Summary
Glucagon plays an important role in glucose homeostasis by regulating hepatic glucose output in both normo- and hypo-glycemic conditions. In this study, we created and characterized α-cell specific insulin receptor knockout (αIRKO) mice to directly explore the role of insulin signaling in the regulation of glucagon secretion in vivo. Adult male αIRKO mice exhibited mild glucose intolerance, hyperglycemia and hyperglucagonemia in the fed state, and enhanced glucagon secretion in response to L-Arginine stimulation. Hyperinsulinemic-hypoglycemic clamp studies revealed an enhanced glucagon secretory response and an abnormal norepinephrine response to hypoglycemia in αIRKO mice. The mutants also exhibited an age-dependent increase in β-cell mass. Furthermore, siRNA-mediated knockdown of insulin receptor in glucagon-secreting InR1G cells promoted enhanced glucagon secretion and complemented our in vivo findings. Together, these data indicate a significant role for intra-islet insulin signaling in the regulation of α-cell function in both normo- and hypo-glycemic conditions.
Introduction
Type 1 diabetes mellitus (T1DM) and T2DM are both characterized by uncontrolled hyperglycemia and maintenance of blood glucose within the physiological range is critical for the prevention of diabetes-related complications. However, tight glycemic control is also associated with an increased incidence of therapy-induced hypoglycemic events (Cryer, 1994; DCCT, 1997).
Glucagon, secreted from the pancreatic α-cells, counters the actions of insulin and corrects hypoglycemia by enhancing hepatic glucose output and gluconeogenesis (Exton et al., 1966; Unger and Orci, 1977). Inappropriate glucagon secretion is often observed in patients with diabetes, and a defective glucagon response to hypoglycemia in hyperinsulinemic states frequently exacerbates hypoglycemic attacks, and limits intensive insulin therapy (Amiel et al., 1988; Gerich et al., 1973). On the other hand, excess glucagon secretion (Unger, 1978) and increase in α-cells in pancreatic islets in type 1 (Orci et al., 1976) and type 2 diabetes (Yoon et al., 2003) have been reported to worsen the hyperglycemia. In addition to its effects on insulin secretion (Scheen et al., 1996), glucagon has also been suggested to play a role in the development of β-cells although the molecular mechanisms underlying these effects are not fully understood (Prasadan et al., 2002; Vuguin et al., 2006). Thus, investigating the mechanism(s) that regulate α-cell secretory function and growth are useful to plan therapeutic approaches for preventing hypoglycemia and improving glucose homeostasis in diabetes.
The secretion of glucagon from α-cells is elevated in states of hypoglycemia and suppressed by hyperglycemia (Gromada et al., 2007). While some studies suggest a direct effect of glucose on α-cell secretory function (Ravier and Rutter, 2005; Vieira et al., 2007) additional regulators include the central and/or autonomic nervous systems (Ahren, 2000; Evans et al., 2004; Marty et al., 2005), and intra-islet paracrine factors including insulin (Diao et al., 2005; Gerich et al., 1975; Greenbaum et al., 1991; Maruyama et al., 1984; Ravier and Rutter, 2005; Stagner and Samols, 1986), somatostatin (Gerich et al., 1974), γ-amino-butyric acid (GABA) (Rorsman et al., 1989), and Zinc ions (Zn) (Ishihara et al., 2003). Zn, co-released with insulin, has been reported to suppress glucagon release (Ishihara et al., 2003), and conversely, to stimulate α-cell secretion when its concentration falls as part of a “switch-off” mechanism (Zhou et al., 2007). Intra-islet insulin has been reported to act either by reducing the sensitivity of K+-ATP channels (Franklin et al., 2005) through phosphatidyl inositol 3-kinase (PI3K) (Leung et al., 2006), or by activating Akt, a critical downstream effector of PI3K, which in turn induces recruitment of the GABA-A receptor to the cellular membrane to allow its ligand, GABA, to inhibit glucagon secretion (Xu et al., 2006). It is likely that one or more of these factors act in concert to regulate α-cell function during different physiological states. However, there are no reports, to our knowledge, that provide direct in vivo evidence for a direct role for intra-islet insulin in the modulation of α-cell function especially when insulin sensitivity is normal in peripheral tissues. Here, we report the creation of a α-cell-specific insulin receptor knock-out mouse and provide genetic evidence that insulin signaling is important for the regulation of glucagon secretion in both normoglycemic and hypoglycemic states in vivo.
Results
Creation of α-cell specific insulin receptor knockout mice
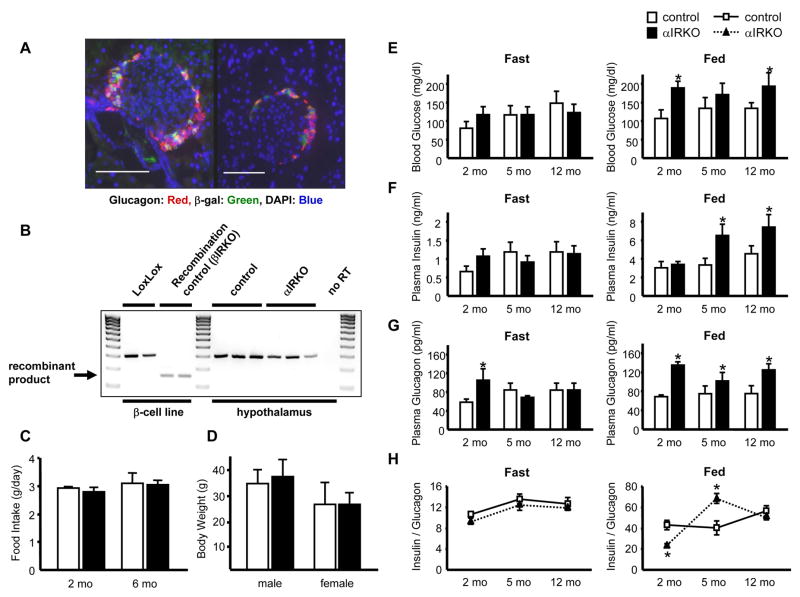
α-cell specific insulin receptor (IR) knockout (αIRKO) mice were created by crossing mice carrying the insulin receptor-floxed gene (Kulkarni et al., 1999a) with mice expressing Cre recombinase driven by the glucagon promoter (Herrera, 2000). αIRKO mice were born in a normal Mendelian pattern and did not exhibit abnormalities in the post-natal period. The specificity of recombination by the glucagon-promoter driven Cre recombinase (Glu-Cre), was confirmed by crossing ROSA26-LacZ reporter mice (Soriano, 1999) with Glu-Cre mice and immuostaining for β-galactosidase (β-gal) in pancreatic islets, hypothalamus, and intestinal L-cells (Gromada et al., 2007; Kieffer and Habener, 1999). Nearly 85 % of the α-cells expressed β-gal, indicating a high rate of recombination and high specificity, since the other islet cell types expressed significantly low levels of β-gal (Fig. 1A; insulin+ cells (0.01 ± 0.01% in 5000 cells), glucagon+ cells (83 ± 4.4 % in 2000 cells), somatostatin+ cells (0.01 ± 0.01% in 200 cells), n=4 mice).
Figure 1. α-cell specific recombination, fed hyperglycemia and hyperglucagonemia in αIRKO mice.
(A) Two individual islets from pancreas sections of glucagon-Cre/ROSA26-LacZ mice are shown. Bar= 100 μm. Magnification: X40. (B) Recombination of insulin receptor in hypothalamus assessed by RT-PCR. Positive control, βIRKO β-cells; Negative control, LoxLox β-cells. (C) Food intake in 2 and 6 month-old male mice. n=6 in each group. (D) Body weights in 6 month-old male and female mice. n=8–12 in each group. (E) Blood glucose, (F) plasma insulin, and (G) plasma glucagon were measured after an overnight (16 h) fast or in random-fed states in 2, 5, and 12 month-old males. n=6–8 in each group. (H) Insulin/glucagon ratio. Control: empty bar; αIRKO: filled bar. Data are expressed as means ± SEM, *, p<0.05; control versus αIRKO.
No significant expression of β-gal was detected in the hypothalamus or other regions of the brain (data not shown). Further, RT-PCR of RNA extracted from hypothalamus revealed no recombination product in the hypothalamus of αIRKO mice (Fig. 1B). Consistent with these data food intake (Fig. 1C) and body weights (Fig. 1D) were not significantly different between groups. Interestingly, we observed β-gal in a small number of cells (<5%) in the small intestine, indicating some recombination in L-cells that are known to secrete glucagon like-peptide-1 (data not shown). However, the plasma GLP-1 levels were unaltered between genotypes (8 month-old males, random fed; control 4.00 ± 1.64 pM, αIRKO 3.61 ± 0.43 pM; female control 3.94 ± 0.87 pM, αIRKO 4.15 ± 0.65 pM; p=NS; n=3–5 in each group), suggesting the small amount of recombination did not impact secretion of GLP-1.
αIRKO mice exhibit hyperglycemia, hyperglucagonemia and glucose intolerance
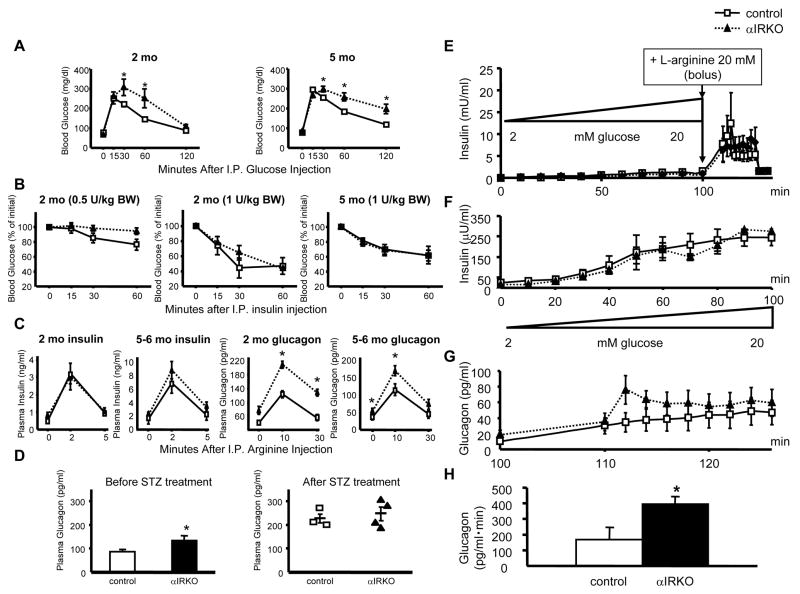
αIRKO mice exhibited up to 50% higher blood glucose levels compared to controls in the fed state, while no significant differences were observed in fasting blood glucose levels between groups (Fig. 1E). Two month-old male αIRKO mice also showed higher levels of plasma glucagon compared to controls in both fasted and fed states, and the hyperglucagonemia persisted only in the fed state at older ages (Fig. 1G). While no significant differences in plasma insulin levels were detected between groups in the fasting state, αIRKO mice exhibited elevated plasma insulin levels at 5 and 12 months of age in the fed state (Fig. 1F). Further, the insulin/glucagon ratio was relatively stable in the controls in both fasted and fed states compared to an altered ratio in the fed state in the αIRKOs (Fig. 1H). Intra-peritoneal (IP) glucose tolerance tests (IPGTT) revealed glucose intolerance in αIRKO mice at 2 and 5 months of age (Fig. 2A). The mutants exhibited normal whole body insulin sensitivity, as assessed by an intra-peritoneal insulin tolerance tests (IPITT) at 1 U/kg BW insulin. However, we observed a tendency to insulin resistance at a lower dose of insulin (0.5 U/kg BW; p=0.07 at 60 min) (Fig. 2B) suggesting an unmasking of glucagon induced hepatic insulin resistance. Further, 30 min after the insulin injection in 2 month-old mice (1 U/kg BW), plasma glucagon was significantly higher in the knockouts (control 219.9 ± 40.2 pg/ml, αIRKO 359.0 ± 41.7 pg/ml n=3–5 in each group; p<0.05), while blood glucose and plasma insulin levels were comparable between groups (data not shown). Together, these data indicate abnormalities in the regulation of glucagon secretion leading to altered glucose homeostasis.
Figure 2. Mild glucose intolerance and enhanced glucagon secretion in αIRKO mice.
(A) Glucose tolerance in 2 and 5 month-old male mice. n=3–12 in each group. (B) Whole body insulin sensitivity (at insulin doses, 0.5 and 1 U/kg BW) in 2 and 5 month-old male mice. n=3–5 in each group. (C) Insulin and glucagon responses to L-arginine were assessed by intra-peritoneal L-arginine stimulation test in 2 and 5–6 month-old male mice. n=4–6 in each group. (D) Plasma glucagon was assessed in the random fed state before and after streptozotocin treatment. n=6–7 in each group before treatment, n=3–4 after treatment. (E, F) Insulin and (G, H) glucagon secretion were examined by ex vivo whole pancreas perfusion. Control: empty square; αIRKO: filled triangle. Data are expressed as means ± SEM, *, p<0.05; control versus αIRKO.
Enhanced L-arginine stimulated glucagon secretion in αIRKO mice
Since L-arginine stimulates both α-cells and β-cells (Unger et al., 1970), we examined hormone secretion in vivo in response to L-arginine stimulation in 2 and 5–6 month-old mice. As expected, L-arginine significantly stimulated insulin secretion in both groups and no significant differences were observed (Fig 2C, left panels). Consistent with loss of insulin signaling in α-cells, we observed significantly higher glucagon secretion in αIRKO mice compared to controls (Fig. 2C, right panels).
To assess the effects of L-arginine, independent of systemic insulin sensitivity and neural influence, we performed ex vivo whole pancreas perfusion experiments. In situ pancreas perfusions were performed in αIRKO mice in which the glucose concentration in the perfusate was increased progressively from 2 to 20 mM over 100 min. At this point 20 mM L-arginine was added to the perfusion in the continued presence of 20 mM glucose for an additional 26 min. We observed no significant difference between groups in glucose-stimulated insulin secretion (Fig. 2E, F). However, the increment in glucagon secretion after L-arginine infusion was significantly higher in the mutant mice from 110 min to 126 min (area under curve; Fig. 2G, H), indicating enhanced L-arginine-stimulated glucagon secretion. These data are consistent with the in vivo findings (Fig. 2C), and likely due to an absence of intra-islet insulin-mediated inhibition of glucagon secretion in αIRKO mice. The glucose concentration used in the perifusion model is relatively high (20 mM); in future studies, it will be useful to test the insulin dependency of glucagon suppression in the αIRKOs using a glucose concentration in the physiological range.
Glucagon secretion in STZ-treated mice
Next, we treated both controls and mutants with streptozotocin (STZ) and induced insulin-deficient diabetes characterized by insulinopenia and hyperglycemia (Supplemental Fig. S1). Plasma glucagon was elevated and reached similar levels in both groups (Fig. 2D) supporting the concept that either the insulinopenia or hyperglycemia, or a combination of both enhances glucagon secretion. These data suggest that, in the controls, imposition of insulinopenia and hyperglycemia lead to a glucagon secretion response (Fig. 2D). In the αIRKOs, who are already blind to ambient insulin, the lack of a further increase in plasma glucagon levels that is even higher than controls in response to the hyperglycemia suggests a dominant role for insulin in the suppression of glucagon secretion (Meier et al., 2006). Future studies aimed at long-term therapy of STZ-treated αIRKO mice, with exogenous insulin, to normalize glycemia followed by assessment of glucagon secretory responses to induction of hypoglycemia will be useful to simulate the scenario observed in human patients with long-standing type 1 diabetes undergoing chronic treatment with insulin.
Enhanced glucagon secretion in hyperinsulinemic-hypoglycemic clamp studies in αIRKO mice
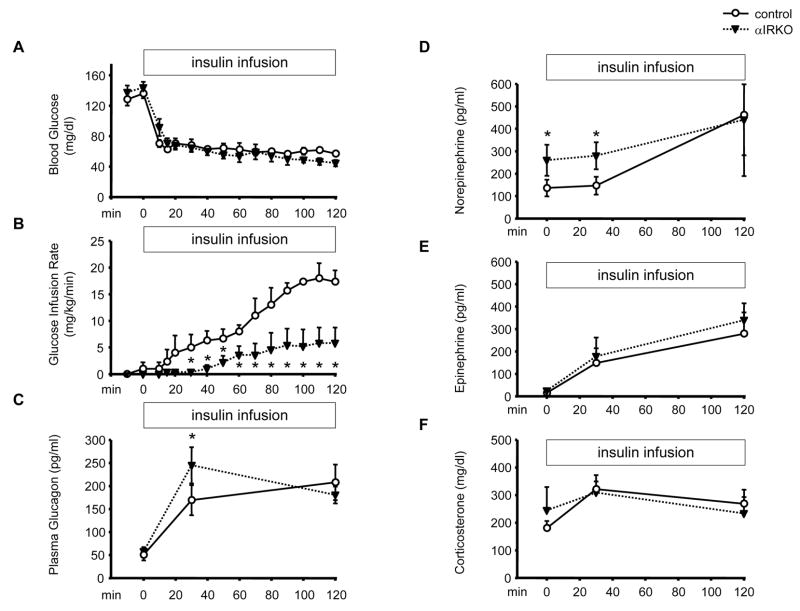
Glucagon plays important roles both in euglycemic and hypoglycemic conditions. To assess the role of insulin signaling in α-cells in vivo during hypoglycemia, we performed hyperinsulinemic-hypoglycemic clamp experiments in 2 month-old αIRKO and control mice (Fig. 3A). Hypoglycemia-stimulated glucagon secretion was significantly higher in αIRKO mice 30 min after initiation of the insulin infusion (Fig. 3C) and may have contributed to the lower glucose infusion rate (GIR) observed in the mutants (Fig. 3B). Furthermore, αIRKO mice exhibited higher norepinephrine levels at baseline and in response to hypoglycemia (Fig. 3D), whereas epinephrine and corticosterone responses to hypoglycemia were not significantly different between groups (Fig. 3E, F). The higher glucagon secretion in αIRKO mice suggests the hyperinsulinemia was unable to suppress glucagon secretion in the mutants to the same extent as the controls due to absence of insulin receptors in the former and supports a direct role for insulin receptors in modulating α-cell secretory function.
Figure 3. Hyperinsulinemic-hypoglycemic clamp and counter-regulatory responses in αIRKO mice.
The mice were continuously infused with insulin and glucose to maintain hypoglycemia. (A) Blood glucose and (B) glucose infusion rate were measured every 10 min. At 0, 30 and 120 min of the clamp experiment, (C) glucagon, (D) norepinephrine, (E) epinephrine and (F) corticosterone response were examined. n=4–5 in each group. Control: empty circle, αIRKO: filled triangle. Data are expressed as means ± SEM. *, p<0.05; control versus αIRKO.
Blunted glucagon response to fasting-induced hypoglycemia in αIRKO mice
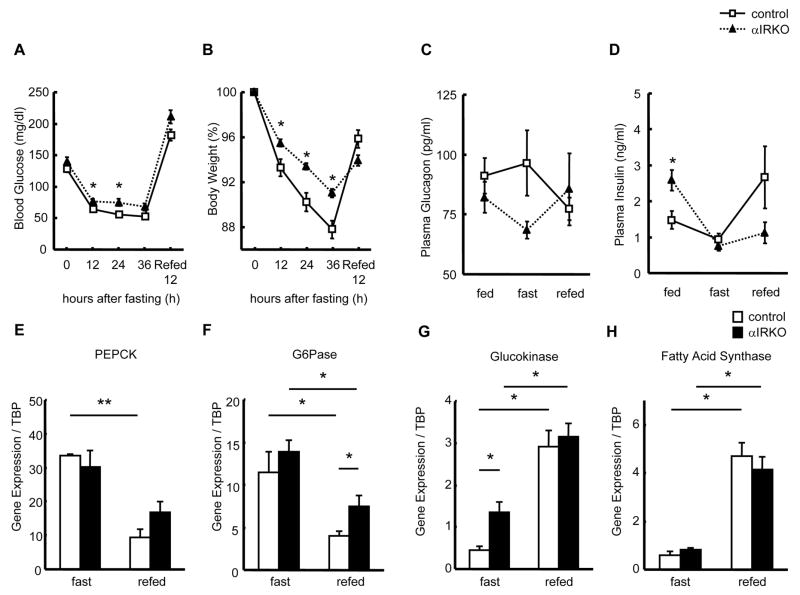
To assess the effect of insulin receptor disruption on α-cell function in response to physiological hypoglycemia, we subjected 6 month-old control and αIRKO mice to fasting and refeeding. Although both groups showed the expected decrease in body weights (Fig. 4B) and blood glucose (Fig. 4A), the αIRKOs exhibited mild hyperglycemia over the fasting period. The control mice exhibited a tendency of increasing plasma glucagon in response to physiological hypoglycemia induced by fasting and decreasing glucagon in the refed state (Fig. 4C) consistent with a decrease in plasma insulin levels during fasting and an increase following refeeding (Fig. 4D). However, the glucagon secretory response to fasting-induced hypoglycemia was not only blunted but was even lower than in the fed state in the αIRKO mice (Fig. 4C), and was followed by a trend to an increase during re-feeding suggesting abnormal secretory responses when insulin signaling is absent in the α-cells (Fig. 4C).
Figure 4. Glycemic parameters and hepatic gene expression in αIRKO mice.
The mice were subjected to 36 h fasting followed with 12 h re-feeding. (A) Blood glucose and (B) body weight were measured every 12 h. n=4–8 in each group. (C) Plasma glucagon and (D) insulin were measured in the fed, 36 h fast, and 12 h re-fed conditions. n=4–8 in each group. Control: empty square, αIRKO: filled triangle. Data are expressed as means ± SEM. *, p<0.05; control versus αIRKO. Hepatic expression of (E) PEPCK, (F) G6Pase, (G) glucokinase, and (H) fatty acid synthase genes were quantified by real-time PCR and normalized to TBP. n=4 in each group. Control: empty bar, αIRKO: filled bar. Data are expressed as means ± SEM, *, p<0.05, **, p<0.01; between indicated groups.
To examine the effect of glucopenia in the absence of hyperglycemia, we treated 6 month-old mice with phloridzin for 14 days. Both groups exhibited a significant decrease in random fed blood glucose levels without a significant alteration in plasma insulin levels (Supplemental Fig. S2). The glucagon response was unaltered between treated and non-treated groups in both groups (Supplemental Fig. S2) suggesting that in the αIRKOs reducing blood glucose levels does not modulate α-cell function further.
Attenuated suppression of hepatic gene expression following fasting and refeeding in αIRKO mice
Since the liver is a major target for glucagon action, we explored alterations in hepatic gene expression patterns in hyperglucagonemic αIRKO mice in fed and fasting conditions, with a focus on hepatic glucose metabolism. Except for a higher glucokinase expression in the αIRKOs, no other significant differences were observed between groups in the expression of genes involved in hepatic glucose metabolism in the fasting state (Fig. 4E–H). Following refeeding, both groups showed the expected patterns of a decrease in phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase) (Fig. 4E, F). However, the decrease in G6Pase was significantly attenuated while suppression of PEPCK was marginal in the αIRKO mice compared to controls (Fig. 4E, F), suggesting one possible explanation for the mild hyperglycemia in αIRKO mice. A significant increase in glucokinase and fatty acid synthase following refeeding (Fig. 4G, H) indicated a largely intact hepatic response in the mutant animals.
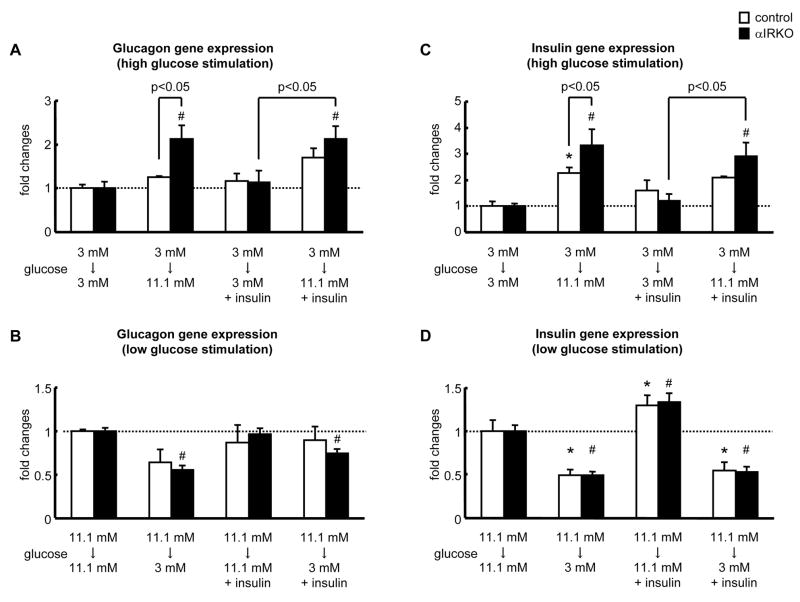
Alterations in glucagon and insulin gene expression in isolated islets from αIRKO mice
Our in vivo results clearly suggest a role for insulin signaling in the regulation of glucagon secretion. To examine the potential effects at the transcriptional level we studied insulin and glucagon gene expression in response to glucose stimulation (3 or 11 mM) of islets isolated from αIRKOs and controls. High glucose stimulated glucagon gene expression significantly in αIRKO islets but not in controls (Fig. 5A) suggesting that glucose-stimulated increase in glucagon gene expression is higher due to lack of a tonic suppressive effect of intra-islet insulin in α-cells (Philippe, 1989). Furthermore, this increase in glucagon gene expression persisted even in the presence of 100 nM insulin only in αIRKO islets (Fig. 5A). On the other hand, glucagon gene expression in response to low glucose stimulation was significantly reduced in αIRKO islets, while causing a non-significant change in the control group (Fig. 5B). Taken together, these data indicate that insulin signaling in the α-cells regulates glucagon gene expression in both high- and low-glucose conditions.
Figure 5. Impact of α-cell specific insulin receptor disruption on insulin and glucagon gene expression.
(A, C) Effect of incubating islets from low (3 mM) to high (11.1 mM) glucose; or (B, D) high (11.1 mM) to low (3 mM) glucose on glucagon (A, B) or insulin gene expression (C, D). Fold changes were calculated relative to controls. n=3 each in each group. Control: empty bar, αIRKO: filled bar. Data are expressed as means ± SEM. *, p<0.05; versus 3 mM -3 mM control group (C), versus 11 mM - 11 mM control group (D). #, p<0.05; versus 3 mM - 3 mM αIRKO group (A, C), versus 11 mM - 11 mM αIRKO group (B, D).
High glucose stimulated and low glucose inhibited insulin gene expression to comparable levels in control and αIRKO islets (Fig. 5C, D). Interestingly, αIRKO islets exhibited a significantly higher insulin gene expression in response to high glucose stimulation, and the addition of 100 nM insulin to the high glucose stimulation media further enhanced insulin gene expression in the αIRKO islets, but not in the controls, suggesting that β-cells in αIRKO islets are more sensitive to insulin (Fig. 5C).
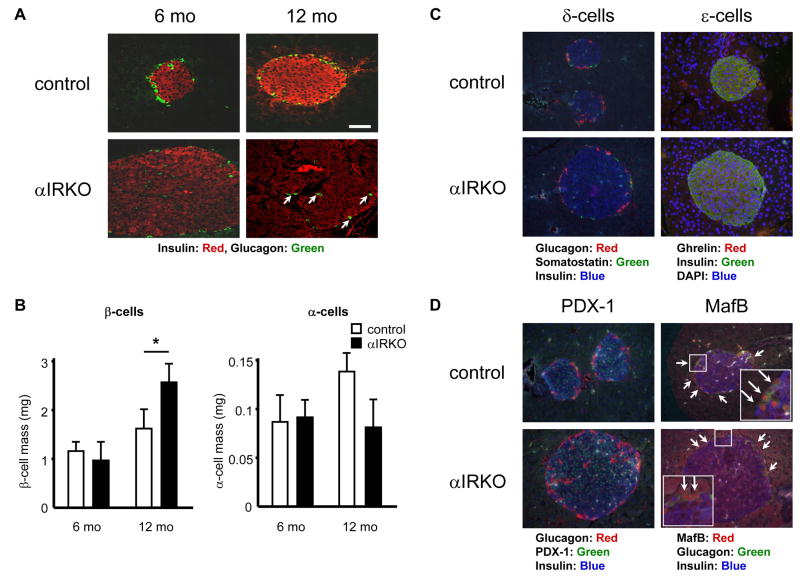
Progressive increase in β-cell mass and relative decrease in α-cell mass in αIRKO mice
To determine if insulin signaling in α-cells plays a role in α-cell growth and proliferation, we assessed pancreatic islet morphology in 6 and 12 month-old animals (Fig. 6A). Although αIRKO mice exhibited a relatively normal islet architecture with α-cells distributed in the periphery, we observed an age-dependent hyperplasia compared to controls (Fig. 6A). Further, the trend to an age-dependent increase in α-cell area in the control islets was absent in the αIRKOs (Fig. 6B). Interestingly, the β-cell area in 12 month-old αIRKO mice was significantly increased compared to controls (Fig. 6B) suggesting that altered insulin signaling in α-cells has indirect effects on β-cell proliferation. The number and distribution of δ-cells were unaltered, and ghrelin positive cells were not detectable in the islets from either control or αIRKO mice (Fig. 6C). The presence of ghrelin positive cells in neonatal pancreas confirmed a working anti-ghrelin antibody (Supplemental Fig. S3). Immunostaining for PDX-1 and MafB, two transcription factors shown to be important in β- and α-cell development, also revealed no significant difference between groups in 12 month-old mice (Fig. 6D). Gene array analyses of islets from 3 and 10 month-old mice using PancChip (Scearce et al., 2002) also revealed no significant differences in the expression of transcription factors known to be important for islet cell development except for a mild increase in Pax4 in old αIRKO mice (Supplemental Table 1).
Figure 6. Pancreas morphometry in αIRKO mice.
Pancreas sections were immunostained as indicated (A, C, D; Bar= 50 μm. Magnification: X40). (B) β- and α-cell mass were quantified at 6 and 12 month-old. n=4–5 in each group. Somatostatin, ghrelin, PDX-1 and MafB were immunostained in 12 month-old controls and mutants. Control: empty bar, αIRKO: filled bar. Data are expressed as means ± SEM, *, p<0.05; control versus αIRKO.
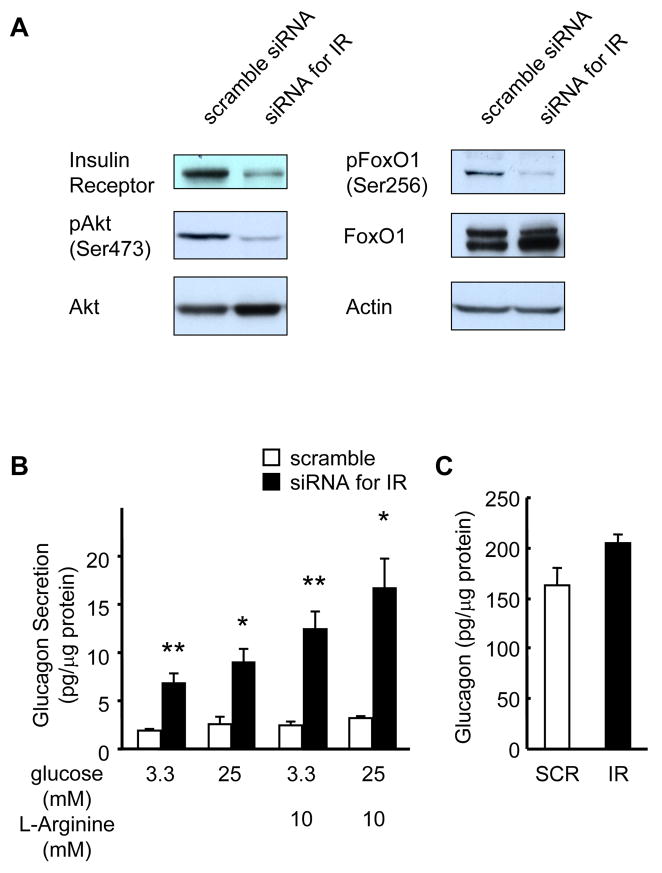
Knockdown of insulin receptors in InR1G cells promotes enhanced glucagon secretion
Glucagon-secreting InR1G cells were transfected with pre-designed siRNA for insulin receptor or scrambled control RNA, leading to ~85% knockdown of insulin receptor protein in the siRNA treated groups (Fig. 7A). Both p-Akt/PKB and p-FoxO1 were significantly reduced in InR1G cells treated with siRNA for insulin receptor consistent with the knockdown (Fig. 7A).
Figure 7. Enhanced glucagon secretion in InR1G cells with insulin receptor knockdown.
(A) Western blots for insulin receptor, total and phospho-Akt and FoxO1 and normalized for actin. Representative of 3 independent experiments. (B) Glucagon secretion was assessed by static incubation for 60 min and expressed per mg of total protein. n=6 in each group. (C) Total protein content and total glucagon content. n=3 in each group. Empty bar: scrambled siRNA; Filled bar: siRNA for insulin receptor. Data are expressed as means ± SEM, *, p<0.05, **, p<0.01; scramble versus siRNA for insulin receptor.
At both low (3.3 mM) and high (25 mM) glucose concentrations, InR1G cells with a knockdown of insulin receptor secreted more glucagon compared to control scramble siRNA transfected cells (Fig. 7B). Next, we examined glucagon secretion in response to L-arginine (10 mM), a known stimulator of glucagon secretion by α-cells (Unger et al., 1970). Again, siRNA for insulin receptor treated InR1G cells secreted significantly more glucagon compared to controls (Fig. 7B), while the glucagon content showed a trend to an increase but did not reach statistical significance compared to controls (Fig. 7C). These findings support a role for insulin signaling in glucagon secretion in response to glucose and L-arginine but not glucagon biosynthesis in InR1G cells, consistent with a direct role for insulin signaling in the regulation of glucagon secretion by α-cells.
Discussion
To directly examine whether insulin signaling modulates glucagon secretion in vivo, we created and characterized mice with a conditional knockout of the insulin receptor in pancreatic α-cells (αIRKO mice). Adult male αIRKO mice exhibited hyperglucagonemia, glucose intolerance, and fed hyperglycemia, and an elevated glucagon response to L-arginine stimulation. Hyperinsulinemic-hypoglycemic clamp studies revealed an enhanced glucagon secretory response in αIRKO mice. These in vivo findings were complemented by enhanced glucagon secretion in siRNA-mediated knockdown of the insulin receptor in InR1G cells. Together, these data provide direct genetic evidence for a significant role for the insulin receptor in the modulation of pancreatic α-cell function.
The regulation of glucagon secretion involves a complex interplay of signals including glucose, intra-islet paracrine factors, and the central and autonomic nervous systems (Gromada et al., 2007). Insulin, secreted from β-cells has been proposed as one of the intra-islet paracrine factors that can modulate the secretion of glucagon by neighboring α-cells (Asplin et al., 1981; Maruyama et al., 1984; Weir et al., 1976). Although several ex vivo (e.g. isolated islets) and/or in vitro (e.g. dispersed islet cells) approaches (reviewed in (Cryer, 1994; Gromada et al., 2007)) have been used to address the role of intra-islet insulin, these studies do not mimic the in vivo situation where the direction of blood flow from β-cells can potentially influence the function of α-cells located downstream (Bonner-Weir and Orci, 1982; Samols and Stagner, 1988). The αIRKO mouse model circumvents these limitations and provides direct in vivo evidence that disrupting insulin signaling in α-cells leads to hyperglucagonemia and glucose intolerance. The relatively mild hyperglucagonemia in the αIRKOs did not translate to major defects in hepatic gene expression patterns in the non-stressed mouse. However, subjecting the αIRKOs to fasting stress led to a significant attenuation of the decrease in expression of hepatic glucokinase, while after refeeding, the decrease in hepatic G6Pase expression was also significantly lower compared to the response in controls. It is possible that a lack of appropriate suppression of glucokinase and G6Pase, that are both important for hepatic glucose metabolism (Barthel and Schmoll, 2003) contributed, in part, to the glycemic perturbations in the αIRKOs.
Intra-islet insulin has been implicated in the glucagon counterregulatory response that is necessary to prevent hypoglycemia (Ahren, 2000; Gromada et al., 2007). To directly assess the role of intra-islet insulin in the αIRKO model we used two paradigms of hypoglycemia. Subjecting the mice to hyperinsulinemic hypoglycemia revealed significantly reduced glucagon release in controls compared to αIRKOs suggesting that the concomitant elevation in insulin suppresses the α-cell secretory response to hypoglycemia in the controls but not in αIRKOs. The lack of suppression in the αIRKOs is likely due to absent insulin signaling in α-cells that in turn allows a greater glucagon secretory response to hypoglycemia despite lower sympathetic (i.e. norepinephrine) tone. The similar rise in epinephrine levels in the two groups indicates the effects of the experimental hypoglycemia on α-cell secretion are independent of circulating epinephrine (Gromada et al., 2007). It is also possible that the additional deficiency in epinephrine-induced counter-regulatory defense against hypoglycemia, that is observed in many patients with type 1 diabetes (Heller and Cryer, 1991; Mokan et al., 1994; Powell et al., 1993), is due to chronic hypoglycemia and is not fully manifest in the αIRKO mice. The lower norepinephrine (basal and hypoglycemic) response in the αIRKOs suggests that insulin signaling in α-cells potentially signals to the brain to recruit the sympathetic nervous system to participate in the hypoglycemic response (Gromada et al., 2007). However, it is worth noting that changes in systemic levels of norepinephrine have been reported to be the result of ‘spill-over’ and are considered non-discriminatory in clamp studies (Evans et al., 2004; McCrimmon et al., 2005). The αIRKO mouse provides a useful model to evaluate if additional neuronal signals can contribute to counter-regulatory glucagon responses in the absence of the effects of intra-islet insulin. Glucose infusion rates (GIR) are typically expected to be low during hypoglycemic clamps when compared to euglycemic clamps (Jacobson et al., 2006), due to the combined effects of the decrease in the mass action of glucose (suppress the liver glucose production and augment muscle glucose uptake), and of the increased counteregulatory hormones opposing insulin action. The lower GIR in the αIRKO mice during the hypoglycemic clamp may be due, in part, to hepatic insulin resistance induced by higher glucagon responses. It is conceivable that insulin sensitivity is lower as well and studies with euglycemic clamps may shed further light on this phenomenon.
In a second paradigm, when hypoglycemia was induced by fasting but without a substantial decrease in insulin levels, the circulating glucagon levels did not drop in controls. These data suggest that a “switch-off” mechanism (Hope et al., 2004; Zhou et al., 2004) may not operate in vivo, and the recognition of a significant decrease in circulating insulin, by insulin receptors in α-cells, is necessary for an appropriate glucagon secretory response to hypoglycemia. The αIRKO mouse would be a useful tool to further distinguish the significance of glucose versus somatostatin in the intra-islet control of α-cell secretion (Greenbaum et al., 1991).
The alterations in glucagon gene expression in the αIRKO islets exposed to different concentrations of glucose indicates a regulatory role for insulin signaling in α-cells at the level of transcription consistent with previous studies (Gonzalez et al., 2008; Philippe, 1989). Since glucose is an important physiological suppressor of glucagon secretion in vivo (Gromada et al., 2007) the paradoxical stimulation of glucagon secretion by high glucose (Salehi et al., 2006) has led to the suggestion that additional modulator(s) exist for glucagon suppression in vivo. Our observations of enhanced glucagon secretion in InR1G cells with a knockdown of insulin receptors complements our in vivo findings and indicates that the additional modulator is insulin itself or that the putative modulator requires a functional insulin receptor for its effects on α-cells. Although InR1G cells do not secrete insulin (Drucker et al., 1988), it is possible that the insulin present in growth media used to routinely culture and maintain the cells has a suppressive effect on glucagon secretion that is unmasked in the controls but not in the knockdown cells. Further support for a direct role for insulin in the regulation of α-cell function is provided by the observation that glucagon secretion is abolished in response to low glucose in isolated islets and in αTC6 cells expressing nsulin receptor siRNA (Diao et al., 2005).
Surprisingly, we observed an increase in β-cell mass and a decrease in α-cell mass in the αIRKO mice. Given the potential role for glucagon in the development of β-cells (Prasadan et al., 2002; Vuguin et al., 2006), it is conceivable that altered α-cell function in the αIRKO mice directly or indirectly influences β-cell proliferation in the mutants. On the other hand, the reduced α-cells in older αIRKO mice may be secondary to attenuation of insulin signaling-mediated anti-apoptosis leading to increased α-cell death, an observation that is similar to the reduced β-cell mass in β-cell specific insulin receptor knockout mice (Kulkarni et al., 1999a). Alternatively, α- to β-cell trans-differentiation, that has been suggested to involve the transcription factor aristaless-related homeobox (Arx) (Collombat et al., 2003), may induce an increase in β-cells at the expense of α-cells. PancChip analyses of islets (Scearce et al., 2002) (Supplemental Table 1) revealed no significant differences between groups in the expression of transcription factors, including PDX-1 and MafB, that are important for proliferation and/or development of islets cells except for a marginal increase in Pax-4 in the αIRKOs. Further investigation during early development and post-natal periods is necessary to examine the potential role of Pax4 in islet cell fate specification in the αIRKO mice. Alternatively, lineage trace analyses could be useful to explore whether the new β-cells in the αIRKOs emerge from irreversibly marked α-cells lacking growth factor signaling. Finally, it will be worth exploring the individual contributions of insulin versus IGF-I receptors and proteins in their signaling pathways to the α-cell hyperplasia that is observed in glucagon resistant models (Chen et al., 2005; Gelling et al., 2003).
In summary, we provide direct genetic evidence for a role for insulin signaling in the regulation of α-cell function in vivo. We propose that insulin resistance in the α-cell contributes to dysregulation of glucagon secretion in altered glycemic states. These data provide a basis for developing therapeutic approaches aimed at modulating α-cell growth and function with the long-term goal of improving glucose homeostasis in patients with T1DM and T2DM.
Experimental Procedures
Animal and physiological experiments
Mice were housed in pathogen-free facilities and maintained on a 12 h light/dark cycle at the Animal Care Facility of Joslin Diabetes Center, Boston, MA, and the Foster Biomedical Research Laboratory, Brandeis University, Waltham, MA. All protocols were approved by the Institutional Animal Care and Use Committee of the Joslin Diabetes Center and Brandeis University and were in accordance with NIH guidelines. Blood glucose was monitored using an automated glucose monitor (Glucometer Elite, Bayer), plasma insulin by ELISA (Crystal Chem), plasma glucagon by RIA (Linco), and plasma GLP-1 by ELISA (Linco). Fasting blood sampling and L-arginine stimulation tests were performed 16 h after fasting. Glucose and insulin tolerance tests were performed as described previously (Kulkarni et al., 1999a). For L-arginine stimulation tests, blood samples were collected before and after I.P. L-arginine injection (3 g/kg b. wt) at 2 and 5 min for insulin, and at 10 and 30 min for glucagon. Streptozotocin (STZ; Sigma, 75 mg/kg. b. wt) was injected in 3 month-old mice for 4 days. Random fed blood samples were monitored one day before, and 7 and 14 days after the first STZ injection.
Insulin secretion from the in situ-perfused pancreas
The pancreas was perfused in situ in a humidified temperature-controlled chamber using a modification of a protocol described previously (Pontoglio et al., 1998). After 100 min, 20 mM L-arginine was perfused in the continued presence of 20 mM glucose for an additional 26 min. The increase in glucagon secretion after L-arginine infusion was calculated as area under curve of the absolute increase in glucagon between 110 min and 126 min.
Hyperinsulinemic-hypoglycemia clamp and assessment of counter-regulatory hormones
The hyperinsulinemic-hypoglycemic clamps were performed using a modification of a protocol described previously (Jacobson et al., 2006). The mice were subjected to clamps under conscious and unstressed conditions after 6 h fasting. Blood glucose level was maintained low with a target value ~50 mg/dl for 120 min with insulin infusion at a rate of 10 mU/kg/min. At 0, 30, and 120 mins, blood was collected for measurement of counter-regulatory hormones for hypoglycemia including glucagon (RIA), epinephrine, norepinephrine (HPLC) and corticosterone (RIA, Linco) (Jacobson et al., 2006).
Fasting experiments
Mice were fasted for 36 h, and blood glucose and body weights were measured every 12 h. Blood samples were obtained before and 36 h after fasting, and 12 h after re-feeding. Thirty-six h after fasting or 12 h after re-feeding, the mice were sacrificed, and livers were harvested, and stored at −80 °C for extraction of RNA.
Assessment of recombination and gene expression
Islets were obtained by collagenase digestion (Kulkarni et al., 1999a). Total RNA from hypothalamus and isolated islets was extracted using RNeasy Mini Kit (QIAGEN). RNA from β-cell lines (Supplemental Methods) and liver was extracted by Trizol method (Invitrogen). cDNA samples were generated by high-capacity cDNA Archive Kit (Applied Biosystems). Recombination of insulin receptor was detected as described previously (Kulkarni et al., 1999a). In isolated islet studies, gene expression was assessed in healthy hand-picked islets, after 48 h culture (Kulkarni et al., 1999a). Batches of 20 healthy size-matched islets were pre-incubated in RPMI-1640 media containing 0.1 % BSA and low (3 mM) or high (11.1 mM) glucose for 2 h. Subsequently, the islets were incubated in media containing 0.1 % BSA, high (preincubation: 3 mM glucose 2 h, stimulation: 11.1 mM glucose 8 h) or low (preincubation: 11.1 mM glucose 2 h, stimulation: 3 mM glucose 8 h) glucose with or without 100 nM insulin. Gene expression for insulin, glucagon and hepatic enzymes was determined by quantitative Real-Time PCR (Raeymaekers, 2000) using appropriate primers (Supplemental Methods), and normalized for TBP (liver) or actin (islet).
Pancreas morphometry
The mice were anesthetized, and pancreas was rapidly dissected, weighed and processed as described previously (Kulkarni et al., 1999a) and immunostained for insulin (Linco), glucagon (Sigma), somatostatin (DAKO), ghrelin (Phoenix Pharmaceuticals), PDX-1 (Chemicon), MafB (Bethyl Laboratories), and β-galactosidase (MP Biomedicals) followed by amplification by TSA Fluorescence systems (Perkin Elmer) (Morioka et al., 2007). α- and β-cell area were analyzed as described previously (Kulkarni et al., 2002).
Cell culture, transfection of siRNA and glucagon secretion analyses
Glucagon secreting InR1G cells (a kind gift from Dr. J. Philippe) were cultured as described (Philippe, 1989) and all experiments were performed in cells between passages 6 through 9. Forty-eight h before transfection, InR1G cells were replated in 12-well plates/60 mm dishes and transfected with siRNA for insulin receptor (SMARTpool; Dharmacon) or scramble controls (Silencer Negative Control #1; Ambion) using LipofectAMINE 2000 reagent (Invitrogen). After a further 48 h culture in RPMI-1640 media cells were harvested for hormone secretion or protein expression studies. Cellular glucagon content was assessed by acid ethanol extraction (Kulkarni et al., 1999b). The protein content was measured by BCA protein assay (Pierce) and glucagon was measured by RIA (Linco).
Western Blotting
Fifty μg each of cellular protein extracts were used (Kawamori et al., 2006). Antibodies used; rabbit anti-insulin receptor β-chain and goat anti-actin antibody (Santa Cruz Biotechnology), rabbit anti-Akt antibody, anti-Ser473-phospho-specific Akt antibody, anti-FoxO1 antibody, and anti-Ser256-phospho-specific FoxO1 antibody (Cell Signaling Technology), anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology), anti-goat IgG HRP-conjugated secondary antibody (Zymed Laboratories). Relative protein amounts were estimated by densitometry (Fluorchem IS-8000 image analyzer; Alpha Innotech).
Statistics
All data are presented as mean ± S.E.M., and analyzed using an unpaired 2-tailed Student’s t test or analysis of variance (ANOVA) and post hoc tests as appropriate. A P value less than 0.05 was considered significant.
Acknowledgments
We thank Dr. Jacques Philippe (Geneva, Switzerland) for InR1G cells, Dr. K.C. Hayes (Brandeis University, MA) for housing of mice, Drs. Pierre Lefèbvre (University of Liège, Belgium) and Ryo Suzuki (Joslin) for discussions, Drs. Wataru Nishimura and Arun J. Sharma (Joslin) for neonatal pancreas sections, Hui Li (Specialized Assay Core, DERC, Joslin) for assays, and Lindsay Huse for excellent assistance with manuscript preparation. We especially acknowledge the valued and continued support of Cathy and Stan Bernstein for the work in the Kulkarni lab. D. K. is the recipient of a Research Fellowship (Manpei Suzuki Diabetes Foundation, Japan), and a JDRF Post-doctoral Fellowship. This work was supported in part by an American Diabetes Association Research Grant 7-04-RA-55 (R.N.K.) and 5P30DK36836 (Joslin DERC Specialized Assay and Advanced Microscopy Cores), the Swiss National Science Foundation (P.L.H.), the NIH/NIDDK (Beta Cell Biology Consortium) (P.L.H.), JDRF (P.L.H.), the European Union (6th F.P.) (P.L.H.), NIH DK31842 and DK56341 (Washington University DRTC) (K.S.P.), UL1RR024992 (Washington University CTSA) (K.S.P.), DK59637 (Vanderbilt MMPC) (O.P.M), and DK20593 (Vanderbilt DRTC) (O.P.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV. Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes. 1988;37:901–907. doi: 10.2337/diab.37.7.901. [DOI] [PubMed] [Google Scholar]
- Asplin CM, Paquette TL, Palmer JP. In vivo inhibition of glucagon secretion by paracrine beta cell activity in man. J Clin Invest. 1981;68:314–318. doi: 10.1172/JCI110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2003;285:E685–692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982;31:883–889. doi: 10.2337/diab.31.10.883. [DOI] [PubMed] [Google Scholar]
- Chen M, Gavrilova O, Zhao WQ, Nguyen A, Lorenzo J, Shen L, Nackers L, Pack S, Jou W, Weinstein LS. Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gs alpha deficiency. J Clin Invest. 2005;115:3217–3227. doi: 10.1172/JCI24196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer PE. Banting Lecture. Hypoglycemia: the limiting factor in the management of IDDM. Diabetes. 1994;43:1378–1389. doi: 10.2337/diab.43.11.1378. [DOI] [PubMed] [Google Scholar]
- DCCT. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46:271–286. [PubMed] [Google Scholar]
- Diao J, Asghar Z, Chan CB, Wheeler MB. Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic alpha-cells. J Biol Chem. 2005;280:33487–33496. doi: 10.1074/jbc.M506276200. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Philippe J, Mojsov S. Proglucagon gene expression and posttranslational processing in a hamster islet cell line. Endocrinology. 1988;123:1861–1867. doi: 10.1210/endo-123-4-1861. [DOI] [PubMed] [Google Scholar]
- Evans ML, McCrimmon RJ, Flanagan DE, Keshavarz T, Fan X, McNay EC, Jacob RJ, Sherwin RS. Hypothalamic ATP-sensitive K + channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes. 2004;53:2542–2551. doi: 10.2337/diabetes.53.10.2542. [DOI] [PubMed] [Google Scholar]
- Exton JH, Jefferson LS, Jr, Butcher RW, Park CR. Gluconeogenesis in the perfused liver. The effects of fasting, alloxan diabetes, glucagon, epinephrine, adenosine 3′,5′-monophosphate and insulin. Am J Med. 1966;40:709–715. doi: 10.1016/0002-9343(66)90151-3. [DOI] [PubMed] [Google Scholar]
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Gerich JE, Lorenzi M, Schneider V, Kwan CW, Karam JH, Guillemin R, Forsham PH. Inhibition of pancreatic glucagon responses to arginine by somatostatin in normal man and in insulin-dependent diabetics. Diabetes. 1974;23:876–880. doi: 10.2337/diab.23.11.876. [DOI] [PubMed] [Google Scholar]
- Gerich JE, Tsalikian E, Lorenzi M, Schneider V, Bohannon NV, Gustafson G, Karam JH. Normalization of fasting hyperglucagonemia and excessive glucagon responses to intravenous arginine in human diabetes mellitus by prolonged infusion of insulin. J Clin Endocrinol Metab. 1975;41:1178–1180. doi: 10.1210/jcem-41-6-1178. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Boer U, Dickel C, Quentin T, Cierny I, Oetjen E, Knepel W. Loss of insulin-induced inhibition of glucagon gene transcription in hamster pancreatic islet alpha cells by long-term insulin exposure. Diabetologia. 2008;51:2012–2021. doi: 10.1007/s00125-008-1134-5. [DOI] [PubMed] [Google Scholar]
- Greenbaum CJ, Havel PJ, Taborsky GJ, Jr, Klaff LJ. Intra-islet insulin permits glucose to directly suppress pancreatic A cell function. J Clin Invest. 1991;88:767–773. doi: 10.1172/JCI115375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40:223–226. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP. Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the “switch-off” hypothesis. Diabetes. 2004;53:1488–1495. doi: 10.2337/diabetes.53.6.1488. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Ansari T, McGuinness OP. Counterregulatory deficits occur within 24 h of a single hypoglycemic episode in conscious, unrestrained, chronically cannulated mice. Am J Physiol Endocrinol Metab. 2006;290:E678–684. doi: 10.1152/ajpendo.00383.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamori D, Kaneto H, Nakatani Y, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem. 2006;281:1091–1098. doi: 10.1074/jbc.M508510200. [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999a;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR. beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat Genet. 2002;31:111–115. doi: 10.1038/ng872. [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Winnay JN, Daniels M, Bruning JC, Flier SN, Hanahan D, Kahn CR. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. J Clin Invest. 1999b;104:R69–75. doi: 10.1172/JCI8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung YM, Ahmed I, Sheu L, Gao X, Hara M, Tsushima RG, Diamant NE, Gaisano HY. Insulin regulates islet alpha-cell function by reducing KATP channel sensitivity to adenosine 5′-triphosphate inhibition. Endocrinology. 2006;147:2155–2162. doi: 10.1210/en.2005-1249. [DOI] [PubMed] [Google Scholar]
- Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I, Binnert C, Beermann F, Thorens B. Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors. J Clin Invest. 2005;115:3545–3553. doi: 10.1172/JCI26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest. 1984;74:2296–2299. doi: 10.1172/JCI111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon RJ, Evans ML, Fan X, McNay EC, Chan O, Ding Y, Zhu W, Gram DX, Sherwin RS. Activation of ATP-sensitive K+ channels in the ventromedial hypothalamus amplifies counterregulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes. 2005;54:3169–3174. doi: 10.2337/diabetes.54.11.3169. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Kjems LL, Veldhuis JD, Lefebvre P, Butler PC. Postprandial suppression of glucagon secretion depends on intact pulsatile insulin secretion: further evidence for the intraislet insulin hypothesis. Diabetes. 2006;55:1051–1056. doi: 10.2337/diabetes.55.04.06.db05-1449. [DOI] [PubMed] [Google Scholar]
- Mokan M, Mitrakou A, Veneman T, Ryan C, Korytkowski M, Cryer P, Gerich J. Hypoglycemia unawareness in IDDM. Diabetes Care. 1994;17:1397–1403. doi: 10.2337/diacare.17.12.1397. [DOI] [PubMed] [Google Scholar]
- Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest. 2007;117:2860–2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Baetens D, Rufener C, Amherdt M, Ravazzola M, Studer P, Malaisse-Lagae F, Unger RH. Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Proc Natl Acad Sci U S A. 1976;73:1338–1342. doi: 10.1073/pnas.73.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe J. Glucagon gene transcription is negatively regulated by insulin in a hamster islet cell line. J Clin Invest. 1989;84:672–677. doi: 10.1172/JCI114214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoglio M, Sreenan S, Roe M, Pugh W, Ostrega D, Doyen A, Pick AJ, Baldwin A, Velho G, Froguel P, et al. Defective insulin secretion in hepatocyte nuclear factor 1alpha-deficient mice. J Clin Invest. 1998;101:2215–2222. doi: 10.1172/JCI2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AM, Sherwin RS, Shulman GI. Impaired hormonal responses to hypoglycemia in spontaneously diabetic and recurrently hypoglycemic rats. Reversibility and stimulus specificity of the deficits. J Clin Invest. 1993;92:2667–2674. doi: 10.1172/JCI116883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasadan K, Daume E, Preuett B, Spilde T, Bhatia A, Kobayashi H, Hembree M, Manna P, Gittes GK. Glucagon is required for early insulin-positive differentiation in the developing mouse pancreas. Diabetes. 2002;51:3229–3236. doi: 10.2337/diabetes.51.11.3229. [DOI] [PubMed] [Google Scholar]
- Raeymaekers L. Basic principles of quantitative PCR. Mol Biotechnol. 2000;15:115–122. doi: 10.1385/MB:15:2:115. [DOI] [PubMed] [Google Scholar]
- Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes. 2005;54:1789–1797. doi: 10.2337/diabetes.54.6.1789. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Berggren PO, Bokvist K, Ericson H, Mohler H, Ostenson CG, Smith PA. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341:233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- Salehi A, Vieira E, Gylfe E. Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes. 2006;55:2318–2323. doi: 10.2337/db06-0080. [DOI] [PubMed] [Google Scholar]
- Samols E, Stagner JI. Intra-islet regulation. Am J Med. 1988;85:31–35. doi: 10.1016/0002-9343(88)90395-6. [DOI] [PubMed] [Google Scholar]
- Scearce LM, Brestelli JE, McWeeney SK, Lee CS, Mazzarelli J, Pinney DF, Pizarro A, Stoeckert CJ, Jr, Clifton SW, Permutt MA, et al. Functional genomics of the endocrine pancreas: the pancreas clone set and PancChip, new resources for diabetes research. Diabetes. 2002;51:1997–2004. doi: 10.2337/diabetes.51.7.1997. [DOI] [PubMed] [Google Scholar]
- Scheen AJ, Castillo MJ, Lefebvre PJ. Assessment of residual insulin secretion in diabetic patients using the intravenous glucagon stimulatory test: methodological aspects and clinical applications. Diabetes Metab. 1996;22:397–406. [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stagner JI, Samols E. Retrograde perfusion as a model for testing the relative effects of glucose versus insulin on the A cell. J Clin Invest. 1986;77:1034–1037. doi: 10.1172/JCI112356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism. 1978;27:1691–1709. doi: 10.1016/0026-0495(78)90291-3. [DOI] [PubMed] [Google Scholar]
- Unger RH, Aguilar-Parada E, Muller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970;49:837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Orci L. The role of glucagon in the endogenous hyperglycemia of diabetes mellitus. Annu Rev Med. 1977;28:119–130. doi: 10.1146/annurev.me.28.020177.001003. [DOI] [PubMed] [Google Scholar]
- Vieira E, Salehi A, Gylfe E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia. 2007;50:370–379. doi: 10.1007/s00125-006-0511-1. [DOI] [PubMed] [Google Scholar]
- Vuguin PM, Kedees MH, Cui L, Guz Y, Gelling RW, Nejathaim M, Charron MJ, Teitelman G. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology. 2006;147:3995–4006. doi: 10.1210/en.2005-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Knowlton SD, Atkins RF, McKennan KX, Martin DB. Glucagon secretion from the perfused pancreas of streptozotocin-treated rats. Diabetes. 1976;25:275–282. doi: 10.2337/diab.25.4.275. [DOI] [PubMed] [Google Scholar]
- Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58. doi: 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, Robertson RP. Regulation of alpha-cell function by the beta-cell during hypoglycemia in Wistar rats: the “switch-off” hypothesis. Diabetes. 2004;53:1482–1487. doi: 10.2337/diabetes.53.6.1482. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhang T, Harmon JS, Bryan J, Robertson RP. Zinc, not insulin, regulates the rat alpha-cell response to hypoglycemia in vivo. Diabetes. 2007;56:1107–1112. doi: 10.2337/db06-1454. [DOI] [PubMed] [Google Scholar]