Abstract
Nearly ubiquitous infection, including central nervous system invasion, with human herpesvirus 6 occurs in childhood. There are two variants, HHV6A and HHV6B. Usually asymptomatic, it is associated with the common, self-limited childhood illness roseola infantum, and rarely with more severe syndromes. In patients with immune compromise, reactivation of viral activity may lead to severe limbic encephalitis. HHV6 has been identified as a possible etiologic agent in multiple sclerosis. A preponderance of evidence supports an association between HHV6 and febrile seizures. An ongoing multicenter study is investigating possible links between HHV6 infection, febrile status epilepticus, and development of mesial temporal sclerosis (MTS). Investigation of temporal lobectomy specimens showed evidence of active HHV6B but not HHV6A replication in hippocampal astrocytes in about two-thirds of patients with MTS, but not other causes of epilepsy. HHV6 has been detected in similar clinical syndromes by other investigators, although less frequently, and additional viruses have been detected as well. It has been suggested that HHV6B may cause ‘excitotoxicity’ by interfering with astrocyte excitatory amino acid transport. Although conventional inflammatory changes are not found in most MTS specimens, inflammatory modulators may play a role in neuronal injury leading to MTS as well. If the link between early viral infection and later development of intractable temporal lobe epilepsy is confirmed, possibly associated with febrile seizures, new therapeutic approaches to a common intractable epilepsy syndrome may be possible.
Introduction
Herpes viruses, such as herpes simplex, Varicella-zoster, human herpes virus type 6 (HHV6), cytomegalovirus (CMV) and Epstein–Barr virus (EBV), in addition to causing acute disease of the nervous system, may be capable of establishing latent infection and reactivating under a variety of stimuli (Eeg-Olofsson et al 2003).
Human herpes virus 6 (HHV6) a β-herpesvirus, is almost universally acquired early in childhood; by age three nearly 90% of children have evidence of exposure (Caserta et al 2001). A double-stranded DNA B-herpes virus, 160 to 162 kb in size, it has two variants: HHV6A and HHV6B (Ablashi et al 1991; De Bolle et al 2005). Some data suggest that HHV6A may be more virulent and neurotropic, while HHV6B is related to (generally benign) childhood infections (Dewhurst et al 1993). Some studies of acute encephalitis have distinguished between HHV6 subtypes. Subsequent PCR on samples from brain sections in a previously reported case of limbic encephalitis confirmed that the HHV6B can cause localized limbic encephalitis in immunosuppressed bone marrow transplant recipients (Wainwright et al 2001, Epstein and Wainwright, unpublished data). HHV-6A has also been shown to cause encephalitis in immunosuppressed patients. (McCullers et al 1995)
Interest in the role of HHV6B in the pathogenesis of seizures has increased with the recent reports of HHV6B detection in tissue of temporal lobectomy specimens resected from patients with intractable mesial temporal lobe epilepsy (Donati et al 2003, Fotheringham et al 2007) as well as its association with prolonged febrile seizures (Hall et al 1994, Dewhurst et al 1993, Epstein et al 2005).
Primary HHV6 infection
Several studies document that HHV6 is a common virus acquired in early childhood and one associated with a variety of childhood febrile illnesses. In a prospective study of initially healthy children, primary HHV-6 infection was detected by polymerase chain reaction in saliva in 130 of 277 children (77 percent of those at risk by 24 months (Zerr et al 2005). In this study, the saliva samples were used to ‘bracket’ the time of infection, rather than document it acutely. Ninety-three percent with a well-defined time of viral infection had symptoms, including fever, roseola, and diarrhea, but none in the prospective study had seizures. Approximately 10% percent of children with acute febrile illnesses had primary HHV-6 infection, documented by viremia and seroconversion (Hall et al 1994, Byington et al 2003).
Disorders associated with primary HHV6 infection include roseola infantum or exanthem subitum, and meningoencephalitis (Yamanishi et al 1988, Yoshikawa et al 2000). CNS invasion appears to be common at the time of primary infection. In contrast to the prospective study of previously healthy children, HHV6B was the cause of one third of all febrile convulsions in children under the age of two presenting to an emergency department with a febrile illness (Hall et al 1994). HHV6B neonatal encephalitis with seizures in an apparently healthy child has been reported (Zerr et al 2002, Lanari et al 2003). This may have been due to congenital or intrauterine infection, as reactivation may occur during pregnancy, HHV 6 DNA has been detected in cord blood specimens of apparently healthy newborns and in fetuses following spontaneous abortions, as well as in cervical swabs from pregnant women (Hall et al 2004; Yamashita and Morishima, 2005). However, DNA PCR found HHV-6 in only 3 of 245 CSF samples from children <2 months of age with possible sepsis or neurologic symptoms (Ansari et al 2004). Immunocompetent adults rarely are affected by clinical disease (Birnbaum et al 2005, Issacson et al 2005). HHV-6 DNA was found on PCR in 6% of immunocompetent patients with focal encephalitis of unknown etiology although this does not prove the etiology (McCullers et al 1995, Whitley and Lakeman 2005).
Establishment of Latency
All herpesviruses (zoster is the best known) can establish lifelong latent infection, with viral genome integrated into host cell DNA (Luppi et al 1993). About 40% of children may have persistent detection of HHV6 DNA in CSF after primary infection (Caserta et al 1993). Both neuronal and glial cells may be infected by HHV6, but a particular predilection for astrocytes has been reported (Lusso et al 1998, Albright et al 1998, He et al 1996, Knox et al 2000, Challoner et al 1995). HHV-6B was particularly prominent in hippocampal astrocytes of patients with limbic encephalitis after stem cell or bone marrow transplants (Tsujimura et al 1998 Wainwright et al 2001).
Acute disease due to reactivation in immunosuppressed patients
HHV6 virus may become reactivated, usually in the setting of immune compromise. Reactivation may result in encephalitis that has a predilection for limbic structures. Adult disease due to HHV6B is almost certainly always due to reactivation. Acute encephalitis and meningoencephalitis have been reported due to viral reactivation in immunosuppressed patients, particularly after allogeneic hematopoietic stem cell transplantation (Singh et al 2000, Wainwright et al 2001; Gorniack et al 2006, Seeley et al 2007). Some patients have had fulminant courses resulting in death, while others experienced more indolent disease, with varying degrees of recovery. It is particularly interesting for the potential role of HHV6B in mesial temporal sclerosis (MTS) that many of the patients had increased MRI T2 signal intensity in limbic structures such as hippocampus and amygdala, followed in some cases by focal atrophy (Wainwright et al 2001; Gorniack et al 2006, Seeley et al 2007) (figure 1). CSF was positive for HHV6 in a 16 year old with acute lymphoblastic leukemia in remission who developed Mollaret's Meningitis. Foscarnet treatment led to clinical improvement and clearance of CSF (Capouya et al 2006). Increased bilateral hippocampal signal may occur during acute disease, and sequelae include hippocampal atrophy and cognitive impairment (Gorniack et al 2006). In one case, treatment with acyclovir in a patient who presented with memory loss one month after bone marrow transplantation led to clearance of previously detected HHV-6 DNA in both cerebrospinal fluid and serum, and improvement of MRI lesions and cognitive symptoms (MacLean and Douen 2002).
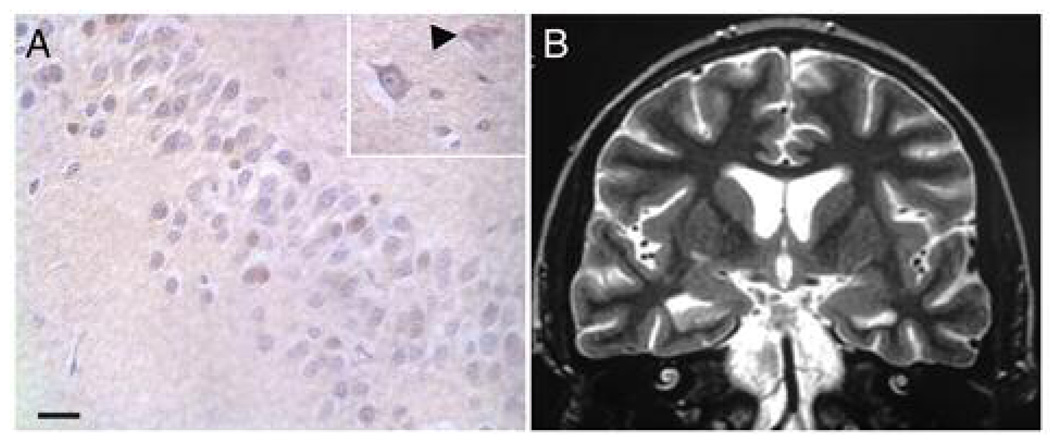
Figure 1.
HHV6 immunohistochemistry (A) and fast spin echo T2-weighted coronal magnetic resonance image (B) from a patient with limbic encephalitis following stem cell transplantation. Images are from patient 4 in the published series of such patients (Wainwright et al., 2001). (A) Hematoxylin-eosin counterstaining from autopsied temporal lobe sections shows brown immunoreactivity for HHV6 P41/P101 proteins in astrocytes and neurons. Within the hippocampal neuronal layer numerous HHV6 immunoreactive neurons were present. In some of the labeled neurons immunoreactivity was most prominent in cytoplasm (inset). Abundant HHV6-immunoreactive astrocytes were also present (inset, arrowhead). (B) Coronal image of hippocampi heads on day 43 following stem cell transplant demonstrates right hippocampal volume loss and increased T2 signal intensity after clinical onset of symptoms on day 24. (A) Bar = 20 µm.
HHV6 reactivation, documented by significant increases in serum antibody titers, and in some cases PCR on peripheral blood, has been has been reported in association with hypersensitivity syndromes in immunocompetent patients (Descamps et al 2001, Aihara et al 2003). Other herpes viruses such as HHV7 may be involved as well, although, based on limited data, less frequently (Seishima et al 2006).
HHV6 and Multiple Sclerosis
The possible association of HHV6 infection with multiple sclerosis (MS) illustrates some of the problems in confirming a pathogenic role in neurologic disease for a ubiquitous virus. Neuroglia, oligodendrocytes and inflammatory cells containing the HHV-6 genome have been found in biopsy specimens from MS patients presenting with acute disease, who had not received immunomodulatory therapy (Goodman et al., 2003). Although HHV-6 DNA was amplified from brains of both MS patients and controls, HHV-6 DNA and mRNA was detected at a significantly higher frequency in MS plaques compared to brain tissue from non-MS neurologic disorders, non-MS inflammatory and normal appearing white matter (NAWM) from MS brains (Cermelli et al., 2003 Opsahl and Kennedy, 2005). However, since HHV6 DNA can be found in normal brain, its presence alone may have no pathological significance (Chan et al 1999, Cuomo et al 2001). The frequency of HHV6 sequences detected in brain varies depending on the methodologies used (chan et al 2001, Cermelli et al 2003).
A significant increase in IgM response to the HHV-6 p41/38 early antigen, suggesting active infection, was demonstrated in patients with relapsing remitting MS (Soldan et al., 1997). Viral sequences in cell free compartments, considered a measure of productive virus, have been found in serum of approximately 23% of MS patients and 0% of controls ( Soldan et al., 1997, Akhyani et al., 2000, Berti et al., 2002). Serum HHV-6 DNA presence may correlate with clinical worsening in some patients (Berti et al., 2002). In contrast, sequences from other herpesviruses (HSV, VZV, EBV, CMV, HHV-7, HHV-8) were not found in increased frequency (Alvarez-Lafuente et al., 2002a, Alvarez-Lafuente et al., 2002b)‥ A significantly higher percentage of MS patients had proliferative responses to HHV-6A (66%) than healthy controls (33%) (Soldan et al., 2000) (Cirone et al., 2002) (Tejada-Simon et al., 2002) (Tejada-Simon et al., 2003). HHV-6 A variant amplification occurs in MS but not control PBMC, serum, and urine (Akhyani et al., 2000, Kim et al., 2000).
None of these studies can prove that HHV6 is an etiologic agent. In MS the virus may be present merely as a fellow traveler whose expression is due to, rather than causes, disease activity. Similar doubts might be raised to the putative association with epilepsy, but some of the available TLE data may answer this objection.
Potential role in epilepsy
Febrile seizures
Several studies implicate HHV6 as a common (but not the only) etiologic agent in febrile seizures. HHV6B and HHV7 primary infection coincides with the peak incidence of febrile seizures, (Barone 1995, Hall 1994, Caserta 1998).
In contrast to the prospective study of Hall and associates (1994), other studies in children with febrile seizures have not shown consistently a higher incidence of seroconversion to HHV6 than to other viruses. Reported detection of HHV6 in children with febrile seizures is very variable, ranging from 8–40% (Millichap and Millichap 2006). Evolving methods of viral detection, as well as geographic variation, may affect these results. It is important to note that HHV6B and HHV7 can cause reactivation of each other. In most cases, later infection with HHV7 reactivates HHV6. Unless the methods for detection include evidence of viral replication using culture or PCR combined with the detection of viral specific antibodies, viremia due to reactivated HHV6 infection cannot be distinguished from primary HHV6 infection. This requirement further complicates the interpretation of many published studies {Caserta et al 2004, Hall et al 2006}
It is interesting that HHV6 has been detected more commonly in the West than in Asia. Higher overall rates for febrile seizures are reported from Japan, however, so the influence of HHV6 might be diluted. Clearly, several factors may be at play in febrile seizures, as well as epilepsy and MTS, including other infections, genetic susceptibility and inflammatory mediators.
The majority of illnesses associated with febrile seizures are of presumed viral origin, and HHV6, though potentially a common cause, is certainly not the only one (Berg et al Epilepsia 1995). HHV7, for example, may play a role as well (Shinnar 2003). It is possible, moreover, that varying viral etiology may influence febrile seizure severity and duration, including the extent of hippocampal injury.
HHV6 DNA has been found in the cerebrospinal fluid of children with roseola and recurrent seizures or additional signs of meningoencephalitis (Yoshikawa et al 1992, Suga et al 1993). CSF tends to be acellular in children with febrile seizures, whether associated with HHV6 infections or not (Frank et al 2006). As HHV-6 is a cell based virus and the appearance of HHV6 in the CSF can be transient, it is difficult to determine purely from CSF specimens whether CNS invasion has occurred or not (Mannonen et al 2007)
Febrile Status Epilepticus (FSE)
Several reports, moreover, suggest that HHV6 and HHV7 infection may be associated with prolonged febrile seizures and status epilepticus (Suga 2000, Ward 2005) . The consequences of febrile status epilepticus (FSE) lasting more than 30 minutes is the subject of a is a multi-site prospective study (FEBSTAT) that will enroll 200 children ages 1 month to 5 years. Interval data analysis from the FEBSTAT study indicates that primary HHV-6B may be an important cause of FSE. Primary infection with HHV6, indicated by HHV6 DNA and RNA (amplified across a spliced junction) at time 0, was found in 21/64 subjects (32.8%) at the time of presentation with FSE (Epstein et al 2005, 2006). There was no associated CSF pleocytosis (Frank et al 2006). These data suggest that HHV6 is a common, but not the only, cause of FSE. Follow up will determine whether FSE caused by HHV-6 is associated with an increased risk of both hippocampal injury and subsequent temporal lobe seizures. Given the known long latency of 8–11 years between the episode of FSE and the onset of TLE (French et al 1993, Mathern et al 1995), it will be some time before we have a definitive answer.
If HHV6B infection associated with febrile SE is associated with an increased risk of subsequent MTS and TLE, it can lead to potentially novel therapeutic targets to prevent epileptogenesis. At this point some caution is required in interpreting the results. HHV6B appears to be the most common cause of febrile SE. However it is still unclear at this time whether this is because, in addition to being a common cause of fever in young infants, the virus also is neuroinvasive which would be expected to result in a higher rate of sequelae including MTS and TLE. Alternatively, HHV6B may simply be a common cause of fever in young infants in the susceptible age group for febrile seizures.
Temporal lobe Epilepsy
Several studies based on tissue removed at surgery for refractory MTLE suggest a potential pathophysiological role of HHV6.
Six patients in a group of 17 with TLE were positive for HHV6 by PCR, and one for herpes simplex virus (Uesugi et al 2000). All the HHV6 positive patients had 'anterior hippocampal sclerosis.' Three of the six HHV6 positive patients had a history of encephalitis that had been attributed to measles in one case. Histopathologically, 'meningeal' inflammatory findings were apparent in only one of the six patients. In addition, one patient who had been diagnosed with measles encephalitis showed positive results for HHV-6 and negative results for measles. It may be possible that latent HHV6 reactivated after primary measles infection.
Eeg-Olofsson et al (2004) used nested PCR in 19 Canadian and 17 Swedish patients operated for intractable epilepsy. HHV6 was found in 4 of 23 patients with 'gliosis.' HHV6 and Cytomegalovirus (CMV) DNA each were found in two of three patients with Rasmussen’s encephalitis. Epstein–Barr virus (EBV) was detected in four of six children with ganglioglioma. CMV was more likely to be found in the Canadian patients (median age 22), and EBV in the Swedish group (median age 14) who were also more likely to have malformations of cortical development. There was no clear association with febrile seizures. Seven of twelve 'reference' patients with a variety of diagnoses were positive (7 for HSV-1, 3 for HHV6, one for EBV).
A subsequent study used virus specific real-time TaqMan PCR assay, Western blot analysis and in-situ immunohistochemistry to detect and characterize HHV6 DNA in a series of epilepsy surgery specimens (Donati et al 2003, Fotheringham et al 2007). HHV6 reactive cells were analyzed for expression of glial fibrillary acidic protein (GFAP) by double immunofluorescence. HHV6B, but not HHV6A, was detected in 15 of 24 patients with mesial temporal sclerosis/ MTLE, in contrast to none of 14 with other localization-related epilepsy syndromes and pathological substrates, including tumors and malformations. All brain regions that tested positive by HHV-6B variant-specific TaqMan PCR were positive for viral DNA by nested PCR. The highest levels of HHV6 were found in hippocampal sections. HHV6 was co-localized to astrocytes, identified by GFAP reactivity and morphology. None of the HHV6B positive patients had conventional evidence for inflammation in their resected temporal lobe specimens. In these data, there was a non-significant trend for patients with a history of febrile seizures to be more likely to be HHV6B positive. They also tended to be more likely to be seizure-free one year after surgery. HHV6B negative patients showed trends toward earlier onset age, longer epilepsy duration, and smaller hippocampi ipsilateral to the seizure focus. These differences support the concept of multiple etiologies for MTLE with MTS.
HHV6, HSV1 and HHV8 were detected in the hippocampus of 3, 2 and 1 patients respectively, in a cohort of 33 patients with MTLE, using real-time PCR (Karatas et al 2007). None of 7 control autopsy specimens were positive for virus. All 3 HHV6 positive patients had both a family history for epilepsy, and a history of febrile seizures.
Some studies have suggested that 'late-onset' TLE in particular may be preceded by 'limbic encephalitis,' although no specific viral etiology has been found in these cases (Bien et al 2007) MRI at seizure onset may show hippocampal swelling, followed by atrophy with FLAIR/T2 signal increase. Bilateral abnormalities may be more frequent in these patients than in those with other TLE etiologies. T cell infiltration and loss of hippocampal neurons was found at onset in one patient (Bien et al 2007).
HHV6 and Potential 'Excitotoxicity'
A wide range of experimental and clinical data suggests that seizures lead to neuronal injury, and that MTS / MTLE is a progressive syndrome (Theodore and Gaillard 2002, Sutula et al 2003). This process may be related to glutamatergic excitotoxicity. Microdialysis studies showed ictal release of glutamate to a greater degree ipsilateral than contralateral to temporal lobe foci (During and Spencer 1993). Higher glutamate levels in sclerotic hippocampus compared with non-sclerotic hippocampus were shown on MRS as well (Petroff et al 2002). TLE patients show higher than baseline levels of extracellular glutamate; levels increase during seizures in humans as well as experimental animals (Meurs et al 2007). These mechanisms may contribute epileptogenesis as well. Seizures and hippocampal injury can induce mossy fiber sprouting that contributes to the emergence of recurrent limbic excitation and epilepsy (Sutula and Dudek 2007).
Disease caused by an environmental toxin supports the hypothesis of excitotoxic injury. Domoic acid, a glutamate analogue produced by Pseudonitzschia australis, caused status epilepticus and hippocampal damage in sea lions and humans (Cendes et al 1995; Silvagni et al 2005). There was evidence for memory loss in both (loss of directional sense in surviving sea lions) and subsequent TLE at least in the human.
HHV6B infection of astrocytes may be of pathophysiological significance in TLE (figure 2). Astrocyte cultures infected in vitro with HHV-6 had a marked decrease in glutamate transporter EAAT2 expression, suggesting a possible mechanisms for viral-induced hippocampal injury (Fotheringham et al 2007). Recent studies lend additional support for an important role of astrocyte dysfunction in epilepsy (Tian et al 2005). On DNA microarray analysis, sclerotic hippocampi from patients with TLE had changes in molecular signaling pathways, including increased expression of genes associated with astrocyte structure and inflammatory responses, possibly indicating molecular pathways that could lead to enhanced glutamate release (Lee et al 2007).
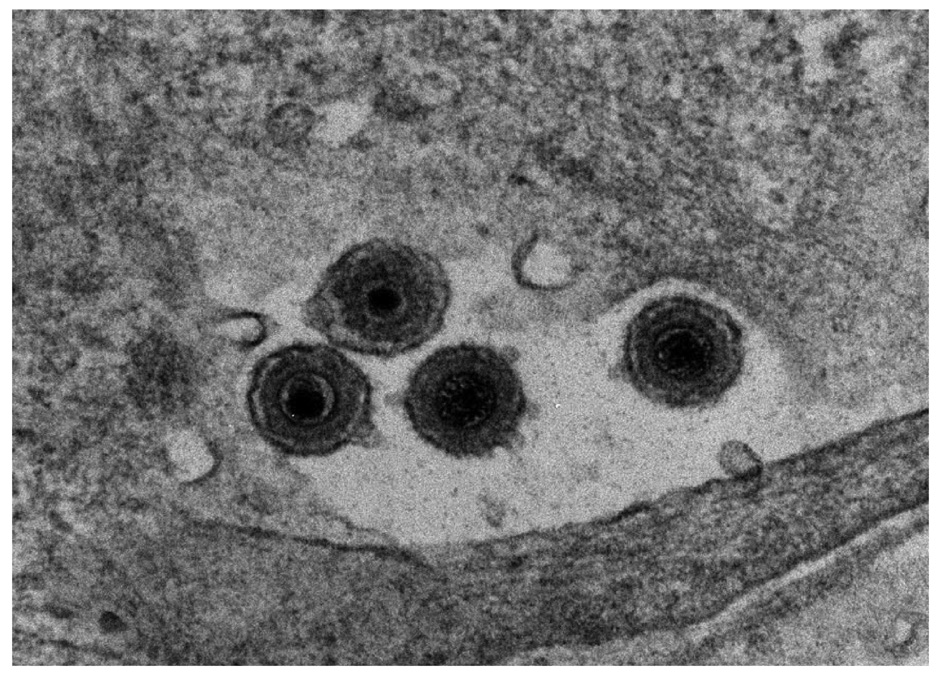
Figure 2.
Electron micrograph of HHV-6 virus particles from human progenitor derived astrocytes infected for 7 days. We thank Jenny Ahlqvist and the NINDS EM facility
The role of inflammation
Other evidence implicates immune factors and response to infection as an important mediator in the pathogenesis of MTLE Most patients with TLE and mesial temporal sclerosis, including those reported to have HHV6B reactivation, show little or no evidence for traditional inflammatory changes on pathological examination of resected tissue (Uesugi et al 2000, Donati et al 2003, Fotheringham et al 2007). However, some studies have shown increased levels of cytokines, interleukins, and other inflammatory mediators in epilepsy (Vezzani and Granata 2005). Astrocytoma cell lines infected by HHV6 show increased cytokine synthesis (Yoshikawa et al 2002), and increased CSF cytokines have been reported in febrile seizures (Virta et al 2002). In focal cortical dysplasia, IL-1β expression was increased, and the number of IL1β and signaling receptor IL1RI-positive neurons positively correlated with seizure frequency (Ravizza et al 2006).
Both human and animal data suggest that pro-inflammatory cytokines are upregulated after seizures, and may themselves contribute to increased cortical excitability (Vezzani and Granata 2005). IL1 and TNFα receptors upregulated in neurons during seizures, may mediate these effects, possibly by inhibiting astrocyte glutamate reuptake or increasing release, and inhibiting GABAergic transmission (Vezzani and Granata 2005).
A rat model of self-sustaining epileptogenesis and resected hippocampal specimens from TLE patients both showed glial and neuronal activation of the IL-1B system (Ravizza et al. 2007), further supporting the potential contribution of neuroinflammation to the mechanisms of epileptogenesis. Notably, this study did not identify cells of adaptive immunity in the brains of either rat or human epileptic tissue. This observation differentiates the brain inflammatory response in the mechanisms of TLE from inflammation in Rasmussen’s encephalitis in which the adaptive immune response is robust.
Human pathologic, in vitro, and in vivo studies have implicated a glia-mediated neuroinflammatory response both in the pathophysiology of Alzheimer’s disease (Mrak and Griffin 2005) and as treatment target (Hu et al. 2007). Microglial activation leading to overexpression of IL-1 has been proposed as the pivotal step in initiating a self-propagating cytokine cycle culminating in neurodegeneration (Mrak and Griffin 2005).
Multiple lines of evidence that glial activation may be a common pathophysiologic mechanism and therapeutic target in diverse forms of neurologic injury (Akiyama et al. 2000; Emsley et al. 2005; Hu et al. 2005; Perry et al. 2007) including epilepsy (Somera-Molina et al. 2007; Vezzani and Baram 2007). The potential of neuroinflammation as a therapeutic target in epilepsy is relatively understudied and is directly relevant to the potential role of HHV6 in the pathogenesis of TLE
These mechanisms may be important as well for Rasmussen's encephalitis, a progressive childhood-onset disorder of unknown etiology, characterized pathologically by T-cell-inflammatory T-cell infiltrates microglial activation, and neuronal loss. The syndrome has been associated with a variety of viruses, and possible antiglutamate receptor antibodies (Bien et al 2005). Recent data suggest that cytotoxic T lymphocytes are responsible for astrocytic apoptotic cell loss (Bauer et al 2007). In contrast to Rasmussen’s however, MTLE is not associated with marked inflammatory pathology.
Conclusions
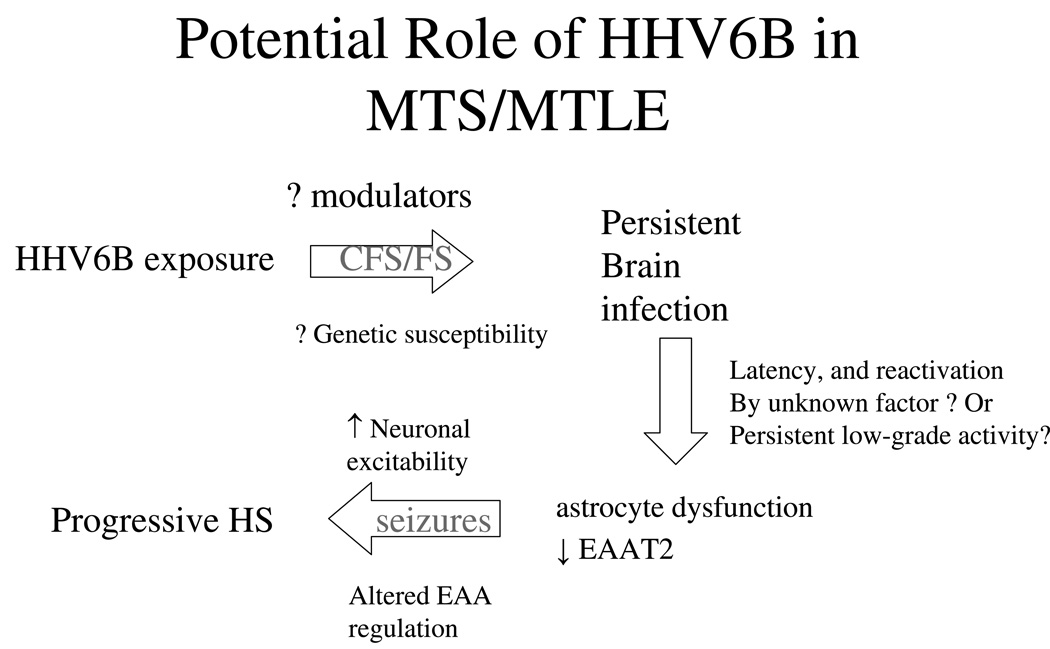
The evidence we have reviewed suggests that HHV6 may play an important pathogenic role in the etiology of MTLE in a substantial portion of patients. In some patients this may result from a reactivation of virus; in others, chronic persistent infection. This model accounts for several observations in MTLE, including early age of acquired risk, a latent period before establishment of habitual seizures, chronic progressive brain injury, and possibly glutamatergic excitatory neurotoxicity (figure 3).
Figure 3.
Possible pathway for association of HHV6B (or other virus) with mesial temporal lobe epilepsy with mesial temporal sclerosis.
Support for a potential etiologic role in MTS and MTLE is strengthened by failure to find virus in temporal tissue from patients with other seizure etiologies, and the fact that HHV6B was present only in a proportion of patients. One possible model would involve primary infection, either asymptomatic or marked by febrile seizures, followed, in some patients, by latency and eventual reactivation. It is possible that HHV6B reactivation could be the result, rather than the cause of intractable seizures. However, the absence of virus in hippocampal tissue from patients with other seizure etiologies but not MTS may suggest that uncontrolled epilepsy itself is not sufficient for viral reactivation despite nearly ubiquitous population exposure. Alternatively, some patients might have continual viral activity after initial infection.
Viral infection of astrocytes may lead to impaired excitatory amino acid reuptake after neuronal release. Increased EAA levels may be associated with the hippocampal atrophy and neuronal loss seen on MRI and pathological examination, as well as the metabolic dysfunction documented by positron emission tomography and magnetic resonance spectroscopy. Increased levels of inflammatory mediators, including cytokines and interleukins, may contribute to this process. If confirmed, the role of HHV6B suggests that specific antiviral interventions or anti-inflammatory medications may be possible, with the goal of impeding epileptogenesis or of developing vaccines that may prevent MTLE.
Acknowledgments
Supported by the NINDS Division of Intramural Research, and NINDS NS 43209 (the FEBSTAT study).
References
- Ablashi DV, Balachandran N, Josephs SF, Hung CL, Krueger GR, Kramarsky B, et al. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology. 1991;184:545–552. doi: 10.1016/0042-6822(91)90424-a. [DOI] [PubMed] [Google Scholar]
- Aihara Y, Ito S-I, Kobayashi Y, Yamakawa Y, M.Aihara M, Yokota S. Carbamazepine-induced hypersensitivity syndrome associated with transient hypogammaglobulinaemia and reactivation of human herpesvirus 6 infection demonstrated by real-time quantitative polymerase chain reaction. British Journal of Dermatology. 2003;149:165–169. doi: 10.1046/j.1365-2133.2003.05368.x. [DOI] [PubMed] [Google Scholar]
- Akhyani N, Berti R, Brennan MB, Soldan SS, Eaton JM, McFarland HF, Jacobson S. Tissue distribution and variant characterization of human herpesvirus (HHV)-6: increased prevalence of HHV-6A in patients with multiple sclerosis. J Infect Dis. 2000;182:1321–1325. doi: 10.1086/315893. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole G, Cooper N, Eikelenboom P, Emmerling M, Fiebich B, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s Disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Lafuente R, Martin-Estefania C, de las Heras V, Castrillo C, Cour I, Picazo JJ, Varela De Seijas E, Arroyo R. Acta Neurol Scand. 2002a;105:95–99. doi: 10.1034/j.1600-0404.2002.1o050.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lafuente R, Martin-Estefania C, de Las Heras V, Castrillo C, Picazo JJ, Varela de Seijas E, Gonzalez RA. Arch Neurol. 2002b;59:929–933. doi: 10.1001/archneur.59.6.929. [DOI] [PubMed] [Google Scholar]
- Albright AV, Lavi E, Black JB, Goldberg S, O'Connor MJ, Gonzalez-Scarano F. The effect of human herpesvirus-6 (HHV-6) on cultured human neural cells: oligodendrocytes and microglia. J Neurovirol. 1998;4:486–494. doi: 10.3109/13550289809113493. [DOI] [PubMed] [Google Scholar]
- Ansari A, Li S, Abzug MJ, Weinberg A. Human herpesviruses 6 and 7 and central nervous system infection in children. Emerg Infect Dis. 2004;10:1450–1454. doi: 10.3201/eid1008.030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone SR, Kaplan MH, Krilov LR. Human herpesvirus-6 infection in children with first febrile seizures. J Pediatr. 1995;127:95–97. doi: 10.1016/s0022-3476(95)70263-6. [DOI] [PubMed] [Google Scholar]
- Berg AT, Shinnar S, Shapiro ED, Salomon ME, Crain EF, Hauser WA. Risk factors for a first febrile seizure: a matched case-control study. Epilepsia. 1995;36:334–341. doi: 10.1111/j.1528-1157.1995.tb01006.x. [DOI] [PubMed] [Google Scholar]
- Berti R, Brennan MB, Soldan SS, Ohayon JM, Casareto L, McFarland HF, Jacobson S. Increased detection of serum HHV-6 DNA sequences during multiple sclerosis (MS) exacerbations and correlation with parameters of MS disease progression. J Neurovirol. 2002;8:250–256. doi: 10.1080/13550280290049615-1. [DOI] [PubMed] [Google Scholar]
- Bien CG, Urbach H, Schramm J, Soeder BM, Becker AJ, Voltz R, Vincent A, Elger CE. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology. 2007;69:1236–1244. doi: 10.1212/01.wnl.0000276946.08412.ef. [DOI] [PubMed] [Google Scholar]
- Bien CG, Granata T, Antozzi C, Cross JH, Dulac O, Kurthen M, Lassmann H, Mantegazza R, Villemure JG, Spreafico R, Elger CE. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain. 2005;128:454–471. doi: 10.1093/brain/awh415. [DOI] [PubMed] [Google Scholar]
- Bauer J, Elger CE, Hans VH, Schramm J, Urbach H, Lassmann H, Bien CG. Astrocytes are a specific immunological target in Rasmussen's encephalitis. Ann Neurol. 2007;62:67–80. doi: 10.1002/ana.21148. [DOI] [PubMed] [Google Scholar]
- Birnbaum T, Padovan CS, Sporer B, Rupprecht TA, Ausserer H, Jaeger G, Pfister HW. Severe Meningoencephalitis Caused by Human Herpesvirus 6 Type B in an Immunocompetent Woman Treated with Ganciclovir. Clinical Infectious Diseases. 2005;40:887–889. doi: 10.1086/427943. [DOI] [PubMed] [Google Scholar]
- Byington CL, Zerr DM, Taggart EW, Nguy L, Hillard DR, Carroll KC, Corey L. Human herpesvirus 6 infection in febrile infants ninety days of age and younger. Pediatric Infectious Disease Journal. 2002;21:996–999. doi: 10.1097/00006454-200211000-00004. [DOI] [PubMed] [Google Scholar]
- Capouya JD, Berman DM, Dumois JA. Mollaret’s Meningitis Due to Human Herpesvirus 6 in an Adolescent. Clin Pediatr. 2006;45:861–863. doi: 10.1177/0009922806295286. [DOI] [PubMed] [Google Scholar]
- Caserta MT, Hall CB, Schnabel K, Long CE, D'Heron N. Primary human herpesvirus 7 infection: a comparison of human herpesvirus 7 and human herpesvirus 6 infections in children. J Pediatr. 1998;133:386–389. doi: 10.1016/s0022-3476(98)70275-6. [DOI] [PubMed] [Google Scholar]
- Caserta MT, Schnabel KS, McIntyre KM, Costanzo M, Hall CB. Central nervous system persistence of human herpesvirus-6 (HHV-6) Clin Infect Dis. 1993;17:557. [Abstract] [Google Scholar]
- Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001;33(6):829–833. doi: 10.1086/322691. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Carpenter S, Zatorre RJ, Cashman NR. Temporal lobe epilepsy caused by domoic acid intoxication: evidence for glutamate receptor-mediated excitotoxicity in humans. Ann Neurol. 1995;37:123–126. doi: 10.1002/ana.410370125. [DOI] [PubMed] [Google Scholar]
- Cermelli C, Berti R, Soldan SS, Mayne M, D'Ambrosia JM, Ludwin SK, Jacobson S. High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J Infect Dis. 2003;187:1377–1387. doi: 10.1086/368166. [DOI] [PubMed] [Google Scholar]
- Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PK, Ng HK, Hui M, Cheng AF. Prevalence and distribution of human herpesvirus 6 variants A and B in adult human brain. J Med Virol. 2001;64:42–46. doi: 10.1002/jmv.1015. [DOI] [PubMed] [Google Scholar]
- Chan PK, Ng HK, Hui M, Ip M, Cheung JL, Cheng AF. Presence of human herpesviruses 6, 7, and 8 DNA sequences in normal brain tissue. J Med Virol. 1999;59:491–495. doi: 10.1002/(sici)1096-9071(199912)59:4<491::aid-jmv11>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Cirone M, Cuomo L, Zompetta C, Ruggieri S, Frati L, Faggioni A, Ragona G. Human herpesvirus 6 and multiple sclerosis: a study of T cell cross-reactivity to viral and myelin basic protein antigens. J Med Virol. 2002;68:268–272. doi: 10.1002/jmv.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo L, Trivedi P, Cardillo MR, Gagliardi FM, Vecchione A, Caruso R, Calogero A, Frati L, Faggioni A, Ragona G. Human herpesvirus 6 infection in neoplastic and normal brain tissue. J Med Virol. 2001;63:45–51. [PubMed] [Google Scholar]
- De Bolle L, Naesens L, De Clercq E. Update on Human Herpesvirus 6 Biology, Clinical Features, and Therapy. Clinical Microbiology Reviews. 2005;18:217–245. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descamps V, Valance A, Edlinger C, Fillet AM, Grossin M, Lebrun-Vignes B, Belaich S, Crickx B. Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch Dermatol. 2001;137:301–304. [PubMed] [Google Scholar]
- Dewhurst S, McIntyre K, Schnabel K, Hall CB. Human herpesvirus 6 (HHV-6) variant B accounts for the majority of symptomatic primary HHV-6 infections in a population of U.S. infants. J Clin Microbiol. 1993;31:416–418. doi: 10.1128/jcm.31.2.416-418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati D, Akhyani N, Fogdell-Hahn A, Cermelli C, Cassiani Ingoni R, Vortmeyer A, Heiss JD, Cogen P, Gaillard WDS, Sato S, Theodore WH, Jacobson S. Detection of Human Herpesvirus 6 (HHV-6) in Mesial Temporal Lobe Epilepsy Surgical Brain Resections. Neurology. 2003;61:1405–1411. doi: 10.1212/01.wnl.0000094357.10782.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati D, Martinelli E, Cassiani-Ingoni R, Ahlqvist J, Hou J, Major EO, Jacobson S. Variant-specific tropism of human herpesvirus 6 in human astrocytes. J Virol. 2005;79:9439–9448. doi: 10.1128/JVI.79.15.9439-9448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Eeg-Olofsson O. Virological and immunological aspects of seizure disorders. Brain & Development. 2003;25(2003):9–13. doi: 10.1016/s0387-7604(02)00162-6. [DOI] [PubMed] [Google Scholar]
- Eeg-Olofsson O, Bergstrom T, Andermann F, Andermann E, Olivier A, Rydenhag B. Herpesviral DNA in brain tissue from patients with temporal lobe epilepsy. Acta Neurol Scand. 2004;109:169–174. doi: 10.1046/j.1600-0404.2003.00238.x. [DOI] [PubMed] [Google Scholar]
- Emsley H, Smith C, Georgiou R, Vail A, Hopkins S, Rothwell N, Tyrrell P. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psych. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. for the IL-1ra Acute Stroke Investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LG, Nordli DN, Hamidullah A, Pellock J, Frank M, Lewis D, Hesdorffer DC, Marmarou A, O'Dell C, Shinnar S the FEBSTAT study team. The Role of Primary Human Herpes Virus 6, 7(HHV-6, HHV-7) Infection in Febrile Status Epilepticus. Ann Neurol. 2005;58(suppl 9):S79–S80. [Google Scholar]
- Epstein LG, Nordli DR, Hamidullah A, Pellock JM, Frank LM, Lewis DV, Hesdorffer DC, Marmarou A, O'Dell C, Shinnar S FEBSTAT study team. The role of primary Human Herpes Virus 6, 7 (HHV-6, HHV-7) Infection in Febrile Status Epilepticus. Clinical Virology. 2006;37(Suppl 1):S116. [Google Scholar]
- Fotheringham J, Donati D, Akhyani N, Vortmeyer A, Heiss JD, Williams E, Weinstein S, Bruce DA, Gaillard WD, Sato S, Theodore WH, Jacobson S. Detection of Human Herpesvirus-6B DNA and Antigen in Primary Astrocyte Cultures from Mesial Temporal Lobe Epilepsy Brain Resections. PLoS Med. 2007;4:e180. doi: 10.1371/journal.pmed.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotheringham J, Akhyani N, Vortmeyer A, Donati D, Williams E, Oh U, Bishop M, Barrett J, Gea-Banacloche J, Jacobson S. Detection of active human herpesvirus-6 infection in the brain: correlation with polymerase chain reaction detection in cerebrospinal fluid. J Infect Dis. 2007;195:450–454. doi: 10.1086/510757. [DOI] [PubMed] [Google Scholar]
- Frank LM, Hesdorffer DC, O'Dell C, Pellock JM, Nordli DR, Lewis DV, Marmarou A, Shinnar S FEBSTAT Study Team. Febrile Status Epilepticus Does Not Cause CSF Pleocytosis: Results of the FEBSTAT Multicenter Trial. Epilepsia. 2006;47(Suppl 4):133–134. [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Goodman AD, Mock DJ, Powers JM, Bake JV, Blumberg BM. Human herpesvirus 6 genome and antigen in acute multiple sclerosis lesions. J Infect Dis. 2003;187:1365–1376. doi: 10.1086/368172. [DOI] [PubMed] [Google Scholar]
- Gorniak RJT, Young GS, Wiese DE, Marty FM, Schwartz RB. MR Imaging of Human Herpesvirus-6–associatedEncephalitis in 4 Patients with Anterograde Amnesia after Allogeneic Hematopoietic Stem-Cell Transplantation AJNR Am J Neuroradiol. 2006;27:887–891. [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, Knott A, Dewhurst S, Insel RA, Epstein LG. Human herpesvirus-6 infection in children: A prospective study of complications and reactivation. N Engl J Med. 1994;331:432–438. doi: 10.1056/NEJM199408183310703. [DOI] [PubMed] [Google Scholar]
- Hall CB, Caserta MT, Schnabel KC, Boettrich C, McDermott MP, Lofthus GK, Carnahan JA, Dewhurst S. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7) Journal of Pediatrics. 2004;145:472–477. doi: 10.1016/j.jpeds.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Hall CB, Caserta MT, Schnabel KC, McDermott MP, Lofthus GK, Carnahan JA, Gilbert LM, Dewhurst S. Characteristics and acquisition of human herpesvirus (HHV) 7 infections in relation to infection with HHV-6. J Infect Dis. 2006;193:1063–1069. doi: 10.1086/503434. [DOI] [PubMed] [Google Scholar]
- He J, McCarthy M, Zhou Y, Chandran B, Wood C. Infection of primary human fetal astrocytes by human herpesvirus 6. J Virol. 1996;70:1296–1300. doi: 10.1128/jvi.70.2.1296-1300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Ralay-Ranaivo H, Roy S, Behanna H, Wing L, Munoz L, Van Eldik L, Watterson D. Development of a novel therapeutic suppressor of brain pro-inflammatory cytokine up-regulation that attenuates synaptic dysfunction and behavioral deficits. Bioorgan Med Chem Lett. 2007;17:414–418. doi: 10.1016/j.bmcl.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Ralay-Ranaivo H, Craft J, Van Eldik L, Watterson D. Validation of the neuroinflammation cycle as a drug discovery target using integrative chemical biology and lead compound development with an Alzheimer's disease-related mouse model. Curr Alzheimer Res. 2005;2:197–205. doi: 10.2174/1567205053585828. [DOI] [PubMed] [Google Scholar]
- Isaacson E, Glaser CA, Forghani B, Amad Z, Wallace W, Armstrong RW, Exner MM, Schmid S. Evidence of Human Herpesvirus 6 Infection in 4 Immunocompetent Patients with Encephalitis Clinical Infectious Diseases. 2005;40:890–893. doi: 10.1086/427944. [DOI] [PubMed] [Google Scholar]
- Karatas H, Gurer G, Pinar A, Soylemezoglu F, Tezel GG, Hascelik G, Akalan N, Tuncer S, Ciger A, Saygi S. Investigation of HSV-1, HSV-2, CMV, HHV-6 and HHV-8 DNA by real-time PCR in surgical resection materials of epilepsy patients with mesial temporal lobe sclerosis. J Neurol Sci. 2007 Sep 3; doi: 10.1016/j.jns.2007.08.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kim JS, Lee KS, Park JH, Kim MY, Shin WS. Detection of human herpesvirus 6 variant A in peripheral blood mononuclear cells from multiple sclerosis patients. Eur Neurol. 2000;43:170–173. doi: 10.1159/000008158. [DOI] [PubMed] [Google Scholar]
- Knox KK, Brewer JH, Henry JM, Harrington DJ, Carrigan DR. Human herpesvirus 6 and multiple sclerosis: systemic active infections in patients with early disease. Clin Infect Dis. 2000;31:894–903. doi: 10.1086/318141. [DOI] [PubMed] [Google Scholar]
- Lanari M, Papa I, Venturi V, Lazzarotto T, Faldella G, Gabrielli L, Guerra B, Landini MP, Salvioli1 GP. Congenital Infection With Human Herpesvirus 6 Variant B Associated With Neonatal Seizures and Poor Neurological Outcome. Journal of Medical Virology. 2003;70:628–632. doi: 10.1002/jmv.10441. [DOI] [PubMed] [Google Scholar]
- Lee T-S, Mane S, Eid T, Zhao H, Lin A, Gua Zn, Kim JH, Schweitzer J, King-Stevens D, Weber P, Spencer SS, Spencer DD, de Laneroll NC. Gene Expression in Temporal Lobe Epilepsy is Consistent with Increased Release of Glutamate by Astrocytes. Mol Med. 2007;13:1–13. doi: 10.2119/2006-00079.Lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi M, Marasca R, Barozzi P, Ferrari S, Ceccherini-Nelli L, Batoni G, Merelli E, Torelli G. Three cases of human herpesvirus-6 latent infection: integration of viral genome in peripheral blood mononuclear cell DNA. J Med Virol. 1993;40:44–52. doi: 10.1002/jmv.1890400110. [DOI] [PubMed] [Google Scholar]
- Lusso P, Markham PD, Tschachler E, di Marzo Veronese F, Salahuddin SZ, Ablashi DV, Pahwa S, Krohn K, Gallo RC. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6) J Exp Med. 1988;167:1659–1670. doi: 10.1084/jem.167.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannonen L, Herrgård E, Valmari P, Rautiainen P, Uotila K, Aine MR, Karttunen-Lewandowski P, Sankala J, Wallden T, Koskiniemi M. Primary human herpesvirus-6 infection in the central nervous system can cause severe disease Ped Neurol. 2007;37:186–191. doi: 10.1016/j.pediatrneurol.2007.05.011. [DOI] [PubMed] [Google Scholar]
- MacLean HJ, Douen AG. Severe amnesia associated with human herpesvirus 6 encephalitis after bone marrow transplantation. Transplantation. 2002;73:1086–1089. doi: 10.1097/00007890-200204150-00012. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius J. The clinical pathogenic mechanisms of hippocampal neuron loss and surgical outcome in temporal lobe epilepsy. Brain. 1995;118:105–118. doi: 10.1093/brain/118.1.105. [DOI] [PubMed] [Google Scholar]
- McCullers JA, Lakeman FD, Whitley RJ. Human herpesvirus 6 is associated with focal encephalitis. Clin Infect Dis. 1995;21:571–576. doi: 10.1093/clinids/21.3.571. [DOI] [PubMed] [Google Scholar]
- Meurs A, Clinckers R, Ebinger G, Michotte Y, Smolders I. Seizure activity and changes in hippocampal extracellular glutamate, GABA, dopamine and serotonin Epilepsy Research. 2007 doi: 10.1016/j.eplepsyres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Mrak R, Griffin W. Glia and cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Opsahl ML, Kennedy PG. Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain. 2005;128:516–527. doi: 10.1093/brain/awh390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Neuronal and glial metabolite content of the epileptogenic human hippocampus. Ann. Neurol. 2002;52:635–642. doi: 10.1002/ana.10360. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Boer K, Redeker S, Spliet WGM, van Rijen PC, Troost D, Vezzani A, Aronica E. The IL-1β system in epilepsy-associated malformations of cortical development. Neurobiology of Disease. 2006;24:128–143. doi: 10.1016/j.nbd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol Disease. 2007 doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Marty FM, Holmes TM, Upchurch K, Soiffer RJ, Antin JH, Baden LR, Bromfield EB. Post-transplant acute limbic encephalitis: Clinical features and relationship to HHV6. Neurology. 2007;69:156–165. doi: 10.1212/01.wnl.0000265591.10200.d7. [DOI] [PubMed] [Google Scholar]
- Seishima M, Yamanaka S, Fujisawa T, Tohyama M, Hashimoto K. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br J Dermatol. 2006;155:344–349. doi: 10.1111/j.1365-2133.2006.07332.x. [DOI] [PubMed] [Google Scholar]
- Shinnar S. Febrile seizures and mesial temporal sclerosis. Epilepsy Currents. 2003;3:115–118. doi: 10.1046/j.1535-7597.2003.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvagni PA, Lowenstine LJ, Spraker T, Lipscomb TP, Gulland FMD. Pathology of Domoic Acid Toxicity in California Sea Lions (Zalophus californianus) Vet Pathol. 2005;42:184–191. doi: 10.1354/vp.42-2-184. [DOI] [PubMed] [Google Scholar]
- Singh N, Paterson DL. Encephalitis caused by human herpesvirus-6 in transplant recipients: relevance of a novel neurotropic virus. Transplantation. 2000;69:2474–2479. doi: 10.1097/00007890-200006270-00002. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Berti R, Salem N, Secchiero P, Flamand L, Calabresi PA, Brennan MB, Maloni HW, McFarland HF, Lin HC, Patnaik M, Jacobson S. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997;3:1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Leist TP, Juhng KN, McFarland HF, Jacobson S. Increased lymphoproliferative response to human herpesvirus type 6A variant in multiple sclerosis patients. Ann Neurol. 2000;47:306–313. [PubMed] [Google Scholar]
- Somera-Molina K, Robin B, Somera C, Anderson C, Stine C, Koh S, Behanna H, Van Eldik L, Watterson D, Wainwright M. Glial activation links early-life seizures and long-term neurologic dysfunction: evidence using a small molecule inhibitor of pro-inflammatory cytokine up-regulation. Epilepsia. 2007;48(9):1785–1800. doi: 10.1111/j.1528-1167.2007.01135.x. [DOI] [PubMed] [Google Scholar]
- Suga S, Suzuki K, Ihira M, Yoshikawa T, Kajita Y, Ozaki T, Iida K, Saito Y, Asano Y. Clinical characteristics of febrile convulsions during primary HHV-6 infection. Arch Dis Child. 2000;82:62–66. doi: 10.1136/adc.82.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga S, Yoshikawa T, Asano Y, et al. Clinical and virological analyses of 21 infants with exanthem subitum (roseola infantum) and central nervous system complications. Ann Neurol. 1993;33:597–603. doi: 10.1002/ana.410330607. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Hagen J, Pitkänen A. Do epileptic seizures damage the brain? Curr Opin Neurol. 2003 Apr 16;16(2):189–195. doi: 10.1097/01.wco.0000063770.15877.bc. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res. 2007;163:541–563. doi: 10.1016/S0079-6123(07)63029-5. [DOI] [PubMed] [Google Scholar]
- Tejada-Simon MV, Zang YC, Hong J, Rivera VM, Killian JM, Zhang JZ. Detection of viral DNA and immune responses to the human herpesvirus 6 101-kilodalton virion protein in patients with multiple sclerosis and in controls. J Virol. 2002;76:6147–6154. doi: 10.1128/JVI.76.12.6147-6154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada-Simon MV, Zang YC, Hong J, Rivera VM, Zhang JZ. Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann Neurol. 2003;53:189–197. doi: 10.1002/ana.10425. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Gaillard WD. Neuroimaging and the progression of epilepsy. Prog Brain Res. 2002;135:305–313. doi: 10.1016/S0079-6123(02)35028-3. [DOI] [PubMed] [Google Scholar]
- Tian G-F, Azmi H, Takano T, et al. An astrocytic basis of epilepsy. Nat. Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura H, Iseki T, Date Y, Watanabe J, Kumagai K, Kikuno K, et al. Human herpesvirus-6 encephalitis after bone marrow transplantation: magnetic resonance imaging could identify the involved sites of encephalitis. Eur J Haematol. 1998;61:284–285. doi: 10.1111/j.1600-0609.1998.tb01718.x. [DOI] [PubMed] [Google Scholar]
- Uesugi H, Shimizu H, Maehara T, Arai N, Nakayama H. Presence of human herpesvirus 6 and herpes simplex virus detected by polymerase chain reaction in surgical tissue from temporal lobe epilepsy patients. Psychiatry and Clinical Neurosciences. 2000;54:589–593. doi: 10.1046/j.1440-1819.2000.00758.x. [DOI] [PubMed] [Google Scholar]
- Virta M, Hurme M, Helminen M. Increased plasma levels of pro-and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia. 2002;43:920–923. doi: 10.1046/j.1528-1157.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Granata T. Brain Inflammation in Epilepsy: Experimental and Clinical Evidence. Epilepsia. 2005;46(11):1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Baram T. New roles for interleukin-1 beta in the mechanisms of epilepsy. Epil Curents. 2007;7:45–50. doi: 10.1111/j.1535-7511.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright MS, Martin PL, Morse RP, Lacaze M, Provenzale JM, Coleman RE, Morgan MA, Hulette C, Kurtzberg J, Bushnell C, Epstein L, Lewis DV. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001;50:612–619. doi: 10.1002/ana.1251. [DOI] [PubMed] [Google Scholar]
- Ward KN, Andrews NJ, Verity CM, Miller E, Ross EM. Human herpesviruses-6 and-7 each cause significant neurological morbidity in Britain and Ireland. Arch Dis Child. 2005;90:619–623. doi: 10.1136/adc.2004.062216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley RJ, Lakeman FD. Human Herpesvirus 6 Infection of the Central Nervous System: Is It Just a Case of Mistaken Association? Clinical Infectious Diseases. 2005;40:894–895. doi: 10.1086/427953. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Morishima T. HHV-6 and seizures. Herpes. 2005;12:46–49. [PubMed] [Google Scholar]
- Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Asano Y, Akimoto S, Ozaki T, Iwasaki T, Kurata T, Goshima F, Ishiyama Y. Latent infection of human herpesvirus 6 in astrocytoma cell line and alteration of cytokine synthesis. J Med Virol. 2002;66:497–505. [PubMed] [Google Scholar]
- Yoshikawa T, Asano Y. Central nervous system complications in human herpesvirus-6 infection. Brain Dev. 2000;22:307–314. doi: 10.1016/s0387-7604(00)00113-3. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Nakashima T, Suga S, Asano Y, Yazaki T, Kimura H, Morishima T, Kondo K, Yamanishi K. Human herpesvirus-6 DNA in cerebrospinal fluid of a child with exanthem subitum and meningoencephalitis. Pediatrics. 1992;89:888–890. [PubMed] [Google Scholar]
- Zerr DM, Yeung LC, Obrigewitch RM, Huang ML, Frenkel LM, Corey L. Case report: primary human herpesvirus-6 associated with an afebrile seizure in a 3-week-old infant. J Med Virol. 2002;66:384–387. doi: 10.1002/jmv.2156. [DOI] [PubMed] [Google Scholar]
- Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang M-L, Wald A, Rhoads MP, Nguy L, Rena Bornemann R, Morrow RA, Corey L. A Population-Based Study of Primary Human Herpesvirus 6 Infection. N Engl J Med. 2005;352:768–776. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]