Abstract
BACKGROUND AND AIM:
Severe bleeding from gastrointestinal ulcers is a life-threatening event that is difficult to manage when endoscopic treatment fails. Transcatheter embolization has been suggested as an alternative treatment in this situation. The present study reports on the efficacy and long-term outcomes of transcatheter embolization after failed endoscopic treatments were assessed in high-operative-risk patients.
METHODS:
A retrospective review of 60 consecutive emergency embolization procedures in hemodynamically unstable patients (41 men, 19 women; mean [±SD] age 69.4±15 years) was conducted. Patients were referred for selective angiography between 1999 and 2008 after failed endoscopic treatment of massive bleeding from gastrointestinal ulcers. Mean follow-up was 22 months.
RESULTS:
Embolization was feasible and successful in 57 patients. Sandwich coiling of the gastroduodenal artery was used in 34 patients, and superselective occlusion of the terminal feeding artery (with glue, coils or gelatin particles) was used in 23 patients. Early rebleeding occurred in 16 patients and was managed with endoscopy (n=8), reembolization (n=3) or surgery (n=5). No major embolization-related complications occurred. Sixteen patients died within 30 days after embolization (including three who died from rebleeding) and 11 died thereafter. No late bleeding recurrences were reported.
CONCLUSIONS:
Selective angiographic embolization is safe and effective for controlling life-threatening bleeding from gastroduodenal ulcers. The procedure usually obviates the need for emergency surgery in these high-risk patients. Survival depends chiefly on underlying conditions.
Keywords: Angiography, Arteries, Embolization, Gastrointestinal bleeding, Peptic ulcer
Abstract
HISTORIQUE ET BUT :
Les hémorragies massives associées aux ulcères des voies digestives sont des événements gravissimes et difficiles à maîtriser après l’échec du traitement endoscopique. L’embolisation transcathéter a été proposée comme solution de rechange dans de tels cas. La présente étude fait le point sur l’efficacité et l’issue à long terme de l’embolisation transcathéter après l’échec des traitements endoscopiques chez des patients exposés à un risque opératoire important.
MÉTHODES :
Les auteurs ont procédé à une analyse rétrospective de 60 embolisations d’urgence consécutives chez des patients hémodynamiquement instables (41 hommes, 19 femmes; âge moyen [± É.-T.] 69,4 ans ± 15 ans). Ces patients avaient été adressés pour angiographie sélective entre 1999 et 2008, après l’échec d’un traitement endoscopique pour hémorragie massive associée à des ulcères des voies digestives. La durée moyenne du suivi a été de 22 mois.
RÉSULTATS :
L’embolisation a été possible et fructueuse chez 57 patients. L’embolisation dite « en sandwich » de l’artère gastroduodénale a été utilisée chez 34 patients et l’occlusion supersélective de l’artère nourricière terminale (par exemple, colle, spirales métaliques, particules de gélatine) a été utilisée chez 23 patients. Le saignement a rapidement repris chez 16 patients et a été corrigé par méthode endoscopique (n = 8), réembolisation (n = 3) ou chirurgie (n = 5). On n’a déploré aucune complication majeure liée à l’embolisation. Seize patients sont décédés dans les 30 jours suivant l’embolisation (dont trois, par suite de la reprise de l’hémorragie) et 11 sont décédés plus tard. Aucune récurrence tardive de l’hémorragie n’a été rapportée.
CONCLUSIONS :
L’embolisation angiographique sélective permet de maîtriser de façon sécuritaire et efficace l’hémorragie gravissime associée aux ulcères gastroduodénaux. L’intervention permet aussi habituellement d’éviter le recours à une chirurgie d’urgence chez ces patients à haut risque. La survie dépend principalement des conditions sous-jacentes.
Acute bleeding is the leading complication of peptic ulcer disease, which contributes to approximately one-half of the cases of upper gastrointestinal bleeding (1,2). Bleeding from peptic ulcers is fatal in 5% to 10% of patients, a proportion that has not changed substantially over the past two decades (3–5). The bleeding persists or recurs in approximately 20% of cases requiring emergency medical treatment or endoscopic intervention (1,5). When these methods fail, either surgery or transcatheter arterial embolization must be performed (6). Bleeding from peptic ulcers occurs selectively in older patients with serious underlying conditions, in whom surgery is associated with a high risk of mortality (40%) and morbidity (45%) (7). Embolization is used at the Bocage Teaching Hospital, Dijon, France, in patients with failed endoscopic treatment for severe peptic ulcer bleeding.
The objective of the present study was to report the safety, efficacy, and short- and long-term outcomes of selective embolization in 60 consecutive patients with failed endoscopic treatment for severe peptic ulcer bleeding between October 1999 and January 2008.
METHODS
Patients
A retrospective chart review was conducted to identify patients admitted to the Department of Interventional Radiology and Endovascular Therapy for transcatheter arterial embolization to treat massive peptic ulcer bleeding (defined as a need for more than four units of blood/24 h), causing hemodynamic instability (defined as clinical hypovolemic shock requiring volume replacement). The present study was approved by the institutional review board. Given the retrospective design, informed consent was not required.
Sixty patients were identified, 41 men and 19 women, with a mean age of 69.4 years (range 29 to 95 years). The operative risk was high in most of the patients as a result of advanced age (older than 70 years in 61.7% of patients and older than 80 years in 23.3%) and serious comorbid conditions (one or more in 90% of patients; two or more in 65% of patients, respectively). Furthermore, 58.3% of patients were on anticoagulant and/or anti-inflammatory treatment at hospital admission.
Emergency endoscopy showed gastric or duodenal peptic ulcers (n=55) or Dieulafoy lesions (n=5) with active bleeding (type 1; n=33), recent bleeding (type 2; n=22), or no bleeding (type 3; n=5) according to Forrest’s classification (8). Endoscopic hemostasis involved noradrenaline injection or placement of metal clips around the bleeding site. Mean time from hypovolemic shock onset to referral was 2.3 days. At the time of embolization, the patients had received a mean of 11 packed red blood cell units (range two to 40 packed red blood cell units). Mean hemoglobin level at admission was 65 g/L (range 30 g/L to 107 g/L).
Technique
All procedures were performed by one of three experienced interventional radiologists. Selective angiography of the celiac trunk, gastroduodenal artery (GDA) and superior mesenteric artery was performed using a 5-Fr Simmons-type 1 catheter (Cook Inc, USA) inserted through a 6-Fr sheath placed in the common femoral artery. In three patients, bleeding was from the right (n=2; extravasation) or left (n=1; no extravasation) gastric artery, which was too thin and tortuous to allow catheterization; the 57 other patients were treated with embolization. Extravasation of contrast medium or a false aneurysm-like lesion was noted at the bleeding site in 38 patients, of whom 17 were managed with embolization after superselective arterial catheterization using a 2.9-Fr coaxial microcatheter (Terumo Medical, Belgium). The 19 remaining patients underwent ‘sandwich’ embolization of the GDA. The 22 patients who had no identifiable bleeding site underwent blind embolization based on the endoscopic findings, using the technique chosen by the interventional radiologist. The left gastroepiploic artery was embolized through the splenic artery in two patients, and the left gastric artery was selectively occluded in six patients.
Overall, embolization involved the GDA and its branches or the pancreaticoduodenal arches in 49 patients, of whom 15 underwent selective embolization of the bleeding branch (Figure 1) and 34 underwent sandwich embolization (Figure 2) as described elsewhere (occlusion of the GDA trunk on either side of the bleeding site) (9). When the sandwich technique was used, the anterior and posterior superior pancreaticoduodenal arteries were routinely embolized; in three of these 34 patients, persistent extravasation prompted selective embolization of the anterior and posterior inferior pancreaticoduodenal arteries through the superior mesenteric artery. Embolization was usually achieved using 0.089 cm steel coils for the sandwich method or 0.046 cm soft platinum multiple-curled microcoils for the superselective method (Cook, USA), mechanically disrupted gelatin powder sheet (CuraMedical, Netherlands), or cyanoacrylate surgical glue (GEM SRL, Italy) mixed with ultrafluid lipiodol (Therapex, E-Z-EM, Canada) in a 1:3 ratio. Microspheres ranging in size from 500 μm to 700 μm in diameter (Biosphere Medical, France) were used in a few patients. In 28 patients, a single material was used (coils, n=15; glue, n=8; gelatin, n=3; microspheres, n=2). In 13 patients, both coils and gelatin sponge were used to occlude the GDA. The coils were released distally to prevent retrograde filling through the right gastroepiploic artery. A gelatin sponge was placed in the arterial trunk, and coils were then released in the proximal part of the GDA. Coils and glue were used in nine patients, gelatin sponge and microspheres in three patients, glue and gelatin in one patient, and coils and microspheres in one patient. In two patients, three embolic agents were used in combination. All agents were released near the bleeding site until angiographic extravasation stopped and/or occlusion of the targeted vessel was achieved, as shown by fluoroscopic guidance. The mean volume of contrast medium was 190 mL per patient, and the mean total procedure time was 70 min.
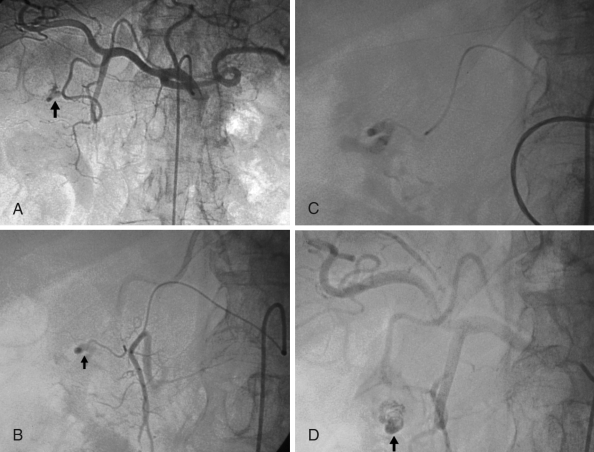
Figure 1).
Arteriogram images of bleeding from a bulbar duodenal ulcer in a 76-year-old man. A, B Arteriogram showing contrast medium extravasated from a slender branch of the gastroduodenal artery into the duodenum (arrows). C, D After microcatheterization, selective glue embolization (radiopaque because of associated lipiodol (arrow) preserving the gastroduodenal artery ensured control of the bleeding, with no early or late recurrences
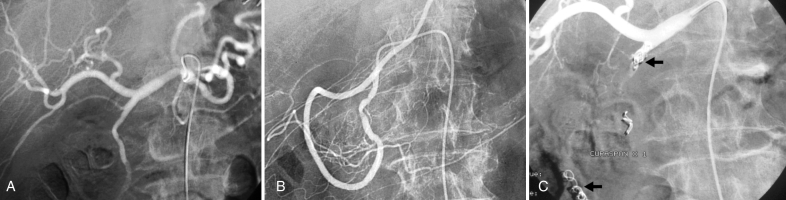
Figure 2).
Typical sandwich embolization in a 75-year-old man with bleeding from a postbulbar duodenal ulcer at endoscopy. A,B Angiography before embolization: No evidence of active bleeding; C Image after coil embolization of the distal and proximal gastroduodenal artery (with gelatin sponge placed in the arterial trunk), including the anterior and posterior superior pancreaticoduodenal arteries and the right gastroepiploic artery, to prevent retrograde flow (arrows). No ischemic complications were reported
Follow-up
Follow-up information was available for all patients. The mean length of hospital stay was 18 days (range one to 98 days) after embolization. Mean follow-up was 22 months (range one day to 103 months). A physical examination was performed during the hospital stay and one month after hospital discharge as part of routine patient care. Data on subsequent events were collected during telephone interviews of patients during follow-up by attending physicians.
Procedural success was defined as the absence of extravasation on postembolization arteriography; early clinical success as absence of rebleeding within one month after embolization; and long-term clinical success as absence of rebleeding throughout follow-up. Rebleeding was defined as bleeding with a greater than 20 g/L decrease in the hemoglobin level and/or failure of conservative medical treatment; early rebleeding was defined as bleeding occurring within 30 days after embolization. Complications were classified as major complications if they required surgery and/or prolonged hospitalization and as minor complications otherwise.
RESULTS
Most patients were at high operative risk because of associated medical conditions (one or more in 54 patients and two or more in 39 patients), including malignancy (n=21), coronary artery disease (n=19), pulmonary embolism or respiratory failure (n=18), hypertension (n=15), severe diabetes mellitus (n=14), heart failure (n=12), chronic renal failure (n=11), cardiac arrhythmia (n=10), stroke within 15 days (n=7), peripheral occlusive arterial disease (n=6), surgery within 15 days (n=4) and cirrhosis (n=3). Furthermore, 36 patients were on anticoagulant treatment at hospital admission, including antiplatelets (n=13), oral vitamin K antagonists (n=8), heparin (n=14) and fibrinolytic agents (n=1). Fifteen patients were taking anti-inflammatory drugs, including nonsteroidal anti-inflammatory drugs (n=8) and corticosteroids (n=7).
A bleeding peptic ulcer was visualized by endoscopy in 55 of the 60 patients (91.7%), in the stomach (n=7), bulbar duodenum (n=35) or postbulbar duodenum (n=13). In the five remaining patients (8.3%), bleeding was from a Dieulafoy lesion in the fundus. Endovascular treatment was feasible in 57 patients. The remaining three patients required surgery to treat bleeding from the right (n=2) or left (n=1) gastric artery, which was too slender and tortuous to allow catheterization.
Table 1 provides a summary of patient management according to angiographic findings. In 22 patients, no extravasation was identified by angiography but the artery supplying the endoscopically identified bleeding site was embolized in 21 patients. In three patients, the left gastric artery was occluded using resorbable or nonresorbable particles, and in 18 patients, treatment of the GDA was achieved using the sandwich technique (n=15) or by selective embolization (n=3). Selective embolization of the bleeding vessel was performed in 17 additional patients. The 19 remaining patients with angiographic extravasation underwent sandwich embolization of the GDA.
Table 1.
Summary of angiographic findings and arterial occlusion methods according to the embolized vessel in the study group
| Occlusion method | Abnormal angiography (presence of extravasation) (n=38) | Normal angiography (absence of extravasation) (n=22) |
|---|---|---|
| Nonfeasible embolization (n=3) | Right gastric artery (n=2) | Left gastric artery (n=1) |
| Feasible embolization (n=57) | ||
| Selective method (n=23) | Gastroduodenal artery and pancreaticoduodenal arteries (n=12); left gastric artery (n=3); left gastroepiploic artery (n=2) | Gastroduodenal artery and pancreaticoduodenal arteries (n=3); left gastric artery (n=3); left gastroepiploic artery (n=0) |
| Sandwich technique (n=34) | Gastroduodenal artery and pancreaticoduodenal arteries (n=19) | Gastroduodenal artery and pancreaticoduodenal arteries (n=15) |
The main results are summarized in Tables 2 and 3. Embolization was consistently effective in stopping the bleeding. However, 16 patients required further treatment within 72 h for recurrent bleeding: eight were managed endoscopically; three, all of whom initially underwent sandwich occlusion of the GDA, were treated with embolization of the inferior pancreaticoduodenal artery; and five underwent duodenotomy. The second treatment was initially successful in all 16 patients but three rapidly experienced fatal rebleeding from the same site. In total, gastrointestinal surgery was needed in eight of the 60 patients (13.3%). Initial embolizations were unsuccessful in 13 of 36 patients (36.1%) who were taking anticoagulant and/or anti-inflammatory drugs at admission and in three of 21 patients (14.3%) without these medications (Table 4).
TABLE 2.
Outcomes after embolization
| Outcome |
Patients |
|
|---|---|---|
| n/n | % | |
| Procedural success | 57/60 | 95.0 |
| Early clinical success | 41/57 | 71.9 |
| Late clinical success | 54/57 | 94.7 |
| Rebleeding | ||
| Early rebleeding | 16/57 | 10.5 |
| Late rebleeding | 0/57 | 0 |
| Complications* | ||
| Major complications | 2/60 | 3.33 |
| Minor complications | 4/60 | 6.67 |
| Mortality | ||
| Within one month | 16/60 | 26.7 |
| After one month | 11/44 | 25.0 |
| Cause | ||
| Recurrent bleeding | 3/27 | 11.1 |
| Underlying illness | 24/27 | 8.89 |
Complications were classified as major complications if they required surgery and/or prolonged hospitalization, and as minor complications otherwise
TABLE 3.
Short-term outcomes according to the embolized bleeding vessel
| Embolized bleeding vessel |
Early clinical success |
Mortality rate within 30 days |
||||
|---|---|---|---|---|---|---|
| Patients, n/n | % | Patients, n/n | % | |||
| GDA and PDA | 34/49 | 69.4 | 13/49 | 26.5 | ||
| Selective occlusion | 12/15 | 80 | 6/15 | 40 | ||
| Sandwich occlusion | 22/34 | 64.7 | 7/34 | 20.6 | ||
| Left gastric artery | 5/7 | 71.4 | 3/7 | 42.9 | ||
| LGEA | 2/2 | 100 | 0/2 | 0 | ||
| Right gastric artery | 0/2 | 0 | 0/2 | 0 | ||
GDA gastroduodenal artery; LGEA left gastroepiploic artery; PDA pancreaticoduodenal arteries;
TABLE 4.
Results of treatment within 30 days of embolization in patients using medications associated with increased bleeding or with gastrotoxic effects before the procedure (n=57)
| Patients | n |
|---|---|
| Successful embolization | 41 |
| Previous AC and/or AI | 23 |
| No previous AC and/or AI | 18 |
| Failed embolization | 16 |
| Previous AC and/or AI | 13 |
| No previous AC and/or AI | 3 |
| Previous AC and/or AI | 36 |
| Died | 11 |
| Lived | 25 |
| Without previous AC and/or AI | 21 |
| Died | 5 |
| Lived | 16 |
| Deceased | 16 |
| Previous AC and/or AI | 11 |
| No previous AC and/or AI | 5 |
Anticoagulant medications (AC) include antiplatelets agents, oral vitamin K antagonists, preventive or curative heparin and fibrinolytic agents; Anti-inflammatory drugs (AI) include nonsteroidal anti-inflammatory drugs and corticosteroids
No serious embolization-related complications occurred. Four patients experienced minor complications. Transient liver enzyme elevation occurred in a patient with proximal celiac trunk occlusion and retrograde filling of the GDA through the inferior pancreaticoduodenal artery, in whom catheterization beyond the bleeding site was not feasible; after verification of portal venous flow, resorbable particles were used to achieve retrograde occlusion of the inferior pancreaticoduodenal artery, GDA and common hepatic artery. A transient increase in serum amylase levels without symptoms was noted in another patient. In two patients, a microcoil migrated into the common hepatic artery during embolization, with no detectable effects. Angiography was responsible for two major complications. A hematoma in the groin area responsible for hemodynamic instability developed in one patient, who required vascular surgery. In the other patient, who was obese (160 kg), a false aneurysm of the femoral artery was identified a few days after the procedure but produced no symptoms; thrombosis was achieved by mechanical compression for 20 min under Doppler sonography guidance. No ischemic gastrointestinal complications were identified by clinical examination.
Of the 57 patients treated with embolization, 16 (28.1%) died within one month after the procedure from multiple organ failure (n=7); hypovolemic shock due to massive rebleeding (n=3); pulmonary embolism (n=2); bloodstream infection (n=2); malignancy (n=1); or myocardial infarction (n=1). No deaths were due to ischemic complications. Eleven of 36 patients (30.5%) who were taking anticoagulant and/or anti-inflammatory drugs at admission and five of 21 patients (23.8%) without these medications died within 30 days of embolization. No significant rebleeding occurred after the first month; mean follow-up was 22 months. Eleven additional patients died during follow-up: 34 days, 42 days, 45 days, 47 days, 50 days, 98 days, six months, seven months, seven months, 18 months and 23 months, respectively, after embolization due to causes unrelated to the procedure (underlying malignancy n=5; malnutrition n=3; bloodstream infection n=2; and cardiorespiratory failure n=1).
DISCUSSION
Endoscopy is the first-line method for diagnosing and treating actively bleeding peptic ulcers because its success rate is high. Endoscopic hemostasis is effective in most patients, thereby obviating the need for surgery, a valuable benefit in patients at high risk for surgical complications as a result of advanced age or comorbid conditions. When endoscopy fails, surgery is associated with high mortality rates of 20% to 40% (7,10). Endovascular embolization is an alternative to surgery in high-risk patients who fail endoscopic treatment. However, most studies of embolization were conducted in small patient populations who had a wide variety of diseases including peptic ulcers, malignant tumours, vascular malformations, trauma-induced lesions and post-inflammatory lesions (11). We focused on patients with bleeding from gastric or duodenal peptic ulcers (n=55) or Dieulafoy lesions (n=5). The bleeding site was identified endoscopically in all of our patients. In a study of 28 patients with bleeding duodenal ulcers (12), celiac arteriography showed extravasation in only 11 patients (39%). We visualized the extravasation site in a higher proportion of patients (38 of 60 [63.3%]), probably because we injected both the celiac trunk and the GDA, followed by the superior mesenteric artery. These results are in concordance with most published series (13–15) reporting a 60% to 80% sensitivity rate of arteriography in the detection of active upper bleeding. Furthermore, our study establishes that embolization can be performed successfully even when angiography fails to visualize the extravasation site. Previous reports (16–18) have found empirical embolization based on endoscopic findings, in the absence of contrast extravasation, to be helpful in controlling hemorrhage, with no difference between patients with negative and positive angiography results, confirming the practice of endoscopy-directed blind embolization.
All but one of the 16 rebleeding events in our study occurred after embolization of the GDA and its branches; three occurred after selective embolization of a pancreaticoduodenal branch and 12 after sandwich embolization of the GDA not including the inferior pancreaticoduodenal artery. The extensive collateral circulation in the duodenum probably increases the risk of rebleeding after embolization in this territory. In addition, rebleeding after selective embolization of a branch of the GDA or a pancreaticoduodenal artery alone, or sandwich embolization of the GDA alone not including the inferior pancreaticoduodenal artery, may be ascribable to retrograde flow into the pancreaticoduodenal arches and right gastroepiploic artery. Complete sandwich occlusion of the GDA including the superior and inferior pancreaticoduodenal arteries has been suggested to decrease the rebleeding rate, even when extravasation is not visualized (19,20). Furthermore, it seems that embolization was more likely to be unsuccessful in patients under anticoagulant and/or anti-inflammatory treatment (36.1%) than in those without (14.3%), confirming that all efforts should be made to stop it early in the course of hemodynamically unstable bleeding patients.
In our study, six of the 15 deaths (40%) recorded within one month after embolization occurred among the 15 patients who underwent selective embolization of a bleeding branch of the GDA. The clinical success rate in this subgroup was higher than in the subgroup of 34 patients treated with sandwich GDA embolization (80% versus 64.7%, respectively). Of these 34 patients, seven (20.6%) died. Two factors may contribute to explain the high mortality rate (six of 15 patients) after selective embolization. First, patients who underwent selective embolization had angiographic evidence of contrast-medium extravasation that is associated with active bleeding, severe blood loss and hypovolemic shock. Second, patients treated with selective embolization were slightly older (mean age 73.1 years) than those treated with sandwich embolization (mean age 68.8 years). Therefore, our data cannot be used to determine whether one of these embolization techniques is superior over the other in terms of 30-day survival.
Finally, three patients with acute bleeding from the pyloric artery (right gastric artery) (n=2) or left gastric artery (n=1) were treated surgically because the bleeding vessel was too slender and tortuous to allow catheterization.
Although rates of procedural success (95%) and early clinical success (71.9%) were high in our study, 26.7% of patients died within the first month. Severe hypovolemic shock at admission, advanced age and comorbid conditions probably contributed to this high mortality rate, which simply reflected the high surgical risk in our population. The impact of medications associated with increased bleeding on the one-month mortality rate was not clear in our study. Only three of the 16 deaths were due to early rebleeding. In the only previously published retrospective study (21) comparing outcomes of embolization (31 patients) and surgery (39 patients) after failed endoscopic treatment of bleeding peptic ulcers, no differences were found regarding rates of rebleeding, surgery or mortality. Unfortunately, the postprocedural morbidity rate was not compared between the two techniques. Few data are available regarding postsurgical morbidity, most notably complications related to the surgical method and infectious complications. In a retrospective study of 49 patients (7), the morbidity rate was 45%. No cases of bowel ischemia were noted in our study. In addition, major complications developed in only 3.3% of patients and were unrelated to vascular occlusion. Puncture-site injuries were the main complications. Using small introducers and catheters (4-Fr), delaying introducer retrieval until the coagulation parameters are normalized, or using vascular closure devices might help to decrease the rate of puncture-site injuries. The low morbidity rate is a major advantage of embolization over surgery. In addition, embolization is less invasive and less aggressive. The most troublesome long-term complication was duodenal stenosis (25%) in a study of 28 patients (22) followed for at least five years after embolization for a bleeding duodenal ulcer. Symptomatic duodenal stenosis was not reported in any of our patients, even those treated with surgical glue, probably because the follow-up was too short. Routine investigations may have identified cases of asymptomatic duodenal stenosis. The potential role for embolization in the occurrence of duodenal stenosis is unclear because duodenal stenosis can occur as a complication of peptic ulcer disease. After the first month, none of our patients experienced severe rebleeding requiring endoscopy or invasive treatment during follow-up, which lasted 22 months on average.
Although the results of the present retrospective study should be interpreted with caution, they indicate that arterial embolization performed by experienced interventional radiologists is effective in controlling bleeding from gastroduodenal ulcers, even when extravasation is not visualized by angiography and does not cause ischemia. At our hospital, this technique is the treatment of choice after failure of endoscopic hemostasis. Embolization usually obviates the need for surgery.
REFERENCES
- 1.Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331:717–27. doi: 10.1056/NEJM199409153311107. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health Consensus Conference Therapeutic endoscopy and bleeding ulcers. JAMA. 1989;262:1369–72. [PubMed] [Google Scholar]
- 3.Goldman ML, Land WC, Bradley EL, Anderson J. Transcatheter therapeutic embolization in the management of massive upper gastrointestinal bleeding. Radiology. 1976;120:513–21. doi: 10.1148/120.3.513. [DOI] [PubMed] [Google Scholar]
- 4.Dousset B, Suc B, Boudet MJ, et al. Traitement chirurgical des hémorragies ulcéreuses graves: Facteurs prédictifs de la mortalité opératoire. Gastroenterol Clin Biol. 1995;19:259–65. [PubMed] [Google Scholar]
- 5.Rollhauser C, Fleischer DE. Nonvariceal upper gastrointestinal bleeding: An update. Endoscopy. 1997;29:91–105. doi: 10.1055/s-2007-1004082. [DOI] [PubMed] [Google Scholar]
- 6.Qvist P, Arnesen KE, Jacobsen CD, Rosseland AR. Endoscopic treatment and restrictive surgical policy in the management of peptic ulcer bleeding: Five years’ experience in a central hospital. Scand J Gastroenterol. 1994;29:569–76. doi: 10.3109/00365529409092474. [DOI] [PubMed] [Google Scholar]
- 7.Cheynel N, Peschaud F, Hagry O, et al. Bleeding peptic ulcer: Results of surgical management. Ann Chir. 2001;126:232–5. doi: 10.1016/s0003-3944(01)00505-3. [DOI] [PubMed] [Google Scholar]
- 8.Forrest JAH, Finlayson NDC, Schearman DJC. Endoscopy of upper gastrointestinal bleeding. Lancet. 1974;2:394–7. doi: 10.1016/s0140-6736(74)91770-x. [DOI] [PubMed] [Google Scholar]
- 9.Loffroy R, Guiu B, Cercueil JP, et al. Refractory bleeding from gastroduodenal ulcers: Arterial embolization in high-operative-risk patients. J Clin Gastroenterol. 2008;42:361–7. doi: 10.1097/MCG.0b013e3180319177. [DOI] [PubMed] [Google Scholar]
- 10.Vellakot KD, Dronfield MW, Atkinson M, Langman MJS. Comparison of surgical and medical management of bleeding peptic ulcers. Br J Med. 1982;284:548–50. doi: 10.1136/bmj.284.6315.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krämer SC, Görich J, Rilinger N, et al. Embolization for gastrointestinal hemorrhages. Eur Radiol. 2000;10:802–5. doi: 10.1007/s003300051007. [DOI] [PubMed] [Google Scholar]
- 12.De Wispelaere JF, De Ronde T, Trigaux JP, et al. Duodenal ulcer hemorrhage treated by embolization: Results in 28 patients. Acta Gastroenterol Belg. 2002;65:6–11. [PubMed] [Google Scholar]
- 13.Sos TA, Jack GL, Wixson D, Sniderman KW. Intermittent bleeding from minute to minute in acute massive gastrointestinal hemorrhage: Arteriographic demonstration. Am J Roentgenol. 1978;131:1015–7. doi: 10.2214/ajr.131.6.1015. [DOI] [PubMed] [Google Scholar]
- 14.Rahn NH, Tishler JM, Han SY, Russinovich NA. Diagnostic and interventional angiography in acute gastrointestinal hemorrhage. Radiology. 1982;143:361–6. doi: 10.1148/radiology.143.2.6978500. [DOI] [PubMed] [Google Scholar]
- 15.Kelemouridis V, Athanasoulis C, Waltman A. Gastric bleeding sites: An angiographic study. Radiology. 1983;149:643–8. doi: 10.1148/radiology.149.3.6606186. [DOI] [PubMed] [Google Scholar]
- 16.Poultsides GA, Kim CJ, Orlando R, III, Peros G, Hallisey MJ, Vignati PV. Angiographic embolization for gastroduodenal hemorrhage: Safety, efficacy, and predictors of outcome. Arch Surg. 2008;143:457–61. doi: 10.1001/archsurg.143.5.457. [DOI] [PubMed] [Google Scholar]
- 17.Walsh RM, Anain P, Geisinger M, et al. Role of angiography and embolization for massive gastroduodenal hemorrhage. J Gastrointest Surg. 1999;3:61–6. doi: 10.1016/s1091-255x(99)80010-9. [DOI] [PubMed] [Google Scholar]
- 18.Schenker MP, Duszak R, Jr, Soulen MC, et al. Upper gastrointestinal hemorrhage and transcatheter embolotherapy: Clinical and technical factors impacting success and survival. J Vasc Interv Radiol. 2001;12:1263–71. doi: 10.1016/s1051-0443(07)61549-8. [DOI] [PubMed] [Google Scholar]
- 19.Toyoda H, Nakano S, Takeda I, et al. Transcatheter arterial embolization for massive bleeding from duodenal ulcers not controlled by endoscopic hemostasis. Endoscopy. 1995;27:304–7. doi: 10.1055/s-2007-1005697. [DOI] [PubMed] [Google Scholar]
- 20.Bell SD, Lau KY, Sniderman KW. Synchronous embolization of the gastroduodenal artery and the inferior pancreaticoduodenal artery in patients with massive duodenal hemorrhage. J Vasc Interv Radiol. 1995;6:531–6. doi: 10.1016/s1051-0443(95)71129-0. [DOI] [PubMed] [Google Scholar]
- 21.Ripoll C, Banares R, Beceiro I, et al. Comparison of transcatheter arterial embolization and surgery for treatment of bleeding peptic ulcer after endoscopic treatment failure. J Vasc Interv Radiol. 2004;15:447–50. doi: 10.1097/01.rvi.0000126813.89981.b6. [DOI] [PubMed] [Google Scholar]
- 22.Lang EK. Transcatheter embolization in management of hemorrhage from duodenal ulcer: Long-term results and complications. Radiology. 1992;182:703–7. doi: 10.1148/radiology.182.3.1535883. [DOI] [PubMed] [Google Scholar]