Abstract
The FK506-binding proteins (FKBPs) are a unique group of chaperones found in a wide variety of organisms. They perform a number of cellular functions including protein folding, regulation of cytokines, transport of steroid receptor complexes, nucleic acid binding, histone assembly and modulation of apoptosis. Many of these functions are mediated by specific domains that adopt distinct tertiary conformations. Using the Threading/ASSEmbly /Refinement (TASSER) approach, tertiary structures were predicted for a total of 45 FKBPs in 23 species. These models were compared with previously characterized FKBP solution structures and the predicted structures were used to identify groups of homologous proteins. The resulting classification may be utilized to infer functional roles of newly discovered FKBPs. The three-dimensional conformations revealed that this family may have undergone several modifications throughout evolution, including loss of N- and C-terminal regions, duplication of FKBP domains as well as insertion of entire functional motifs. Docking simulations suggest that additional sequence segments outside FKBP domains may modulate the binding affinity of FKBPs to immunosuppressive drugs. The docking models also indicate the presence of a helix-loop-helix (HLH) region within a subset of FKBPs, which may be responsible for the interaction between this group of proteins and nucleic acids.
Introduction
Immunophilins are a diverse family of chaperones found throughout all known taxonomic groups. These proteins are also known as peptidyl-prolyl cis-trans isomerases (PPIases) for their ability to convert proline bonds from cis to trans form, a rate-limiting step in protein folding (Harding et al., 1989; Standaert et al., 1990; Galat, 1993; Kay, 1996; Schiene and Fisher, 2000; Davies and Sanchez, 2005). In addition, immunophilins can be divided into two subfamilies based on their ability to bind specific immunosuppressive drugs. Those that bind cyclosporin are known as cyclophilins while those that associate with FK506, FK1706, rapamycin and other derivatives are known as the FK506-binding proteins (FKBPs).
In humans, 15 FKBPs have been identified thus far (Rulten et al., 2006). The smallest and most comprehensively studied of these is FKBP1 (also known as FKBP12), which is 108 amino acids in length (12 kDa) and contains just one FKBP domain. Other FKBPs possess up to four FKBP domains along with several additional functional motifs, including nucleic acid binding domains (Riviere et al., 1993), tetratricopeptide repeat (TPR) motifs (Davies and Sanchez, 2005), Ef-hand (EfH) calcium-binding domains (Nakamura et al., 1998), as well as transmembrane (Rulten et al., 2006), nuclear localization (Jin et al., 1992; Riviere et al., 1993) and endoplasmic reticulum (ER) signal sequences (Jin et al., 1991).
FKBPs from numerous other organisms also possess variable numbers and types of domains, which enable this protein family to perform a wide variety of cellular functions in addition to protein folding. For example, in mammals, the FK506-FKBP1 complex binds to and inhibits calcineurin, a serine-threonine phosphatase and activator of NFAT (nuclear factors of activated T-cells) (Liu et al., 1991; Galat, 1993). The NFAT group of transcription factors regulates production of a number of T-cell specific activators, including cytokines IL-2 and IL-5, as well as cell surface receptors and signaling proteins (Rao et al., 1997; Li et al. 2002). FKBP1 also mediates intracellular calcium release through its interaction with the ryanodine receptors (RyR) in skeletal and cardiac muscle (Marks, 1996; Morita et al., 2006 and included references).
In addition, FKBP3 (FKBP25) from humans, FKBP46 from Spodoptera frugiperda (fall army worm), FKBP45 from Bombyx mori (silk moth), FKBP39 in Drosophila melanogaster, FKBP3 in Saccharomyces cerevisiae and others (Table 1) possess signals for nuclear localization as well as nucleic acid binding domains and may play a role in nucleosome assembly and/or pre-mRNA splicing (Riviere et al., 1993; Alnemri et al., 1994; Xiao et al., 2006; Somarelli, et al., 2007).
Table 1.
FKBPs used in TASSER predictions.
| Accession # a | Species b | Protein | Sequence identity c | Template d | C-score e |
|---|---|---|---|---|---|
| P54397 | Dm | FKBP39 | 30.3% (201/357) | 1fd9 | -0.737 |
| A | 19.2% (104/135) | 1xo9 | -2.058 | ||
| B | 29.4% (201/222) | 1fd9 | 0.730 | ||
| NP524895 | Dm | FKBP59 | 44.4% (351/439) | 1kt0 | 1.021 |
| P48375 | Dm | FKBP1 | 39.0% (105/108) | 1fd9 | 3.330 |
| AAF16717 | Ms | FKBP | 36.2% (105/108) | 1fd9 | 3.350 |
| AAY86706 | Bm | FKBP45 | 30.6% (193/402 ) | 1fd9 | -2.562 |
| A | 21.6% (190/205) | 1u00 | -3.190 | ||
| B | 30.7% (192/197) | 1fd9 | 1.175 | ||
| Q26486 | Sf | FKBP46 | 27.7% (191/412) | 1fd9 | -2.444 |
| A | 18.6% (188/218) | 1kt0 | -3.366 | ||
| B | 27.9% (190/194) | 1fd9 | 0.988 | ||
| EAL41402 | Ag | FKBP | 45.3% (349/396) | 1kt0 | 2.117 |
| EAA06906 | Ag | FKBP | 25.9% (263/318) | 1kt0 | 1.128 |
| EAA08436 | Ag | FKBP | 34.1% (132/137) | 1fd9 | 2.876 |
| EAA10152 | Ag | FKBP | 44.6% (130/211) | 1jvw | -0.665 |
| EAA44266 | Ag | FKBP | 24.5% (323/405) | 1kt0 | -0.017 |
| A | 14.9% (67/67) | 1e32 | -0.764 | ||
| B | 38.2% (55/55) | 2awg | 1.044 | ||
| C | 26.9% (208/283) | 1kt0 | -0.603 | ||
| EAA00094 | Ag | FKBP | 23.9% (272/325) | 1kt0 | 1.510 |
| EAA00155 | Ag | FKBP | 40.0% (105/108) | 1fd9 | 3.340 |
| XP972491 | Tc | FKBP39 | 30.4% (191/349) | 1fd9 | -2.084 |
| A | 19.0% (216/233) | 1kt0 | -3.777 | ||
| B | 37.2% (113/116) | 1fd9 | 3.075 | ||
| BAD90849 | Bm | FKBP59 | 47.2% (356/451) | 1kt0 | 1.055 |
| P62942 | Hs | FKBP1 | 36.2% (10 5/108) | 1fd9 | 3.282 |
| AAH91475 | Hs | FKBP2 | 30.5% (128/142) | 1fd9 | 2.664 |
| NP002004 | Hs | FKBP3 | 29.0% (200/224) | 1fd9 | 2.119 |
| Q02790 | Hs | FKBP4 | 60.3% (353/459) | 1kt0 | 0.747 |
| AAI11051 | Hs | FKBP5 | 98.0% (353/457) | 1kt0 | 0.821 |
| A | 28.5% (137/157) | 1fd9 | 1.711 | ||
| B | 99.1% (117/117) | 1kt0 | 1.281 | ||
| C | 28.1% (135/183) | 1ihg | 0.435 | ||
| O75344 | Hs | FKBP6 | 31.1% (254/327) | 1kt0 | 1.077 |
| Q9Y680 | Hs | FKBP7 | 32.3% (127/259) | 2f4e | -0.516 |
| NP036313 | Hs | FKBP8 | 24.8% (330/413) | 1kt0 | 0.024 |
| NP_0090210 | Hs | FKBP9 | 26.3% (346/570) | 1kt0 | -0.932 |
| 21.1% (261/269) | 1kt0 | 1.501 | |||
| 31.3% (115/119) | 1jvw | 2.032 | |||
| 36.0% (89/182) | 1a7x | -0.609 | |||
| NP068758 | Hs | FKBP10 | 27.1% (277/582) | 1kt0 | -2.058 |
| A | 30.0% (207/280) | 1kt0 | -0.349 | ||
| B | 25.8% (244/264) | 1kt0 | 0.820 | ||
| C | 21.1% (38/38) | 1kt0 | 0.588 | ||
| Q9NYL4 | Hs | FKBP11 | 32.5% (120/201) | 1fd9 | 0.590 |
| NP060416 | Hs | FKBP14 | 34.3% (143/211) | 1jvw | -0.155 |
| P38911 | Sc | FKBP | 23.9% (201/411) | 1fd9 | -2.503 |
| A | 17.6% (204/209) | 1fgg | -3.059 | ||
| B | 24.0% (200/202) | 1fd9 | 0.842 | ||
| Q6CWE8 | Kl | FKBP | 29.5% (193/418) | 1fd9 | -2.742 |
| A | 19.5% (215/223) | 1zjc | -1.960 | ||
| B | 29.0% (193/195) | 1fd9 | 0.861 | ||
| EAL64753 | Dd | FKBP | 32.5% (197/364) | 1fd9 | -1.398 |
| A | 25.2% (123/158) | 1xo9 | -2.275 | ||
| B | 32.7% (196/206) | 1fd9 | 0.775 | ||
| CAB81345 | At | FKBP | 29.5% (190/487) | 1fd9 | -2.418 |
| A | 20.3% (256/291) | 1kt0 | -3.205 | ||
| B | 29.6% (189/196) | 1fd9 | 1.148 | ||
| NP957178 | Dr | FKBP8 | 25.7% (342/406) | 1kt0 | 0.691 |
| A | 19.4% (103/103) | 1ign | -2.292 | ||
| B | 19.0% (105/115) | 1kt0 | 1.286 | ||
| C | 30.9% (136/1 88) | 1ihg | 0.387 | ||
| AAB05213 | Sm | FKBP | 36.3% (336/430) | 1kt0 | 0.380 |
| A | 30.1% (133/152) | 1fd9 | 1.375 | ||
| B | 21.7% (106/109) | 1kt0 | 0.044 | ||
| C | 33.1% (136/169) | 1kt0 | 0.266 | ||
| AAQ84562 | Mm | FKBP8 | 22.8% (337/402) | 1kt0 | -0.035 |
| A | 26.3% (95/103) | 1ign | -1.834 | ||
| B | 18.6% (97/114) | 1kt0 | 1.132 | ||
| C | 27.2% (136/185) | 1ihg | 0.127 | ||
| NP_001032257 | Rn | FKBP8 | 24.2% (335/403) | 1kt0 | 0.036 |
| A | 25.8% (97/104) | 1ign | -1.785 | ||
| B | 20.0% (95/114) | 1kt0 | 1.139 | ||
| C | 27.9% (136/185) | 1ihg | 0.304 | ||
| AAF08340 | Br.m | FKBP | 25.4% (142/164) | 1jvw | 2.441 |
| AAD01596 | Ov | FK BP | 35.8% (134/137) | 1jvw | 2.598 |
| AAD01595 | Br.m | FKBP | 35.7% (115/137) | 1fd9 | 2.493 |
| AAD01594 | Di | FKBP | 30.5% (131/137) | 1fd9 | 2.653 |
| CAA53594 | Bs | FKBP | 33.9% (115/134) | 1fd9 | 2.677 |
| AAB65470 | Ce | FKBP | 29.3% (188/261) | 1fd9 | -0.181 |
| A | 9.4% (53/56) | 1ugj | -0.204 | ||
| B | 28.9% (187/205) | 1fd9 | 1.068 | ||
| AAD01597 | Br.m | FKBP | 42.8% (353/426) | 1kt0 | 1.749 |
| NP417806 | Ec | FKBP | 37.1% (202/270) | 1fd9 | 1.169 |
| A64403 | Mj | FKBP | 44.7% (150/240) | 1ix5 | -0.241 |
| A | 45.0% (149/158) | 1ix5 | 1.628 | ||
| B | 20.7% (82/82) | 1ogc | -0.424 | ||
| XP713635 | Ca | FKBP | 25.4% (189/426) | 1fd9 | -2.579 |
| A | 17.0% (224/235) | 1p35 | -2.047 | ||
| B | 25.6% (180/191) | 1fd9 | 0.871 | ||
Genbank accession num bers for all FKBPs used in the TASSER predictions are listed. For proteins with C-scores below 0, the structure was divided into A, B and C domains, as described in e.
Species include: Dm (Drosophila melanogaster), Ms (Manduca sexta), Bm (Bombyx mori), Sf (Spodoptera frugiperda), Ag (Anopheles gambiae), Tc (Tribolium castaneum ), Hs (Homo sapiens), Sc (Saccharomyces cerevisiae), Kl (Kluyveromyces lactis), Dd (Dictyostelium discoideum), At (Arabidopsis thaliana), Dr (Danio rerio), Sm (Schistosoma mansoni), Mm (Mus musculus), Rn (Rattus norvegicus), Br.m (Brugia m alayi), Ov (Onchocerca volvulus), Di (Dirofilaria immitis), Bs (Botryllus schlosseri), Ce (Caenorhabditis elegans), Ec (Escherichia coli), Mj (Methanococcus jannaschii), Ca (Candida albicans).
Sequence identity is calculated between the top 1 template and the target sequence, listed here as a percentage. The coverage is indicated in parentheses as the number of residues from the top 1 template over the number of residues in each target sequence.
The PDB ID is listed for each template used in the predictions.
The C-score is the confidence score used to evaluate the certainty of the TASSER structure prediction.
C-score = ln (( M / R * Mtot) * Z ), where M represents the multiplicity of structures in a SPICKER cluster, Mtot is the total number of structures submitted for clustering and R is the average relative mean standard deviation (RMSD) of the structures relative to the cluster centroid. Predictions with low C-scores indicate that the foldable fraction is low and models become more unreliable.
Highlighted rows indicate that empirically determined structures are available in the PDB.
A different group of FKBPs containing TPR (tetratricopeptide repeat) domains are involved in transport of a number of high molecular weight complexes, including transient receptor potential-like (TRPL) proteins in insects (Goel et al., 2001) and high-affinity steroid receptor complexes in both plants and vertebrates (Kurek et al., 2002; Sinars et al., 2003). For example, FKBP4s and FKBP5s promote and inhibit the formation of glucocorticoid receptor (GR) and progesterone receptor (PR) complexes, respectively (Tranguch et al., 2005). FKBP8 (FKBP38) orthologues contain an N-terminal FKBP domain and a single TPR motif. These proteins regulate apoptosis via formation of an active Ca2+/calmodulin (CaM)/Bcl-2 complex (Edlich et al., 2005; Wang et al., 2005; Maestre-Martinez et al., 2006). Subsequent to binding Ca2+ and CaM, FKBP8 undergoes conformational changes, which enables the complex to interact with and sequester Bcl-2 from the mitochondria, thus promoting cell death (Maestre-Martinez et al., 2006).
In humans, FKBP2, 7, 9, 10, 11, and 14 possess variable numbers of FKBP domains and are associated with the endoplasmic reticulum (ER). Unlike the other ER localized proteins in this family, FKBP7, FKBP9 and FKBP10 contain Ca2+ binding Ef-H regions. In spite of these differences, it is likely that all of these proteins have similar functions as mediators of protein folding and secretion (Rulten et al., 2006).
Despite the rather large body of work surrounding the functional characterization of FKBPs in several organisms, relatively few tertiary structures exist for this protein family. Most of the crystallographic and/or NMR data regarding FKBPs on the Protein Data Bank (PDB) concerns FKBP1 and its interactions with various small molecules including the immunosuppressants FK506 (Wilson et al., 1995), rapamycin (Van Duyne et al., 1991; Wilson et al., 1995), FK1012 (Schultz and Clardy, 1998) as well as other ligands. Comparison of FKBP1 in association with 16 distinct ligands suggests that different conformational changes are involved with each complex (Wilson et al., 1995). Aside from FKBP1 (PDB ID: 1FKK), three-dimensional configurations are available for a limited number of other FKBPs, including one each from Methanococcus thermolithotrophicus (PDB ID: 1IX5), Escherichia coli (PDB ID: 1Q6U), and Caenorhabditis elegans (PDB ID: 1R9H), two from Arabidopsis thaliana (PDB ID: 2IF4 and 1U79), as well as for FKBP4 (PDB ID: 1N1A) and FKBP5 (PDB ID: 1KT0) from humans. Wu et al. (2004) found that differences in both the orientations and sequences of FKBP4 and FKBP5 may be responsible for the distinct cellular roles of these proteins. In addition, individual FKBP domains from FKBP3 (PDB ID: 1PBK) and FKBP8 (PDB ID: 2F2D and 2AWG) are also available.
Utilizing the protein tertiary structure prediction algorithm TASSER (Zhang et al., 2005), tertiary structures were predicted for 45 FKBPs in 23 species. Generating three-dimensional models for a comprehensive number of FKBPs provides information on the structure-function relationships as well as the different functional roles adopted by FKBPs throughout evolution. Some of the structures represent a first approximation of several uncharacterized domains within specific FKBPs. The predicted structures also revealed significant similarity at the level of tertiary conformations among groups of orthologous FKBPs, suggesting gene duplications and domain insertion events early in evolution. Docking simulations suggest that additional incorporation of domains may reduce the affinity of FKBP domains for FK506 and/or create novel active sites for drug binding, possibly by altering the steric hindrance surrounding the active site.
Materials and Methods
Selection of FKBP sequences for tertiary structure analyses
Amino acid sequences from a total of 45 FKBPs were obtained from the National Center for Bioinformatics (NCBI) database (http://www.ncbi.nlm.nih.gov/) and examined with the PROSPECTOR_3 algorithms, an iterative threading program that combines both close and distant sequence alignments as well as secondary structure predictions to create a subset of targets for use in subsequent identification of pair interactions (Skolnick et al., 2004). In order to understand the complexity of folding patterns within the entire FKBP family, sequences were chosen representing a range of sizes and domains found within these proteins. Genbank accession numbers for all sequences are listed in Table 1. In order to assess the accuracy of our predictions, three FKBP sequences with complete tertiary structures from empirically determined data were included (highlighted in gray in Table 1).
Prediction of FKBP tertiary structures
All FKBP folding patterns were predicted using the TASSER methodology as previously described (Zhang et al., 2005). Briefly, TASSER utilizes the PROSPECTOR_3 threading algorithm to identify empirically determined protein templates (Skolnick et al., 2004), followed by tertiary structure assembly using Monte Carlo sampling and clustering with the program SPICKER (Zhang and Skolnick, 2004). This data set was further refined by generating a set of consensus contacts for each of four threading iterations. Finally, sets of predicted side chain contacts, continuous local fragments and folding templates were constructed. The conditions and parameters for this group of programs were based on a template library consisting of 3,575 representative structures obtained from the PDB http://www.pdb.org and clustered into representative structural families.
Parallel hyperbolic Monte Carlo sampling (PHS) was utilized to position the continuous aligned protein segments and assemble a full protein model from the threading templates. A total of 40 replicas were generated by PHS and the 14 low-temperature replica trajectories were clustered by SPICKER, from which the five highest structural density clusters were selected (Zhang and Skolnick, 2004). The top model for each FKBP was used in all subsequent steps described below.
A confidence score (C-score) was also generated to evaluate the certainty of the TASSER predictions: C-score = ln (( M / R * Mtot) * Z ), where M represents the multiplicity of structures in a SPICKER cluster, Mtot is the total number of structures submitted for clustering and R is the average relative mean standard deviation (RMSD) of the structures relative to the cluster centroid (Table 1). Predictions with negative C-scores are unreliable, while those with positive C-scores are more likely to represent authentic native protein conformations (Zhang and Skolnick, 2004). For cases in which the C-score was below 0, the protein was divided into multiple segments (labeled A, B and C in Table 1) and TASSER was rerun for each domain. This generates greater reliability for specific domains within a protein and identifies regions of low certainty. All 45 predicted structures are available as supplementary .tar compressed file on the Proteins: Structure, Function and Bioinformatics website (Online Supplementary File 1).
Alignments and superposition of predicted structures
Conformational differences among FKBPs were compared by alignment and superposition using the Java-based STRAP program available at: http://www.charite.de/bioinf/strap/ (Gille and Frommel, 2001). Structure predictions were aligned and superimposed using ClustalW_3D and Superimpose3D_CE, respectively, within STRAP. FKBPs were divided into groups based on 1) their molecular weight, 2) the number and type of motifs within each protein, and 3) their predicted tertiary structures. Figures 2 and 3 were generated in PyMol.
Figure 2. FKBP tertiary structures.
Tertiary structures were predicted using the TASSER set of programs and assembled into groups of potential homologues. Models were rendered using PyMol. Figures 1A through 1I correspond to Homo sapiens FKBP1, FKBP2, FKBP3, FKBP4/5, FKBP6, FKBP7, FKBP8, FKBP9/10 and FKBP11/14, respectively. FKBPs from other organisms were placed into groups of orthologues based on structural similarity to one or more of the Homo sapiens FKBPs.
Figure 3. Superposition of Homo sapiens FKBP1 with insect homologues.
Putative orthologues were superimposed in a pairwise fashion using the STRAP program to assess the degree of structural similarity among groups of FKBPs. FKBP1 from Homo sapiens (red) is superimposed with FKBPs from (A) Anopheles gambiae (yellow) and (B) Manduca sexta (green). Although FKBPs exist in all known organisms, their cellular roles have not been identified in a number of taxonomic groups. Structural similarity suggests that functional parallelisms may exist in a variety of organisms and the superpositions enable us to hypothesize about the utility of the FKBPs in species for which no function has been characterized.
Docking simulations
A three dimensional model of FK506 was extracted from a solution structure of the FKBP1-FK506 complex (PDB ID: 1FKF). A three-dimensional model of a stem-loop RNA was obtained from a U1A-RNA complex (PDB ID: 1AUD). FK506 or stem-loop RNA was input as the ligand into the ZDOCK server (http://zdock.bu.edu/), with each FKBP structure input as the receptor. No residues were selected to either force into or block from the active site. Ligand/receptor complexes were generated using the Create Complexes Java script (http://zdock.bu.edu/cgi/help.cgi).
Prediction of molecular weight and pI
The theoretical molecular weight and isoelectric point (pI) were calculated for each FKBP sequence using the Compute pI/MW tool available on the Expert Protein Analysis System (ExPASy) proteomics server (http://ca.expasy.org/tools/pi_tool.html) (Bjellqvist et al., 1993; Gasteiger et al., 2005).
Results
Generation of three-dimensional models and comparison with crystal structures
The amino acid sequences of 45 FKBPs among 23 species representing the five taxonomic kingdoms, including Archaea, Bacteria, Fungi, Plantae and Animalia were selected from the NCBI protein database. Complete, empirically determined solution structures are available on the PDB for three of the 45 proteins, including one from Escherichia coli (PDB ID: 1Q6U), and two from Homo sapiens (FKBP1 with PDB ID: 1FKK and FKBP5 with PDB ID: 1KT0) (highlighted in gray in Table 1). These were utilized as comparative controls in the TASSER predictions. Solution structures are also available for N- and C-terminal domains of FKBP4 (PDB ID: 1Q1C and 1QZ2), as well as the FKBP domain of FKBP8 (PDB IDs: 2F2D and 2AWG) in humans.
To understand how well our best models fit the known folding properties of FKBPs, both empirically-determined and predicted FKBPs were superimposed using the Superimpose_CE algorithm within the Java-based STRAP software (Gille and Frommel, 2001). With few exceptions, the predicted structures were nearly identical to their empirically generated counterparts (Online Supplementary File 2). Furthermore, the predicted structure of FKBP5 from humans is more complete than the FKBP5 crystallographic structure of Sinars et al. (2003), with additional residues at the N- and C- termini (Met1-Val33 and Met413-Ala459, respectively). Similarly, the Escherichia coli FKBP model contains additional amino acids at the N- and C-termini when compared to its counterpart in the PDB (Met1-Ala39 and Lys253-Lys270, respectively). Pairwise structural alignments between TASSER generated models and complete FKBPs from the PDB further demonstrate the high degree of similarity between simulated and empirically determined conformations (Figure 1).
Figure 1. FKBP models closely represent previously determined crystal structures.
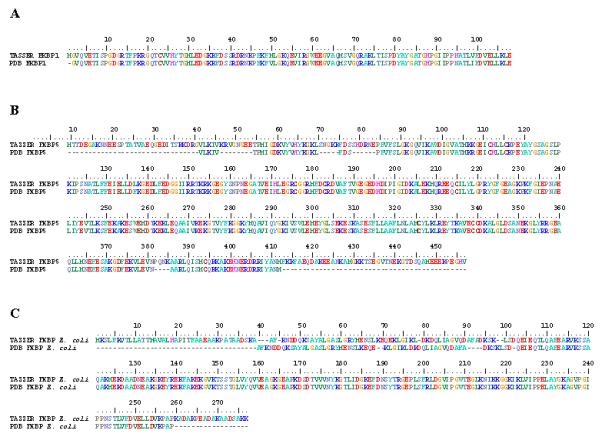
Pairwise structural alignments using ClustalW_3D between empirically generated protein structures and in silico derived models revealed that the TASSER set of programs creates complete protein representations that match closely the known crystal structures. TASSER models are on the top, with the PDB structures at the bottom of each pair. The number bar at the top indicates position of amino acids. A. TASSER generated model of FKBP1 from H. sapiens matches exactly to that of its corresponding conformation in the PDB (PDB ID: 1FKK), with the exception of a single Met in the PDB structure. B. Both N- and C-terminal regions of FKBP5 from H. sapiens are missing in the crystallograph (dashes) compared to the TASSER model. The structural alignment delineates 4 additional internal regions that are incomplete in the PDB conformation. C. Although the N- and C-termini of the E. coli FKBP from TASSER is more complete, the first 102 amino acids are shifted by 1 to 4 positions compared to the PDB structure.
Assignment of FKBPs into groups
Overall sequence identity between FKBP models and PDB templates ranges from 9.4% to 99.1%, with an average identity of 30.8%. Overall coverage of PDB templates to predicted FKBP targets ranges from 44.4% to 100% with an average of 81.8%. A total of 44% (20/45) of predicted structures had negative C-scores in one or more domains; yet, comparisons with empirically determined conformations and the assembly of FKBPs into groups of orthologues allowed inferences to be made regarding the overall structure and function of many of these proteins. The creation of these groups may provide information regarding the potential function of newly discovered FKBPs.
The 42 previously uncharacterized FKBPs were subdivided into six major groups, based on their size and structural characteristics (Table 2). Using the STRAP program, best models for members of each group were superimposed in a pairwise fashion in order to understand subtle differences within the tertiary conformations of FKBP orthologues. The first group of proteins consists of FKBP1 orthologues. These proteins are made up of a single FKBP domain. Figure 2A illustrates the human FKBP1 (PDB ID: 1FKK) tertiary structure.
Table 2.
FKBP orthologues grouped by structural and physico-chemical characteristics
| Accession Number | Protein | Species | FKBP domains |
HLH domains |
TPR domains |
EfH domains |
Predicted Size (kDa) |
Predicted pI |
|
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | P48375 | FKBP1 | Drosophila melanogaster | 1 | - | - | - | 11.7 | 7.85 |
| AAF16717 | FKBP | Manduca sexta | 1 | - | - | - | 11.8 | 7.86 | |
| EAA00155 | FKBP | Anopheles gambiae | 1 | - | - | - | 11.6 | 7.90 | |
| P62942 | FKBP1 | Homo sapiens | 1 | - | - | - | 12.0 | 7.89 | |
| Group 2 | A64403 | FKBP | Methanococcus jannaschii | 1 | - | - | - | 27.0 | 5.80 |
| AAD01596 | FKBP | Onchocerca volvulus | 1 | - | - | - | 15.4 | 5.46 | |
| AAD01595 | FKBP | Brugia malayi | 1 | - | - | - | 15.3 | 5.86 | |
| AAD01594 | FKBP | Dirofilaria immitis | 1 | - | - | - | 15.2 | 4.88 | |
| CAA53594 | FKBP | Botryllus schlosseri | 1 | - | - | - | 14.8 | 7.64 | |
| AAF08340 | FKBP | Brugia malayi | 1 | - | - | - | 18.0 | 5.92 | |
| EAA08436 | FKBP | Anopheles gambiae | 1 | - | - | - | 15.1 | 7.74 | |
| Q9NYL4 | FKBP11 | Homo sapiens | 1 | - | - | - | 22.2 | 9.44 | |
| NP_060416 | FKBP14 | Homo sapiens | 1 | - | - | - | 24.2 | 5.70 | |
| AAH91475 | FKBP2 | Homo sapiens | 1 | - | - | - | 15.6 | 9.00 | |
| Group 3 | NP_417806 | FKBP | Escherichia coli | 1 | 1 | - | - | 28.9 | 8.39 |
| EAL64753 | FKBP | Dictyostelium discoideum | 1 | 1 | - | - | 40.0 | 4.57 | |
| P38911 | FKBP | Saccharomyces cerevisiae | 1 | 1 | - | - | 46.6 | 4.36 | |
| Q6CWE8 | FKBP | Kluyveromyces lactis | 1 | 1 | - | - | 47.3 | 4.39 | |
| XP13635 | FKBP | Candida albicans | 1 | 1 | - | - | 47.7 | 4.33 | |
| CAB81345 | FKBP | Arabidopsis thaliana | 1 | 1 | - | - | 53.3 | 4.91 | |
| P54397 | FKBP39 | Drosophila melanogaster | 1 | 1 | - | - | 39.3 | 4.69 | |
| AAY86796 | FKBP45 | Bombyx mori | 1 | 1 | - | - | 44.7 | 4.75 | |
| Q26486 | FKBP46 | Spodoptera frugiperda | 1 | 1 | - | - | 45.8 | 4.68 | |
| XP972491 | FKBP39 | Tribolium castaneum | 1 | 1 | - | - | 38.9 | 4.79 | |
| NP_002004 | FKBP3 | Homo sapiens | 1 | 1 | - | - | 25.2 | 9.29 | |
| Group 4 | EAA10152 | FKBP | Anopheles gambiae | 1 | - | - | - | 23.3 | 4.64 |
| EAA 00094 | FKBP | Anopheles gambiae | 1 | - | 1 | - | 38.1 | 7.68 | |
| EAA44266 | FKBP | Anopheles gambiae | 1 | - | 1 | - | 44.7 | 5.01 | |
| EAA06906 | FKBP | Anopheles gambiae | 1 | - | 1 | - | 36.9 | 6.35 | |
| NP_957178 | FKBP8 | Danio rerio | 1 | - | 1 | - | 43.8 | 5.13 | |
| NP_001032257 | FKBP8 | Rattus norvegicus | 1 | - | 1 | - | 43.6 | 5.13 | |
| AAQ84562 | FKBP8 | Mus musculus | 1 | - | 1 | - | 43.5 | 5.08 | |
| NP_036313 | FKBP8 | Homo sapiens | 1 | - | 1 | - | 44.6 | 4.78 | |
| Q75344 | FKBP6 | Homo sapiens | 1 | - | - | - | 37.2 | 6.48 | |
| Group 5 | Q9Y680 | FKBP7 | Homo sapiens | 1 | - | - | 1 | 30.0 | 6.09 |
| EAL24461 | FKBP9 | Homo sapiens | 4 | - | - | 1 | 63.0 | 4.91 | |
| NP_068758 | FKBP10 | Homo sapiens | 4 | - | - | 1 | 64.3 | 5.36 | |
| Group 6 | AAB65370 | FKBP | Caenorhabditis elegans | 2 | - | - | - | 29.0 | 5.30 |
| AAD01597 | FKBP | Brugia malayi | 2 | - | 1 | - | 47.5 | 5.76 | |
| AAB05213 | FKBP | Schistosoma mansonii | 1 | - | 1 | - | 48.2 | 5.61 | |
| NP_5248 95 | FKBP59 | Drosophila melanogaster | 2 | - | 1 | - | 48.8 | 5.31 | |
| EAL41402 | FKBP | Anopheles gambiae | 2 | - | 1 | - | 43.9 | 6.15 | |
| BAD90849 | FKBP59 | Bombyx mori | 2 | - | 1 | - | 51.0 | 8.00 | |
| Q02790 | FKBP4 | Homo sapiens | 2 | - | 1 | - | 51.2 | 5.71 | |
| AAI11051 | FKBP5 | Homo sapiens | 2 | - | 1 | - | 51.8 | 5.35 | |
In comparison to FKBP1, proteins in the second group possess one FKBP domain, as well as an additional N-terminal alpha helix and extra C-terminal amino acids. This group of proteins includes orthologues to human FKBP2 (Figure 2B). The N-terminal helix contains a signal peptide responsible for membrane association while the additional C-terminal amino acids are essential for ER localization (Jin et al., 1991). Two FKBPs from Brugia malayi (GA#: AAD01595 and AAF08340) as well as FKBPs from Onchocerca volvulus (GA#: AAD01596), Dirofilaria immitis (GA#: AAD01594), Botryllus schlosseri (GA#: CAA53594) and Anopheles gambiae (GA#: EAA08436) closely fit the structure of FKBP2 (FKBP13) from humans. The archaeal FKBP from Methanococcus jannaschii also appears to be most closely related to this group despite several distinct differences. Particularly, the N-terminal signal sequence is missing and two loop structures protrude from the FKBP domain at Glu34-Tyr50 and Lys90-Glu142. The M. jannaschii FKBP contains two additional small C-terminal helices at Asp159-Lys240. An empirically determined structure from Methanococcus thermolithotrophicus reveals a similar pattern, with an insertion of 3 alternating β sheets and a single, small α-helix extending from the FKBP domain (Suzuki et al., 2003) (Online Supplementary File 1). This FKBP, however, does not contain the C-terminal helix motif found in the M. jannaschii FKBP. FKBP11 and FKBP14 from humans (Figure 2I) also possess this C-terminal helical structure and both are targeted for the ER (Rulten et al., 2006); however, the two loops within the M. jannaschii and M. thermolithotrophicus FKBP domains seem to have been lost in these two human orthologues as well as in all other members assigned to this group (Table 2). Alternatively, these sequences may represent lineage-specific acquired characteristics incorporated subsequent to the separation from other taxa. FKBP14 appears to be more similar than FKBP11 to the M. jannaschii FKBP, with an 11 amino acid C-terminal helix-loop region.
The FKBP3 (FKBP25) homologues comprise the third group, which contain just one C-terminal FKBP domain, a central helix-loop-helix (HLH) motif, which is thought to bind nucleic acid (Riviere et al., 1993; Alnemri et al., 1994; Somarelli, et al., 2007), and an N-terminal area of low structural complexity (Figure 2C). These proteins range in size from 224 to 487 amino acids and differ in their size and three-dimensional arrangement at the N-terminal low complexity region; however, the tertiary structure of both the central α-helix and the FKBP domain maintains a striking similarity among all proteins in this group. The smallest of these proteins, Homo sapiens FKBP3 and Escherichia coli FKBP, lack the low complexity N-terminal region.
The fourth group contains FKBP6 and FKBP8 orthologues, with a single C-terminal FKBP domain (Figure 2E). Although all of the members of this group possess a similar tertiary structure, the FKBP6s lack an N-terminal TRP motif (Table 1). The fifth group is distinguished by the presence of an EfH domain in the C-termini and one to four FKBP domains at the N-termini (Figure 2F and G). The final group identified includes human FKBP4 and 5 orthologues with two FKBP motifs and a single TPR region (Figure 2D). Another member of this group, FKBP3 from Caenorhabditis elegans, is missing the TPR domain, and may represent a protein whose sequence has been incompletely characterized or became truncated at some point throughout evolution. Additionally, based on sequence alignments, the Schistosoma mansonii FKBP in group four appears to have just one FKBP domain along with the TPR motif (data not shown); however, superposition of this protein model with other members of its group suggests that it maintains the same tertiary conformation as other FKBP4 or FKBP5 orthologues.
Discussion
Evaluation of TASSER generated models
Three of the 45 FKBPs reported here (highlighted in gray in Table 1) have been previously characterized by empirical techniques. To assess the accuracy of the TASSER predictions, the crystal structures of these FKBPs were compared to their corresponding TASSER models. Both structural alignments (Figure 1) and superpositions (Online Supplementary File 2) revealed significant overlap between between PDB structures and TASSER conformations. In addition, these models all have positive C-scores. Although it is possible that some of the predicted configurations, in particular those with negative C-scores, do not represent biologically realistic conformations, our comparisons suggest that the in silico derived models may closely represent the native folding characteristics of these proteins.
Assembly of FKBP structures into groups
Using the TASSER threading methodology, an assembly of 45 FKBP tertiary structure predictions was created. Although the interactions of several FKBPs have been well documented, the cellular role(s) of FKBPs in a number of organisms remains to be elucidated. By utilizing a combination of functionally characterized structures as well as several empirically determined three-dimensional conformations, we were able to place FKBPs of unknown configuration into groups of potential homologues (Table 1). This information should provide valuable insight into the structure-function relationships of this diverse group of proteins. Characteristics such as molecular weight, pI, and protein sequence can be highly variable within groups (Table 2). However, the structure predictions are strikingly similar among the members of each group. For example, small molecular weight FKBPs from Manduca sexta (GA#: AAF16717) and Anopheles gambiae (GA#: EAA00155) maintain nearly identical folding patterns when superimposed upon FKBP1 from humans (Figure 3). The highly conserved nature of FKBP1 orthologues throughout a wide range of taxonomic groups at both the sequence and higher structural levels suggests that these proteins play a critical role in cellular processes, possibly as chaperones (Somarelli and Herrera, 2007). In addition, it is likely that these proteins also mediate folding in Manduca sexta and Anopheles gambiae. The partitioning of these structures into groups with seemingly analogous functions may aid in assigning cellular roles to newly identified FKBPs as they emerge.
FKBP models suggest both gains and losses of motifs throughout evolution
Interestingly, FKBP1 and FKBP2 orthologues in organisms belonging to some of the older phylogenetic branches (e.g. Methanococcus jannaschii, Brugia malayi) possess additional N- and C-terminal regions of low complexity that younger lineages lack. The absence of these regions in more modern taxa may have resulted from relaxed selection pressure and evolutionary streamlining of the essential components of the protein over time, leading to the loss of these low complexity regions. In this scenario, FKBP11 and FKBP14 from Homo sapiens, which contain similar extensions of the N- and C-termini as those FKBPs found in more basal taxa may represent the retention of the ancestral condition.
The presence of several groups of FKBPs, including FKBP1, FKBP2, FKBP3 and FKBP4/5 in the more ancestral lineages indicates an ancient origin for the FKBP motif and suggests several independent duplication events early in evolution. In addition, the structural similarity between FKBPs from distantly related taxa supports the hypothesis that the FKBP region duplicated independently multiple times. In addition, insertion events of various types of domains within FKBP genes may have taken place in basal phylogenetic branches and have subsequently been conserved throughout evolution.
Docking simulations reveal variable drug-ligand conformations
Despite various single amino acid substitutions among members of the FKBP1 and FKBP2 groups, all of the FKBP1 and FKBP2 orthologues maintain the typical half β-barrel shape characteristic of FKBP domains (Van duyne et al., 1991; Maestre-Martinez et al., 2006). Docking simulations also suggest that many of the structural elements necessary for the interaction between FKBPs and FK506 remain intact among groups and members of FKBP groups in all organisms examined. Docking models accurately predict the active site for FK506 within FKBP1 in humans, although the docking results place FK506 further inside the binding pocket and rotated downward compared to the crystal structure (Figure 4). Minor variations in the orientation of the drug are also observed among members of the FKBP1 and FKBP2 groups, which may suggest that each protein has a slightly different affinity for FK506.
Figure 4. Docking simulations suggest that FKBP orthologues may have differential affinity for FK506.
FK506-FKBP interactions were modeled using the ZDOCK server and compared with previously determined FK506-FKBP solution structures. Docking simulations using Homo sapiens FKBP1 and FK506 correctly predicted the drug active site; however, ZDOCK showed FK506 bound more internally (A) within the active site when compared to the solution structure complex (B). These models revealed the potential for different orthologues to bind FK506 in slightly different orientations, which may reflect subtle differences in affinity among FKBPs.
It is known that human FKBP1, FKBP2 and FKBP3 demonstrate different binding capacities for FK506 (Kd = 0.4 nM, 55 nM and 160 nM, respectively) (Braun et al., 1995), with the Kd increasing in relation to the total size of the protein. Although there appear to be only slight structural differences among the active sites themselves, differential affinity of these proteins for FK506 may be attributable in part to the extra domains found within FKBP2 and FKBP3. Additional peptide segments within these proteins, such as those observed in the Methanococcus jannaschii FKBP2 appear to affect drug docking, placing the drug outside the previously established active site when compared to other FKBP2s from different species. As reflected in the docking predictions, external helices and other structures may also modulate the interactions through steric hindrance and repulsion near the active site. These additional sequences may also create novel, lower affinity drug binding sites.
TASSER models provide a first look at FKBP45 and its orthologues
FKBP45 (Group 3) from Bombyx mori is of particular interest due to its potential role in pre-mRNA splicing (Somarelli et al., 2007). This protein and most of its orthologues appear to be comprised of three major structural regions. Residues Met1-Leu90 corresponds to a tightly folded region of low complexity. Amino acids Asp91-Glu287 consist of alternating E-D and K-R rich repeats approximately 10 to 30 residues in length, which make up several looping α-helices containing nuclear localization signals (Somarelli et al., 2007). The remaining 114 residues, Lys288 to Lys402 correspond to the FKBP domain. The N-terminal region exhibits a unique folding pattern, however, the FKBP motif maintains the same conformation as the archetypal FKBP1 counterpart in humans. Although docking simulations place FK506 outside of the active site (indicated in brackets in Figure 5A) and along the folded alpha helices from Glu108-Lys139 (Figure 5A), the active site of the FKBP domain within FKBP45 in the silk moth is nearly identical to FKBP1 in humans. Several closely related counterparts of FKBP45 in other species bind FK506, including FKBP46 from Spodoptera frugiperda (Alnemri et al., 1994), FKBP3 in humans (Jin et al., 1992), and the FKBP45 in Saccharomyces cerevisiae (Manning-Krieg et al., 1994), suggesting that FKBP45 also possesses drug-binding ability. It is possible that this additional N-terminal helical region may affect the drug’s access to the predicted active site, and/or creates a novel drug-binding motif.
Figure 5. FKBP45 from Bombyx mori may possess an overlapping active site for both FK506 and RNA.
Identification of the potential active site for FK506 (A) and stem-loop RNA (B) within Bombyx mori FKBP45 indicates that the protein may associate with the drug and RNA in the same region. The characteristic FK506- and rapamycin-binding domain is outlined in white brackets. These simulations also suggest that additional domains surrounding the FKBP drug binding pocket may reduce the protein’s affinity for FK506 and/or create novel sites for drug interactions.
Docking experiments, as well as structural superpositions among FKBP45 orthologues also revealed an RNA-binding domain within FKBP45. Previous sequence comparisons between FKBP45 and RNA-binding domains suggested that the FKBP45 RNA-binding region may reside in the N-terminal 225 amino acids (Somarelli et al., 2007). Utilizing the tertiary structures of FKBP45 and an RNA stem-loop (PDB ID: 1AUD), docking simulations placed the active site for stem-loop RNA among four N-terminal helices, Asp96-Val105, Ala114-Lys135, Ala141-Asp152 and Lys245-Lys261 (Figure 5B). Moreover, RNA interacts with the same HLH region within all other members of this FKBP group, as expected from docking predictions. This region was previously predicted to be the DNA-binding region for FKBP3 (Riviere et al., 1993) and FKBP46 (Alnemri et al., 1994). As indicated by the docking simulations, the binding sites of FK506 and RNA overlap and therefore, they may compete for access to the FKBP motif. It is noteworthy that FKBP45 shares considerable structural similarity with the DNA binding region of human FKBP3, especially within regions that are thought to be of functional importance. Given the high degree of structural similarity between these two proteins, it is possible that FKBP3 is also capable of associating with U1 snRNA.
FKBP3s from different species possess N-terminal domains of low complexity capable of adopting different conformations. Although this may be the result of inaccurate folding predictions due to the lack of comparable structures in the PDB, it is also possible that these regions are not functionally necessary and are not subject to the same level of selection pressure as, for example, the PPIase and HLH domains, both of which have well defined functional roles and maintain consistent shapes through evolution (Riviere et al., 1993; Alnemri et al., 1994; Somarelli et al., 2007). The fact that species from early and recent lineages possess FKBP3 orthologues without the N-terminal low complexity domain suggests that gains and losses of this variable region do not strongly affect some functional aspect of this protein.
Analysis of multi-FKBP domain proteins
Models of the FKBP4 and FKBP5 members possess the same overall structural arrangement consisting of two FKBP domains and 3-4 pairs of helical TPR motifs. The FKBP domains are twisted approximately 90 degrees about a 10 residue loop. In general, the folding predictions are nearly identical to the crystal structures of FKBP4 and FKBP5 (Wu et al., 2004). Yet, unlike the crystal structures, where the TPR motif of FKBP5 is packed more closely to the FKBP domains than in FKBP4 (Wu et al., 2004), the TPR domains from the predicted FKBP4 and FKBP5 topologies can be superimposed completely. Wu et al. (2004) suggested that the differences in orientation of the TPR domain may be attributed to variations in crystal packing. It is possible that the flexibility in the TPR region contributes to the ability of these proteins to assemble into high molecular weight complexes through conformational changes that reduce steric hindrance between multiple protein aggregates (Stanley et al., 2007).
Based on sequence alignments, FKBP9 and FKBP10 from humans appear to contain four FKBP domains (data not shown); however, the predicted structure reveals only two N-terminal regions that maintain the half β-barrel conformation of the FKBP domain, while the more C-terminal FKBP motifs have lost this typical morphology (Figure 2H). It is possible that the TASSER models for these two proteins were improperly paired with templates. FKBP5 was used as the template for both of these proteins, and it may be that the C-terminal TPR domain within FKBP5 did not fit the target proteins in these regions. This may also be due to amino acid substitutions within the FKBP domain that have compromised its structure, although no significant differences exist among these domain sequences. Docking simulations using the ZDOCK server indicate that the immunosuppressive drug FK506 binds preferentially to the most C-terminal FKBP domain of both FKBP9 and FKBP10 (data not shown).
Conclusions
Functional interactions among biological molecules are tightly linked to their three-dimensional structures. The FKBPs represent a unique protein family consisting of several, multi-domain proteins with a variety of cellular roles. An understanding of structural relationships among these proteins is necessary to address questions involving their evolution and assortment of functions. Sequence and topological variations among these proteins resulting in different affinities for ligands may allow for modulation and diversity of function.
Supplementary Material
Acknowledgments
RJH acknowledges U.S. Public Health Service Grant SO6 GM08205 for support of this work. JAS acknoweldges EPA fellowship number 91670801-0. JS acknowledges NIH grants NO GM-37408 and GM-48835 for partial support of this research and SY Lee acknowledges the Korean Research Foundation Grant funded by Korea Government (MOEHRD, Basic Research Promotion Fund) (KRF-2005-214-C00146).
Reference
- 1.Alnemri ES, Fernandes-Alnemri T, Pomerenke K, Robertson NM, Dudley K, DuBois GC, Litwack G. FKBP46, a novel Sf9 insect cell nuclear immunophilin that forms a protein-kinase complex. J. Biol. Chem. 1994;269:30828–30834. [PubMed] [Google Scholar]
- 2.Bjellqvist B, Hughes GJ, Pasquali C, Paquet N, Ravier F, Sanchez J.-Ch., Frutiger S, Hochstrasser DF. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis. 1993;14:1023–1031. doi: 10.1002/elps.11501401163. [DOI] [PubMed] [Google Scholar]
- 3.Braun W, Kallen J, Mikol V, Walkinshaw MD, Wuthrich K. Three-dimensional structure and actions of immunosuppressants and their immunophilins. FASEB J. 1995;9:63–72. doi: 10.1096/fasebj.9.1.7529736. [DOI] [PubMed] [Google Scholar]
- 4.Bush KT, Hendrickson BA, Nigam SK. Induction of the FK506-binding protein, FKBP13, under conditions which misfold proteins in the endoplasmic reticulum. Biochem J. 1994;303:705–708. doi: 10.1042/bj3030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crackower MA, Kolas NK, Noguchi J, Sarao R, Kikuchi K, Kaneko H, Kobayashi E, Kawai Y, Kozieradzki I, Landers R, Mo R, Hui CC, Nieves E, Cohen PE, Osborne LR, Wada T, Kunieda T, Moens PB, Penninger JM. Essential role of Fkbp6 in male fertility and homologous chromosome pairing in meiosis. Science. 2003;300:1291–1295. doi: 10.1126/science.1083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edlich F, Weiwad M, Erdmann F, Fanghanel J, Jarczowski F, Rahfeld JU, Fischer G. Bcl-2 regulator FKBP38 is activated by Ca2+/calmodulin. EMBO J. 2005;24:2688–99. doi: 10.1038/sj.emboj.7600739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer G, Aumuller T. Regulation of peptide bond cis/trans isomerization by enzyme catalysis and its implication in physiological processes. ReV. Physiol. Biochem. Pharmacol. 2003;148:105–150. doi: 10.1007/s10254-003-0011-3. [DOI] [PubMed] [Google Scholar]
- 8.Galat A. Peptidylproline cis-trans isomerases: immunophilins. Eur. J. Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- 9.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. Protein Identification and Analysis Tools on the ExPASy Server. In: Walker John M., editor. The Proteomics Protocols Handbook. Humana Press; 2005. [Google Scholar]
- 10.Gille C, Frommel C. STRAP: editor for STRuctural Alignments of Proteins. Bioinformatics. 2001;17:377–378. doi: 10.1093/bioinformatics/17.4.377. [DOI] [PubMed] [Google Scholar]
- 11.Harding MW, Galat A, Uehling DE, Schrieber SL. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- 12.Hutchison KA, Scherrer LC, Czar MJ, Ning Y, Sanchez ER, Leach KL, Deibel MR, Pratt WB. FK506 binding to the 56-kilodalton immunophilin (hsp56) in the glucocorticoid receptor heterocomplex has no effect on receptor folding or function. Biochemistry. 1993;32:3953–3957. doi: 10.1021/bi00066a015. [DOI] [PubMed] [Google Scholar]
- 13.Jin YJ, Albers MW, Lanei WS, Beirer BE, Schreiber SL, Burakoff SJ. Molecular cloning of a membrane-associated human FK506- and rapamycin-binding protein, FKBP-13 (rotamase/T-cefl activation/mast cell) Immunology. 1991;88:6677–6681. doi: 10.1073/pnas.88.15.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin YJ, Burakoff SJ, Bierer BE. Molecular cloning of a 25-kDa high affinity rapamycin binding protein, FKBP25. J Biol Chem. 1992;267:10942–10945. [PubMed] [Google Scholar]
- 15.Kay JE. Structure-function relationships in the FK506-binding protein (FKBP) family of peptidylprolyl cis-trans isomerases. Biochem. J. 1996;314:361–385. [PMC free article] [PubMed] [Google Scholar]
- 16.Kurek I, Dulberger R, Azem A, Tzvi BB, Sudhakar D, Christou P, Breiman A. Deletion of the C-terminal 138 amino acids of the wheat FKBP73 abrogates calmodulin binding, dimerization and male fertility in transgenic rice. Plant. Mol. Biol. 2002;48:369–381. doi: 10.1023/a:1014023329807. [DOI] [PubMed] [Google Scholar]
- 17.Li TK, Baksh S, Cristillo AD, Bierer BE. Calcium- and FK506-independent interaction between the immunophilin FKBP51 and calcineurin. J. Cell. Biochem. 2002;84:460–471. doi: 10.1002/jcb.10026. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Farmer JD, Jr., Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 19.Manning-Krieg UC, Henriquez R, Cammas F, Graff P, GavCriaux S, Movva NR. Purification of FKBP-70, a novel immunophilin from Saccharomyces cerevisiae, and cloning of its structural gene, FPR3. FEBS Letters. 1994;352:98–103. doi: 10.1016/0014-5793(94)00927-9. [DOI] [PubMed] [Google Scholar]
- 20.Marks AR. Cellular functions of immunophillins. Physiol. Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 21.Morita K, Kitayama T, Kitayama S, Dohi T. Cyclic ADP-ribose requires FK506-binding protein to regulate intracellular Ca2+ dynamics and catecholamine release in acetylcholine-stimulated bovine adrenal chromaffin cells. Journal of Pharmacological Science. 2006;101:40–51. doi: 10.1254/jphs.fp0050991. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Yabe D, Kanazawa N, Tashiro K, Sasayama S, Honjo T. Molecular cloning, characterization, and chromosomal localization of FKBP23, a novel FK506-binding protein with Ca2+-binding ability. Genomics. 1998;54:89–98. doi: 10.1006/geno.1998.5571. [DOI] [PubMed] [Google Scholar]
- 23.Oubridge C, Ito N, Evans PR, Teo C-H, Nagai K. Crystal structure at 1.92 Å resolution of the RNA-binding domain of the U1A spliceosomal protein complexed with an RNA hairpin. Nature. 2002;372:432–438. doi: 10.1038/372432a0. [DOI] [PubMed] [Google Scholar]
- 24.Rao A, Luo C, Hogan PG. Transcription factors of the NF-AT family: regulation and function. Annu. Rev. Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 25.Renoir JM, Le Bihan S, Mercier-Bodard C, Gold A, Arjomandi M, Radanyi C, Baulieu EE. Effects of immunosuppressants FK506 and rapamycin on the heterooligomeric form of the progesterone receptor. J. Steroid Biochem. Mol. Biol. 1994;48:101–110. doi: 10.1016/0960-0760(94)90256-9. [DOI] [PubMed] [Google Scholar]
- 26.Riviere S, Menez A, Galat A. On the localization of FKBP25 in T-lymphocytes. FEBS Letters. 1993;315:247–251. doi: 10.1016/0014-5793(93)81173-w. [DOI] [PubMed] [Google Scholar]
- 27.Rulten SL, Kinloch RA, Tateossian H, Robinson C, Gettins L, Kay JE. The human FK506-binding proteins: characterization of human FKBP19. Mamm. Genome. 2006;17:322–331. doi: 10.1007/s00335-005-0127-7. [DOI] [PubMed] [Google Scholar]
- 28.Schiene C, Fisher G. Enzymes that catalyze the restructuring of proteins. Curr. Opin. Struct. Biol. 2000;10:40–45. doi: 10.1016/s0959-440x(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 29.Schultz LW, Clardy J. Chemical inducers of dimerization: the atomic structure of FKBP12-FK1012A-FKBP12. Bioorg.Med.Chem.Lett. 1998;8:1–6. doi: 10.1016/s0960-894x(97)10195-0. [DOI] [PubMed] [Google Scholar]
- 30.Skolnick J, Kihara D, Zhang Y. Development and large scale benchmark testing of the PROSPECTOR_3 threading algorithm. Proteins: Structure, Function and Bioinformatics. 2004;56:502–518. doi: 10.1002/prot.20106. [DOI] [PubMed] [Google Scholar]
- 31.Somarelli JA, Coll JC, Velandia A, Martinez L, Herrera RJ. Characterization of immunophilins in the silkmoth Bombyx mori. Archives of Insect Biochemistry and Physiology. 2007;65:195–209. doi: 10.1002/arch.20177. [DOI] [PubMed] [Google Scholar]
- 32.Somarelli JA, Herrera RJ. Evolution of the 12kDa FK506-binding protein gene. Biology of the Cell. 2007;99:311–321. doi: 10.1042/BC20060125. [DOI] [PubMed] [Google Scholar]
- 33.Standaert RF, Galat A, Verdine GL, Schreiber SL. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature. 1990;346:671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- 34.Stanley WA, Pursiainen NV, Garman EF, Juffer AH, Wilmanns M, Kursula P. A previously unobserved conformation for the human Pex5p receptor suggests roles for intrinsic flexibility and rigid domain motions in ligand binding. BMC Struct. Biol. 2007;7:1472–6807. doi: 10.1186/1472-6807-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki R, Nagata K, Yumoto F, Kawakami M, Nemoto N, Furutani M, Adachi K, Maruyama T, Tanokura M. Three-dimensional Solution Structure of an Archaeal FKBP with a Dual Function of Peptidyl Prolyl cis—trans Isomerase and Chaperone-like Activities. J. Mol. Biol. 2003;328:1149–1160. doi: 10.1016/s0022-2836(03)00379-6. [DOI] [PubMed] [Google Scholar]
- 36.Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA. 2005;102:14326–31. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. Atomic structure of FKBP-FK506, an immunophilin-immunosuppressant complex. Science. 1991;252:839–842. doi: 10.1126/science.1709302. [DOI] [PubMed] [Google Scholar]
- 38.Van Duyne GD, Standaert RF, Schreiber SL, Clardy J. Atomic Structure of the Rapamycin Human Immunophilin Fkbp-12 Complex. J.Am.Chem.Soc. 1991;113:7433–7434. [Google Scholar]
- 39.Wang HQ, Nakaya Y, Du Z, Yamane T, Shirane M, Kudo T, Takeda M, Takebayashi K, Noda Y, Nakayama KI, Nishimura M. Interaction of presenilins with FKBP38 promotes apoptosis by reducing mitochondrial Bcl-2. Hum. Mol. Genet. 2005;14:1889–1902. doi: 10.1093/hmg/ddi195. [DOI] [PubMed] [Google Scholar]
- 40.Weiwad M, Edlich F, Kilka S, Erdmann F, Jarczowski F, Dorn M, Moutty MC, Fischer G. Comparative Analysis of Calcineurin Inhibition by Complexes of Immunosuppressive Drugs with Human FK506 Binding Proteins. Biochemistry. 2006;45:15776–15784. doi: 10.1021/bi061616p. [DOI] [PubMed] [Google Scholar]
- 41.Wilson KP, Yamashita MM, Sintchak MD, Rotstein SH, Murcko MA, Boger J, Thomson JA, Fitzgibbon MJ, Black JR, Navia MA. Comparative X-ray structures of the major binding protein for the immunosuppressant FK506 (tacrolimus) in unliganded form and in complex with FK506 and rapamycin. Acta Cryst. 1995;D51:511–521. doi: 10.1107/S0907444994014514. [DOI] [PubMed] [Google Scholar]
- 42.Xiaoa H, Jackson V, Leia M. The FK506-binding protein, Fpr4, is an acidic histone chaperone. FEBS Letters. 2006;580:4357–4364. doi: 10.1016/j.febslet.2006.06.093. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Arakaki AK, Skolnick J. TASSER: an automated method for the prediction of protein tertiary structures in CASP6. Proteins: Structure, Function and Bioinformatics Suppl. 2005;7:91–98. doi: 10.1002/prot.20724. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Skolnick J. SPICKER: a clustering approach to identify near-native protein folds. J. Comput. Chem. 2004;25:865–871. doi: 10.1002/jcc.20011. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Skolnick J. Automated structure prediction of weakly homologous proteins on a genomic scale. Proc. Natl. Acad. Sci. USA. 2004;101:7594–7599. doi: 10.1073/pnas.0305695101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






