Abstract
A spatial cuing task was used to identify two types of readers, those with a relatively fast and those with a relatively slow buildup of inhibition of return (IOR). Backward-directed eye movements (regressions) during sentence reading were then examined as a function of the two IOR types. The results revealed that readers with fast IOR executed larger regressions than readers with slow IOR, as they directed the eyes away from the most recently attended area of text. Forward-directed eye movements (saccades), by contrast, were not a function of IOR type. Ease of sentence comprehension influenced the size of regressions, but this effect was also independent of IOR type. Multiple mechanisms of spatial attention, including IOR, bias eye movements toward upcoming words in the text during reading.
Effective reading requires linguistic knowledge and the mastery of task-specific skills. Written linguistic symbols are spatially defined and spatially arranged, and readers must establish word order so that sentence and passage meaning can be determined. Because high-acuity vision is confined to a relatively small spatial area, task-specific perceptual skills need to be coordinated with motor skills that move the eyes to upcoming words in the text when they become relevant for sentence comprehension.
Although the vast majority of eye movements (saccades) progress with the text, readers do not necessarily look at (fixate) each word. Skipping short words is relatively common, and some long words receive more than one fixation. Approximately 10% to 25% of saccades (called regressions) move the eyes in the direction opposite to word order (Rayner & Pollatsek, 1989). A covert processing mechanism thus appears to intervene between linguistic processes and overt eye movements. The critical function of this mechanism is to direct attention and linguistic processing to relevant words in the text.
Effective perception during a fixation is biased toward the next words on a line of text (i.e., the words that are next according to the ordering, left to right or right to left, used for reading in the language in question; Inhoff, Pollatsek, Posner, & Rayner, 1989; Pollatsek, Bolozky, Well, & Rayner, 1981), indicating the involvement of forward-directed covert shifts of spatial attention. Information available at adjacent lines is not extracted (Inhoff & Briihl, 1991; Inhoff & Topolski, 1994; Pollatsek, Raney, Lagasse, & Rayner, 1993), further indicating that forward selection of pertinent words goes hand in hand with the rejection of other spatially near but irrelevant information. The current study was motivated by the assumption that the spatial selection of upcoming words during a fixation works in tandem with a complementary backward-inhibiting mechanism that prevents spatial attention from sliding backward. Together, the two covert attentional mechanisms generate a directional preference that propels overt saccades toward new text.
Posner and Cohen (1984) were the first to report evidence for covert spatial attraction and avoidance mechanisms. They observed that a peripheral cue, the brief brightening of one of two square-shaped frames to the right and left of fixation, led to faster detection of the visual target at the cue’s location when the cue-target interval was relatively short. Critically, the peripheral cue impeded target detection at longer intervals when a second, intervening cue was shown prior to the target. This second cue appeared to pull attention away from—and to inhibit—the initially attended location. Such inhibitory cuing effects, referred to as inhibition of return (IOR), have been obtained in detection and recognition tasks under a wide range of viewing conditions, and for manual and oculomotor responses (Reuter-Lorenz, Jha, & Rosenquist, 1996; see Klein, 2000, for a comprehensive review). IOR is not confined to a single previously attended location, but rather can be applied to several previously cued locations (Snyder & Kingstone, 2000). During reading, IOR could push attention away from previously attended word locations, thereby biasing eye movements toward upcoming text.
Recent research has shown that an inhibitory control mechanism is effective in saccadic eye guidance. Specifically, saccade latencies are longer when the eyes return to a previously fixated target location than when they move to a novel location if attention had been shifted elsewhere in the meantime (Hooge & Frens, 2000; Klein & MacInnes, 1999; Ro, Pratt, & Rafal, 2000). Some saccade latency effects in reading also suggest the presence of saccadic IOR. Specifically, fixation durations preceding regressions are longer when the eyes move to a previously fixated word than when they move to a previously skipped word (Rayner, Juhasz, Ashby, & Clifton, 2003). A study by Spalek and Hammad (2005) provides further indirect evidence for a linkage between IOR and reading. Canadian speakers of English showed larger IOR when the direction of cue presentation was from left to right rather than right to left, whereas Egyptian speakers of Arabic showed the opposite directional bias. Because consecutive words are ordered from left to right in English and from right to left in Arabic, familiarity with written word order could have introduced the spatial IOR bias that was observed.
Despite these intriguing findings, the linkage between IOR and eye movements in reading remains tentative. In the study by Rayner et al. (2003), regressions to previously skipped words and to previously fixated words could have served radically different processing purposes. These differences could have influenced preregression fixation durations. Regressions to skipped words could support the ongoing recognition of these words, whereas regressions to previously fixated words could involve error correction. In Spalek and Hammad’s (2005) study, the interaction of cue direction and language type was relatively small in comparison with IOR effects that were independent of cue direction. Critically, the small directional IOR effect in the study does not reveal how IOR influences eye movements in reading. IOR could influence the frequency of regressions, the size of regressions, or the size of forward-directed movements.
In the current study, we built on and systematically extended earlier work by effectively combining two experimental approaches: examining eye movements in reading as a function of hypothesized IOR effects (Rayner et. al, 2003) and examining IOR effects for different groups of readers (Spalek & Hammad, 2005). This study constitutes the first direct testing of the hypothesized linkage between IOR and eye movements in reading.
All participants performed two tasks: an IOR task used to identify a group-specific temporal IOR profile and a reading task in which the group-specific IOR pattern was used to predict eye movements. Specifically, readers with a relatively fast buildup of IOR were predicted to avoid small intraline regressions to the left to avoid directing the eyes to a region that has just been attended. Conversely, readers with slow IOR were predicted to prefer smaller regressions because the area immediately to the left of fixation has not yet been inhibited (see Fig. 1). Crucially, if IOR during reading is a functionally distinct spatial avoidance mechanism that inhibits eye movements to previously attended text locations, then IOR type (fast vs. slow buildup) should not influence forward-directed eye movements. We tested our hypothesis using an individual differences approach that correlated temporal patterns of IOR with the size of regressions.
Fig. 1.
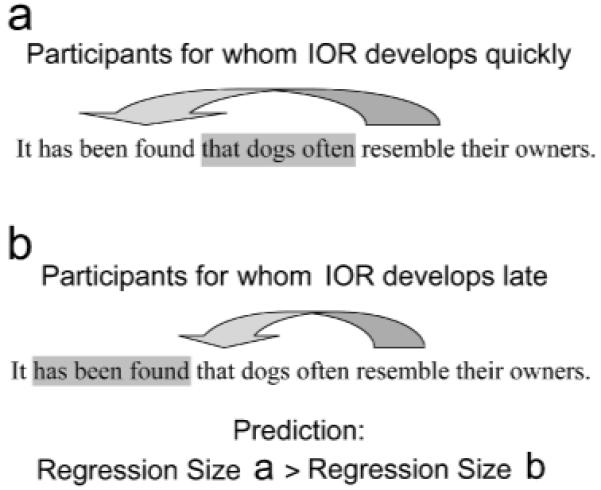
Illustration of the predicted effect of inhibition of return (IOR) on regression size. Inhibited locations are highlighted in gray. If IOR develops quickly (a), locations next to fixation should be inhibited, but if IOR develops more slowly (b), the inhibited area will be further from fixation. Thus, readers with fast IOR buildup should make larger regressions than readers with slow IOR buildup.
METHOD
Participants
Thirty-two State University of New York undergraduate students participated after giving informed consent.
Materials for the Reading Task
One hundred four declarative one-line sentences were used for the reading task. Twenty sentences contained a local lexical ambiguity with subsequent disambiguating text that referred to the nondominant meaning of the ambiguous word. The reading of disambiguating text is often difficult and accompanied by regressions (Carpenter & Daneman, 1981; Duffy, Morris, & Rayner, 1988; Folk & Morris, 1995).
Apparatus and Procedure
Sentences were shown in black font on a gray background on a 21-in. Illama flat-panel monitor. Eye movements were recorded with an Eyelink II system at 500 HZ; horizontal accuracy was approximately 15 min of arc. Reading was self-paced, and participants were instructed to read for meaning. Comprehension questions were asked after approximately every 10th sentence. The vast majority of questions, more than 95%, were answered correctly.
Sentence reading was followed by the IOR task. Two boxes, shown 5.6° to the left and to the right of a central fixation cross, identified the target areas. These three objects were drawn in black font on a white background and appeared at the beginning of each trial; they remained in place throughout the trial. After 300 ms, one of the peripheral boxes brightened for 100 ms (the first cue). After a 100-ms blank interval, the central fixation cross brightened for 100 ms to reorient attention to the center. The peripheral target (another brightening of a box) was presented either 550 ms or 950 ms after the offset of the second (central) cue so that temporal IOR effects could be determined. Initial cues that matched the location of the target were considered valid; initial cues that did not match the location of the target were considered invalid. Participants were instructed to keep the eyes at the central fixation cross throughout each trial and to press a left- or right-side key in response to a left- or right-side target, respectively (i.e., mapping between response keys and target locations was spatially congruent).
Measurement
To be included in the analysis, an eye movement had to extend across more than one letter space (LS). Directional properties, the size of regressions, and the size of forward-directed saccades were of primary interest. Supplementary measures were regression frequency, skipping rate, and the total word-viewing time during reading.
In the IOR task, average response times (RTs) to valid targets (which occupied the initial cue location and were thus subject to IOR) were subtracted from average RTs to invalid targets. A positive value thus indicated IOR. Incorrect responses and outliers (RTs below 100 ms or above 1,000 ms) were excluded from the RT analyses (6.6%).
RESULTS
RTs in the IOR task revealed robust inhibition for the short and long stimulus onset asynchronies (SOAs), t(31) = 4.26, prep 5.994, d = 1.5, and t(31) = 2.71, prep = .948, d = 0.97, respectively. One quarter of the participants showed relatively large IOR in the short-SOA condition and relatively little or no IOR in the long-SOA condition (fast-IOR group), and one quarter of the participants showed the opposite temporal pattern (slow-IOR group). A contrast of these groups revealed a highly significant interaction of IOR type (readers with fast vs. slow IOR buildup) and SOA (short vs. long), F(1, 14) = 123.4, prep = 1, Á2 = .51 (see Table 1).
TABLE 1. Mean Manual Reaction Times (in Milliseconds) in the Visual Target Detection Task.
| Overall (n 32) |
Fast-IOR group |
Slow-IOR group |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SOA onset | Valid cue | Invalid cue | Difference | Valid cue | Invalid cue | Difference | Valid cue | Invalid cue | Difference |
| Short | 473 (16) | 451 (17) | 22 | 492 (23) | 450 (20) | 42 | 503 (22) | 508 (28) | -5 |
| Long | 437 (16) | 422 (16) | 15 | 430 (19) | 448 (21) | -18 | 471 (21) | 429 (25) | 42 |
Note. Standard errors are shown in parentheses. Positive difference scores indicate inhibition of return (IOR).
SOA = stimulus onset asynchrony.
Examination of eye movements across all participants revealed that the mean frequency of forward saccades was 6.7 per sentence and that the mean size of forward saccades 7.4 LS. The mean frequency of regressions was 1.8, and they had a mean size of 12.5 LS. The effect of IOR type on regression size was of primary theoretical interest. As predicted, readers with a fast IOR executed larger regressions, 15.9 LS, than readers with slow IOR, 10.9 LS, t(14) = 2.47, prep = .914, d = 1.32 (see Table 2). Relatively fast buildup of IOR was thus associated with fewer regressions to areas of text that were attended immediately prior to the regressions.
TABLE 2. Regression Parameters as a function of IOR Group in the Visual Target Detection Task.
| Number of regressions |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regression size |
Forward-saccade size |
First-pass skipping rate |
≤ 10 letter spaces |
> 10 letter spaces |
||||||
| IOR group | M | SE | M | SE | M | SE | M | SE | M | SE |
| Fast | 15.9 | 1.7 | 6.8 | 0.29 | 0.33 | 0.02 | 0.97 | 0.12 | 0.52 | 0.05 |
| Slow | 10.9 | 1.08 | 7.1 | 0.29 | 0.32 | 0.02 | 1.65 | 0.21 | 0.49 | 0.05 |
Note. The fast- and slow-IOR groups included 8 subjects each.
Readers with relatively fast IOR also launched fewer regressions than readers with slow IOR (Table 2); mean frequencies were 1.49 and 2.15 per sentence, respectively, t(14) = 2.79, prep = .939, d = 1.49. This result indicates that IOR was generally more effective for readers with fast IOR. Notably, readers with fast IOR also launched fewer small regressions (2 through 10 LS long) than readers with slow IOR (mean frequencies of 0.97 and 1.65 regressions per sentence, respectively), t(14) = 2.70, prep = .933, d = 1.44. Conversely, readers with fast IOR tended to execute slightly more large regressions (0.52 per sentence) than readers with slow IOR (0.49), although this small numeric difference did not approach statistical significance, t(14) = 0.38.
In striking contrast, IOR type had virtually no effect on forward-directed eye movements. The size of forward-directed saccades was 6.8 and 7.1 LS for readers with fast and slow IOR, respectively, t(14) = 0.67, prep = .494, d = 0.36. The skipping rates of words to the right of fixation were virtually identical for the two types of readers, 33% and 32%, t(14) = 0.27, prep = .286, d = 0.144 (see Table 2).
Overall, readers with fast IOR tended to respond somewhat faster than readers with slow IOR. Their mean RTs were somewhat shorter in the target detection task, 455 ms versus 478 ms, as were their total word-viewing times during sentence reading, 359 ms versus 401 ms. The variability within each group was considerable, however, and neither effect was reliable, t(14) = 0.76 and t(14) = 1.39, respectively, both preps < .738, both ds < 0.74. IOR type influenced regression size in two analyses of covariance that used IOR RT and total word-viewing time as covariates, F(1, 13) = 4.95 and F(1, 13) = 6.33, respectively, both preps > .885, both Á2s > .247.
Large regressions are often launched when readers encounter unexpected information, as when text following an ambiguous word refers to its nondominant meaning. Smaller regressions are typically executed to increase the time spent in support of ongoing word identification (Vitu & McConkie, 2000). Readers with fast and slow IOR could have different processing strategies. That is, readers with fast IOR may regress primarily in response to comprehension difficulties, whereas readers with slow IOR may regress primarily in response to word identification difficulties. To examine this possibility, we analyzed the regression size of fast- and slow-IOR readers for sentences with and without lexical ambiguities. The results revealed larger regressions from ambiguous than from nonambiguous sentence regions, 16.6 LS versus 13.2 LS, F(1, 14) = 4.34, prep = .87, Á2 = .073, and the familiar effect of IOR type, F(1, 14) = 6.05, prep = .913, Á2 = .206. Sentence type and IOR type did not interact, F(1, 14) = .33, prep = .446, Á2 ∼ 0, indicating that IOR influenced regression size irrespective of the linguistic processing difficulty.
DISCUSSION
The current study examined the association between a functionally distinct backward-inhibiting attentional mechanism, IOR, and eye guidance in reading. It applied a novel individual differences approach that identified the temporal IOR profile of research participants and then used this profile to predict eye movement patterns. The results revealed that readers with fast IOR executed larger regressions than readers with slow IOR, as they sought to avoid the refixation of words that were read immediately prior to a regression. Conversely, readers with slow IOR avoided large regressions. Regression size reflected readers’ temporal IOR profile even when general processing differences between fast- and slow-IOR readers were factored out and when linguistic processing demands were taken into account. Critically, the time course of IOR buildup did not influence readers’ forward-directed saccades. Together, these findings reveal that a functionally distinct spatial IOR mechanism participates in the control of eye movements during reading.
Spalek and Hammad’s (2005) results, which showed larger IOR for English-speaking readers when cue direction progressed from left to right rather than right to left, and the reversed directional cuing effects for Arab-speaking readers, are consistent with a theoretical conception according to which eye movement habits during reading influence IOR. That is, the moving of the eyes in the direction of word order may be the source of a corresponding directional bias of IOR in reading and in other visual tasks. Our study neither supports nor rejects this conjecture, because the established linkage between readers’ temporal IOR profile and their regressions during reading is correlational.
Several considerations suggest, however, that it is IOR that influences eye movements in reading and perhaps in other visual tasks. The joint effects of cue direction and reading direction on IOR were relatively small in Spalek and Hammad’s (2005) study when compared with direction-independent effects of IOR. As noted before, IOR is a general spatial processing mechanism that is present in a wide range of task and response conditions. Developmentally, IOR has been found in children that cannot— or can barely—read (MacPherson, Klein, & Moore, 2003), and IOR has been observed in the auditory dimension, which should be independent of reading skill (Reuter-Lorenz et al., 1996).
Reading, though a young skill when seen from an evolutionary perspective, thus appears to take advantage of attentional mechanisms with much older roots (see Posner & Petersen, 1990). Established backward-inhibiting and forward-directed spatial selection mechanisms cooperate to generate an oculomotor movement pattern that biases the eyes toward upcoming words in the text.
Acknowledgments
This work was supported by National Institutes of Health Grant HD043405 to Albrecht Inhoff. We would like to thank Peter Gordon, Les Lochsky, Cynthia Connine, Brianna Eiter, and an anonymous reviewer for comments. We are also grateful to Daniel Feiler and MacKenzie Thompson for their help. Portions of these data were presented at the 13th European Conference on Eye-Movement Control in Bern, Switzerland (August 2005).
REFERENCES
- Carpenter PA, Daneman M. Lexical retrieval and error recovery in reading: A model based on eye fixations. Journal of Verbal Learning and Verbal Behavior. 1981;20:137–160. [Google Scholar]
- Duffy SA, Morris RK, Rayner K. Lexical ambiguity and fixation times in reading. Journal of Memory and Language. 1988;27:429–446. [Google Scholar]
- Folk JR, Morris RK. Multiple lexical codes in reading: Evidence from eye movements, naming time, and oral reading. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:1412–1429. [Google Scholar]
- Hooge ITC, Frens MA. Inhibition of saccade return (ISR): Spatio-temporal properties of saccade programming. Vision Research. 2000;40:3415–3426. doi: 10.1016/s0042-6989(00)00184-x. [DOI] [PubMed] [Google Scholar]
- Inhoff AW, Briihl D. Semantic processing of unattended text during selective reading: How the eyes see it. Perception & Psychophysics. 1991;49:289–294. doi: 10.3758/bf03214312. [DOI] [PubMed] [Google Scholar]
- Inhoff AW, Pollatsek A, Posner MI, Rayner K. Covert attention and eye movements during reading. Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 1989;41:63–89. doi: 10.1080/14640748908402353. [DOI] [PubMed] [Google Scholar]
- Inhoff AW, Topolski R. Lack of semantic activation from unattended text during passage reading. Bulletin of the Psychonomic Society. 1994;30:365–366. [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Klein RM, MacInnes WJ. Inhibition of return is a foraging facilitator in visual search. Psychological Science. 1999;10:346–352. [Google Scholar]
- MacPherson AC, Klein RM, Moore C. Inhibition of return in children and adolescents. Journal of Experimental Child Psychology. 2003;85:337–351. doi: 10.1016/s0022-0965(03)00104-8. [DOI] [PubMed] [Google Scholar]
- Pollatsek A, Bolozky S, Well AD, Rayner K. Asymmetries in the perceptual span for Israeli readers. Brain and Language. 1981;14:174–180. doi: 10.1016/0093-934x(81)90073-0. [DOI] [PubMed] [Google Scholar]
- Pollatsek A, Raney GE, Lagasse L, Rayner K. The use of information below fixation in reading and in visual search. Canadian Journal of Experimental Psychology. 1993;47:179–200. doi: 10.1037/h0078824. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis DG, editors. Attention and performance X: Control of language processes. Erlbaum; Hillsdale, NJ: 1984. pp. 531–556. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Rayner K, Juhasz B, Ashby J, Clifton C., Jr. Inhibition of saccade return in reading. Vision Research. 2003;43:1027–1034. doi: 10.1016/s0042-6989(03)00076-2. [DOI] [PubMed] [Google Scholar]
- Rayner K, Pollatsek A. The psychology of reading. Prentice Hall; Engle-wood Cliffs, NJ: 1989. [Google Scholar]
- Reuter-Lorenz PA, Jha AP, Rosenquist JN. What is inhibited in inhibition of return? Journal of Experimental Psychology: Human Perception and Performance. 1996;22:367–378. doi: 10.1037//0096-1523.22.2.367. [DOI] [PubMed] [Google Scholar]
- Ro T, Pratt R, Rafal RD. Inhibition of return in saccadic eye movements. Experimental Brain Research. 2000;130:264–268. doi: 10.1007/s002219900257. [DOI] [PubMed] [Google Scholar]
- Snyder JJ, Kingstone A. Inhibition of return and visual search: How many separate loci are inhibited? Perception & Psychophysics. 2000;62:452–458. doi: 10.3758/bf03212097. [DOI] [PubMed] [Google Scholar]
- Spalek TM, Hammad S. The left-to-right bias in inhibition of return is due to the direction of reading. Psychological Science. 2005;16:15–18. doi: 10.1111/j.0956-7976.2005.00774.x. [DOI] [PubMed] [Google Scholar]
- Vitu F, McConkie GW. Regressive saccades and word perception in adult reading. In: Kennedy A, Radach R, Heller D, Pynte J, editors. Reading as a perceptual process. Elsevier; Oxford, England: 2000. pp. 301–326. [Google Scholar]


