INTRODUCTION
The utility of an animal model is predicated on its ability to incorporate essential features of the human phenomenon it is modeling in a way that permits systematic investigation of those features. For this reason, investigators who study the neurobiological mechanisms of addictive drugs such as opiates and stimulants are making extensive use of self-administration models as a way to more closely mimic the manner in which drugs of abuse are experienced by humans (Bozarth, Murray, & Wise, 1989; Caggiula, Donny, White, Chaudhri, Booth, Gharib, Hoffman, Perkins, & Sved, 2001; Carroll, Krattiger, Gieske, & Sadoff, 1990; Corrigall & Coen, 1989; Donny, Caggiula, Knopf, & Brown, 1995; Johanson, 1981; Roberts, 1992; Shaham & Stewart, 1995). A basic tenet of the self-administration model is that a drug, acting as a primary reinforcer, will increase the future occurrence of a response if its administration is contingent on that response (Meisch, 1993). Nicotine, like other drugs of abuse, is self-administered by a variety of animal species (Corrigall & Coen, 1989; Goldberg, Spealman, & Goldberg, 1981; Henningfield & Goldberg, 1983; Rose & Corrigall, 1997). Nicotine self-administration is dose- and schedule-dependent (Corrigall & Coen, 1989; Donny, Caggiula, Mielke, Jacobs, Rose, & Sved, 1998; Donny, Caggiula, Rowell, Gharib, Maldovan, Booth, Mielke, Hoffman, & McCallum, 2000; Shoaib, Schindler, & Goldberg, 1997), extinguishes when nicotine is removed (Corrigall & Coen, 1989; Taylor & Jentsch, 2001), and is dependent on nicotine delivery being response-contingent (Donny et al., 1998). Models of nicotine self-administration are being used to investigate the behavioral, environmental and neurophysiological underpinnings of nicotine reinforcement (e.g., Caggiula et al., 2001; Corrigall, 1992; Picciotto, Zoli, Rimondini, Lena, Marubio, Pich, Fuxe, & Changeux, 1998) and to aid in the development of novel pharmacotherapies for smoking cessation.
However, there is mounting evidence that a simple, primary reinforcement model of nicotine self-administration fails to fully explain existing data from both the animal self-administration and human smoking literatures. We have recently proposed a “dual-reinforcement” model (Chaudhri, Caggiula, Donny, Palmatier, Liu, & Sved, 2006b; Donny, Chaudhri, Caggiula, Evans-Martin, Booth, Gharib, Clements, & Sved, 2003) that is designed to more fully capture the relationship between nicotine and self-administration in animals and smoking in humans. This model incorporates a large body of evidence that emphasizes the importance of non-nicotine stimuli that accompany nicotine delivery and contribute to the overall level of reinforcement afforded to the behavior. The model addresses the nature of the relationship between such stimuli and nicotine, and it postulates that the resulting behavior is a function of nicotine acting as both a primary reinforcer and an enhancer of the incentive motivational and reinforcing effects of accompanying stimuli. We will first briefly discuss the importance of non-nicotine stimuli in self-administration and smoking. We will then outline our model describing the two ways in which nicotine interacts with those stimuli and discuss the research conducted to test the major tenets of the model (see Table 6.1).
TABLE 6.1.
DUAL-REINFORCEMENT MODEL
| Response-dependent Nicotine (Contingent) | Response-independent Nicotine (Non-contingent) | |
|---|---|---|
| Primary Reinforcement | Can maintain operant behavior in the absence of non-nicotine stimuli | Cannot maintain operant behavior in the absence of non-nicotine stimuli |
| Can establish concurrent neutral environmental stimuli as conditioned reinforcers* | Cannot establish concurrent neutral environmental stimuli as conditioned reinforcers* | |
| Reinforcement Enhancement | Can elevate behavior maintained by reinforcing stimuli ** | Can elevate behavior maintained by reinforcing stimuli ** |
Pavlovian conditioning can theoretically be established when a neutral stimulus is temporally paired with discrete infusions of either self-administered or experimenter-administered (non-contingent) nicotine. We have opted to focus on the former because it is a better model of conditioning that may occur when humans smoke cigarettes.
This applies to stimuli that are unconditioned reinforcers or that have been established as conditioned reinforcers through prior pairing with nicotine or some other unconditioned reinforcer.
ROLE OF NON-NICOTINE STIMULI IN SELF-ADMINISTRATION AND SMOKING
Smokers do not just self-administer nicotine, they take the drug within a context of multiple environmental stimuli, including the sight, smell and taste of cigarette smoke, the oropharyngeal consequences of smoking, and external contextual stimuli that are associated with the behavior. There is a substantial literature showing increases in craving or desire to smoke when smokers are exposed to smoking cues, such as a lit cigarette resting in an ashtray (Conklin & Tiffany, 2001; Sayette, Martin, Wertz, Shiffman, & Perrott, 2001). Furthermore, research from several laboratories, most notably that of Rose and his colleagues, has demonstrated that the administration of non-nicotine smoking stimuli can increase subjective reports of liking and satisfaction, and decrease craving and withdrawal in dependent smokers (reviewed in Caggiula et al., 2001). For example, smoking a denicotinized cigarette increased satisfaction in briefly deprived smokers, while intravenous nicotine alone had no effect. Both nicotine and cues were necessary for complete reduction of craving (Rose, Behm, Westman, & Johnson, 2000). Furthermore, daily smokers will continue to smoke nicotine-free cigarettes over an 11-day period with only small decreases in the number of cigarettes smoked and in the motivation to smoke (as measured by a progressive ratio schedule of reinforcement; Donny, Houtsmuller, & Stitzer, 2007). These studies suggest that non-nicotine stimuli are extremely important in both motivating and maintaining smoking behavior.
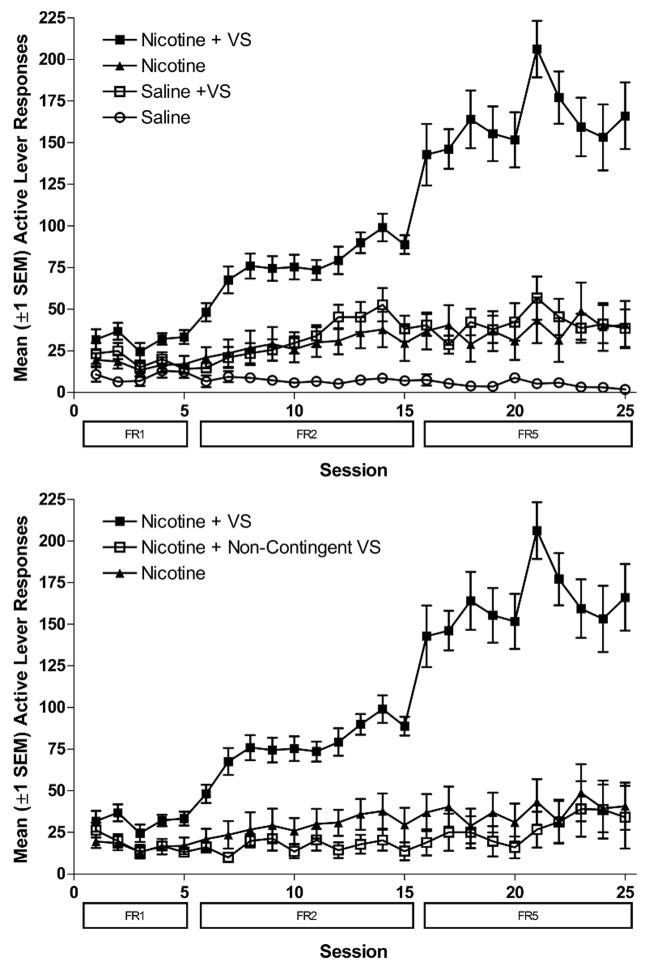
It is now widely accepted that environmental stimuli associated with drug delivery can influence self-administration in animals of several drugs of abuse (Arroyo, Markou, Robbins, & Everitt, 1998; de Wit & Stewart, 1981; Goldberg et al., 1981; Markou, Weiss, Gold, Caine, Schulteis, & Koob, 1993; Robinson & Berridge, 1993; Schenk & Partridge, 2001), including nicotine (Balfour, Wright, Benwell, & Birrell, 2000; Di Chiara, 2000; Donny, Caggiula, Mielke, Booth, Gharib, Hoffman, Maldovan, Shupenko, & McCallum, 1999; Donny, Caggiula, Rose, Jacobs, Mielke, & Sved, 2000; Rose & Corrigall, 1997) and contribute to drug dependence and relapse in humans (Childress, 1992; Juliano, Donny, Houtsmuller, & Stitzer, 2006; Margolin, 1992; O’Brien, Childress, Ehrman, & Robbins, 1998). In a direct test of the hypothesis that environmental stimuli accompanying nicotine delivery become an important part of the stimulus complex that sustains nicotine self-administration, we conducted a series of experiments in male rats in which nicotine infusions and environmental stimuli were independently manipulated (Caggiula, Donny, Chaudhri, Perkins, Evans-Martin, & Sved, 2002a; Caggiula et al., 2001; Caggiula, Donny, White, Chaudhri, Booth, Gharib, Hoffman, Perkins, & Sved, 2002b). A compound visual stimulus (VS: the onset of a 1-sec cue light and the offset of a chamber light for 1-min) that accompanied lever pressing and nicotine infusions was found to be at least as important as nicotine in the rapid acquisition of self-administration, in the degree to which withdrawing nicotine extinguished the behavior, and in the reacquisition of lever pressing after extinction (Caggiula et al., 2002a; Caggiula et al., 2001, 2002b). These studies also demonstrated that the effectiveness of this stimulus in promoting self-administration was dependent on it being contingently related to the rat’s responding and/or nicotine infusions, since the VS presented non-contingently did not enhance self-administration (Donny et al., 2003). A critical feature of this work must be emphasized, namely, the relationship between the VS and nicotine was synergistic; response rates generated by the combination of VS and nicotine were more than twice the sum of response rates produced by either the VS or nicotine alone (e.g., Figure 6.1 upper panel). These results illustrate the importance of environmental stimuli in the acquisition of nicotine self-administration and indicate that such stimuli can combine with nicotine to potentiate the behavior (Caggiula et al., 2002a; Caggiula et al., 2001, 2002b).
Figure 6.1.
Mean (+SE) responses on the active lever for NIC+VS peaks at more than twice the sum of NIC alone plus VS alone. For clarity the lower panel reproduces the NIC and NIC + VS data of the upper panel and shows non-contingent VS exposure (controlled by rats in the NIC+VS group through yoking) does not synergize with contingent NIC. N=8-12/group. Data derived from Donny et al (2003).
DUAL REINFORCING EFFECTS OF NICOTINE
The above findings that environmental stimuli play a critical role in nicotine self-administration raise the important question: what is the nature of the synergism between these stimuli and nicotine? One possible answer is that the VS, acting as a neutral stimulus, develops conditioned reinforcing properties over time by virtue of its close temporal association with nicotine, acting as a primary reinforcer. While Pavlovian conditioning is an important part of our dual-reinforcement model (and will be addressed later), it is not a viable explanation for the synergism initially seen between nicotine and the VS. The VS is not a neutral stimulus but rather an unconditioned reinforcer. Rats exhibited response rates on the active lever for contingent VS --- without nicotine --- that were significantly higher than control values (non-contingent VS presentations or inactive lever responses). This finding is consistent with a wealth of data from an older literature on sensory reinforcement (Fowler, 1971; Harrington, 1963). In fact, the VS-alone and nicotine-alone often support similar rates of operant behavior (e.g., Figure 1; see also Palmatier, Evans-Martin, Hoffman, Caggiula, Chaudhri, Donny, Liu, Booth, Gharib, Craven, & Sved, 2006a), suggesting that they are of similar strength as unconditioned reinforcers. Elemental conditioning models (e.g., Rescorla & Wagner, 1972) predict little or no change in the behavior evoked by a putative conditioned stimulus (in this case the VS) paired with an equally salient unconditioned stimulus, nicotine. Thus, the increased responding is probably not based on associative learning. A more likely explanation for the synergism emerged from our experiments manipulating the contingency between both reinforcers and the animal’s behavior (Caggiula, Donny, White, Chaudhri,, Gharib, Booth, Sved, 2001; Donny et al., 2003).
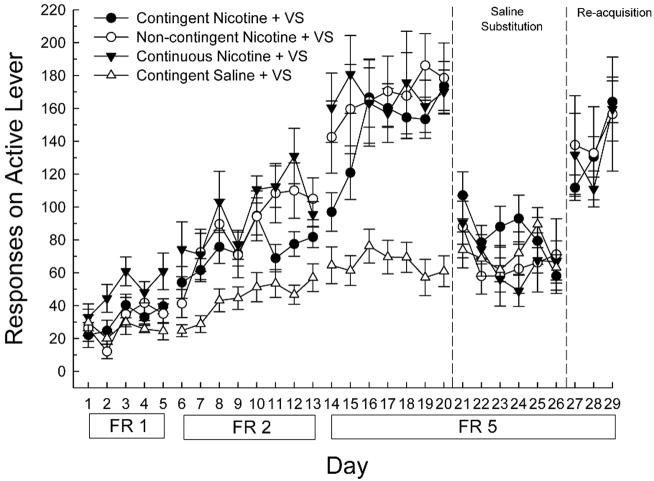
To address this issue, we asked whether the enhancement of operant behavior seen when both the VS and nicotine infusions are contingent on the rat’s lever pressing, could occur when the VS was contingent on the animal’s behavior but nicotine administration was response-independent (i.e., either controlled by another rat in a yoked design or continuously infused). The results of the first study and multiple replications (Chaudhri et al., 2006b; Donny et al., 2003) were that yoked nicotine infusions enhanced bar pressing for the VS to levels that were statistically indistinguishable from contingent nicotine (Figure 6.2). This effect of nicotine occurred in naive rats during acquisition without previous association between nicotine and the VS, across a range of nicotine doses, and on both fixed ratio (FR) and progressive ratio (PR) reinforcement schedules (Chaudhri, Caggiula, Donny, Booth, Gharib, Craven, Palmatier, Liu, & Sved, 2007). The enhancement of bar pressing for the VS produced by non-contingent nicotine was also obtained by continuously infused nicotine (Figure 6.2), but not by non-contingent food presentation (Donny et al., 2003), suggesting that not all primary reinforcers produce the enhancing effect. The enhanced lever pressing for the VS was equally attenuated and reinstated by the removal and subsequent replacement of contingent and non-contingent nicotine (Figure 6.2). These data suggest that nicotine can enhance the reinforcing properties of other stimuli by a mechanism that does not require a discrete temporal relationship with either the stimuli or the behavior. The demonstration that nicotine produces only modest primary reinforcing effects, but potent enhancement of the reinforcing effects of other stimuli, may help to resolve a long-standing paradox regarding how a drug with relatively modest primary reinforcing properties can support the establishment and maintenance of such a persistent behavior (i.e., smoking; Rose & Corrigall, 1997).
Figure 6.2.
Effects of response-contingent, yoked, or continuous nicotine or responding for VS. Acquisition data (days1-20) were followed by 6 days in which saline was substituted for nicotine (days 21-26) and then three days in which nicotine was replaced (days 27-29). Results are mean + SEM of data obtained from 7-9 animals per group (From Donny et al. 2003. Copyright © 2003 by Springer-Verlag. Reprinted with permission)
PREDICTIONS OF THE DUAL-REINFORCEMENT MODEL: REINFORCING STRENGTH OF THE NONPHARMACOLOGICAL STIMULUS
The dual reinforcement model makes important predictions about the relationship between the reinforcing value of nonpharmacological stimuli and the reinforcement-enhancing effects of nicotine. Specifically, nicotine should have a more pronounced enhancing effect on more reinforcing stimuli when compared to stimuli with little incentive value. Ultimately, if the stimulus is neither an unconditioned nor conditioned reinforcer (i.e., neutral), this enhancing effect of nicotine should be absent. We have used two approaches to test this hypothesis.
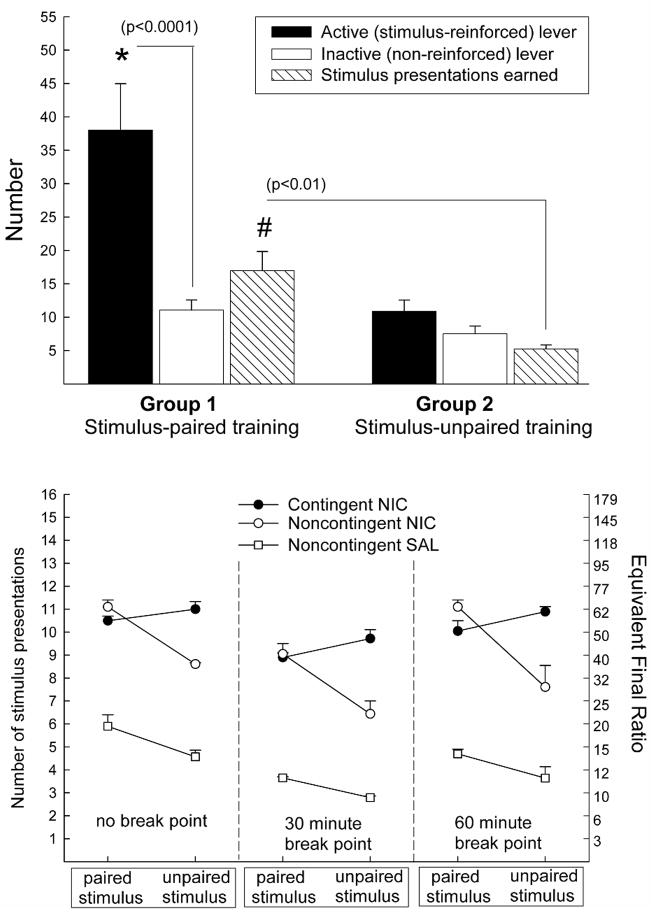
In the first approach (Chaudhri, Caggiula, Donny, Booth, Gharib, Craven, Palmatier, Liu, & Sved, 2006a) we compared the effects of contingent and non-contingent intravenous nicotine on the same stimulus that was rendered more or less reinforcing by prior Pavlovian conditioning. A weakly reinforcing light-tone stimulus was established as a conditioned reinforcer by repeated pairings with sucrose. A control group received equal exposure to the stimulus and sucrose in a temporally unpaired manner. Subsequently, both groups lever pressed for the stimulus with contingent nicotine, non-contingent nicotine, or non-contingent saline on FR and PR reinforcement schedules. Repeated pairing with sucrose established the light-tone stimulus as a robust conditioned reinforcer, as evidenced by more active-lever responding in the Paired group (Figure 6.3, upper panel). As predicted, contingent and non-contingent nicotine elevated responding equally for this conditioned stimulus. In contrast, for the less reinforcing (sucrose-unpaired) version of the same stimulus, contingent nicotine more effectively elevated behavior compared to non-contingent nicotine on both reinforcement schedules (Figure 6.3, lower panel; see Chaudhri et al., 2006a for complete data). These data are consistent with other reports that nicotine can enhance responding for a stimulus that has been established as a conditioned reinforcer by prior association with a non-nicotine primary reinforcer (Olausson, Jentsch, & Taylor, 2004a, 2004b).
Figure 6.3.
(a) Responses on the active lever, inactive lever and number of stimulus presentations (mean + SEM) during a 30-minute test for conditioned reinforcement by rats trained previously with a stimulus that was paired with sucrose (n=41), or the same stimulus that was explicitly unpaired with sucrose (n=25). (b) Interaction between stimulus-training condition (sucrose-paired vs. sucrose-unpaired) and drug contingency (contingent NIC vs. non-contingent NIC). Data from non-contingent SAL + stimulus conditions are also shown for comparison. Panels represent data from either the entire 180 minute test session (no break point), or after a 30 minute or 60 minute break point was imposed. Data are mean (* SEM) stimulus presentations earned on the last 2 days of the progressive ratio schedule. All interactions are significant at P<0.05 (From Chaudhri et al. 2006a. Copyright © 2006 by Springer-Verlag. Reprinted with permission)
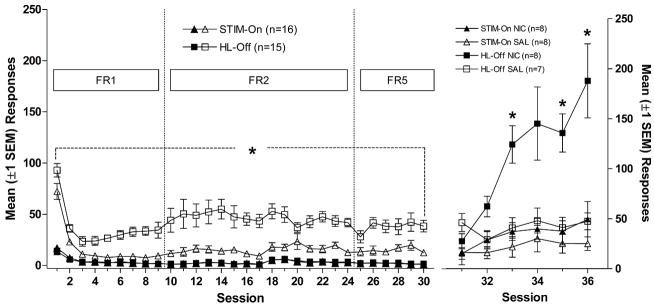
In the second approach (Palmatier, Matteson, Black, Liu, Caggiula, Craven, Donny, & Sved, 2007), we compared the effects of subcutaneous non-contingent nicotine injections on two stimuli that differed in their unconditioned reinforcing effects. Across daily 1-h sessions, rats responded at higher rates for the House-Light off stimulus (5-s extinction of a house light + 83 dB tone) than for the Lever-Light on stimulus (5-s onset of a stimulus light + the same tone), confirming that the former was more reinforcing. After responses stabilized, rats in each group were randomly assigned to one of two drug conditions; nicotine or saline injections were given 5-min before each of the remaining test sessions. Saline injections did not affect responding for either stimulus. The House-Light off group showed increased responding following nicotine administration, whereas response rates for the Lever-Light on group were unchanged by nicotine (Figure 6.4). Both studies strongly support our prediction that the relative reinforcing value of non-pharmacological stimuli determines the degree to which nicotine exerts its reinforcement enhancing effects.
Figure 6.4.
Left panel shows the mean (±1 SEM) number of active- and inactive-lever responses made during the stimulus comparison phase. Active-lever responses were significantly higher in the House-Light off than the Lever-Light on condition. Right panel illustrates the mean (±1 SEM) active-lever responses made during nicotine/saline testing sessions. Active-lever responding in House-Light NIC condition was significantly greater than the House-Light Sal condition. The number of reinforcements earned in this study was previously reported in Palmatier et al. (2007).
PREDICTIONS OF THE DUAL-REINFORCEMENT MODEL: BEHAVIORAL DISSOCIATION
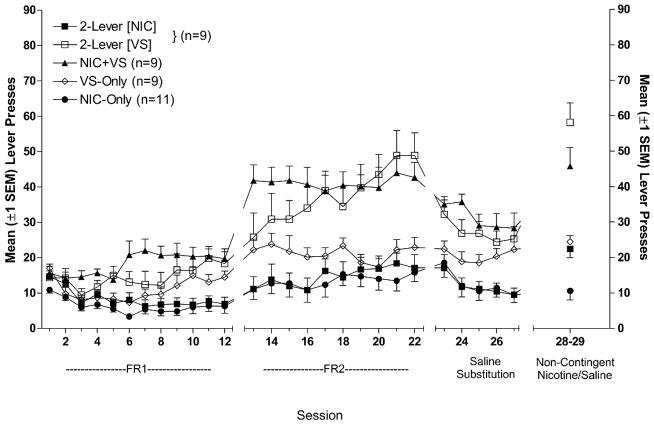
Up to this point, we inferred that self-administered nicotine possesses both primary reinforcing and reinforcement-enhancing effects by comparing self-administered (contingent) with response-independent (non-contingent) nicotine using a standard self-administration model with only one active lever delivering the reinforcer(s). A different model was needed to determine whether rats can behaviorally distinguish between these two effects of nicotine and, ultimately, whether they are mediated by the same or different mechanisms. For this purpose, we adopted a concurrent reinforcement paradigm (Palmatier et al., 2006a). For the key group (2-Lever), pressing one lever delivered the same VS we used in earlier studies and pressing the other lever delivered infusions of nicotine. For comparison, another group (NIC+VS) received standard self-administration training; one lever controlled both the VS and nicotine. Control groups received either nicotine infusions (NIC-Only) or VS presentations (VS-Only) for pressing the active lever. Nicotine alone and VS alone maintained relatively modest levels of responding (Figure 6.5). When these two reinforcers were combined (NIC+VS), response rates were substantially higher, as shown previously. For the 2-Lever group, response rates on the nicotine lever were relatively low, matching the response rates of rats receiving nicotine alone (NIC-Only). However, responding on the VS lever was potently enhanced in this group, equaling the response rates for rats receiving both reinforcers for making a single response (NIC+VS). Moreover, responding in the concurrent reinforcement paradigm is truly under the independent control of each reinforcer; when the outcome associated with each lever is reversed, response rates on each lever change to match the reinforcement earned (Palmatier et al, unpublished). These data indicate that the reinforcement enhancing effects of nicotine are very potent even when only moderate quantities of the drug are self-administered. Moreover, they provide the first demonstration that the reinforcement enhancing and primary reinforcing effects of nicotine can be dissociated behaviorally.
Figure 6.5.
Mean (+1 SEM) active lever responding for rats in the VS-Only, NIC-Only, NIC+VS and 2-Lever groups. For the 2-Lever group, responding on the infusion lever (■) is depicted separately from responding on the VS lever (□). Time-out responding is excluded. For the 2-Lever group, responding for the VS lever exceeded responding in the VS-Only group for FR2 sessions and did not differ from VS+NIC rats. Saline substitution abolished, and NIC replacement reinstated the difference between 2-Lever VS and VS-Only responding. See Palmatier et al, 2006 for details of procedures and statistical analyses. (From Palmatier et al. 2006. Copyright © 2005 by Springer-Verlag. Reprinted with permission)
PREDICTIONS OF THE DUAL-REINFORCEMENT MODEL: PHARMACOLOGICAL DISSOCIATION
Previous research has utilized the standard (single active lever) self-administration paradigm in studying the effects of pharmacological probes on nicotine reinforcement for the purpose of inferring the underlying neurobiological mechanisms. For example, mice lacking the β2 subunit of nicotinic acetylcholine receptors (nAChR) self-administer much less nicotine than wild-type controls (Picciotto et al., 1998), suggesting that nicotinic receptors containing this subunit (e.g., α4β2) are critical to nicotine-derived reinforcement. Although this approach has been useful for describing the neurobiological systems that participate in nicotine’s actions, it is unable to dissociate primary reinforcement from the reinforcement-enhancing functions of nicotine. For example, the difference observed between β2-knock-outs and wild-type controls could have been based on a difference in the primary reinforcing and/or reinforcement enhancing actions of nicotine. By contrast, the concurrent reinforcement paradigm enables us to ask specific questions about the neurobiological basis of these two effects of nicotine.
In a recent study (Palmatier, Evans-Martin, Hoffman, Caggiula, Chaudhri, Donny, Liu, & Sved, 2005) we sought to investigate the usefulness of this concurrent reinforcement paradigm for testing potential pharmacotherapies for smoking. The clinical efficacy of nicotine replacement therapy may lie, in part, in its ability to maintain the reinforcement-enhancement normally resulting from the nicotine in tobacco; in the absence of these enhancing effects, abstinent smokers may experience a loss of reinforcement that contributes to the mood disruptions associated with nicotine withdrawal (Hughes, Hatsukami, Pickens, Krahn, Malin, & Luknic, 1984). If a novel compound that selectively targets the primary reinforcing effects of nicotine was identified, it might be useful in combination with nicotine replacement therapy to decrease the motivation to smoke without altering the mood-related effects of nicotine replacement. Recent studies suggest that metabotropic glutamate 5 receptors (mGluR5) may be a target system for such treatment. For example, the mGluR5 antagonist MPEP decreases nicotine self-administration (Paterson, Semenova, Gasparini, & Markou, 2003; Tessari, Pilla, Andreoli, Hutcheson, & Heidbreder, 2004), but does not alter nicotine’s ability to decrease intracranial self-stimulation thresholds (Harrison, Gasparini, & Markou, 2002). This latter effect of nicotine may be related to the enhanced responding for the visual stimulus induced by nicotine in our laboratory. We found that MPEP decreased the primary reinforcing effects of nicotine, as reflected by reduced responding on the nicotine lever of the two-lever group, but did not alter the enhancing effects of nicotine, since responding on visual stimulus lever continued to be elevated above controls. The results suggest that mGluR5 antagonists may decrease the incentive value of nicotine, without affecting its ability to enhance responding for other reinforcers.
PREDICTIONS OF THE DUAL-REINFORCEMENT MODEL: ROLE OF CONDITIONING
There are two critical predictions of the model that we have only recently begun to address. First, nicotine, acting as a primary reinforcer, can establish a concurrent neutral stimulus as a conditioned reinforcer through Pavlovian associations. Second, nicotine, acting as a reinforcement enhancer, can then magnify the incentive value of such a nicotine-associated, conditioned reinforcer.
Modern theories of tobacco dependence have placed increasing emphasis on the role of nicotine/tobacco associated stimuli or ‘cues’ for the pharmacological effect of nicotine (Rose & Levin, 1991). Many contend that the effects of nicotine become associated with various non-nicotine stimuli and these stimuli acquire conditional value or serve as cues for future nicotine delivery. As a result, the conditional stimuli for tobacco can alter behavior in a manner that maintains smoking or results in lapse/relapse after sustained abstinence. Thus, proximal stimuli normally associated with smoking, such as a lit cigarette, can induce reports of craving in smokers but not in non-smokers (Carter & Tiffany, 1999). Despite this increased emphasis on nicotine-related stimuli, there are no appropriately controlled experimental demonstrations that nicotine can associatively increase the reinforcing value of other discrete non-nicotine stimuli. This is not to say that there are no studies in which stimuli accompanying nicotine self-administration have likely taken on conditioned reinforcing properties (e.g., Cohen, Perrault, Griebel, & Soubrie, 2005; Goldberg et al., 1981). However, at least two critical controls have been missing from this literature and are required before the second hypothesis --- nicotine can enhance the incentive value of a nicotine-established, conditioned reinforcer --- can be systematically tested. One control relates to the fact that laboratory rats find some sensory stimuli intrinsically reinforcing and this may be true of the cues used in some nicotine self-administration models. There is a substantial literature on “sensory reinforcement” (Fowler, 1971) that our recent findings on the reinforcing capabilities of the VS have confirmed. Thus in any conditioning study, the putative conditioned reinforcer must be tested for reinforcing properties independent of any association with nicotine. Second, an explicitly unpaired control group, in which animals are exposed to the same number of nicotine infusions and stimulus presentations as the conditioning group, is required to show that the putative conditioned reinforcer becomes reinforcing because of its specific, temporal association with nicotine and not simply because of the animal’s past experience with the stimulus and the drug in the same context.
A study we recently completed (Palmatier, Donny, Liu, Matteson, Caggiula, & Sved, 2007) employed the acquisition of a novel response technique to investigate whether nicotine could establish a conditioned reinforcer. Experimentally naïve rats (no prior operant training) were randomly assigned to one of 3 groups: Paired, Unpaired, or CS-Only. Paired rats self-administered nicotine accompanied by a putative conditional stimulus (CS; 15-s illumination of a stimulus light) via nose-poke. For CS-Only rats, nose-poke resulted in CS presentation with a saline infusion. For Unpaired rats, CS presentations and nicotine infusions were equated to the Paired group but each event was passively received and separated by a minimum of 70 s (nose pokes were not possible). After conditioning, all rats were tested for acquisition of a novel response (lever pressing) reinforced by the CS. Responding by the Paired rats was significantly higher than Unpaired or CS-Only rats, which did not differ from each other, thus confirming the prediction that the stimulus light became a nicotine-related conditioned reinforcer.
Paired rats were then assigned to one of 3 self-administration conditions; NIC+CS (nicotine infusions accompanied CS), NC-NIC/CS and NC-SAL/CS. For the latter two groups, lever pressing resulted in CS presentations but nicotine or saline infusions were yoked (therefore non-contingent; NC) to the NIC+CS group. The remaining rats (CS-Only and Unpaired) also received nicotine infusions yoked to the NIC+CS group during a second phase of the novel response test. When the CS had acquired value (i.e., previously paired with nicotine) non-contingent nicotine increased responding and CS presentations earned relative to the same (paired) CS with non-contingent saline, or to the other, unpaired control conditions. Thus, non-contingent infusions of nicotine may have enhanced the reinforcing strength of the CS and/or retarded the development of extinction. For Paired NIC+CS rats, there was further acquisition of the lever press response, or acquisition of an association between contingent nicotine and the CS. This study demonstrates that nicotine can conditionally increase the motivational valence of non-nicotine stimuli. Moreover, once this conditioned value has accrued, the reinforcement enhancing effects of nicotine can sustain or promote more responding for the stimulus (also see Donny et al, 2003; Bevins and Palmatier, 2004).
NATURE AND TEMPORAL DYNAMICS OF NICOTINE’S REINFORCEMENT-ENHANCING EFFECTS
Data presented to this point confirm a central prediction from the dual-reinforcement model, namely, that nicotine enhances responding for both unconditioned reinforcing stimuli (e.g., the VS in earlier studies) and a nicotine-established, conditioned reinforcing stimulus. The latter finding is consistent with research from other laboratories showing that nicotine also increases responding for conditioned reinforcing stimuli established by other primary reinforcers, such as water (Brunzell, Chang, Schneider, Olausson, Taylor, & Picciotto, 2006; Olausson et al., 2004a, 2004b). The convergence of these findings might suggest that nicotine is capable of equally enhancing all reinforcers, a conclusion that is at odds with our own finding, described earlier, that the magnitude of nicotine’s enhancing effects depends on the reinforcing strength of the non-nicotine stimulus. More importantly, the conclusion is implausible as applied to smoking, since it is unlikely that nicotine equally enhances proximal conditioned stimuli such as the sight and taste of the cigarette; more remote contextual conditioned or unconditioned stimuli such as the settings within which smoking occurs or other co-abused drugs like alcohol; and remote, weakly reinforcing, conditioned or unconditioned stimuli. Translating these concerns into the present model, we can ask if the reinforcement-enhancing effects of nicotine are identical for unconditioned and conditioned reinforcing stimuli in terms of the magnitude of the effect and its temporal dynamics?
Two lines of investigation bear on this question. In the first, the enhancing effects of repeated nicotine exposure on a water-associated conditioned reinforcer persisted for an extended period after termination of nicotine treatment (Olausson et al., 2004a, 2004b). This persistence contrasts with a finding from our laboratory (Palmatier, Liu, Caggiula, Donny, & Sved, 2006b), in which we used the concurrent reinforcement paradigm to estimate the time course of nicotine’s enhancing effects on an unconditioned reinforcing stimulus (VS) after nicotine withdrawal (saline substitution) or pharmacological antagonism of nAChRs by mecamylamine. For the 2-Lever group, acute mecamylamine challenge (or saline substitution) immediately and totally reduced the reinforcement enhancing effects of nicotine. In contrast, responding on the nicotine-lever decreased gradually over the seven days of testing, as would be expected of extinction learning. At least two, non-mutually exclusive hypotheses can be proposed to account for the discrepancy between the persistent effects reported by others (; Brunzell et al., 2006; Olausson et al., 2004a, 2004b) and the short time frame of our enhancing effects. First, the duration of nicotine’s enhancing effects may depend on the strength of the reinforcing stimulus; the stimulus is likely to have been stronger (i.e., more reinforcing) in the Olausson et al. (2004a, 2004b) and Brunzell et al. (2006) studies than the VS in our experiment. Second, the difference may relate to a difference in the enhancing effects of nicotine on a conditioned (Brunzell et al., 2006; Olausson et al., 2004a, 2004b) versus an unconditioned (Palmatier et al., 2006a; Palmatier et al., 2007) reinforcer. We have not yet determined whether nicotine’s enhancing effects on a nicotine-related conditioned reinforcer persist after termination of treatment and whether this temporal dimension is influenced by the strength of that conditioned reinforcer. Experiments designed to make such a determination by manipulating the nicotine-CS conditioning process are currently underway.
SIGNIFICANCE OF THE DUAL-REINFORCEMENT MODEL
The research reviewed above indicates that the primary reinforcing actions of nicotine are not sufficient to explain the high rates of self-administration exhibited by laboratory animals or cigarette smoking by humans. Two additional factors must be considered, namely the conditioned and unconditioned reinforcing stimuli that accompany nicotine intake and the capacity of nicotine to enhance the reinforcing effects of such stimuli. Initial studies from our laboratory suggest that this reinforcement-enhancing effect of nicotine may be dissociable from its primary reinforcing component.
The dual-reinforcement model may have relevance at both the pre-clinical animal research level, in pursuit of neurobiological mechanisms of nicotine reinforcement, and at the level understanding nicotine’s role in smoking. For the former, reinforcement-enhancing effects are not unique to nicotine; they have also been shown for psychostimulants such as cocaine and amphetamine (Chaudhri et al., 2006b; Phillips & Fibiger, 1990; Taylor & Jentsch, 2001). This fact may be relevant to understanding the actions of an effective pharmacotherapy for smoking cessation, sustained release bupropion (Jorenby, Leischow, Nides, Rennard, Johnston, Hughes, Smith, Muramoto, Daughton, Doan, Fiore, & Baker, 1999). Bupropion antagonizes nAChRs, but can also increase extracellular dopamine and norepinephrine (NE) (Li, Perry, & Wong, 2002), suggesting that while reduced nAChR action may decrease the primary reinforcing effects of nicotine, other pharmacological actions of bupropion may lead to a reinforcement-enhancing effect. Indeed, we (Mays, Levin, Bak, Palmatier, Liu, Caggiula, Donny, Craven, & Sved, 2007) have found that bupropion pretreatment dose-dependently increased responding for a moderately reinforcing sensory stimulus and, like nicotine, this effect sensitized over repeated daily tests. Moreover, the enhancing effects of nicotine, but not bupropion, were blocked by mecamylamine, whereas the enhancing effects of bupropion , but not nicotine, were blocked by prazosin, an α-NE antagonist. Propranolol, a β-NE antagonist, had no detectable effects on responding for either. The results of this study indicate that bupropion has reinforcement-enhancing effects similar to nicotine. Furthermore, the reinforcement-enhancing effects of bupropion and nicotine are pharmacologically dissociable. The emerging pattern suggests that bupropion may ‘replace’ a reinforcement enhancing effect of nicotine. For example, in nicotine self-administration studies with rodents bupropion can increase (Rauhut, Neugebauer, Dwoskin, & Bardo, 2003) responding for intravenous nicotine infusions that are accompanied by non-nicotine stimuli. Also, acute treatment with bupropion increased ad-lib smoking as well as ratings of positive mood and euphoria in people that were not intending to quit (Cousins, Stamat, & de Wit, 2001). While human studies have not investigated the impact of bupropion with and without non-nicotine reinforcers, the ‘replacement’ hypothesis outlined here argues that bupropion would eliminate some of the negative impact of quitting by sustaining and/or promoting other forms of reinforcement.
At the human level, recent evidence suggests that nicotine can modulate hedonic tone and reactivity to external rewards (Dawkins, Powell, West, Powell, & Pickering, 2006). This study reported that abstinent smokers “… expect to derive less enjoyment from a range of ordinary events and activities … ” and “ … showed virtually no reward responsivity …”. Both effects were reversed by nicotine delivered via lozenges. Other data suggested that these positive effects of nicotine on hedonic tone were distinct from its effects on the “… general symptoms of nicotine withdrawal”.
SUMMARY AND CONCLUSIONS
Models of intravenous nicotine self-administration in laboratory animals are being used to investigate the behavioral and neurobiological consequences of nicotine reinforcement, and to aid in the development of novel pharmacotherapies for smoking cessation. Central to these models is the principle of primary reinforcement, which posits that response-contingent presentation of a primary reinforcer, nicotine, engenders robust operant behavior, whereas response-independent drug delivery does not. This dictum of nicotine as a primary reinforcer has been widely used to explain why people smoke tobacco--smoking results in the rapid delivery of nicotine to the brain, setting up a cascade of neurobiological processes that strengthen subsequent smoking behavior. However, there is mounting evidence that the primary reinforcement model of nicotine self-administration fails to fully explain existing data from both the animal self-administration and human smoking literatures. We have recently proposed a “dual reinforcement” model developed to more fully capture the relationship between nicotine and self-administration, including smoking. Briefly, the “dual reinforcement” model posits that nicotine acts as both a primary reinforcer and a reinforcement enhancer. The latter action of nicotine had originally been uncovered by showing that a reinforcing visual stimulus, which accompanies nicotine delivery, synergizes with nicotine in the acquisition and maintenance of self-administration; and that this synergism can be reproduce by combining operant responding for the reinforcing stimulus with non-contingent (response-independent) nicotine. Thus, self-administration (and smoking) is sustained by three actions: 1) nicotine, acting as a primary reinforcer, can sustain behavior that leads to its delivery; 2) nicotine, acting as a primary reinforcer, can establish neutral environmental stimuli as conditioned reinforcers through Pavlovian associations; and 3) nicotine, acting as a reinforcement enhancer, can magnify the incentive value of accompanying stimuli, be they conditioned or unconditioned reinforcers.
Acknowledgments
We thank Sheri Booth, Maysa Gharib, Laure Craven, Kara Mays, Gina Matteson, Kasia Bak, Melissa Levin, and Emily Kraus for their assistance conducting the research and performing analyses. Research conducted in our laboratory followed the NIH Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee (Assurance #: A3187-01). This research was supported by NIH grants DA-10464, DA-12655, DA-17288, and DA-19278 and by a Howard Hughes Predoctoral Research Fellowship awarded to N. Chaudhri.
References
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology (Berl) 1998;140(3):331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Wright AE, Benwell ME, Birrell CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res. 2000;113(12):73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behavioral and Cognitive Neuroscience Review. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Murray A, Wise RA. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol Biochem Behav. 1989;33(4):903–907. doi: 10.1016/0091-3057(89)90490-5. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berl) 2006;184(34):328–338. doi: 10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002a;77(45):683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70(4):515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002b;163(2):230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Gharib M, Booth S, Sved AF. Non-contingent nicotine enhances responding maintained by behaviorally contingent environmental cues. Paper presented at the Society for Neuroscience.2001. [Google Scholar]
- Carroll ME, Krattiger KL, Gieske D, Sadoff DA. Cocaine-base smoking in rhesus monkeys: reinforcing and physiological effects. Psychopharmacology (Berl) 1990;102(4):443–450. doi: 10.1007/BF02247123. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006a;189(1):27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berl) 2007;190(3):353–362. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006b;184(34):353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, Rohsenow DJ, Robbins SJ, O’Brien CP. Classically conditioned factors in drug dependence. In: Lowinson JH, Ruiz P, Millman RB, editors. Substance abuse: a comprehensive textbook. Baltimore: Williams and Wilkins; 1992. pp. 55–69. [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30(1):145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Exp Clin Psychopharmacol. 2001;9(4):399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. A rodent model for nicotine self-administration. In: Boulton A, Baker G, Wu P, editors. Neuromethods: animal models of drug addiction. Vol. 24. Totowa: The Humana Press; 1992. pp. 315–344. [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99(4):473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology (Berl) 2001;157(3):243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I--effects on incentive motivation. Psychopharmacology (Berl) 2006;189(3):355–367. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75(2):134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Behavioural pharmacology and neurobiology of nicotine rewrd and dependence. In: Clementi F, Fomasari D, Gotti C, editors. Handbook of experimental pharmacology: neuronal nicotinic receptors. Berlin: Springer-Verlag; 2000. [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122(4):390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147(2):135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136(1):83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rose C, Jacobs KS, Mielke MM, Sved AF. Differential effects of response-contingent and response-independent nicotine in rats. Eur J Pharmacol. 2000;402(3):231–240. doi: 10.1016/s0014-2999(00)00532-x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151(4):392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169(1):68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Fowler H. Implications of sensory reinforcement. In: Glaser R, editor. The Nature of Reinforcement A Symposium of The Learning Research and Development Center. New York: Academic Press; 1971. pp. 151–195. [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214(4520):573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Harrington GM. Stimulus intensity, stimulus satiation, and optimum stimulation with light-contingent bar-press. Psychological Reports. 1963;13:107–111. [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160(1):56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Control of behavior by intravenous nicotine injections in human subjects. Pharmacol Biochem Behav. 1983;19(6):1021–1026. doi: 10.1016/0091-3057(83)90409-4. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Pickens RW, Krahn D, Malin S, Luknic A. Effect of nicotine on the tobacco withdrawal syndrome. Psychopharmacology (Berl) 1984;83(1):82–87. doi: 10.1007/BF00427428. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. Animal models of drug self-administration. Adv Sub Abuse. 1981;2:219–97. [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. The New England Journal of Medicine. 1999;340(9):685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. J Abnorm Psychol. 2006;115(1):166–173. doi: 10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- Li SX, Perry KW, Wong DT. Influence of fluoxetine on the ability of bupropion to modulate extracellular dopamine and norepinephrine concentrations in three mesocorticolimbic areas of rats. Neuropharmacology. 2002;42:181–190. doi: 10.1016/s0028-3908(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Margolin A, Avants SK. Cue-reactivity and cocaine addiction. In: Kosten TR, Kleber HD, editors. Clinicians guide to cocaine addiction. 109-27. New York: Guilford Press; 1992. [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112(23):163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Mays KL, Levin ME, Bak KM, Palmatier MI, Liu X, Caggiula AR, Donny EC, Craven L, Sved AF. Nicotine and bupropion have similar effects on responding for reinforcing non-drug stimuli. Paper presented at the Society for Research on Nicotine and Tobacco; Austin, TX. 2007. [Google Scholar]
- Meisch RA, Lemaire GA. Drug self-administration. In: van Haaren F, editor. Methods in behavioral pharmacology. BV: Elsevier Science Publications; 1993. pp. 257–300. [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12(1):15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004a;171(2):173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004b;173(12):98–104. doi: 10.1007/s00213-003-1702-9. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Donny EC, Liu X, Matteson GL, Caggiula AR, Sved AF. Conditioned reinforcement established with self-administered nicotine: Motivational effects of stimuli associated with different unit nicotine doses. Paper presented at the Society for Neuroscience; San Diego. 2007. [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006a;184(34):391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Sved AF. Self-administered nicotine enhances responding for a concurrently available visual reinforcer. Paper presented at the Society for Neuroscience; Washington, D.C. 2005. [Google Scholar]
- Palmatier MI, Liu X, Caggiula AR, Donny EC, Sved AF. The Role of Nicotinic Acetylcholine Receptors in the Primary Reinforcing and Reinforcement-Enhancing Effects of Nicotine. Neuropsychopharmacology. 2006b doi: 10.1038/sj.npp.1301228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend. 2007 doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167(3):257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Fibiger HC. Role of reward and enhancement of conditioned reward in persistence of responding for cocaine. Behav Pharmacol. 1990;1(4):269–282. doi: 10.1097/00008877-199000140-00002. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology (Berl) 2003;169(1):1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement. In: Black AH, editor. Classical Conditioning II: Current Research and Theory. New York: Appleton Century Crofts; 1972. pp. 64–99. [Google Scholar]
- Roberts DCS, Richardson NR. Self-administration of psychomotor stimulants using progressive ratio schedules of reinforcement. In: Boulton A, Baker G, Wu PH, editors. Neuromethods: animal models of drug addiction. Clifton: Humana Press; 1992. pp. 233–269. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67(1):71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology (Berl) 1997;130(1):28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Rose JE, Levin ED. Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. Br J Addict. 1991;86(5):605–609. doi: 10.1111/j.1360-0443.1991.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multidimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96(10):1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Influence of a conditioned light stimulus on cocaine self-administration in rats. Psychopharmacology (Berl) 2001;154(4):390–396. doi: 10.1007/s002130000608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995;119(3):334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129(1):35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine (“Ecstasy”) Biol Psychiatry. 2001;50(2):137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499(12):121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]