Abstract
We recently demonstrated that the hepatic cytochrome P-450 (CYP) isoform 1A2 is downregulated in sepsis, which appears to play an important role in the inflammatory response and liver injury. However, the mechanism responsible for the decreased CYP1A2 remains unknown. Since the transcription factor aryl hydrocarbon receptor (AhR) regulates the expression of CYP1A2 and the disruption of the AhR gene causes liver injuries, we hypothesized that downregulation of AhR plays an important role in the reduced hepatic CYP1A2 during sepsis. Adult male rats were subjected to sepsis by cecal ligation and puncture (CLP). Hepatic tissues were collected at 5, 10, and 20 h after CLP or sham-operation. The gene expression of AhR was assessed by RT-PCR technique. Its protein was determined by Western blot analysis. In addition, subcellular localization of AhR was examined by immunohistochemical staining. The results indicate that hepatic AhR gene expression decreased at 5 h and remained downregulated at 10-20 h after CLP. AhR protein levels were significantly reduced at 10-20 h after CLP. Immunohistochemical examination showed that AhR was mainly located in hepatocyte cytoplasm in sham animals. The translocation of AhR from the cytoplasm to the nucleus was observed in septic animals. The downregulation of hepatic AhR and CYP1A2 observed in septic animals does not appear to be due to the elevated endotoxin levels since administration of polymyxin B (an endotoxin-binding agent) did not affect AhR and CYP1A2 gene expression. However, proinflammatory cytokines tumor necrosis factor-α and interleukin-1ß decreased AhR and CYP1A2 expression. As AhR activates the specific gene expression by binding to the target genes, the translocation of AhR to the nucleus in sepsis would suggest that alterations at AhR binding sites may also contribute to the downregulated CYP1A2 expression in sepsis. Since AhR gene expression decreased earlier than the occurrence of depression of CYP1A2 (CYP1A2 decreased at 10-20 h post CLP), the decreased AhR may play an important role in downregulating hepatic CYP1A2 during the progression of sepsis.
Keywords: liver, hepatoma cells, cecal ligation and puncture, tumor necrosis factor-α, interleukin-1ß, hepatic cytochrome P-450 isoform 1A2
Introduction
The incidence of sepsis and septic shock has increased significantly despite improvement in the management of septic patients with systemic antibiotics, surgical intervention, aggressive fluid resuscitation, and careful monitoring (1,2). There is a progressive deterioration of cell and organ functions and even death in the septic host, which might be delayed or even prevented by intervention with pharmaceutical agents against and/or modulating various proinflammatory mediators. The animal model of polymicrobial sepsis induced by cecal ligation and puncture (CLP) mimics various features of clinical sepsis-peritonitis. The CLP model of sepsis is associated with an early, hyperdynamic phase (characterized by increased cardiac output and tissue perfusion, and decreased vascular resistance) followed by a late, hypodynamic phase (characterized by reduced cardiac output and tissue perfusion, as well as increased vascular resistance) (3-5). This model of sepsis has been used extensively to study the pathophysiological and immunologic alterations in sepsis (6).
Cytochrome P-450 (CYP) enzymes are a superfamily of heme proteins which is responsible for the metabolic activation or inactivation of most clinically used drugs and many toxins. CYP isoforms can be found in most tissues, but the largest concentrations of P-450 are found in the liver. The expression of P-450 enzymes is regulated by a variety of factors such as genetic polymorphism, drugs, hormones, development and diet (7). Inflammatory mediators can cause changes in the activities and expression of the P-450 enzyme system. A large number of reports have shown that CYP isoforms and their activities are, for the most part, suppressed in animal models of endotoxemia as well as in cultured hepatocytes stimulated by endotoxin (7-11). In this regard, a recent study from our laboratory showed that hepatic CYP1A2, the most predominant CYP isoform in the rat liver (12), is downregulated during the progression of polymicrobial sepsis induced by CLP (13).
Transcriptional activation of CYPs involves the aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor as well as its nuclear translocator (Arnt) and a chaperone protein, heat shock protein 90 (HSP90) (14,15). Using a Kupffer cell-hepatocyte co-culture system, we recently showed that reduction in nuclear receptor AhR, due to the increased production of Kupffer cell-derived proinflammatory cytokines, tumor necrosis factor (TNF)-α and interleukin (IL)-1ß, plays an important role in suppressing hepatic CYP1A2 expression in sepsis (16). However, the mechanism responsible for suppression of hepatic CYP1A2 in polymicrobial sepsis warrants additional investigations. Therefore, the objective of the present study was to further study the underlying mechanism responsible for the downregulation of CYP1A2 during polymicrobial sepsis. Various experiments were performed to determine whether sepsis-induced CYP1A2 downregulation was associated with and possibly caused by the reduction of AhR expression under such conditions.
Materials and methods
Animal model of sepsis
Male Sprague-Dawley rats (275-325 g), purchased from Charles River Laboratories (Wilmington, MA), were housed in a temperature-controlled room on a 12-h light/dark cycle and fed a standard Purina rat chow diet. Prior to induction of sepsis, rats were fasted overnight but allowed water ad libitum. Rats were then anesthetized with isoflurane inhalation, and the abdomen was shaved and washed with 10% povidone iodine. Cecal ligation and puncture (CLP) was performed as we previously described (4,17), and the animals were randomly assigned to various experimental groups. Hepatic tissues were collected at 5, 10, and 20 h after CLP or sham-operation for determination of CYP1A2 and AhR expression. In separate experimental groups, polymyxin B (PMB, an endotoxin-binding agent) was administered to observe its effect on hepatic AhR and CYP1A2 gene expression. At 0.5 h before CLP as well as 9 h after CLP, PMB (Sigma, St. Louis, MO) was administered intramuscularly at a total dose of 4000 U/kg (2000 U/kg each injection). This dosage was determined as optimal according to our previous experiment (18). Hepatic tissues were collected at 20 h after the onset of sepsis or sham-operation (5-6 rats/group). PMB is an endotoxin-binding antibiotic, which binds and detoxifies lipid A. In vitro studies have confirmed that PMB neutralizes E. coli endotoxin activity (19). All animal experiments were performed in accordance with the NIH guidelines for the use of experimental animals. This project was approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Determination of AhR and CYP1A2 gene expression
The gene expression of AhR and CYP1A2 was determined using RT-PCR technique. The primers were as follows: 5′ GGG ATC GAT TTC GAA GAC ATC AG 3′ (sense), 5′ AAC GCC TGG GAG CCT GGA ATC TC 3′ (antisense) for AhR (NM-013149); and 5′ GAA TCG GTG GCT AAC GTC AT 3′ (sense), 5′ CCA GAA GAT GGC TGT TGT GA 3′ (antisense) for CYP1A2 (NM-012541). Rat glyceraldehyde 3-phosphate dehydrogenase (G3PDH, M17701: sense, TGA AGG TCG GTG TCA ACG GAT TTG GC and antisense, CAT GTA GGC CAT GAG GTC CAC CAC) served as a housekeeping gene. PCR was carried out in a Bio-Rad thermal cycler. PCR cycles were carefully tested in order to avoid the plateau phase of the amplification. Following the RT-PCR procedure, the reaction products were separated in 1.6% TBE-agarose gel containing 0.22 μg/ml ethidium bromide by electrophoresis. The gel was then photographed and the band density was determined by a digital image system (Bio-Rad, Hercules, CA). Target/housekeeping gene band density ratios were then calculated.
Determination of AhR and CYP1A2 proteins
The protein expression of AhR and CYP1A2 was measured by Western blot analysis. Briefly, proteins (25 μg) were fractionated on 4-12% Bis-Tris gel and transferred to 0.2-μm nitrocellulose membranes. Nitrocellulose blots were blocked by incubation in TBST (10 mM Tris-HC1, pH 7.5, 150 mM NaC1, 0.1% Tween-20) containing 5% milk for 1 h. Blots were incubated with rabbit anti-rat AhR polyclonal antibody (1:600; Biomol Research Laboratories Inc., Plymouth Meeting, PA), or goat anti-rat CYP1A2 serum (1:600; Daiichi Pure Chemicals, Tokyo, Japan) overnight at 4°C, respectively. The blots were then washed in TBST for 5×10 min. Blots were incubated with HRP labeled anti-rabbit (1:10,000) or anti-goat (1:4000) IgG for 1 h at room temperature. The blots were then washed in TBST for 5×10 min. To reveal the reaction bands, the membrane was reacted with the ECL Western blot detection system (Amersham, Piscataway, NJ) and exposed on X-ray film. The Bio-Rad GS-800 Calibrated Densitometer analysis system was used to quantitate the Western blots. This system selects the contour of the band, subtracts the background and calculates the density.
Immunohistochemistry
The liver tissues were collected 20 h after CLP or sham-operation (n=4 rats/group) and immediately fixed in the neutralized 4% paraformaldehyde solution overnight. They were then dehydrated and embedded in paraffin through the standard histology procedure. The paraffin sections were dewaxed and rehydrated followed by the microwave antigen retrieval procedures. To retrieve the antigen, slides were soaked in 20% citric acid buffer, pH 6.0 (Vector Labs, Burlingame, CA) and kept at 95°C for 15 min. The slides were cooled at room temperature for 5 min and rinsed with TBS. Endogenous peroxidase was blocked by reaction with 2% H2O2. The slides were then incubated in 2% normal goat serum for 1 h to block the nonspecific binding followed by incubation in 1:100 rabbit anti-AhR polyclonal antibodies (Biomol) overnight at 4°C. After washing, the sections were reacted with 1:200 biotinylated anti-rabbit IgG secondary antibodies (Vector Labs). The Vectastain ABC and DAB substrate kits (Vector Labs) were used to reveal the immunohistochemical reaction. Normal rabbit IgG was substituted for the primary antibody as the negative control.
Hepatoma H-4-II-E cell culture and stimulation
Rat hepatoma cell line H-4-II-E cells (ATCC, Manassas, VA) were cultured in Eagle’s minimum essential medium (ATCC) with 10% fetal bovine serum and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA). H-4-II-E cells were stimulated with proinflammatory cytokines, TNF-α (5.0 ng/ml) or IL-1ß (2.5 ng/ml) (both from Biosource International, Camarillo, CA) for 48 h. Proteins isolated from these cells were used for Western blot analysis.
Statistical analysis
Data are presented as means ± SE. One-way analysis of variance (ANOVA) and Tukey’s test were utilized for the comparison among different groups. The difference was considered significant at P≤0.05.
Results
Alterations in hepatic AhR expression in septic animals
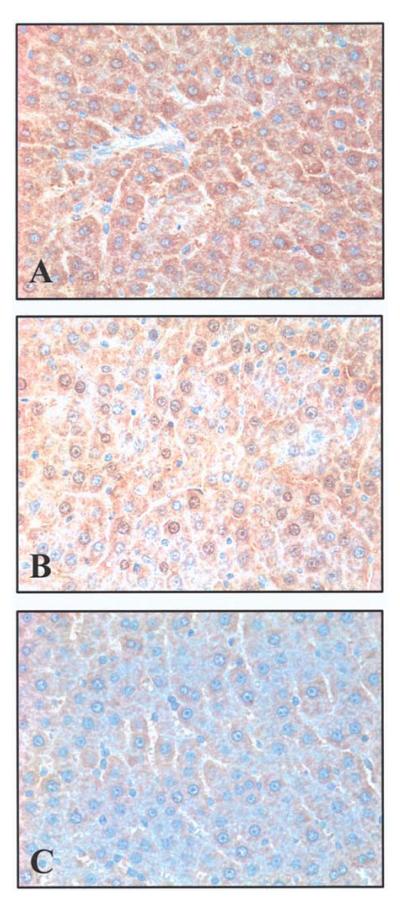
The expression of both the AhR gene and protein in the liver was assessed at 5, 10, and 20 h after the onset of sepsis. The results indicated that hepatic AhR gene expression decreased by 43% at 5 h (P<0.05), and remained significantly reduced at 10-20 h after CLP (Fig. 1). Similarly, AhR protein levels were also significantly reduced at 5, 10, and 20 h after CLP (by 30-59%, Fig. 2). The finding that the AhR gene and protein expression decreased significantly as early as 5 h after the onset of sepsis suggests that the downregulation may have occurred even earlier than 5 h post CLP. Our previous studies showed that hepatic CYP1A2 expression decreased at 10-20 h after CLP (13). The fact that AhR downregulation occurs earlier than that of CYP1A2 may suggest a cause and effect relationship. Immunohistochemical examination showed that AhR protein staining was mainly located in the hepatocyte cytoplasm in sham animals (Fig. 3A). The translocation of AhR from the cytoplasm to the nucleus was observed in septic animals at 20 h after CLP (Fig. 3B). As AhR activates the specific gene expression by binding to the target genes, the translocation of AhR to the nucleus in sepsis would suggest that alterations at AhR binding sites may also contribute to the downregulated CYP1A2 expression in sepsis.
Figure 1.
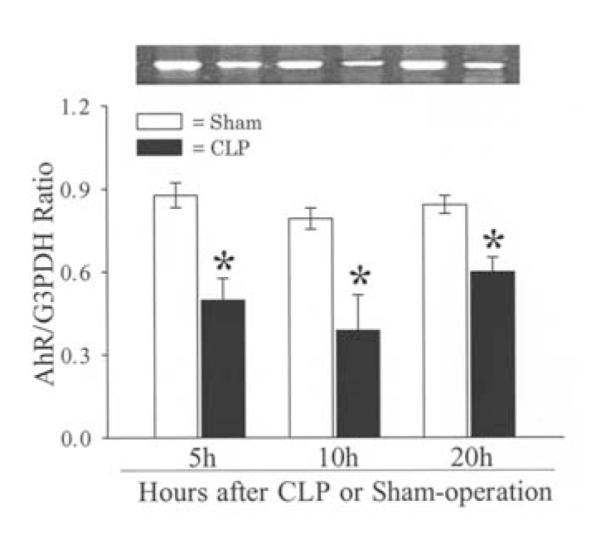
Alterations in gene expression of AhR in the rat liver at 5, 10 and 20 h after CLP or sham-operation. A representative blot is also shown. Data are presented as means ± SE (n=5-6/group) and were compared by one-way ANOVA and Tukey’s test; *P<0.05 versus respective shams.
Figure 2.
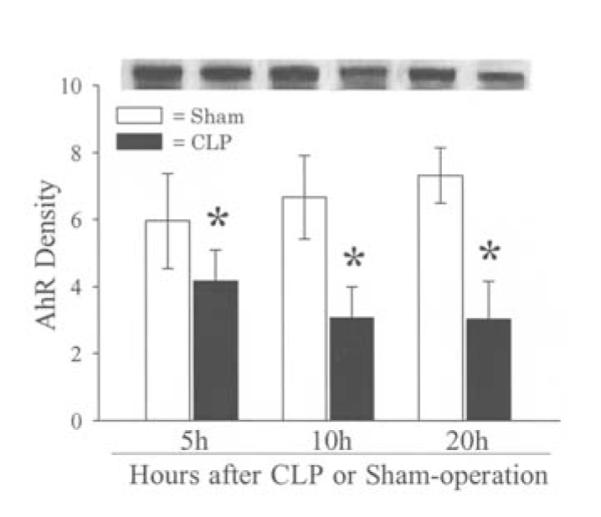
Alterations in protein levels of AhR in the rat liver at 5, 10 and 20 h after CLP or sham-operation. A representative blot is also shown. Data are presented as means ± SE (n=5-6/group) and were compared by one-way ANOVA and Tukey’s test; *P<0.05 versus respective shams.
Figure 3.
Immunohistochemical staining of AhR in the rat liver at 20 h after CLP or sham-operation. (A) AhR was located in the hepatocyte cytoplasm in a sham animal. (B) The translocation of AhR from the cytoplasm to the nucleus was observed in a septic animal. (C) The staining was minimal in the negative control section.
Effects of endotoxin neutralization on hepatic AhR and CYP1A2 gene expression in sepsis
To determine whether endotoxin plays a role in downregulating AhR and CYP1A2 expression in sepsis, PMB was administered to neutralize circulating endotoxin in septic animals. The gene expression of AhR and CYP1A2 was assessed in the liver at 20 h after the onset of sepsis. The results indicated that AhR gene expression decreased significantly 20 h after CLP, and PMB treatment did not affect the diminished expression (Fig. 4A). Likewise, the downregulated CYP1A2 gene expression after sepsis was not affected by PMB treatment (Fig. 4B). These results suggest that AhR and CYP1A2 downregulation in sepsis may not be directly caused by the increased endotoxin.
Figure 4.
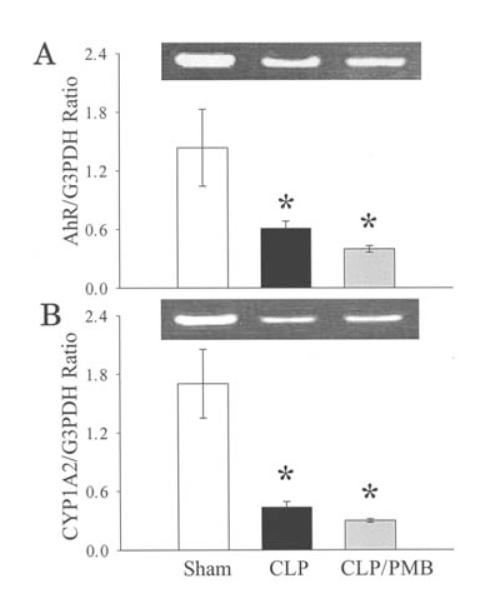
Effects of endotoxin inhibition on AhR (A) and CYP1A2 (B) gene expression in the rat liver at 20 h after CLP. Polymyxin B (PMB, 4000 U/kg) was used to neutralize circulating endotoxin. Representative blots of CYP1A2 and AhR are also presented. Data are expressed as means ± SE (n=6/group) and were compared by one-way ANOVA and Tukey’s test; *P<0.05 versus shams.
Effects of TNF-α and IL-1β on AhR and CYP1A2 in hepatoma cell culture
Since administration of PMB in sepsis did not prevent the downregulation of hepatic AhR and CYP1A2 gene expression, we hypothesized that the reduced AhR and CYP1A2 expression may be caused by proinflammatory cytokines. To study this, hepatoma cells (H-4-II-E) were cultured with or without proinflammatory cytokine TNF-α (5.0 ng/ml) or IL-1ß (2.5 ng/ml) for 48 h. The results indicated that incubation of the hepatoma cells with TNF-α decreased AhR protein expression by 36% (P<0.05, Fig. 5) and CYP1A2 protein expression by 40% (P<0.05, Fig. 6). Similarly, incubation of the hepatoma cells with IL-1ß for 48 h significantly reduced AhR (Fig. 5) and CYP1A2 protein expression (Fig. 6). These results suggest that the elevated levels of proinflammatory cytokines in sepsis may play an important role in producing the downregulation of hepatic AhR and CYP1A2 expression.
Figure 5.
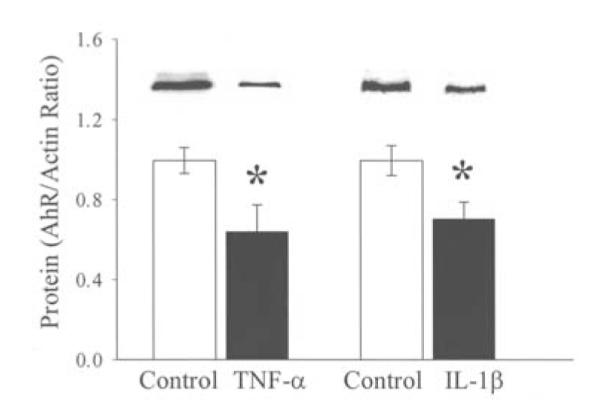
Alterations in AhR protein levels in hepatoma cell (H-4-II-E) culture stimulated with 5.0 ng/ml of TNF-α and 2.5 ng/ml of IL-1ß. Representative blots are also presented. Data are expressed as means ± SE (n=6/group) and were compared by one-way ANOVA and Tukey’s test; *P<0.05 versus controls.
Figure 6.
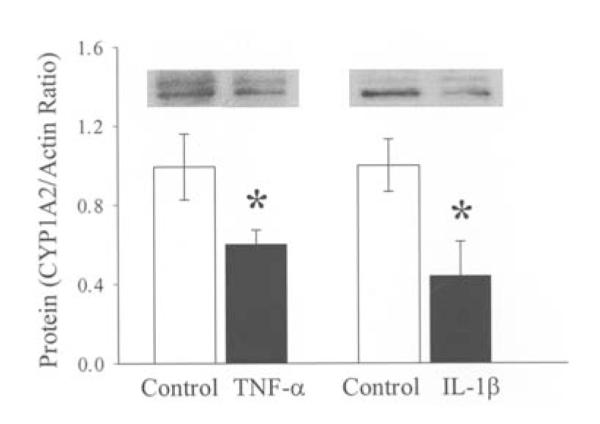
Alterations in CYP1A2 protein levels in hepatoma cell (H-4-II-E) culture stimulated with TNF-α (5.0 ng/ml) and IL-1ß (2.5 ng/ml). Representative blots are also presented. Data are expressed as means ± SE (n=6/grup) and were compared by one-way ANOVA and Tukey’s test; *P<0.05 versus controls.
Discussion
By using an animal model of polymicrobial sepsis, we demonstrated that the AhR gene and protein expression in the liver decreased significantly after the onset of sepsis (i.e. 5-20 h after CLP). It was also observed that the translocation of AhR from the cytoplasm to the nucleus occurred in septic animals. Furthermore, the downregulation of hepatic AhR and CYP1A2 observed in septic animals did not appear to be due to the elevated endotoxin levels, but appeared to be caused by proinflammatory cytokines TNF-α and IL-1ß. As AhR gene expression decreased earlier than the occurrence of depression of CYP1A2 (CYP1A2 decreased at 10-20 h post CLP), the decreased AhR may play an important role in the downregulation of hepatic CYP1A2 in polymicrobial sepsis.
Nuclear receptors such as AhR represent a superfamily of ligand-modulated transcription factors. They mediate a variety of physiological responses to steroids, retinoids, thyroid hormones, and various xenobiotics (20). These receptors play a key role in development and cell differentiation, as well as organ physiology (21). Steroid hormones can enter the cell by simple or facilitated diffusion and then transduce their signals to the genome via intracellular receptors (22). After binding to its receptor, the hormone-receptor complex moves from the cytoplasm to the nucleus, undergoing allosteric changes that enable the complex to bind to high affinity sites in the chromatin and modulate gene transcription (23,24). Several studies have indicated that the transcription of the CYP1A2 gene is mediated at least in part through the AhR signaling pathway (25-27). This observation is further supported by the finding that the human hepatoma cell line SK-Hep-1, which expresses defective AhR, is associated with the lack of CYP1A2 expression (28). In the absence of stimulation, AhR exists as a non-DNA-binding, ∼300 kDa heteromeric complex, associated with the molecular chaperone HSP90 in a 1:2 ratio in the cytosol (29). Upon stimulation, the AhR-HSP90 complex enters the nucleus and subsequently dissociates, enabling AhR to be phosphorylated by tyrosine kinase. Activated AhR then forms a heterodimer complex with its nuclear translocator (Arnt) (30). Within the nucleus, the AhR-Arnt complex recognizes and binds to the specific regulatory sequences known as the dioxin responsive element (DRE) at the promoter region, and initiates the transcription of the CYP1A2 gene (31-34). Using a polymicrobial sepsis model, we recently showed that CYP1A2 gene expression and its protein decreased significantly only at 10-20 h after CLP (13). In the present study, we further extended the observation and found that AhR gene expression and protein were decreased earlier. These observations point to a possible cause and effect relationship of the downregulation of AhR and CYP1A2 during the progression of polymicrobial sepsis.
Increasing interest has been focused on the alteration of cytochrome P-450 contents and activities in inflammation and related pathophysiological conditions (35,36). We focused on the changes of the isoform CYP1A2 after the onset of sepsis in the present as well as the previous study (13). CYP1A2 was chosen because it is the most predominant isoform of the P-450 enzyme system in the rat liver. CYP1A2 is involved in the metabolism of various exogenous agents such as theophylline, imipramine, and naproxen and is inhibited by chemicals such as cimetidine and fluroquinolones (7). Our results showed that both CYP1A2 and AhR gene expression was reduced at 20 h post CLP. Moreover, the downregulated AhR and CYP1A2 gene expression was not reversed by inhibition of endotoxin with PMB. This would suggest that endotoxin by itself does not appear to directly reduce AhR and CYP1A2 gene expression after CLP. In contrast, CYP1A2 and AhR protein expression decreased significantly following the stimulation with TNF-α and IL-1ß in hepatoma cells. Therefore, the decrease in CYP1A2 and possibly AhR can be partially explained by the observation that proinflammatory cytokines such as TNF-α, IL-1ß, and IL-6 may signal the decreased expression of CYP1A2 mRNA in hepatocytes as well as in hepatoma cell cultures (7,36-38). Previous studies have clearly indicated that the levels of these proinflammatory cytokines increase significantly in sepsis (39). Studies have suggested that proinflammatory cytokines released from Kupffer cells may directly downregulate P-450 enzymes in hepatocytes (40). It is therefore possible that gut-derived mediators, which stimulate Kupffer cell TNF-α production and/or release in sepsis, could have a downregulatory effect on the hepatic AhR and P-450 enzyme system. Although our study was not designed to determine other mechanisms that regulate P-450, the close correlation between the increased proinflammatory cytokines and the decreased CYP1A2 proteins in hepatoma cells indicates the notion of a drug-cytokine interaction following inflammation (7,41). Nonetheless, our present study suggests that AhR downregulation plays at least a partial role in reducing CYP1A2 expression in the liver during sepsis.
In summary, our results indicate that proinflammatory cytokines, but not the direct effects of endotoxin, play an important role in downregulating hepatic CYP1A2 expression in sepsis. The reduction of AhR expression may be the underlying mechanism responsible for such downregulation.
Acknowledgements
This investigation was supported by National Institutes of Health (NIH) grants R01 GM053008 and R01 GM057468 (P. Wang). The authors sincerely thank Dr H. Hank Simms and Dr Yan J. Jiang for their excellent assistance.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Baue AE. Multiple organ failure, multiple organ dysfunction syndrome, and systemic inflammatory response syndrome: Why no magic bullets? Arch Surg. 1997;132:703–707. doi: 10.1001/archsurg.1997.01430310017002. [DOI] [PubMed] [Google Scholar]
- 3.Wang P, Chaudry IH. Mechanism of hepatocellular dysfunction during hyperdynamic sepsis. Am J Physiol. 1996;270:R927–R938. doi: 10.1152/ajpregu.1996.270.5.R927. [DOI] [PubMed] [Google Scholar]
- 4.Wang P, Chaudry IH. A single hit model of polymicrobial sepsis: Cecal ligation and puncture. Sepsis. 1998;2:227–233. [Google Scholar]
- 5.Yang S, Cioffi WG, Bland KI, Chaudry IH, Wang P. Differential alterations in systemic and regional oxygen delivery and consumption during the early and late stages of sepsis. J Trauma. 1999;47:706–712. doi: 10.1097/00005373-199910000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Deitch EA. Animal models of sepsis and shock: A review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Morgan ET. Regulation of cytochrome P450 by inflammatory mediators: why and how? Drug Metab Dispos. 2001;29:207–212. [PubMed] [Google Scholar]
- 8.Monshouwer M, McLellan RA, Delaporte E, Witkamp RF, VanMiest AS, Renton KW. Differential effect of pentoxifylline on Lipopolysaccharide-induced downregulation of cytochrome P450. Biochem Pharmacol. 1996;52:1195–2000. doi: 10.1016/0006-2952(96)00468-6. [DOI] [PubMed] [Google Scholar]
- 9.McKindley DS, Boulet J, Sachdeva K, Wang P, Chichester C. Endotoxic shock alters the pharmacokinetics of lidocane and monoethylglycinexylidide. Shock. 2002;17:199–204. doi: 10.1097/00024382-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Iber H, Sewer MB, Barclay TB, Mitchell SR, Li T, Morgan ET. Modulation of drug metabolism in infectious and inflammatory diseases. Drug Metab Rev. 1999;31:29–41. doi: 10.1081/dmr-100101906. [DOI] [PubMed] [Google Scholar]
- 11.Shedlofsky SI, Israel BC, McClain CJ, Hill DB, Blouin RA. Endotoxin administration to humans inhibits hepatic cytochrome P450-mediated drug metabolism. J Clin Invest. 1994;94:2209–2214. doi: 10.1172/JCI117582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spatzenegger M, Horsmans Y, Verbeeck RK. Differential activities of CYP1A isozymes in hepatic and intestinal microsomes of control and 3-methylcholanthrene-induced rats. Pharmacol Toxicol. 2000;86:71–77. doi: 10.1034/j.1600-0773.2000.d01-14.x. [DOI] [PubMed] [Google Scholar]
- 13.Crawford JH, Yang S, Zhou M, Simms HH, Wang P. Down-regulation of hepatic CYP1A2 plays an important role in inflammatory responses in sepsis. Crit Care Med. 2004;32:502–508. doi: 10.1097/01.CCM.0000109453.57709.E2. [DOI] [PubMed] [Google Scholar]
- 14.Honkakoski P, Negishi M. Regulation of cytochrome P450 (CYP) genes by nuclear receptors. Biochem J. 2000;341:321–337. doi: 10.1042/0264-6021:3470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waxman DJ. P450 gene induction by structurally diverse xenochemicals: central role of nuclear CAR, PXR, and PPAR. Arch Biochem Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- 16.Wu R, Cui X, Dong W, Zhou M, Simms H, Wang P. Suppression of hepatocyte CYP1A2 expression by Kupffer cells via AhR pathway: The central role of proinflammatory cytokines. Int J Mol Med. 2006;18:339–346. [PubMed] [Google Scholar]
- 17.Wu R, Cui X, Lim YP, Bendelja K, Zhou M, Simms HH, Wang P. Delayed administration of human inter-alpha inhibitor proteins reduces mortality in sepsis. Crit Care Med. 2004;32:1747–1752. doi: 10.1097/01.ccm.0000132903.14121.0e. [DOI] [PubMed] [Google Scholar]
- 18.Yang S, Zhou MN, Chaudry IH, Wang P. The role of lipopolysaccharide in stimulating Adrenomedullin production during polymicrobial sepsis. Biochim Biophys Acta. 2001;1537:167–174. doi: 10.1016/s0925-4439(01)00069-2. [DOI] [PubMed] [Google Scholar]
- 19.Morrison DC, Jacobs DM. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 20.Greschik H, Moras D. Structure-activity relationship of nuclear receptor-ligand interactions. Curr Top Med Chem. 2003;3:1573–1599. doi: 10.2174/1568026033451736. [DOI] [PubMed] [Google Scholar]
- 21.Beato M, Herrlich P, Schultz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 22.Mangelsdorf DJ, Thimmel C, Beato M, Herrlich P, Schultz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forman BM, Chen J, Evans RM. The peroxisome proliferators-activated receptors: ligands and activators. Ann NY Acad Sci. 1996;804:266–275. doi: 10.1111/j.1749-6632.1996.tb18621.x. [DOI] [PubMed] [Google Scholar]
- 24.Handschin C, Meyer UA. Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev. 2003;55:649–673. doi: 10.1124/pr.55.4.2. [DOI] [PubMed] [Google Scholar]
- 25.Hankakoski P, Negishi M. Regulation of cytochrome P450 (CYP) genes by nuclear receptors. Biochem J. 2000;347:321–337. doi: 10.1042/0264-6021:3470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waxman DJ. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR and PPAR. Arch Biochem Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- 27.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 28.Roberts EA, Harper PA, Wong JM, Wang Y, Yang S. Failure of Ah receptor to mediate induction of cytochromes P450 in the CYP1 family in the human hepatoma line SK-Hep-1. Arch Biochem Biophys. 2000;3384:190–198. doi: 10.1006/abbi.2000.2059. [DOI] [PubMed] [Google Scholar]
- 29.Grandin K, McGuire J, Wenger RH, Kvietikova I, Fhitelaw ML, Toftgard R, Tora L, Gasssmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomita S, Signal CJ, Yim SH, Gonzalez FJ. Conditional disruption of the Aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor lalpha. Mol Endocrinol. 2000;14:1674–1681. doi: 10.1210/mend.14.10.0533. [DOI] [PubMed] [Google Scholar]
- 31.Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the AH receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 32.Poellinger L. Ligand-dependent recruitment of the Arnt coregulator determines DNA recognition by the dioxin receptor. Mol Cell Biol. 1993;13:2504–2514. doi: 10.1128/mcb.13.4.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coumailleau P, Poellinger L, Gustafsson JA, Whitelaw ML. Definition of a minimal domain of the dioxin receptor that is associated with Hsp90 and maintains wild type ligand binding affinity and specificity. J Biol Chem. 1995;270:5291–5300. doi: 10.1074/jbc.270.42.25291. [DOI] [PubMed] [Google Scholar]
- 34.Whitelaw ML, McGuire J, Picard D, Gustafsson JA, Poellinger L. Heat shock protein hsp90 regulates dioxin receptor function in vivo. Proc Natl Acad Sci USA. 1995;92:4437–4441. doi: 10.1073/pnas.92.10.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spatzenegger M, Horsmans Y, Verbeeck RK. Differential activities of CYP1A isozymes in hepatic and intestinal microsomes of control and 3-methylcholanthrene-induced rats. Pharmacol Toxicol. 2000;86:71–77. doi: 10.1034/j.1600-0773.2000.d01-14.x. [DOI] [PubMed] [Google Scholar]
- 36.Morgan ET. Regulation of cytochromes P450 during inflammation and infection. Drug Metab Rev. 1997;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- 37.Barker CW, Fagan JB, Pasco DS. Interleukin-1 beta suppresses the induction of P4501A1 mRNAs in isolated hepatocytes. J Biol Chem. 1992;267:8050–8055. [PubMed] [Google Scholar]
- 38.Nicholson TE, Renton KW. Role of cytokines in the lipopolysaccharide-evoked depression of cytochrome P450 in the brain and liver. Biochem Pharmacol. 2001;62:1709–1717. doi: 10.1016/s0006-2952(01)00859-0. [DOI] [PubMed] [Google Scholar]
- 39.Ertel W, Morrison MH, Wang P, Ba ZF, Ayala A, Chaudry IH. The complex pattern of cytokines in sepsis: Association between prostaglandins, cachectin and interleukins. Ann Surg. 1991;214:141–148. doi: 10.1097/00000658-199108000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milosevic N, Schawalder H, Maier P. Kupffer cell-mediated differential down-regulation of cytochrome P450 metabolism in rat hepatocytes. Eur J Pharmacol. 1999;368:75–87. doi: 10.1016/s0014-2999(98)00988-1. [DOI] [PubMed] [Google Scholar]
- 41.Piscitelli SC, Reiss WG, Figg WD, Petros WP. Pharmacokinetic studies with recombinant cytokines. Scientific issues and practical considerations. Clin Pharmacokinet. 1997;32:368–381. doi: 10.2165/00003088-199732050-00003. [DOI] [PubMed] [Google Scholar]