neurons in hippocampal slice cultures were co-transfected with PSD95-YFP with either CFP or CFP-AIP and treated with control FCM or Gbz, as described in Methods. (A) Representative sets of 2D projection images showing a segment of dendrite under different conditions. The actual quantitation was done using 3D stacks. The left panel shows YFP fluorescence, the middle panel shows CFP fluorescence and the right panel shows the merged images with the YFP in yellow and the CFP in green. The red arrows and circles in the upper-left panel correspond to those in the panels in B and distinguish shaft (arrows) and spine (circles) PSDs. (B) The image stack used to construct the projection for control images shown in (A) is shown to demonstrate how spine and shaft PSDs were distinguished. For example, the red arrows and circles indicate four PSDs that all appear to be on the shaft in the 2D projection images in (A). Scrutiny of the entire image stack reveals the location of PSDs in the z-axis, revealing that the two PSDs identified by arrows are on dendritic shaft but the two circled PSDs are not in the same plane as the dendritic shaft but are on projections from the shaft and were consequently classified as spine PSDs.
Raw data obtained in Scion Image or ImageJ was exported to Microsoft Excel for further analysis. When analyzing spine length, spines with length <0.5 µm were not scored because it is typically difficult to get a reliable measurement of such small spines at the light microscope level of resolution. Analyses of spines and PSD95 clusters were performed by an individual blinded to the experimental conditions. Unless otherwise noted, statistical comparisons were done by ANOVA with a Bonferroni posthoc correction (normality was confirmed by a Kolmogorov-Smirnoff test).
Western Blot
Hippocampal slices were lysed in lysis buffer (50 mM Tris, pH 6.8, 2% SDS, 50 mM EDTA) in the presence of 0.1 µM calyculin A (Cell Signaling Technology), phosphatase inhibitor cocktail (Roche), protease inhibitor cocktail (Roche), and 1 mM sodium vanadate. Lysates were sonicated briefly and boiled for 10 min before loading. Approximately 20 µg of protein was loaded into each lane. Proteins were separated on 10% polyacrylamide SDS gel and transferred to an ECL nitrocellulose membrane (Amersham Pharmacia) or PVDF membrane after electrophoresis. Antibodies used: anti-CaMKIIα antibody 1:4000; anti-phosphoCaMKIIα antibody (Affinity Bioreagents 1:1500; Promega 1:5000); and anti-actin antibody 1:600. HRP-conjugated secondary antibodies were used at 1:20,000. Chemiluminescent reaction and detection was performed with an ECLplus kit (Amersham Pharmacia) or a Westpico kit (Pierce), according to the manufacturer’s instruction. Quantitation was done using ImageJ software (NIH).
Results
Epileptiform activity activates CaMKII
We have previously shown that epileptiform activity (EA) reduces the number of dendritic spines and of spine PSDs on pyramidal neurons in hippocampal slice cultures (Zha et al. 2005). In these experiments EA was induced by inhibiting GABAA receptors with gabazine (Gbz) and was verified by observation of repetitive synchronized [Ca2+]i transients in hippocampal pyramidal neurons (Zha et al. 2005). Similar approaches have been used to study EA-induced changes in hippocampal slices (Jiang et al. 1998; Swann et al. 2007; Thompson et al. 1996; Zha et al. 2005).
Inhibiting GABAA receptors for up to 7 days has no significant effect on cell density, pyramidal cell number, or pyramidal cell layer volume (Swann et al. 2007). Similarly, we detected no significant changes in the morphology of Gbz-treated slices, relative to control slices, after up to 48 hrs of Gbz. On western blots, total CaMKII levels were also unchanged in Gbz-treated slices (Figure 1), indicating no extensive neuronal death. These observation are consistent with previous studies showing that developing hippocampal slices are resistant to seizure-induced cell death (Cavalheiro et al. 1987; Lee et al. 1995; Nitecka et al. 1984; Okada et al. 1984).
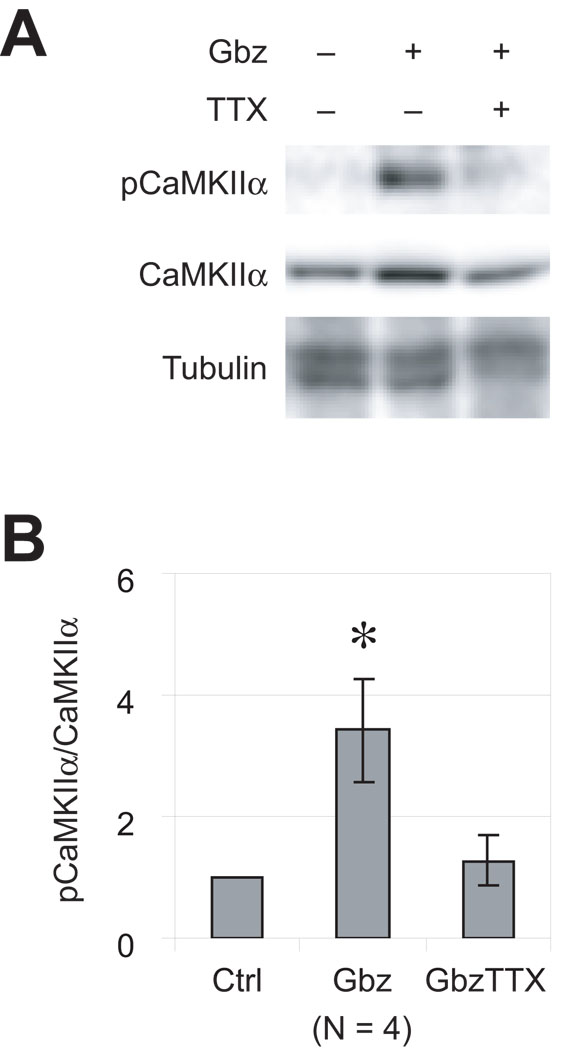
Figure 1. CaMKIIα phosphorylation is induced by epileptiform activity.
Hippocampal slices were cultured for 4–5 days, then maintained for an additional 12 hrs in various conditions: control, 10 µM Gbz, or 10 µM Gbz plus 0.2 µM TTX. Lysates were prepared and probed for CaMKIIα and for phosphorylated CaMKIIα (pCaMKIIα) by western blot. (A) A representative set of blots is shown. Immunoblotting with actin antibody verified that equal amounts of protein were loaded for each of the conditions. (B) Quantitation of the ratio of pCaMKIIα to CaMKIIα immunoreactivity from 4 separate experiments. The pCaMKIIα to CaMKIIα ratio under control condition was arbitrarily set to 1. Gbz treatment significantly (p<0.05) increased CaMKIIα phosphorylation and this is blocked by TTX.
Because CaMKII is a prominent Ca2+-regulated intracellular signaling molecule (Soderling et al. 2001) we asked whether CaMKII activity is regulated by EA. Phosphorylation on Thr286 is correlated with CaMKII activity and is sufficient for the activation of CaMKIIα (reviewed by (Hudmon and Schulman 2002; Lisman et al. 2002). We therefore examined the activation of CaMKIIα by immunoblot using an antibody that specifically recognizes Thr286 phosphorylated form of CaMKIIα. As shown in Figure 1, phosphorylated CaMKIIα (pCaMKII) immunoreactivity was increased with Gbz treatment, showing that EA induces CaMKIIα activation. Inhibiting EA with TTX blocked the increase in CaMKIIα phosphorylation (Figure 1).
CaMKII inhibition blocks EA-induced loss of dendritic protrusions
Previous studies have shown a reduction in dendritic spine density in epileptiform models in vivo and in vitro (Isokawa 2000; Jiang et al. 1998; Muller et al. 1993; Swann et al. 2000; Zha et al. 2005). Because EA causes spine loss while increasing CaMKII activity we hypothesized that CaMKII activity is causal to the reduction in spine density. To test this hypothesis, we used a genetically-encoded specific inhibitor of CaMKII, GFP-AIP (Bok et al. 2007), which is a GFP protein fused to the CaMKII inhibitor peptide AIP (Ishida et al. 1998). The peptide substrate mimetic minimizes nonspecific effects likely with pharmacological inhibitors and the presence of GFP allows direct visualization of transfected neurons for spine analysis. Because GFP-labeled dendrites without adjacent GFP-labeled axons were analyzed, CaMKII inhibition was restricted to the postsynaptic cell.
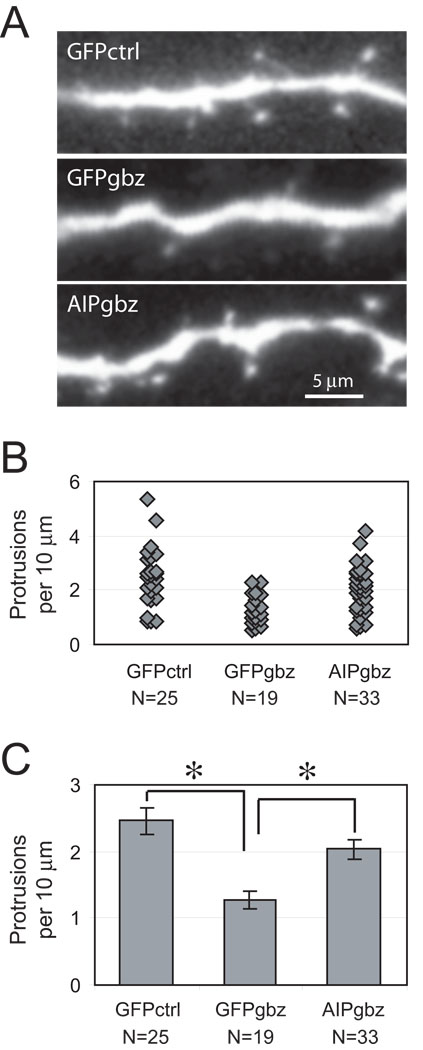
Hippocampal slices were biolistically transfected with either control GFP- or GFP-AIP-encoding plasmids. The slices were then maintained for 48 h in either Gbz-containing or control culture medium before fixation and imaging. Dendrites from transfected pyramidal neurons were imaged (Figure 2A) and dendritic protrusions (spines and filopodia) quantified. As shown in Figure 2, the density of dendritic protrusions in Gbz-treated slices was reduced by ∼50%, from 2.46 ± 0.22/10 µm to 1.28 ± 0.13/10 µm. Expression of GFP-AIP blocked Gbz-induced reduction in the density of dendritic protrusions. In Gbz-treated slices, neurons expressing GFP-AIP had 2.04 ± 0.15 dendritic protrusions per 10 µm, significantly (P<0.001) higher than neurons transfected with GFP.
Figure 2. Inhibition of CaMKII activation by AIP blocked Gbz-induced loss of dendritic protrusions.
Hippocampal pyramidal neurons were transfected with GFP or GFP-AIP expression vectors 9–12 hrs before a 48 hr treatment in control or 10 µM Gbz-containing medium. (A) Representative set of images showing a segment of apical dendrites of neurons transfected with GFP or GFP-AIP. (B) Distribution of dendritic protrusion density of neurons maintained in various conditions. Each diamond represents the protrusion density of one neuron. All neurons from three separate experiments were pooled together. (C) Mean dendritic protrusion density (mean ± S.E.) under different conditions: there was a significant (P < 0.001, Student's t-test) decrease in mean protrusion density of GFP-transfected neurons in Gbz-treated slices. In Gbz, there was a significant (P < 0.001) increase in protrusion density in neurons expressing GFP-AIP relative to neurons expressing GFP. N is the total number of neurons (three separate experiments) analyzed for the corresponding condition.
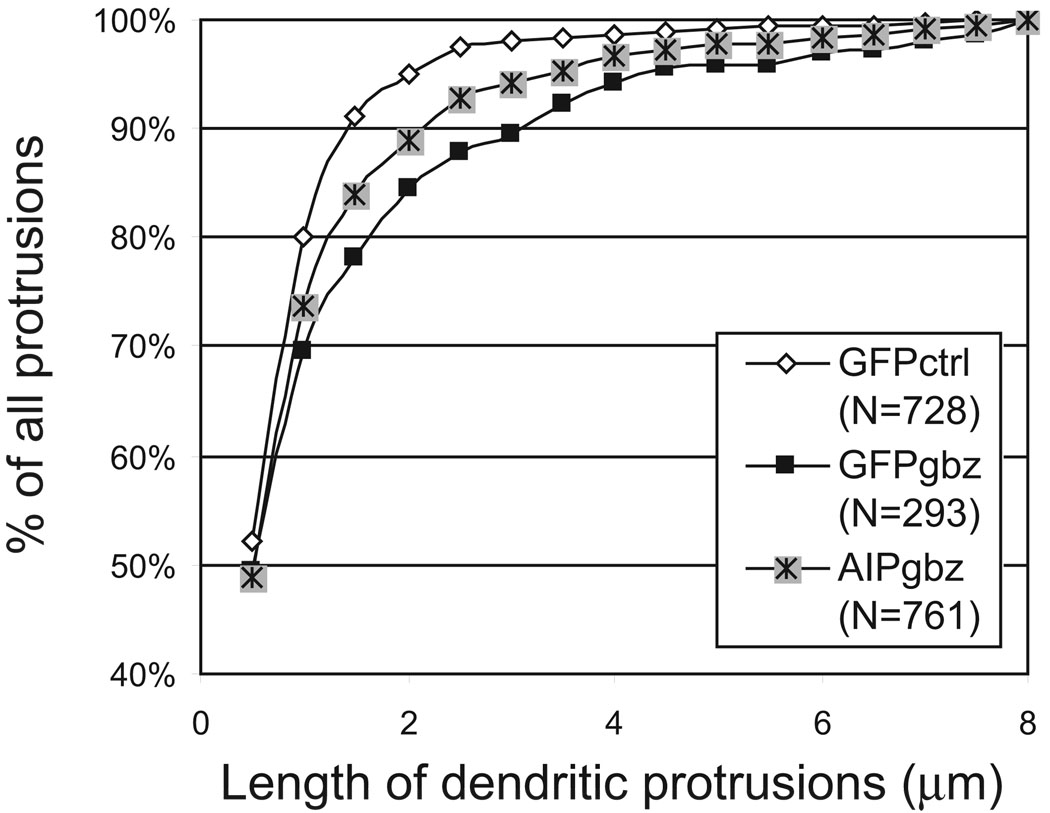
Previously we showed that Gbz-induced spine loss parallels an increase in spine length (Zha et al. 2005). To address whether AIP is responsible for the changes in spine length, we analyzed the length of dendritic protrusions under EA. Both GFP and GFP-AIP transfected pyramidal neurons showed significantly (P < 0.01, χ2 test) increase in the length of dendritic protrusions under EA than that of pyramidal neurons in control slices (Figure 3). There was no significant difference in protrusion length between GFP and GFP-AIP expressing neurons under EA conditions. This increase in protrusion length could be due to increased persistence or length of filopodia in EA conditions (Zha et al. 2005) or increased ratio of filopodia to spines (see below).
Figure 3. AIP did not inhibit the epileptiform activity-induced increase in protrusion length.
Shown is a cumulative histogram plot of dendritic protrusion length. N indicates the total number of spines analyzed for each condition obtained from three individual experiments: GFP-expressing neurons maintained in control (GFPctrl) or Gbz-containing medium (GFPgbz) or GFP-AIP-expressing neurons maintained in Gbz containing medium (AIPgbz). There was a significant (χ2 test) difference in distribution between GFPctrl and GFPgbz (P < 0.001), and between GFPctrl and AIPgbz (P < 0.01). No significant difference was seen between GFPgbz and AIPgbz (P = 0.145).
CaMKII activity contributes to stability of dendritic spines and spine PSDs
The results above do not necessarily imply that CaMKII is required for EA-induced reduction in the density of synapse-bearing spines. Labeling dendrites with Golgi or other conventional stains, with fluorescent dyes or GFP expression, does not distinguish between mature spines that contain PSDs and filopodia that do not. For this reason, we refer to structures labeled with GFP simply as dendritic protrusions. We previously showed that EA caused a loss of mature spines but had little effect on the number of filopodia (Zha et al. 2005). We therefore asked whether CaMKII inhibition during EA has different effects on spines vs. filopodia.
To distinguish spines and filopodia, we biolistically transfected YFP-tagged PSD-95 to label PSDs. PSD-95-YFP was co-transfected with CFP-AIP or control CFP only. Replacement of GFP with CFP in the AIP construct facilitates distinguishing between the two fluorescent proteins in the confocal microscope. The CFP or CFP-AIP fills the dendrite (Figure 4A) while the PSD-95-YFP allows the PSDs to be visualized as PSD-95-YFP clusters (Figure 4). One potential concern is that overexpression of PSD-95-YFP might itself increase spine density (El-Husseini et al. 2000). However, there is no significant difference between the number of dendritic protrusions counted in dendrites expressing GFP only (Figure 2C, 2.46 ± 0.22/10 µm) and dendrites expressing PSD-95-YFP (Figure 5D, sum of filopodia + spines, 2.59 ± 0.14/10 µm). This result indicates that, with the level and duration of PSD-95-YFP expression employed here, there is no effect on the number of dendritic protrusions. This is consistent with other studies showing that transient (48–72 hr) expression of PSD-95 does not affect synapse or spine density in hippocampal slices (Ehrlich and Malinow 2004; Marrs et al. 2001; Zha et al. 2005).
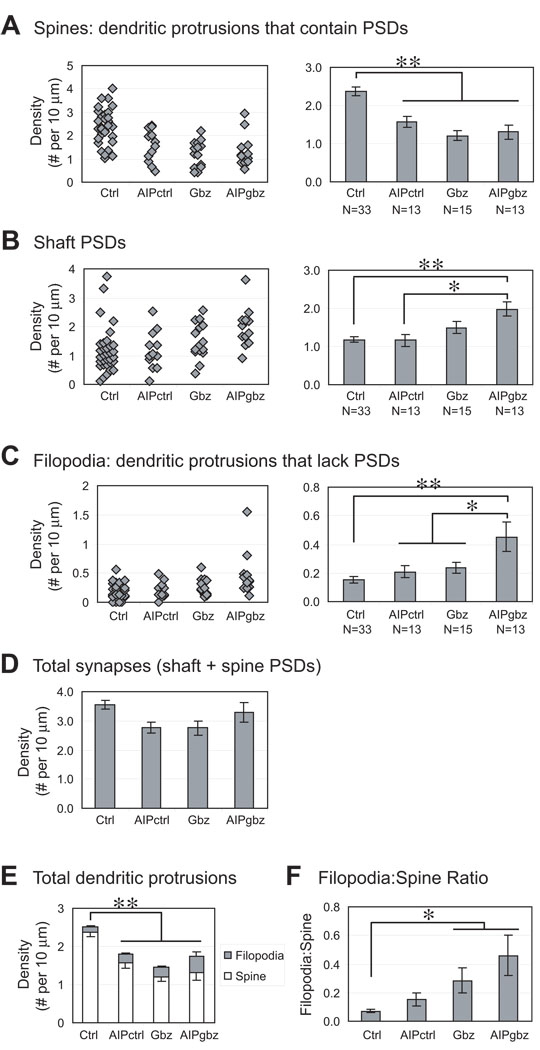
Figure 5. Quantification of spines, filopodia and shaft PSDs under control and EA condition.
Hippocampal slice culture and transfection were done as described in Figure 3. A-C show distribution and mean density of (A) spines (dendritic protrusions that contain PSDs); (B) shaft PSDs; and (C) filopodia (dendritic protrusions that lack PSDs). Each diamond represents the density of PSD-95 clusters in one neuron. All neurons from three separate experiments were pooled together. N = 33 (Ctrl); 13 (AIP); 15 (Gbz); 13 (AIPgbz). (D) Total PSDs (shaft + spine). (E) All dendritic protrusions counted: spines (white) or filopodia (gray). (F) Filopodia:Spine ratio calculated from data in A-C. Significant differences are indicated by * P < 0.05 or ** P < 0.01 (Student's t-test).
Expression of CFP-AIP led to a modest but significant (p<0.01) reduction in the density of spine PSDs to 1.75 ± 0.17/10 µm from the control value of 2.36 ± 0.12/10 µm (Figure 5A), showing that basal levels of CaMKII activity promotes the maintenance of mature spines. This is consistent with previous studies indicating that CaMKII activity contributes to stability of dendritic spines and spine PSDs (Asrican et al. 2007; Jourdain et al. 2003; Okamoto et al. 2007), and suggests that the increased CaMKII activity in EA is unlikely to be causal to the EA-induced loss of mature spines.
Spine reduction caused by EA is independent of CaMKII
Consistent with our previous study (Zha et al. 2005), EA significantly reduced the density of spine PSDs to 1.17 ± 0.13/10 µm (Figure 5A). However, expression of CFP-AIP did not significantly affect this reduction. Spine PSD density for CFP-AIP-expressing neurons in Gbz-treated slice cultures was 1.38 ± 0.22, not significantly different from that of CFP-expressing neurons. Thus, the reduction in spine PSDs caused by EA is independent of CaMKII.
We also asked whether EA or CFP-AIP affected the density of PSDs on the shafts of the pyramidal neuron dendrites (“shaft PSDs,” Figure 5B). Neither EA nor AIP alone had a significant effect on the density of shaft PSDs. However, when we inhibited CaMKII activity during EA, we saw a significant increase in the density of shaft PSDs above control. When we compared the total number of PSDs (shaft+spine), we did not see significant differences between groups (Figure 5D).
CaMKII inhibition during EA increases the number of filopodia
Because CaMKII inhibition increased the number of dendritic protrusions in cultures maintained in Gbz (Figure 2) but did not prevent Gbz-induced spine loss, we surmised that the number of filopodia – i.e., dendritic protrusions lacking PSDs – had increased. We counted filopodia in pyramidal neurons in hippocampal slices cotransfected with PSD-95-YFP and either CFP-AIP or CFP. As above, CFP or CFP-AIP filled the cytoplasm allowing filopodia to be visualized as protrusions lacking PSD-95-YFP clusters.
As predicted, inhibition of CaMKII in Gbz-treated slices caused a significant increase in filopodia density (Figure 5C). Filopodia density in CFP-AIP-transfected neurons was 0.21 ± 0.03/10 µm in Gbz-treated slices and 0.16 ± 0.02/10 µm in control (no Gbz) slices (not significantly different.) While CaMKII inhibition by CFP-AIP had little effect in control slices, expression of CFP-AIP in Gbz-treated slices resulted in a significant (P < 0.05) increase in filopodia density to 0.38 ± 0.05/10 µm. Thus, the apparent reversal of EA-induced loss of protrusions by AIP (Figure 2B) coincides with a shift in the relative density of spine and filopodial protrusions (Figure 5E, F). As shown earlier (Zha et al. 2005), Gbz also caused a significant increase in the filopodia:spine ratio, as a result of the loss of spines (Figure 5F).
The conditions, GFPgbz and AIPgbz, that resulted in an increased ratio of filopodia to spines also resulted in an increase in protrusion length (Figure 3). This would be predictable if filopodia, protrusions lacking PSDs, were, on average, longer than spines, protrusions containing PSDs. We quantified the length of filopodia and spines and found that this was the case. Filopodia (N = 77 from 16 neurons in control conditions) had an average length of 1.48 ± 0.08 µm, significantly (P = 0.035, Student’s t-test) longer than spines (1.30 ± 0.03 µm for 506 spines). Thus, conversion of filopodia to spines is correlated with a net reduction in average length of protrusions. Conversely, the increase in protrusion length observed in EA appears to be correlated with an increase in the number of filopodia relative to spines.
DISCUSSION
We show here that EA causes an increase in CaMKII activity. Inhibiting CaMKII during EA increases the number of filopodia, defined here as dendritic protrusions lacking PSDs, and increases the number of shaft PSDs. However, inhibiting CaMKII does not prevent EA-induced loss of mature spines, defined here as dendritic protrusions containing PSDs. This latter observation is consistent with our data showing that CaMKII activity is required for maintaining mature spines under control condition. These data imply that during EA-dependent synaptic remodeling CaMKII activity serves to facilitate conversion of filopodia into mature spines, presumably by stabilizing existing spine PSDs and facilitating the translocation of PSDs from the dendritic shaft into filopodia.
Activation of CaMKII by EA
Our data are consistent with previously reported Ca2+ increase in neurons during persistent EA (Badea et al. 2001; Dailey 1996; Scanziani et al. 1994; Zha et al. 2005). Nevertheless, other studies have found transient or even persistent decrease in CaMKII activity – evident as a decrease in Thr286 phosphorylation – in some examples of drug-induced EA. For example, there is a decrease in Thr286 phosphorylation following a transient seizure event (low Mg2+) in dissociated cultured hippocampal neurons (Blair et al. 1999) or an acute transient (<4 h) decrease in Thr286 phosphorylation in vivo following a single dose of the convulsant pentylenetetrazol (Blair et al. 1999; Dong and Rosenberg 2004). In addition to these instances of acute reductions in CaMKII activity, a persistent decrease was reported in a pilocarpine model of epilepsy in vivo (Churn et al. 2000). In contrast, in a mouse model for Angelman Mental Retardation Syndrome, a disorder characterized by retardation and epilepsy and a non-drug-induced example of epilepsy, there is a significant increase in CaMKII phosphorylation on Thr286 (Weeber et al. 2003). Therefore, CaMKII activation is variably associated with EA, possibly the cause or frequency of seizures may determine the extent to which CaMKII is activated.
CaMKII-dependent maintenance of dendritic spines
We showed here that CaMKII inhibition results in a reduced number of mature spines and spine PSDs (Figure 4A). This observation may help explain the increase in seizures observed in CaMKIIα null mice (Butler et al. 1995). Because CaMKII is involved in the maintenance of mature spines, it is possible that abnormal development of neural circuitry contributes to the increased seizure susceptibility in CaMKII null animals. This speculation is further supported by previous studies showing the involvement of CaMKII in the control of axonal and dendritic arborization (Hansen et al. 2003; Redmond et al. 2002; Vaillant et al. 2002; Zou and Cline 1996; Zou and Cline 1999) and possibly synapse formation (Ahmed et al. 2006).
Actions of CaMKII during EA
We show here that during EA in hippocampal slices, there is a reduction in the density of spines on pyramidal neuron dendrites concomitant with an activation of CaMKII. Inhibition of CaMKII does not prevent the loss of spines but does result in an increase in the number of filopodia and of shaft PSDs during EA.
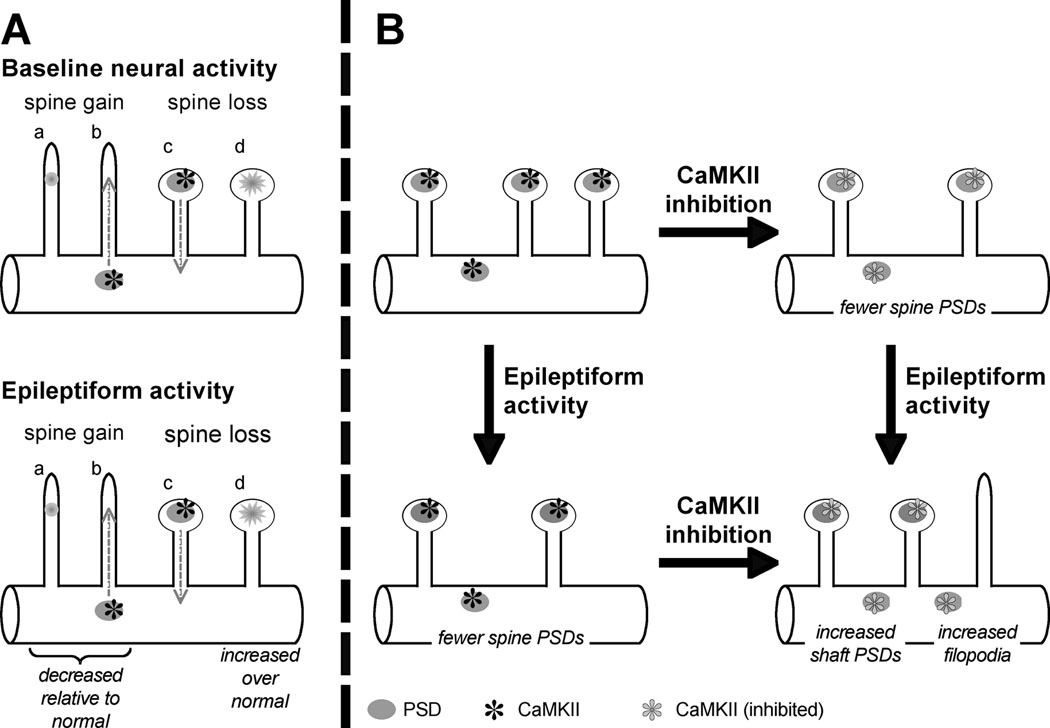
Our previous and current results suggest a model of how spine and synapses are regulated by activity and CaMKII (diagrammed in Figure 6). Spines are dynamic and are simultaneously being gained and lost on dendrites (Dailey and Smith 1996; Dunaevsky et al. 2001; Dunaevsky et al. 1999; Ziv and Smith 1996). During development, the rate of gain exceeds that of loss and spine number increases. Similarly, by directly visualizing spines and PSDs, we have shown that under baseline activity conditions the rate of spine PSD formation exceeds that of spine PSD loss in slice cultures from developing hippocampus (Marrs et al. 2001; Zha et al. 2005). As diagrammed in Figure 6A, spine formation involves either a de novo formation of PSDs in filopodia or a translocation of PSDs from the dendritic shaft into filopodia to form protospines and, ultimately, spines (Marrs et al. 2001; Okabe et al. 2001). Conversely, spine loss involves both disassembly of spine PSDs and translocation of PSDs from spine to shaft (Marrs et al. 2001). Spine formation depends, at least in part, on CaMKII (Ahmed et al. 2006; Asrican et al. 2007; Jourdain et al. 2003; Okamoto et al. 2007). Under epileptiform conditions, there is a moderate decrease in the rate of gain of spine PSDs over baseline conditions and a moderate increase in spine loss, particularly an increase in disassembly of spine PSDs (Zha et al. 2005). The result of synaptic remodeling during EA is a gradual decrease in the number of mature spines, a moderate increase in the number of shaft PSDs, and the appearance of persistent filopodia (Zha et al. 2005). A similar increase in the rate of spine loss during epileptic seizures is observed in vivo in mouse hippocampus by multiphoton imaging of GFP-expressing neurons (Mizrahi et al. 2004).
Figure 6. Summary diagram showing effects of EA and of CaMKII inhibition on spine PSDs.
A. Previous observations (Marrs et al. 2001; Okabe et al. 2001) indicate spines are gained by de novo formation of PSDs (a) or translocation of PSDs from shaft to filopodium (b). Data presented here implicate postsynaptic CaMKII in spine gain and/or spine maintenance. Spines are lost by translocation of PSDs from spine to shaft (c) or disassembly of shaft PSDs (d). EA results in a gradual net loss of spines mainly due to a small decrease in the rate of spine gain and a small increase in the rate of spine disassembly (Zha et al. 2005). B. Diagram of spine remodeling during normal and EA and role of CaMKII: EA results in a net loss of spines and spine PSDs, as does CaMKII inhibition. CaMKII inhibition results in increased dendritic protrusions in EA but this is due to increased numbers of filopodia, not of spines. A parallel increase in shaft PSDs suggests that a role of CaMKII is to promote translocation of shaft PSDs into filopodia to generate mature spines.
Figure 6B summarizes the consequences of CaMKII inhibition on synaptic remodeling based on the data presented here. CaMKII colocalizes with PSD95 on both spine and shaft synapses (Ahmed et al. 2006). With baseline activity, CaMKII inhibition results in a decrease in the number of spine, but not of shaft, PSDs: there are fewer spine PSDs relative to control. This suggests that CaMKII has a special role in stabilizing or promoting formation of spine PSDs. Observation of the effect of CaMKII inhibition during EA-induced synaptic remodeling reveals a more specific role for CaMKII. During EA there is a small increase in the number of shaft PSDs and in the number of filopodia. CaMKII inhibition results in a further increase in the number of shaft PSDs and of filopodia in GFP-AIP-expressing neurons in Gbz-treated slices relative to control neurons. Thus, CaMKII activity during reduces the effect of EA on filopodia and PSDs. One possibility is that CaMKII activity stabilizes spines. Thus, in the absence of CaMKII activity, EA-induced spine shrinkage results in a PSD on a very short stubby spine or on the shaft. This is consistent with the stabilization of spines by CaMKII in baseline activity but does not explain the increased numbers of filopodia. An alternative, not exclusive, possibility is that CaMKII promotes translocation of PSDs from shaft to spine. Thus, if CaMKII is inhibited during EA-induced synaptic remodeling, translocation of PSDs to filopodia will be reduced resulting in increased numbers of filopodia and shaft PSDs at the expense of spine PSDs, which is what we observed.
These data indicate that, while CaMKII may be activated during EA (although, as indicated above, this is not a necessary outcome of EA), the loss of spines induced by EA is not mediated by CaMKII but, rather, partially antagonized by CaMKII. Thus, other activity-dependent signals must be responsible for the spine loss. These might include calcineurin (Halpain et al. 1998) or calpain (Siman et al. 1989). Because spine loss was observed on dendrites that did not exhibit beading, excitotoxicity is not necessarily the cause of spine loss. Swann et al. (2000) have similarly argued that because epileptic seizures occuring in early life result in a decrease in spine density but not dendritic beading in vivo, mechanisms other than excitotoxicity are likely to be involved.
Relevance to epilepsy in vivo
Epileptic seizure activity is associated with loss of dendritic spines (Swann et al. 2000). Moreover, spine remodeling resulting in long dendritic protrusions has been observed in several neurological disease conditions associated with seizures (for reviews see (Fiala et al. 2002; McKinney 2005). For example, increased numbers of long dendritic protrusions have been reported in models of fragile-X syndrome, which is also characterized by increased seizure activity. In mice in which multiphoton time-lapse observations were made of fluorescently-labeled hippocampal neurons in vivo(Mizrahi et al. 2004), epileptic seizures caused gradual spine loss similar to that described here and in Zha et al. (2005). Although caution must be applied in relating in vitro to in vivo observations, these morphological similarities between hippocampal pyramidal cell dendritic protrusions in epilepsy in vivo and those in epileptiform conditions in vitro, such as those used here, suggests that the in vitro model may be useful in elucidating the molecular mechanisms that link EA to spine loss. The roles of CaMKII and other activity-dependent signals in controling spine and PSD assembly or disassembly are likely to be similar and may provide a possible therapeutic target for protection of dendritic spines in some neurological conditions.
These data also imply that caution must be exercised in interpreting studies of “spine” counts under various conditions in vivo or in vitro where spines are visualized by Golgi-like stain or with fluorescent labels including GFP. Not all dendritic protrusions visualized by these means correspond to synapses. We show here that dendritic protrusions containing synapses and those that do not are differentially affected by EA and by CaMKII activity. Distinction between these by labeling synapses directly is necessary to reveal the effect of these perturbations of neural activity on synaptic structures.
ACKNOWLEDGEMENTS
This research was supported by American Heart Association grant #0655764Z (MED), NIH grant DC002961 (SHG), and a UI Biosciences Initiative Pilot Grant (MED and SHG).
Grants: American Heart Association grant #0655764Z (MED), NIH grant DC002961 (SHG), and a UI Biosciences Initiative Pilot Grant (MED and SHG).
REFERENCES
- Ahmed R, Zha XM, Green SH, Dailey ME. Synaptic activity and F-actin coordinately regulate CaMKIIalpha localization to dendritic postsynaptic sites in developing hippocampal slices. Mol Cell Neurosci. 2006;31(1):37–51. doi: 10.1016/j.mcn.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Asrican B, Lisman J, Otmakhov N. Synaptic strength of individual spines correlates with bound Ca2+-calmodulin-dependent kinase II. J Neurosci. 2007;27(51):14007–14011. doi: 10.1523/JNEUROSCI.3587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea T, Goldberg J, Mao B, Yuste R. Calcium imaging of epileptiform events with single-cell resolution. J Neurobiol. 2001;48(3):215–227. doi: 10.1002/neu.1052. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411(6839):801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Blair RE, Churn SB, Sombati S, Lou JK, DeLorenzo RJ. Long-lasting decrease in neuronal Ca2+/calmodulin-dependent protein kinase II activity in a hippocampal neuronal culture model of spontaneous recurrent seizures. Brain Res. 1999;851(1–2):54–65. doi: 10.1016/s0006-8993(99)02100-9. [DOI] [PubMed] [Google Scholar]
- Bok J, Wang Q, Huang J, Green SH. CaMKII and CaMKIV mediate distinct prosurvival signaling pathways in response to depolarization in neurons. Mol Cell Neurosci. 2007;36(1):13–26. doi: 10.1016/j.mcn.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell S, Meredith GE, Phillips J, Staunton H, Doherty C, Grigorenko E, Glazier S, Deadwyler SA, O′Donovan CA, Farrell M. Neuronal hypertrophy in the neocortex of patients with temporal lobe epilepsy. J Neurosci. 2001;21(13):4789–4800. doi: 10.1523/JNEUROSCI.21-13-04789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler LS, Silva AJ, Abeliovich A, Watanabe Y, Tonegawa S, McNamara JO. Limbic epilepsy in transgenic mice carrying a Ca2+/calmodulin-dependent kinase II alpha-subunit mutation. Proc Natl Acad Sci U S A. 1995;92(15):6852–6855. doi: 10.1073/pnas.92.15.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA, Silva DF, Turski WA, Calderazzo-Filho LS, Bortolotto ZA, Turski L. The susceptibility of rats to pilocarpine-induced seizures is age-dependent. Brain Res. 1987;465(1–2):43–58. doi: 10.1016/0165-3806(87)90227-6. [DOI] [PubMed] [Google Scholar]
- Churn SB, Kochan LD, DeLorenzo RJ. Chronic inhibition of Ca2+/calmodulin kinase II activity in the pilocarpine model of epilepsy. Brain Res. 2000;875(1–2):66–77. doi: 10.1016/s0006-8993(00)02623-8. [DOI] [PubMed] [Google Scholar]
- Dailey ME. Dynamic optical imaging of neuronal structure and physiology: Confocal fluorescence microscopy in living brain slices. In: Toga A, Mazziotta J, editors. Brain Mapping: The Methods. Academic Press; 1996. pp. 29–54. [Google Scholar]
- Dailey ME. Optical Imaging of neural structure and physiology: confocal fluorescence microcopy in live brain slices. In: Arthur W, Toga JCM, editors. Brain Mapping: The Methods. 2nd edition. Academic Press; 2002. pp. 49–76. 2 edition. [Google Scholar]
- Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16(9):2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Rosenberg HC. Prolonged changes in Ca2+/calmodulin-dependent protein kinase II after a brief pentylenetetrazol seizure; potential role in kindling. Epilepsy Res. 2004;58(2–3):107–117. doi: 10.1016/j.eplepsyres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Dunaevsky A, Blazeski R, Yuste R, Mason C. Spine motility with synaptic contact. Nat Neurosci. 2001;4(7):685–686. doi: 10.1038/89460. [DOI] [PubMed] [Google Scholar]
- Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci U S A. 1999;96(23):13438–13443. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24(4):916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290(5495):1364–1368. [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399(6731):66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39(1):29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Fink CC, Meyer T. Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol. 2002;12(3):293–299. doi: 10.1016/s0959-4388(02)00327-6. [DOI] [PubMed] [Google Scholar]
- Gleason MR, Higashijima S, Dallman J, Liu K, Mandel G, Fetcho JR. Translocation of CaM kinase II to synaptic sites in vivo. Nat Neurosci. 2003;6(3):217–218. doi: 10.1038/nn1011. [DOI] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18(23):9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MR, Bok J, Devaiah AK, Zha XM, Green SH. Ca2+/calmodulin-dependent protein kinases II and IV both promote survival but differ in their effects on axon growth in spiral ganglion neurons. J Neurosci Res. 2003;72(2):169–184. doi: 10.1002/jnr.10551. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Ishida A, Shigeri Y, Tatsu Y, Uegaki K, Kameshita I, Okuno S, Kitani T, Yumoto N, Fujisawa H. Critical amino acid residues of AIP, a highly specific inhibitory peptide of calmodulin-dependent protein kinase II. FEBS Lett. 1998;427(1):115–118. doi: 10.1016/s0014-5793(98)00405-0. [DOI] [PubMed] [Google Scholar]
- Isokawa M. Remodeling dendritic spines of dentate granule cells in temporal lobe epilepsy patients and the rat pilocarpine model. Epilepsia. 2000;41(Suppl 6):S14–S17. doi: 10.1111/j.1528-1157.2000.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset epilepsy. J Neurosci. 1998;18(20):8356–8368. doi: 10.1523/JNEUROSCI.18-20-08356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Fukunaga K, Muller D. Calcium/calmodulin-dependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J Neurosci. 2003;23(33):10645–10649. doi: 10.1523/JNEUROSCI.23-33-10645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CL, Hrachovy RA, Smith KL, Frost JD, Jr, Swann JW. Tetanus toxin-induced seizures in infant rats and their effects on hippocampal excitability in adulthood. Brain Res. 1995;677(1):97–109. doi: 10.1016/0006-8993(95)00127-c. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1999;96(6):3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3(3):175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283(5409):1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Marrs GS, Green SH, Dailey ME. Rapid formation and remodeling of postsynaptic densities in developing dendrites. Nat Neurosci. 2001;4(10):1006–1013. doi: 10.1038/nn717. [DOI] [PubMed] [Google Scholar]
- McKinney RA. Physiological roles of spine motility: development, plasticity and disorders. Biochem Soc Trans. 2005;33(Pt 6):1299–1302. doi: 10.1042/BST0331299. [DOI] [PubMed] [Google Scholar]
- Mizrahi A, Crowley JC, Shtoyerman E, Katz LC. High-resolution in vivo imaging of hippocampal dendrites and spines. J Neurosci. 2004;24(13):3147–3151. doi: 10.1523/JNEUROSCI.5218-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Gahwiler BH, Rietschin L, Thompson SM. Reversible loss of dendritic spines and altered excitability after chronic epilepsy in hippocampal slice cultures. Proc Natl Acad Sci U S A. 1993;90(1):257–261. doi: 10.1073/pnas.90.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitecka L, Tremblay E, Charton G, Bouillot JP, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. II. Histopathological sequelae. Neuroscience. 1984;13(4):1073–1094. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- Okabe S, Miwa A, Okado H. Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J Neurosci. 2001;21(16):6105–6114. doi: 10.1523/JNEUROSCI.21-16-06105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada R, Moshe SL, Albala BJ. Infantile status epilepticus and future seizure susceptibility in the rat. Brain Res. 1984;317(2):177–183. doi: 10.1016/0165-3806(84)90095-6. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007;104(15):6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Watt AJ, Griffith LC, Nelson SB, Turrigiano GG. Activity-dependent remodeling of presynaptic inputs by postsynaptic expression of activated CaMKII. Neuron. 2003;39:269–281. doi: 10.1016/s0896-6273(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34(6):999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Debanne D, Muller M, Gahwiler BH, Thompson SM. Role of excitatory amino acid and GABAB receptors in the generation of epileptiform activity in disinhibited hippocampal slice cultures. Neuroscience. 1994;61(4):823–832. doi: 10.1016/0306-4522(94)90405-7. [DOI] [PubMed] [Google Scholar]
- Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6(4):277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284(5411):162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Siman R, Noszek JC, Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J Neurosci. 1989;9(5):1579–1590. doi: 10.1523/JNEUROSCI.09-05-01579.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37(2):173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Swann JW, Al-Noori S, Jiang M, Lee CL. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus. 2000;10(5):617–625. doi: 10.1002/1098-1063(2000)10:5<617::AID-HIPO13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Swann JW, Le JT, Lam TT, Owens J, Mayer AT. The impact of chronic network hyperexcitability on developing glutamatergic synapses. Eur J Neurosci. 2007;26(4):975–991. doi: 10.1111/j.1460-9568.2007.05739.x. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Fortunato C, McKinney RA, Muller M, Gahwiler BH. Mechanisms underlying the neuropathological consequences of epileptic activity in the rat hippocampus in vitro. J Comp Neurol. 1996;372(4):515–528. doi: 10.1002/(SICI)1096-9861(19960902)372:4<515::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402(6760):421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Vaillant AR, Zanassi P, Walsh GS, Aumont A, Alonso A, Miller FD. Signaling mechanisms underlying reversible, activity-dependent dendrite formation. Neuron. 2002;34(6):985–998. doi: 10.1016/s0896-6273(02)00717-1. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Jiang YH, Elgersma Y, Varga AW, Carrasquillo Y, Brown SE, Christian JM, Mirnikjoo B, Silva A, Beaudet AL, Sweatt JD. Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J Neurosci. 2003;23(7):2634–2644. doi: 10.1523/JNEUROSCI.23-07-02634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274(5289):972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- Zha XM, Green SH, Dailey ME. Regulation of hippocampal synapse remodeling by epileptiform activity. Mol Cell Neurosci. 2005;29(4):494–506. doi: 10.1016/j.mcn.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci U S A. 2006;103(44):16556–16561. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17(1):91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Cline HT. Expression of constitutively active CaMKII in target tissue modifies presynaptic axon arbor growth. Neuron. 1996;16(3):529–539. doi: 10.1016/s0896-6273(00)80072-0. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Cline HT. Postsynaptic calcium/calmodulin-dependent protein kinase II is required to limit elaboration of presynaptic and postsynaptic neuronal arbors. J Neurosci. 1999;19(20):8909–8918. doi: 10.1523/JNEUROSCI.19-20-08909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]