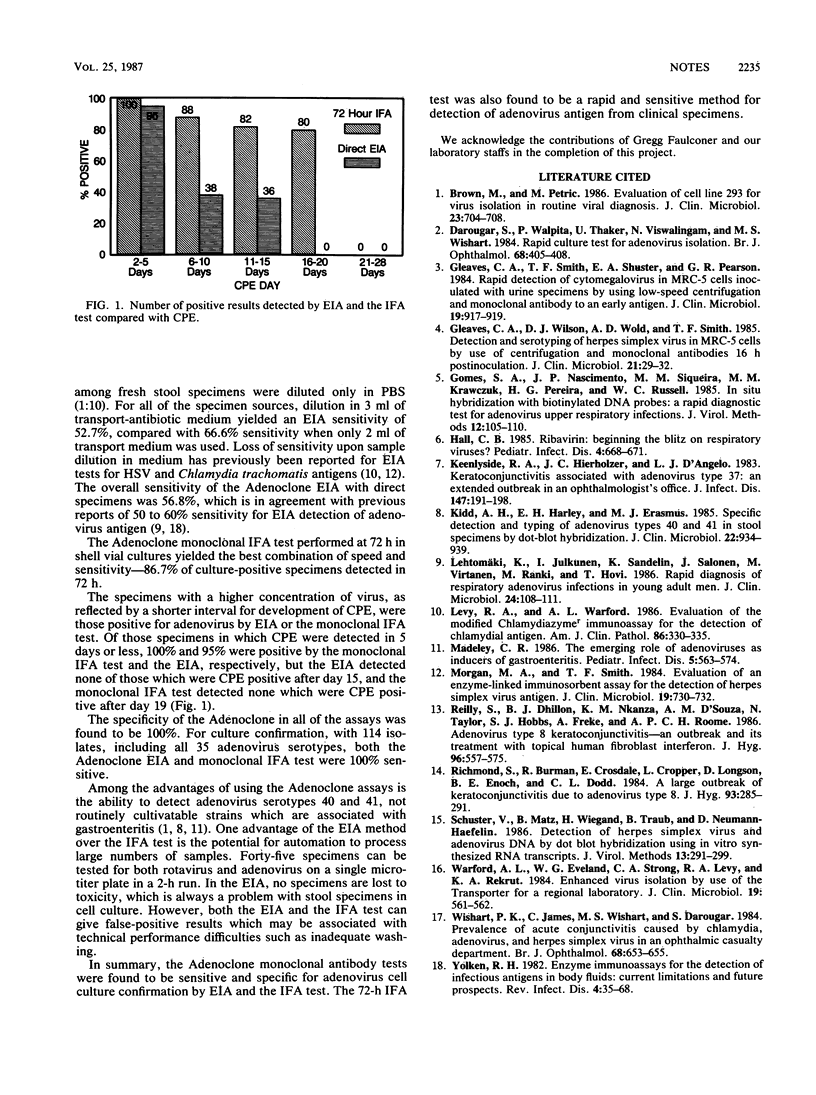
Abstract
The Adenoclone monoclonal antibody for detection of adenovirus antigen was evaluated by enzyme immunoassay and a 72-h indirect fluorescent-antibody test in shell vials and compared with standard culture. The sensitivity and specificity of the Adenoclone test were both 100% with 114 cell culture isolates. With 310 direct clinical specimens, the Adenoclone enzyme immunoassay sensitivity varied from 14.3 to 66.6%, but the Adenoclone indirect fluorescent-antibody test sensitivity was 86.7%, with 100% specificity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M., Petric M. Evaluation of cell line 293 for virus isolation in routine viral diagnosis. J Clin Microbiol. 1986 Apr;23(4):704–708. doi: 10.1128/jcm.23.4.704-708.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darougar S., Walpita P., Thaker U., Viswalingam N., Wishart M. S. Rapid culture test for adenovirus isolation. Br J Ophthalmol. 1984 Jun;68(6):405–408. doi: 10.1136/bjo.68.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleaves C. A., Smith T. F., Shuster E. A., Pearson G. R. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol. 1984 Jun;19(6):917–919. doi: 10.1128/jcm.19.6.917-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleaves C. A., Wilson D. J., Wold A. D., Smith T. F. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 h postinoculation. J Clin Microbiol. 1985 Jan;21(1):29–32. doi: 10.1128/jcm.21.1.29-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes S. A., Nascimento J. P., Siqueira M. M., Krawczuk M. M., Pereira H. G., Russell W. C. In situ hybridization with biotinylated DNA probes: a rapid diagnostic test for adenovirus upper respiratory infections. J Virol Methods. 1985 Oct;12(1-2):105–110. doi: 10.1016/0166-0934(85)90012-6. [DOI] [PubMed] [Google Scholar]
- Hall C. B. Ribavirin: beginning the blitz on respiratory viruses? Pediatr Infect Dis. 1985 Nov-Dec;4(6):668–671. doi: 10.1097/00006454-198511000-00014. [DOI] [PubMed] [Google Scholar]
- Keenlyside R. A., Hierholzer J. C., D'Angelo L. J. Keratoconjunctivitis associated with adenovirus type 37: an extended outbreak in an ophthalmologist's office. J Infect Dis. 1983 Feb;147(2):191–198. doi: 10.1093/infdis/147.2.191. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Harley E. H., Erasmus M. J. Specific detection and typing of adenovirus types 40 and 41 in stool specimens by dot-blot hybridization. J Clin Microbiol. 1985 Dec;22(6):934–939. doi: 10.1128/jcm.22.6.934-939.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtomäki K., Julkunen I., Sandelin K., Salonen J., Virtanen M., Ranki M., Hovi T. Rapid diagnosis of respiratory adenovirus infections in young adult men. J Clin Microbiol. 1986 Jul;24(1):108–111. doi: 10.1128/jcm.24.1.108-111.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. A., Warford A. L. Evaluation of the modified Chlamydiazyme immunoassay for the detection of chlamydial antigen. Am J Clin Pathol. 1986 Sep;86(3):330–335. doi: 10.1093/ajcp/86.3.330. [DOI] [PubMed] [Google Scholar]
- Morgan M. A., Smith T. F. Evaluation of an enzyme-linked immunosorbent assay for the detection of herpes simplex virus antigen. J Clin Microbiol. 1984 Jun;19(6):730–732. doi: 10.1128/jcm.19.6.730-732.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S., Dhillon B. J., Nkanza K. M., D'Souza A. M., Taylor N., Hobbs S. J., Freke A., Roome A. P. Adenovirus type 8 keratoconjunctivitis--an outbreak and its treatment with topical human fibroblast interferon. J Hyg (Lond) 1986 Jun;96(3):557–575. doi: 10.1017/s0022172400066365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond S., Burman R., Crosdale E., Cropper L., Longson D., Enoch B. E., Dodd C. L. A large outbreak of keratoconjunctivitis due to adenovirus type 8. J Hyg (Lond) 1984 Oct;93(2):285–291. doi: 10.1017/s0022172400064810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster V., Matz B., Wiegand H., Traub B., Neumann-Haefelin D. Detection of herpes simplex virus and adenovirus DNA by dot blot hybridization using in vitro synthesized RNA transcripts. J Virol Methods. 1986 Jul;13(4):291–299. doi: 10.1016/0166-0934(86)90054-6. [DOI] [PubMed] [Google Scholar]
- Warford A. L., Eveland W. G., Strong C. A., Levy R. A., Rekrut K. A. Enhanced virus isolation by use of the transporter for a regional laboratory. J Clin Microbiol. 1984 Apr;19(4):561–562. doi: 10.1128/jcm.19.4.561-562.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart P. K., James C., Wishart M. S., Darougar S. Prevalence of acute conjunctivitis caused by chlamydia, adenovirus, and herpes simplex virus in an ophthalmic casualty department. Br J Ophthalmol. 1984 Sep;68(9):653–655. doi: 10.1136/bjo.68.9.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H. Enzyme immunoassays for the detection of infectious antigens in body fluids: current limitations and future prospects. Rev Infect Dis. 1982 Jan-Feb;4(1):35–68. doi: 10.1093/clinids/4.1.35. [DOI] [PubMed] [Google Scholar]


