Abstract
Purpose
Abnormal regulation of the HPA axis and diurnal cortisol rhythms are associated with several pain and chronic inflammatory conditions. Chronic stress may play a role in the disorder of CP/CPPS related to initiation or exacerbation of the syndrome. We tested the hypothesis that men with CPPS have associated disturbances in psychosocial profiles and HPA axis function.
Methods
45 men with CPPS and 20 age-matched, asymptomatic controls completed psychometric self-report questionnaires: Type A personality test; Perceived Stress Scale, Beck Anxiety Inventory; and Brief Symptom Inventory [BSI] for distress from physical symptoms. Saliva samples were collected on two consecutive days at 9 specific times with strict reference to time of morning awakening for evaluation of free cortisol reflecting secretory activity of the HPA axis. We quantified cortisol variations as 2-day average slope of the awakening cortisol response and the subsequent diurnal levels.
Results
CPPS men had more perceived stress and anxiety than controls (p < 0.001). BSI scores were significantly elevated in all scales (somatization, obsessive/compulsive behavior, depression, anxiety, hostility, interpersonal sensitivity, phobic anxiety, paranoid ideation, psychoticism) for CPPS; Global Severity Index rank for CPPS was 93rd versus 48th centile for controls (p < 0.0001). CPPS men had significantly elevated awakening cortisol responses, mean slope of 0.85 versus 0.59 for controls (p < 0.05).
Conclusions
CPPS men scored exceedingly high on all psychosocial variables and showed evidence of dysfunctional HPA axis function reflected in augmented awakening cortisol responses. Observations suggest variables in biopsychosocial interaction that suggest opportunities for neurophysiologic study of relationships of stress and CPPS.
Keywords: Prostatitis, Chronic Pelvic Pain Syndrome, Stress, Salivary Cortisol, Psychosocial, Adrenocortical Activity
INTRODUCTION
In most people the diurnal rhythm of the cortisol cycle is marked by two main characteristics: 1) a peak awakening cortisol response within 30 minutes after awakening in the morning; and 2) a gradually decreasing slope throughout the day, reaching the lowest levels late in the evening. These endocrine patterns are consistent, show high intra-individual stability across time and appear to be markers for subtle changes in HPA axis regulation.1
Stress triggers a cascade of pathophysiological events that involve activation of two pathways: the HPA axis and the autonomic nervous system. Chronic activation of the physiologic stress response induces putative glucocorticoid resistance and altered immunity, release of proinflammatory cytokines and prostaglandins that may contribute to pain syndromes and, ultimately, to cycling psychological distress. Clinicians have anecdotally observed that stress exacerbates symptoms of prostatitis,2 and chronic stress experiments in rats can specifically induce histological inflammation in the prostate.3 Genetic factors and psychological variables, e.g., personality traits, modulate reactivity and vulnerability to stress 4–6 Evidence from our case studies shows that manual physiotherapy with myofascial trigger point release and paradoxical relaxation therapy, both physical and cognitive behavioral therapy, can provide pain relief in some CPPS sufferers.7 Once it is understood how stress-induced neurobiochemical changes may relate to pelvic pain in men, we may modulate these effects and develop new and innovative approaches for the prevention and treatment of CPPS. Despite the likely relevance of stress-related neurohormones in the disease process of CPPS, these factors have not been systematically evaluated.
The primary aim of the study was to test the hypothesis that CPPS is associated with psychological dysfunction as well as endocrine dysregulation of the HPA axis.
PATIENTS AND METHODS
Patients
Men referred to the Stanford University Hospital Urology Clinic from October 2005 to May 2007 with symptoms of CPPS (NIH category IIIA & IIIB) for at least 3 months within the last 6 months were invited to participate in the study. Participants were required to be 18 years of age or older, have a NIH-CPSI total score of 12 or greater (scale of 0–43), and a non-zero pain score at time of enrollment. Approximately 90% of men with CPPS who met the entry criteria agreed to participate in the study. Healthy men with no history or evidence of GU disease or chronic pain conditions were recruited as controls by newspaper advertisement from the same socio-economic community and prospectively age-matched to the CPPS cohort. No analgesics, psychotropic drugs or systemic corticosteroids were allowed for at least 2 weeks before study entry. The protocol was reviewed and approved by the IRB. Subjects received monetary compensation for participation.
Symptom assessments
The NIH-CPSI questionnaire was used to quantify pelvic pain and urinary symptoms at the screening visit and immediately before the diurnal cortisol study.
Psychometric assessments
Baseline psychological and psychosocial stress self-reported questionnaires were answered in the home setting. We measured psychological distress with the Brief Symptom Inventory (BSI) of the SCL-90R, a 53-item inventory that scores nine symptom dimensions: depression, anxiety, somatization, obsessive-compulsive behavior, interpersonal sensitivity, hostility, phobic activity, paranoid ideation, psychoticism (social alienation), and the Global Severity Index (GSI), an overall measure of distress.8 The BSI surveys patient problems and how bothersome during the past 7 days. Responses are a 5-point Likert scale of 0 (not at all) to 4 (extremely bothersome). Raw scores convert to normalized T scores with equivalents based on adult male, non-patient (non-psychiatric) profiles from a reference population. The T-scores are matched with centile ranks for comparison with published norms. The BSI has well established norms, reliability and validity.
The Bortner Type A Personality Test categorized personality based on response to stress; scores between 85 and 154 denote a Type A behavior pattern.
The Beck Anxiety Inventory (BAI), a 21-item inventory assessed symptoms of anxiety which are minimally shared with depression; scores range 0 to 63.
The Perceived Stress Scale (PSS), a 14-item questionnaire, measured perception of stress, feelings and thoughts during the past month. The PSS assesses the degree to which subjects perceive their lives as unpredictable, uncontrollable and with burden overload and includes direct inquiries about current levels of experienced stress; scores range from 0–40.
Procedures for salivary cortisol collection and assessment
The measurement of cortisol in salivary samples accurately reflects levels of physiologically active unbound (free) cortisol in the blood, which diffuses from the blood to saliva. Cortisol secretion correlates with serum ACTH with an approximate 15 minute delay and is considered to accurately reflect secretory activity of the HPA axis.9 Patients collected saliva on two consecutive days at nine time points with strict reference to time of morning awakening: immediately upon waking, at 15-minute intervals for the next hour, and then an additional four samples at 3-hour intervals throughout the day. Awakening was either spontaneous or by alarm clock. To assure compliance with the collection procedures, the participants were given an electronic watch that beeps at designated times for saliva collection. Because cortisol levels vary during the day subjects were informed it was essential to collect samples at the designated time intervals and to enter collection times on a daily log. Subjects collected saliva in sterile Salivette tubes (Sarsted Inc., Newton, NC) with small cotton swabs in capped plastic vials. This noninvasive technique was used for at-home or work-based collections to interfere minimally with normal daily routines. Participants were instructed to refrain from eating, drinking, chewing gum, smoking, brushing teeth or using mouthwash for 30 minutes before collections and to refrigerate samples immediately or place them in an insulated cold bag until refrigeration for return to the clinic.
Saliva samples were stored at −70C prior to laboratory centrifugation and assayed for salivary cortisol by luminescence immunoassay with reagents provided by Immuno-Biological Laboratories, Inc., Hamburg, Germany. Samples from each subject were assayed in duplicate in the same batch by the Stanford GCRC laboratory. Assay sensitivity was 0.015μg/dL. Intra-assay variation on low, medium and high controls averaged 15.5%, 10.2% and 7.6%, respectively. The mean values of the low, medium, and high controls were 0.077μg/dL, 0.24μg/dL and 0.93μg/dL, respectively. The inter-assay coefficients of variation for the low, medium and high controls were 9.8%, 8.8% and 6.6%, respectively.
Data analyses
We used SPSS software (SPSS Inc., Version 13.0, Chicago, Illinois) for all statistical tests. We examined differences in psychosocial measures, medical variables and salivary cortisol with the Mann-Whitney U test (two-tailed) and Student’s t test, and demographics with multivariate logistic regression and Chi square analysis. Salivary cortisol data were log transformed to stabilize the variance. Although we log-transformed cortisol values for all statistical analyses, the means and SD/SEM of untransformed values are presented. Diurnal or daytime cortisol slope was calculated by regressing cortisol values at time of awakening as the baseline, the 60 minute and all subsequent time points for 2 days. Waking cortisol rise was the 2-day average of the difference between the cortisol levels at waking plus 30 minutes. Correlations were determined with the Spearman rank test. For all analyses, significance was at α = 5%.
RESULTS
A total of 65 subjects, 45 men with CPPS and 20 controls were enrolled in the study; all participants completed questionnaires and collected salivary samples as requested. Demographic characteristics are presented in Table 1. Subjects in both groups were successfully age-matched (p = 0.82); 60–70% were white. They were highly educated; approximately 70% had college or graduate/professional degrees. The groups showed no differences in distribution based on demographic variables (all p>0.05). CPPS patients had a mean NIH-CPSI total score of 25 (± 6.7) and a median symptom duration of 39 months.
Table 1.
Characteristics of CPPS patients and healthy pain-free controls
| CPPS | Controls | p Value* | |
|---|---|---|---|
| Number of Subjects | 45 | 20 | |
| Mean age in years, (range) | 43 (21–70) | 44 (23–66) | 0.82 |
| Medical Status | |||
| Mean duration of CPPS, Months (range) | 69 (4–336) | 0 | < 0.0001 |
| Median | 39 | 0 | |
| Mean NIH-CPSI total score | 25 (12–41) | 1.2 (0–5) | <0.0001 |
| Mean NIH-CPSI pain subscore | 12 (3–20) | 0.1 (0–2) | <0.0001 |
| Ethnicity/race, % of sample | 0.18 | ||
| White | 71 | 60 | |
| Asian | 11 | 25 | |
| Afro-American | 4 | 5 | |
| Hispanic | 4 | 10 | |
| Other | 9 | 0 | |
| Level of education, % of sample | 0.53 | ||
| Less than college degree | 31 | 25 | |
| College graduate | 51 | 45 | |
| Graduate/professional | 18 | 30 | |
| Marital status, % of sample | 0.17 | ||
| Never married (single) | 40 | 25 | |
| Married | 49 | 70 | |
| Divorced | 11 | 5 | |
| Employment, % of sample | 0.18 | ||
| Full Time | 58 | 85 | |
| Retired | 15 | 5 | |
| On disability | 7 | 0 | |
| Student | 11 | 5 | |
| Unemployed | 9 | 5 | |
Mann-Whitney U test (2-tailed) for age and medical status; Chi square test for ethnicity, education, marital and employment status.
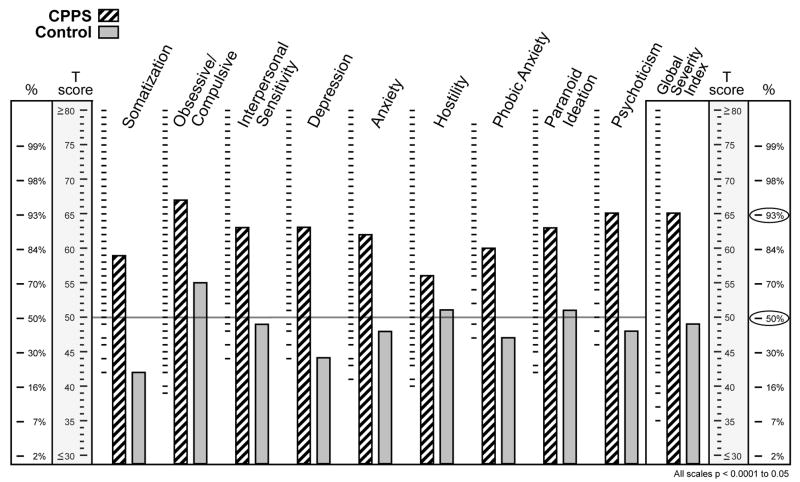
The two groups were discriminate on psychological variables. CPPS patients had significantly more perceived stress and anxiety than controls (p ≤ 0.0004, both PSS and BAI). Both cohorts had similar Type A behavior pattern scores. Psychometric evaluation scores are shown in Table 2. BSI scores were significantly elevated in each of the 9 dimensional scales for CPPS men, ranging from median 73rd to 95th centile ranks, compared with controls, ranging from median 23rd to 70th centile ranks (all dimensions, range p = 0.001 to ≤0.02), as shown in Figure 1. The GSI of the BSI, which provides one summary score as an indicator of the current degree of each subject’s distress, showed median scores for CPPS men in the 93rd centile and 48th centile for controls (p<0.0001).
Table 2.
Psychometric evaluations in CPPS patients and asymptomatic controls, median scores (25th and 75th percentiles)
| Psychometric Test (Number of Patients) | CPPS (45) | Controls (20) | p Value* |
|---|---|---|---|
| Bortner Type A Behavior | 101 (85/115) | 98 (88/110) | 0.83 |
| Perceived Stress Scale | 19 (14/23) | 12 (11/15) | 0.0003 |
| Beck Anxiety Inventory | 8 (5/15) | 1.5 (0.25/4.75) | 0.0004 |
| Global Severity Index of BSI, T scores, centile rank | 65 (55/73) 93% | 49.5 (44/58) 47% | <0.0001 |
Mann-Whitney U test (2-tailed) and Student’s t test
Fig. 1.
Brief Symptom Index clinical profile for men with CPPS and controls (T scores and centile ranks) for the nine dimensional scales and the Global Severity Index.
There was a significant inverse relationship between the GSI and age (r= −0.36, p=0.006) but not between duration of disease or CPSI total or pain subscores.
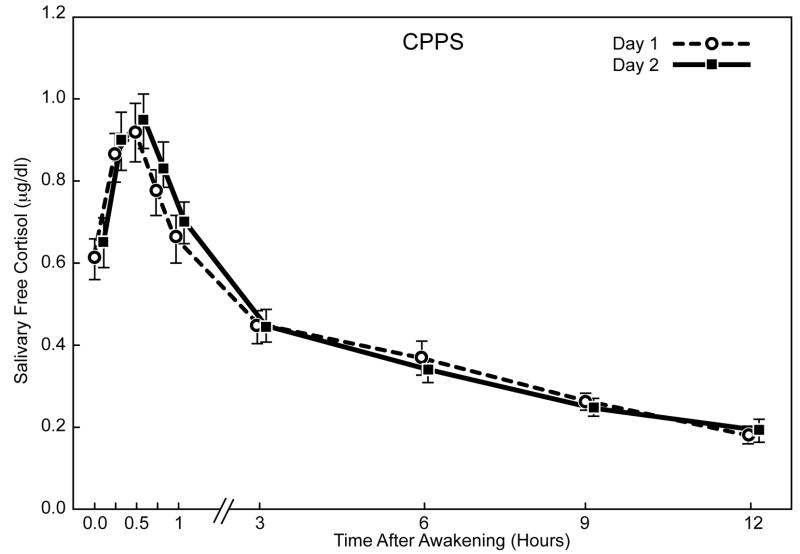
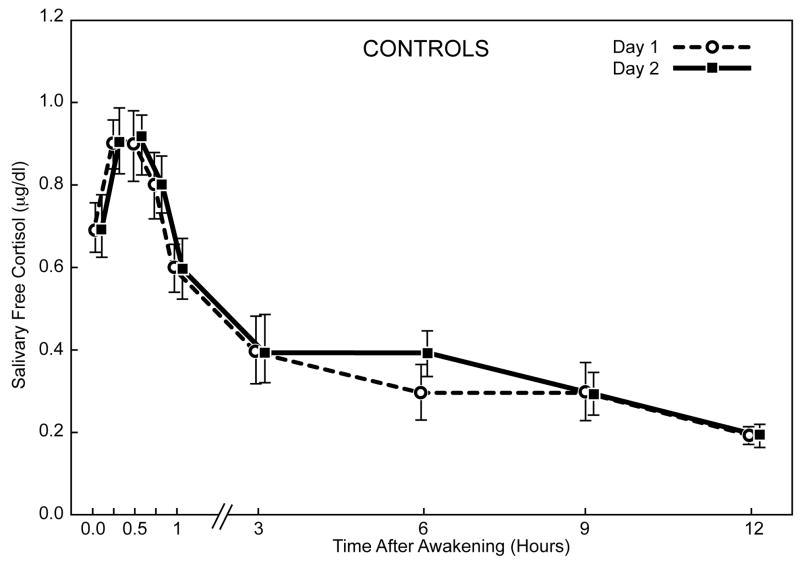
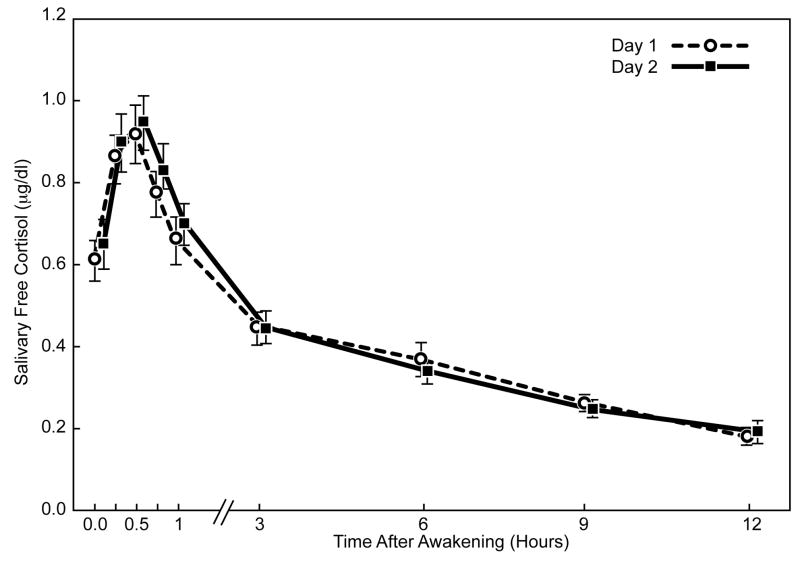
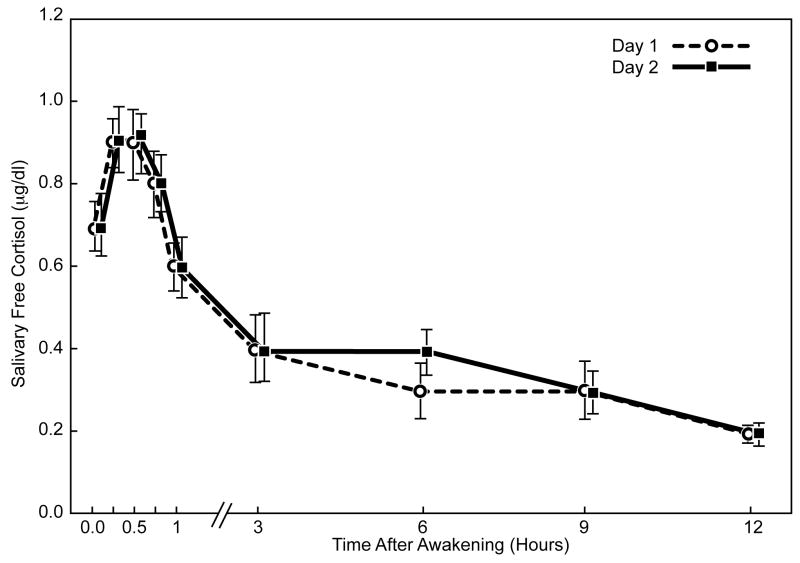
The diurnal cortisol profiles showed good intra-individual stability within the CPPS and control cohorts for saliva sampling on two days (Figure 2). Cortisol levels increased significantly during the first 30 minutes after awakening in both groups (p ≤ 0.001). The awakening cortisol response represents a discrete and distinct part of the cortisol circadian cycle. We looked at responses in two ways: absolute values in the whole period or as the dynamic increase (slope) between awakening and the peak after 30 minutes. In CPPS men, the mean (± SEM) awakening cortisol level was 0.64μg/dL (± 0.03); the level increased approximately 47% to 0.94μg/dL (± 0.05) at 30 minutes post-awakening. For control subjects, the mean awakening level was 0.72μg/dL (± 0.05), increasing approximately 31% to 0.94μg/dL (± 0.05) after 30 minutes (Figure 3). The estimated mean slope of log-cortisol levels for the awakening cortisol response was significantly greater for the CPPS group, 0.85 compared to 0.59 for controls (p = 0.05, Mann-Whitney U test).
Fig. 2.
Mean salivary free cortisol levels (± SEM) on two consecutive sampling days after morning awakening in men with CPPS and control men.
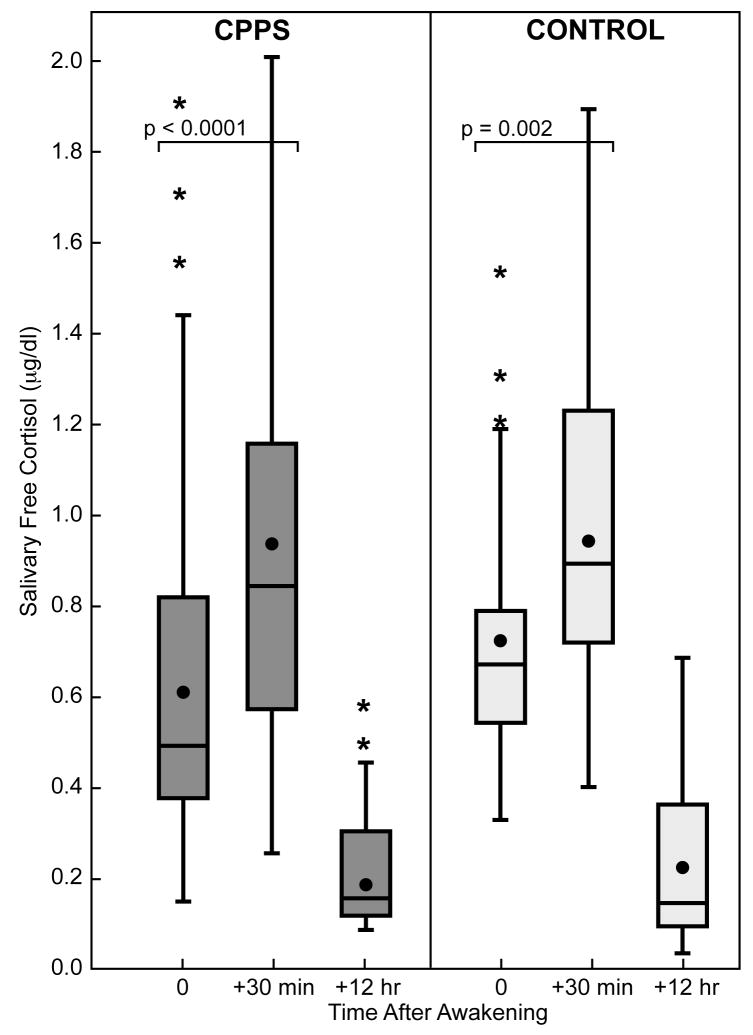
Fig. 3.
Box plot shows awakening cortisol responses (wake + 30 minutes) and daytime decrease (wake + 12 hours) for men with CPPS (shaded bars) and controls (open bars). The dot indicates mean; line in box indicates median. Boundary closest to zero indicates 25th percentile and farthest from zero indicates 75th percentile. Whiskers indicate 90th and 10th percentiles.
The daytime log-cortisol slope (decrease from morning until evening) in both cohorts declined to a stable base with similar mean slopes: CPPS slope, − 0.127; control slope, − 0.127 (p > 0.05).
A multivariate logistic regression was done with the GSI and the slope of the awakening cortisol response as independent variables in conjunction with NIH-CPSI total scores for CPPS men and controls. These two continuous variables were included in a single analysis is to determine whether the CPPS men can be more precisely understood by incorporating the psychometric and the physiological variables into one model. Due to high variance and small sample size, only the GSI proved to be significantly different for CPPS and the control groups (p=0.001)
DISCUSSION
The psychometric profiles derived from the PSS, BAI and BSI domains of the CPPS men showed significantly higher levels of self-reported perceived stress, feelings of depression and anxiety, somatization, obsessive-compulsive behavior and other psychological symptoms than the age-matched sample of men. The GSI of the BSI provides a single composite score indicative of a respondent’s distress level, featuring quantity of symptoms as well as intensity of distress.8 The median GSI for the CPPS cohort was at the 94th centile affirming the patients’ high levels of psychosocial distress. The psychometric profiles confirmed our expectations and also concur with several earlier reports of psychological disturbances (e.g., depression, catastrophic thinking, anxiety, elevated scores on somatic scales, anger-hostility) observed in conjunction with chronic CPPS.2, 4 The correlation between psychosocial distress and CPPS should not be interpreted as causal; indeed, psychosocial distress is associated with chronic pain in general and could be interpreted as a result of the pain or a contributory factor in the patient’s distress regarding their perceived pain. The feed-forward relationship between chronic pain and psychopathology is described by the diathesis-stress model.10 This model illustrates how the stress of coping with chronic pain exacerbates an individual’s semi-dormant but pre-existing characteristics (diatheses), eventually resulting in psychopathology.
Pain scores and psychometric scores were consistently higher in the cohort of CPPS men. Interestingly, within the CPPS cohort, pain scores or duration of CPPS did not correlate with scores on psychometric tests. This lack of correlation may reflect the variability in CPPS patients’ perceptions and psychological responses/adaptations to pain. In addition, we found that as the age of the men increased, the psychometric scores decreased, perhaps reflecting an overall adaptation to a chronic pain syndrome.
In this study we provide evidence that men with CPPS have HPA dysregulation as specifically shown by significantly greater slope of the awakening cortisol response compared with healthy, pain-free controls. The awakening cortisol response serves as a useful index of adrenocortical activity which provides important information on the activation of HPA axis. The relatively high intra-individual stability of the awakening cortisol response when measured over repeated days partly reflects a personal trait. However, HPA axis responses can be influenced by environmental factors including stress and anxiety and serve as an indicator of allostatic load. Allostatic load can take the form of alteration in diurnal cortisol rhythm. Systemic cortisol modulates several systems including the balance between cellular and humoral immunity, or HPA feedback system. HPA axis dysregulation can be manifested either as pathologically decreased or increased cortisol responses. Some abnormalities may lead to dysregulation of inflammatory responses, resulting in chronic inflammatory and pain conditions such as fibromyalgia11 and interstitial cystitis.12 These symptomatically varied conditions share a common feature of lowered activity of the HPA axis. Blunted awakening cortisol responses also are reported in subjects suffering from “burnout”13 or persistent sciatic pain after discectomy.14 These conditions are opposite to the augmented awakening cortisol response that we have observed in CPPS patients. Several other studies also have shown an association between elevated cortisol levels after awakening and psychological variables in chronically stressed individuals due to work overload.5, 15 Chronic stress and depression appear to have a similar pattern of HPA axis dysregulation. While hypercortisolism is associated with clinical depression, this observation also has been made in the nonclinical range of depression in healthy, young men.16 The consistent observation that hypercortisolism and depression co-appear has led many authors to suggest that elevated cortisol levels can cause depressive symptoms, and that depressive symptoms can be reversed with anti-depressant actions on HPA regulation.16 Psychological stress has long been implicated as contributory to the etiology and or exacerbation of CPPS.2, 17 Prospective studies associate perceived stress longitudinally with pain intensity18 and have examined cognitive/behavioral variables, such as catastrophizing, as predictors of greater pain intensity and disability.19 Stress management therapy has been shown to provide improvements in these individuals.2, 17 We recently reported on a neurobehavioral and physiotherapeutic approach to manage CPPS using a paradoxical relaxation therapy in conjunction with release of myofascial trigger points.7
The present study prospectively evaluated psychosocial profiles and HPA axis activity in CPPS, and is the first to expose dysregulation of the awakening cortisol response in this chronic pain condition. The morning rise of cortisol is a discrete and distinctive portion of the circadian cortisol cycle, thought to be under a distinct regulatory influence from the rest of the secretory cycle.9 Several studies have shown the association between the awakening cortisol response, its role in regulation of physiological functions (e.g., the immune system), and sensitivity to psychosocial variables.20 These observations have implications for a greater understanding of the complexities of the psychoneuro-immunological interactions linking the mind and body in CPPS.
Acknowledgments
We thank Ben Varasteh, Stanford GCRC for conducting hormone assays, and Janine Giese-Davis, Ph.D. and Eric Neri, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine for consultation regarding the analyses and interpretation of our cortisol results.
Supported by NIDDK grant U01 DK065297 and partially from 5 M01 RR00070 from the National Center for Research Resources, National Institutes of Health
Abbreviations and Acronyms
- ACTH
Adrenocorticotropin
- BAI
Beck Anxiety Inventory
- BSI
Brief Symptom Index
- CP
Chronic prostatitis
- CPPS
Chronic pelvic pain syndrome
- NIH-CPSI
National Institutes of Health-Chronic Prostatitis Symptom Index
- GCRC
General Clinical Research Center
- GSI
Global Severity Index
- GU
Genitourinary
- HPA axis
Hypothalamus-pituitary-adrenal axis
- IRB
Internal Review Board
- PSS
Perceived Stress Scale
- r
Correlation Coefficient
- SCL
Symptom Checklist
- SD
Standard Deviation
- SEM
Standard Error of the Mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.PruessnerJCWolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- 2.Miller HC. Stress prostatitis. Urology. 1988;32:507. doi: 10.1016/s0090-4295(98)90030-9. [DOI] [PubMed] [Google Scholar]
- 3.Gatenbeck L, Aronsson A, Dahlgren S, Johansson B, Stromberg L. Stress stimuli-induced histopathological changes in prostate: an experimental study in the rat. Prostate. 1987;11:69. doi: 10.1002/pros.2990110109. [DOI] [PubMed] [Google Scholar]
- 4.de la Rosette JJMCH, Ruijgrok MCM, Jeuken JMG, Karthaus HFM, Debruyne FMJ. Personality variable involved in chronic prostatitis. Urology. 1993;42:654. doi: 10.1016/0090-4295(93)90529-j. [DOI] [PubMed] [Google Scholar]
- 5.Wüst S, Federenko I, Helhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- 6.Flaa A, Ekeberg O, Kjeldsen SE, Rostrup M. Personality may influence reactivity to stress. Biopsychosoc Med. 2007;1:5. doi: 10.1186/1751-0759-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson RU, Wise D, Sawyer T, Chan C. Integration of myofascial trigger point release and paradoxical relaxation training for treatment of chronic pelvic pain in men. J Urol. 2005;174:155. doi: 10.1097/01.ju.0000161609.31185.d5. [DOI] [PubMed] [Google Scholar]
- 8.Derogatis LR. Brief Symptom Inventory (BSI), administration, scoring and procedure manual. Minneapolis MN: National Computer Systems, Inc.; 1993. [Google Scholar]
- 9.Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001;68:2093. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- 10.Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychobiology: research findings and theoretical considerations. Psychosom Med. 2002;64:773. doi: 10.1097/01.psy.0000024232.11538.54. [DOI] [PubMed] [Google Scholar]
- 11.Crofford LJ, Pillemar SR, Kalogeras KT, Cash JM, Michelson D. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum. 1994;37:1583. doi: 10.1002/art.1780371105. [DOI] [PubMed] [Google Scholar]
- 12.Lutgendrof SK, Kreder KJ, Rothrock NE, Hoffman A, Kirschbaum C, Sternberg EM, et al. Diurnal cortisol variations and symptoms in patients with interstitial cystitis. J Urol. 2002;167:1338. [PubMed] [Google Scholar]
- 13.Pruessner JC, Helhammer DH, Kirschbaum C. Burnout, perceived stress and cortisol responses to awakening. Psychosom Med. 1999;61:197. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Geiss A, Varadi E, Steinbach K, Bauer HW, Anton F. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neurosc Let. 1997;237:65. doi: 10.1016/s0304-3940(97)00810-0. [DOI] [PubMed] [Google Scholar]
- 15.Schultz P, Kirschbaum C, Pruessner J, Helhammer D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med. 1998;14:91. [Google Scholar]
- 16.Pruessner M, Helhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosom Med. 2003;65:92. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- 17.McNaughton Collins M, Meigis JB, Barry MJ, Walker Corkey E, Giovannucci E, Kawachi I. Prevalence and correlates of prostatitis in the health professionals follow-up study cohort. J Urol. 2002;167:1363. [PubMed] [Google Scholar]
- 18.Ullrich PM, Turner JA, Ciol M, Berger R. Stress is associated with subsequent pain and disability among men with nonbacterial prostatitis/pelvic pain. Ann Behav Med. 2005;30:112. doi: 10.1207/s15324796abm3002_3. [DOI] [PubMed] [Google Scholar]
- 19.Tripp DA, Nickel CJ, Wang Y, Litwin MS, McNaughton-Collins M, Landis JR, et al. Catastrophizing and pain-contingent rest as predictors of patient adjustment in men with chronic prostatitis/chronic pelvic pain syndrome. J Pain. 2006;7:697. doi: 10.1016/j.jpain.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]