Abstract
Conjugated polyamines are potential carriers for biotherapeutics targeting the central nervous system. We describe an efficient synthesis of a polyamine-based amino acid, Lysine-trimethylene(diNosyl)-spermine(triBoc) with Dde or Fmoc orthogonal protecting groups. This nonnatural amino acid was incorporated into a neurotensin analogue using standard Fmoc-based protocols. The analogue maintained high affinity and agonist potency for neurotensin receptors and exhibited dramatically improved analgesia in mice. Our work provides a basis for use of polyamine amino acids in polypeptides.
Polyamines and polyamine-based compounds are ubiquitous in nature; these compounds are also broadly applicable for drug delivery, therapeutics and engineering nanomaterials. Polyamine-modified polypeptides exhibit a significant increase in penetration across the blood-nerve and blood-brain barriers (BBBa).1,2 Our recent study showed that a combination of cationization and lipidization applied to truncated galanin analogues improved their brain penetration, yielding very potent anticonvulsant compounds.3 Furthermore, cell penetrating peptides and peptidic vectors that transfer conjugated cargos into the nervous system contain a large number of cationic amino acid residues. Although the mechanism by which cationized peptides can penetrate biological membranes is not fully understood, several potential pathways, including internalization and adsorptive-mediated endocytosis (AME) have been discussed.4 Despite a potential of improving penetration of bioactive peptides into the nervous system by cationization, no reports exist on employing polyamine-based amino acids into neuropeptides.
To investigate how the structural addition of polyamines to the active moiety could improve pharmacological properties of neuroactive peptides, we selected neurotensin (NT) as the model peptide. NT is an endogenous neuropeptide that produces potent analgesic activity mediated spinally and/or supraspinally by NT receptors.5,6 Two subtypes of NT receptors, NTS1 and NTS2, have been demonstrated to mediate the various antinociceptive effects, including stress-induced antinociception, or analgesia in acetic acid writhing and hot plate tests.5 The spinal NTS1 was suggested to play an important role in reducing inflammatory response in the formalin test, a model of persistent pain.6 The naturally-occurring NT analogue, contulakin-G, containing site-specific O-glycosylation, exhibited picomolar potency in a rat model of inflammatory pain following intrathecal injection.7,8 Therefore, NT is a convenient model peptide for improving its central nervous system (CNS) bioavailability, since NT analogues with improved BBB penetration might be expected to produce enhanced antinociceptive effects in animals.9-11 Numerous strategies have been attempted to improve the BBB permeability of NT analogues. These include backbone and/or side chain modifications to cyclic and mimetic analogues.10,12-17 One of the modified NT(8-13) analogues that penetrates the BBB, NT69L ((N-methyl-Arg)-Lys-Pro-(L-neo-Trp)-(tert-Leu)-Leu), is a potent analgesic and it appeared a very useful pharmacological tool to study the role of neurotensin receptors in the CNS.6,11,18,19 In the research presented here, we synthesized and characterized pharmacological properties of an NT analogue containing Lysine-CH2CH2CH2-spermine, with the main hypothesis that the polyamine-modified compound will exhibit improved analgesic properties.
Our design strategy was to introduce a polyamine amino acid into the truncated NT/contulakin-G analogue (figure 1). In both, NT and contulakin-G, the active fragment comprises the last six amino acid residues (RRPYIL and KKPYIL, respectively).7 Replacements of Arg residues in NT(8-13) with many cationic amino acids did not change receptor binding properties.10,16 Since contulakin-G contains a bulky moiety β-D-Gal-(1→3)-α-D-GalNAc-(1→) disaccharide attached to Thr10 that was apparently benign to the bioactivity of this NT analogue, we designed the truncated contulakin-G analogue containing the polyamine amino acid instead of glycoamino acid. Thus, our lead compound, named polyamine-NT, was based on the truncated contulakin-G analogue, cont-G(10-16), in which the glycoamino acid was replaced by a polyamine amino acid.
Figure 1.
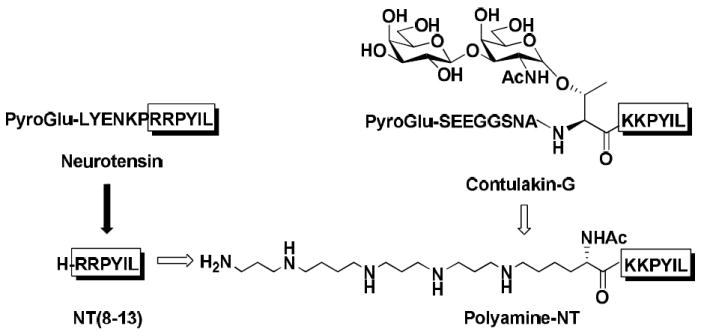
Design of polyamine-NT analogue. C-terminal fragments of NT and Contulakin-G (shown in boxes) are critical for binding to neurotensin receptors. In the truncated Contulakin-G analogue, a naturally-occurring glycoamino acid was replaced with a polyamine-based amino acid residue.
Because of natural biogenic polyamines almost exclusively incorporate 1,3-diaminopropyl or 1,4-diaminobutyl units, the spacer -CH2CH2CH2- between lysine and spermine was selected to maintain the physicochemical properties of the side chain for our construct. Structure 1 was designed to be compatible with solid-phase peptide synthesis (SPPS, Scheme 1). Nosyl (ortho-nitrobenzenesulfonyl, Ns) strategy was used to conjugate lysine and spermine moieties because of synthetic accessibility, no undesired tertiary amine and/or quaternary ammonium salt formation, and highly efficient alkylation with RX or ROH.20 Ns strategy had been successfully applied to the synthesis of polyamine toxins such as spider venom HO-416b,21 and philanthotoxin-343.20
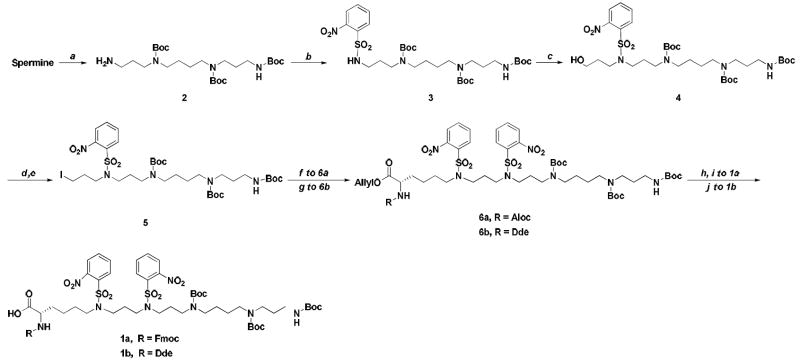
Scheme 1. Synthesis of polyamine amino acidsa.
aReagents and conditions: (a) CF3COOEt, -78 °C to 4 °C; Boc2O, 4 °C; K2CO3, MeOH/H2O (51%); (b) 2-nitrobenzenesulfonyl chloride, Et3N, 0 °C; (c) 3-bromo-1-propanol, Cs2CO3, 50 °C (76% from 2 to 4); (d) MeSO2Cl; DIPEA, 0 °C; (e) NaI, acetone, reflux (74%); (f) Alloc-Lys(Ns)-OAll, Cs2CO3, 50 °C (72%); (g) Dde-Lys(Ns)-OAll, Cs2CO3, 50 °C (64%); (h) Pd(PPh3)4, pyrrolidine (48%); (i) FmocOSu, NaHCO3 (76%); (j) Pd(PPh3)4, pyrrolidine (87%).
Compound 2 was prepared by selective trifluoroacetyl monoprotection of spermine, followed by Boc derivitazation of the remaining amino groups. Subsequent hydrolysis of the trifluoroacetyl functionality was accomplished with methanolic K2CO3, which gave better results than other published methods (NaOH/H2O/MeOH or MeOH/NH4OH).22, 23 Nosylation of 2 gave 3, which yielded 4 after alkylation with 3-bromo-1-propanol (overall 76% yield for the two steps). Mitsunobu coupling of 4 with Alloc-Lys(Ns)-OAll to form 6a directly gave low yields and inseparable by-products. The problem was circumvented via 5, which was prepared conventionally, in good yield by mesylation of 4 followed by displacement with iodide.
Alloc-Lys(Ns)-OAll was synthesized smoothly from Fmoc-Lys-OAll in three steps (see supporting information). However, attempts to synthesize Nα-Fmoc protected 1a by way of 5 and Alloc-Lys(Ns)-OAll gave mixed results. Although alkylation of 5 with Alloc-Lys(Ns)-OAll proceeded well, simultaneous deprotection of both Alloc and allyl groups with catalytic Pd(PPh3)4 in the presence of pyrrolidine gave low yields (48%). Other scavengers such as morpholine24 or barbituric acid25 produced similar or lower yields. Carrasco’s one-pot strategy for Alloc/allyl removal followed by Fmoc protection without purification gave only 10% yield.26
Due to relatively low yields of the above methods, 1b with Dde protection at Nα-position of polyamine acid was designed. To this end, Dde-Lys(Ns)-OAll was synthesized in four steps from H-Lys(Boc)-OH: Nα-Dde protection, allyl ester formation, Nε-Boc deprotection and Nε-nosylation (see supporting information). Dde-Lys(Ns)-OAll was then coupled with 5 to give 6b. Finally allyl deprotection of 6b with Pd(PPh3)4/pyrrolidine afforded 1b in good yield (87%).
The polyamine-NT was synthesized on preloaded Wang-resin using PyBop reagent. Amino acid coupling was performed on an automated peptide synthesizer. The final addition of 1b was done manually. Common Dde deprotection with hydrazine could not be used due to the Ns nitro groups. An alternate deprotection method27 accomplished in 2 h. The peptide was cleaved from the resin with reagent K and purified by HPLC (overall yield 12%).
Polyamine-NT was first tested for its ability to bind and activate the NT receptors, as described previously.29 This analogue exhibited subnanomolar affinity (Ki 0.25nM), as well as low nanomolar agonist potency (EC50 1.4 nM) for the human NTS1 (figure 2a, 2b). These values were similar to those for the full-length, unmodified NT (Ki 0.5 nM and EC50 1.1 nM),29 suggesting that the polyamine moiety did not affect the interactions between the polyamine-NT and the NTS1. This finding is in agreement with other reports (e.g. demotensin analogues30) and our recent study on glycosylated NT analogues, e.g., the presence of N-terminal extensions in NT(8-13), including addition of various glycoamino acids did not change the affinity of the analogues toward NTS1.29 The subnanomolar affinity of polyamine-NT is comparable with another BBB-permeable NT analogue, such as NT67L,9 and with some of Arg8 substituted NT(8-13) analogues.10,31 Two other previously described analgesic NT analogues, NT69L and JMV2012 exhibited Kd of 1.55 nM and 150 nM, respectively, for human NTS1.11, 12
Figure 2.
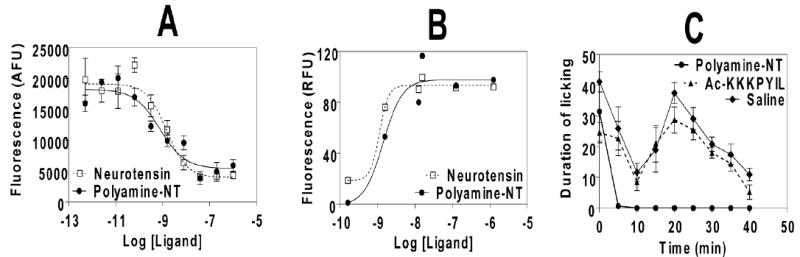
Pharmacological properties of polyamine-NT. (a) Competitive binding assay for the human NTS1 (membrane preparations containing the recombinant NTS1 used in the binding assays were derived from HEK-293T cells). (b) Agonist activity determined by stimulation of intracellular Ca2+ mobilization in HEK-293T cells expressing human NTS1. (c) Analgesic activity in the formalin pain assay in mice (i.p, 4 mg/kg, injected 1 h prior to formalin injection in a paw). Paw-licking (expressed in sec) was monitored continuously for 2 min every 5 min. Increased duration of licking between 0-5 min reflects the phase I response (acute pain), whereas that between 15-30 min is defined as the phase II response (inflammatory pain).
In order to test our main hypothesis that the polyamine-containing NT analogue may have improved CNS bioavailability, we compared the analgesic activity of polyamine-NT with the control analogue missing the spermine side chain, Ac-KKKPYIL (as referenced above,30 the presence of the N-acetyl-Lys moiety preceding -KKPYIL is unlikely to affect the NT receptor binding properties). Polyamine-NT exhibited a pronounced analgesic effect in the mouse formalin pain model at a single bolus dose of 4 mg/kg following intraperitoneal (i.p.) administration. The inflammatory phase was completely eliminated at this dose, which did not induce any apparent motor impairment (figure 2c). In contrast, the control analogue missing the (NH2OH·HCl/imidazole in NMP/CH2Cl2) was applied successfully, followed by N-terminal acetylation. Ns deprotection on the resin using (p-methoxythiophenol/K2CO3 in CH3CN/DMSO28) was spermine side chain was inactive as an analgesic, and did not differentiate itself from the saline control.
Our work shows that incorporation of polyamine amino acid residues during SPPS is feasible, and the resulting neurotensin analogue displayed analgesic properties. Although the antinociception of polyamine-NT is likely mediated by NTS1 located in the spinal cord,6 we cannot rule out an additional role of NTS2 in producing the apparent analgesia. More detail studies are required, including measuring interactions between polyamine-NT analogue and NTS2, to address these pharmacological aspects. Nonetheless, an immediate outcome of our work is that polyamine amino acids should be explored more frequently when attempting to improve the CNS bioavailability of peptides. There are multiple mechanisms by which modified peptides can penetrate the BBB, and the polyamine-conjugated compounds are more likely to be transported by AME.32 Other applications for polyamine amino acids are to explore them as substitutes for cell-penetrating peptides,33 or for engineering biosilica-based nanomaterials.34 Taken together, our results support the use of polyamine amino acids to engineer novel properties of biopolymers assembled via SPPS.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the NIH (R21 NS059669), the Epilepsy Therapy Project, the Epilepsy Research Foundation and the University of Utah Startup Funds. GB also acknowledges a financial support from the NIH Program Project GM 48677. We would like to thank Dr. J. Hinshaw and C.R. Robertson for a critical reading of the manuscript and helpful suggestions. GB and HSW are scientific cofounders of NeuroAdjuvants, Inc.
Footnotes
Supporting Information Available: Experimental details for synthetic procedures, full characterization of all compounds and NMR spectra. This material is available free of charge via the internet at http://pubs.acs.org.
Abbreviations: AME, absorptive-mediated endocytosis; BBB, blood-brain barrier; CNS, central nervous system; Dde, 1-(4,4-dimethyl-2,6-dioxacyclohexylidene)ethyl; DIPEA, N,N-diisopropylethylamine; i. p., intraperitoneal; Ns, ortho-nitrobenzenesulfonyl; NT, neurotensin; NTS1, neurotensin receptor subtype 1; NTS2, neurotensin receptor subtype 2; PyBop, (Benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate; SPPS, solid-phase peptide synthesis.
References
- 1.Poduslo JF, Curran GL. Polyamine modification increases the permeability of proteins at the blood-nerve and blood-brain barriers. J Neurochem. 1996;66:1599–1609. doi: 10.1046/j.1471-4159.1996.66041599.x. [DOI] [PubMed] [Google Scholar]
- 2.Poduslo JF, Curran GL, Gill JS. Putrescine-modified nerve growth factor: bioactivity, plasma pharmacokinetics, blood-brain/nerve barrier permeability, and nervous system biodistribution. J Neurochem. 1998;71:1651–1660. doi: 10.1046/j.1471-4159.1998.71041651.x. [DOI] [PubMed] [Google Scholar]
- 3.(a) Bulaj G, Green BR, Lee HK, Robertson CR, White K, Zhang L, Sochanska M, Flynn SP, Scholl EA, Pruess TH, Smith MD, White HS. Design, synthesis, and characterization of high-affinity, systemically-active galanin analogues with potent anticonvulsant activities. J Med Chem. 2008;51:8038–8047. doi: 10.1021/jm801088x. [DOI] [PubMed] [Google Scholar]; (b) Zhang L, Robertson CR, Green BR, Pruess TH, White HS, Bulaj G. Structural Requirements for a Lipoamino Acid in Modulating the Anticonvulsant Activities of the Systemically-Active Galanin Analogs. J Med Chem. doi: 10.1021/jm801397w. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev. 2005;57:529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Dobner PR. Neurotensin and pain modulation. Peptides. 2006;27:2405–2414. doi: 10.1016/j.peptides.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Roussy G, Dansereau MA, Dore-Savard L, Belleville K, Beaudet N, Richelson E, Sarret P. Spinal NTS1 receptors regulate nociceptive signaling in a rat formalin tonic pain model. J Neurochem. 2008;105:1100–1114. doi: 10.1111/j.1471-4159.2007.05205.x. [DOI] [PubMed] [Google Scholar]
- 7.Craig AG, Norberg T, Griffin D, Hoeger C, Akhtar M, Schmidt K, Low W, Dykert J, Richelson E, Navarro V, Mazella J, Watkins M, Hillyard D, Imperial J, Cruz LJ, Olivera BM. Contulakin-G, an O-glycosylated invertebrate neurotensin. J Biol Chem. 1999;274:13752–13759. doi: 10.1074/jbc.274.20.13752. [DOI] [PubMed] [Google Scholar]
- 8.Allen JW, Hofer K, McCumber D, Wagstaff JD, Layer RT, McCabe RT, Yaksh TL. An assessment of the antinociceptive efficacy of intrathecal and epidural Contulakin-G in rats and dogs. Anesth Analg. 2007;104:1505–1513. doi: 10.1213/01.ANE.0000219586.65112.FA. [DOI] [PubMed] [Google Scholar]
- 9.Tyler BM, Douglas CL, Fauq A, Pang YP, Stewart JA, Cusack B, McCormick DJ, Richelson E. In vitro binding and CNS effects of novel neurotensin agonists that cross the blood-brain barrier. Neuropharmacology. 1999;38:1027–1034. doi: 10.1016/s0028-3908(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 10.Kokko KP, Hadden MK, Price KL, Orwig KS, See RE, Dix TA. In vivo behavioral effects of stable, receptor-selective neurotensin[8-13] analogues that cross the blood-brain barrier. Neuropharmacology. 2005;48:417–425. doi: 10.1016/j.neuropharm.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Boules M, Fredrickson P, Richelson E. Bioactive analogs of neurotensin: focus on CNS effects. Peptides. 2006;27:2523–2533. doi: 10.1016/j.peptides.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Bredeloux P, Cavelier F, Dubuc I, Vivet B, Costentin J, Martinez J. Synthesis and biological effects of c(Lys-Lys-Pro-Tyr-Ile-Leu-Lys-Lys-Pro-Tyr-Ile-Leu) (JMV2012), a new analogue of neurotensin that crosses the blood-brain barrier. J Med Chem. 2008;51:1610–1616. doi: 10.1021/jm700925k. [DOI] [PubMed] [Google Scholar]
- 13.Sefler AM, He JX, Sawyer TK, Holub KE, Omecinsky DO, Reily MD, Thanabal V, Akunne HC, Cody WL. Design and structure-activity relationships of C-terminal cyclic neurotensin fragment analogues. J Med Chem. 1995;38:249–257. doi: 10.1021/jm00002a006. [DOI] [PubMed] [Google Scholar]
- 14.Bittermann H, Einsiedel J, Hubner H, Gmeiner P. Evaluation of lactam-bridged neurotensin analogues adjusting psi(Pro10) close to the experimentally derived bioactive conformation of NT(8-13) J Med Chem. 2004;47:5587–5590. doi: 10.1021/jm049644y. [DOI] [PubMed] [Google Scholar]
- 15.Couder J, Tourwe D, Van Binst G, Schuurkens J, Leysen JE. Synthesis and biological activities of psi (CH2NH) pseudopeptide analogues of the C-terminal hexapeptide of neurotensin. Int J Pept Protein Res. 1993;41:181–184. doi: 10.1111/j.1399-3011.1993.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 16.Lundquist JTt, Dix TA. Synthesis and human neurotensin receptor binding activities of neurotensin(8-13) analogues containing position 8 alpha-azido-N-alkylated derivatives of ornithine, lysine, and homolysine. J Med Chem. 1999;42:4914–4918. doi: 10.1021/jm9903444. [DOI] [PubMed] [Google Scholar]
- 17.Hong F, Zaidi J, Cusack B, Richelson E. Synthesis and biological studies of novel neurotensin(8-13) mimetics. Bioorg Med Chem. 2002;10:3849–3858. doi: 10.1016/s0968-0896(02)00342-5. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, Boules M, Gollatz E, Williams K, Tiner W, Richelson E. Effects of 5 daily injections of the neurotensin-mimetic NT69L on the expression of neurotensin receptors in rat brain. Brain Res Mol Brain Res. 2005;138:24–34. doi: 10.1016/j.molbrainres.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Tyler-McMahon BM, Stewart JA, Farinas F, McCormick DJ, Richelson E. Highly potent neurotensin analog that causes hypothermia and antinociception. Eur J Pharmacol. 2000;390:107–111. doi: 10.1016/s0014-2999(99)00877-8. [DOI] [PubMed] [Google Scholar]
- 20.Kan T, Fukuyama T. Ns strategies: a highly versatile synthetic method for amines. Chem Commun (Camb) 2004:353–359. doi: 10.1039/b311203a. [DOI] [PubMed] [Google Scholar]
- 21.Hidai Y, Kan T, Fukuyama T. Total synthesis of polyamine toxin HO-416b and agel-489 using a 2-nitrobenzenesulfonamide strategy. Chemical & Pharmaceutical Bulletin. 2000;48:1570–1576. doi: 10.1248/cpb.48.1570. [DOI] [PubMed] [Google Scholar]
- 22.Geall AJ, Taylor RJ, Earll ME, Eaton MA, Blagbrough IS. Synthesis of cholesteryl polyamine carbamates: pK(a) studies and condensation of calf thymus DNA. Bioconjug Chem. 2000;11:314–326. doi: 10.1021/bc990115w. [DOI] [PubMed] [Google Scholar]
- 23.Wellendorph P, Jaroszewski JW, Hansen SH, Franzyk H. A sequential high-yielding large-scale solution-method for synthesis of philanthotoxin analogues. Eur J Med Chem. 2003;38:117–122. doi: 10.1016/s0223-5234(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 24.Bregant S, Tabor AB. Orthogonally protected lanthionines: synthesis and use for the solid-phase synthesis of an analogue of nisin ring C. J Org Chem. 2005;70:2430–2438. doi: 10.1021/jo048222t. [DOI] [PubMed] [Google Scholar]
- 25.Allart B, Lehtolainen P, Yla-Herttuala S, Martin JF, Selwood DL. A stable bis-allyloxycarbonyl biotin aldehyde derivative for biotinylation via reductive alkylation: application to the synthesis of a biotinylated doxorubicin derivative. Bioconjug Chem. 2003;14:187–194. doi: 10.1021/bc0255992. [DOI] [PubMed] [Google Scholar]
- 26.Carrasco MR, Brown RT, Serafimova IM, Silva O. Synthesis of N-Fmoc-O-(N’-Boc-N’-methyl)-aminohomo-serine, an amino acid for the facile preparation of neoglycopeptides. J Org Chem. 2003;68:195–197. doi: 10.1021/jo026641p. [DOI] [PubMed] [Google Scholar]
- 27.Diaz-Mochon JJ, Bialy L, Bradley M. Full orthogonality between Dde and Fmoc: the direct synthesis of PNA-peptide conjugates. Org Lett. 2004;6:1127–1129. doi: 10.1021/ol049905y. [DOI] [PubMed] [Google Scholar]
- 28.Narayan RS, Vannieuwenhze MS. Versatile and stereoselective syntheses of orthogonally protected beta-methylcysteine and beta-methyllanthionine. Org Lett. 2005;7:2655–2658. doi: 10.1021/ol0507930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H, Zhang L, Smith MD, White HS, Bulaj G. Glycosylated neurotensin analogs exhibit the subpicomolar anticonvulsant potency in the pharmacoresistant model of epilepsy. ChemMedChem. 2009 doi: 10.1002/cmdc.200800421. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nock BA, Nikolopoulou A, Reubi JC, Maes V, Conrath P, Tourwe D, Maina T. Toward stable N4-modified neurotensins for NTS1-receptor-targeted tumor imaging with 99mTc. J Med Chem. 2006;49:4767–4776. doi: 10.1021/jm060415g. [DOI] [PubMed] [Google Scholar]
- 31.Kokko KP, Hadden MK, Orwig KS, Mazella J, Dix TA. In vitro analysis of stable, receptor-selective neurotensin[8-13] analogues. J Med Chem. 2003;46:4141–4148. doi: 10.1021/jm0300633. [DOI] [PubMed] [Google Scholar]
- 32.Egleton RD, Davis TP. Development of neuropeptide drugs that cross the blood-brain barrier. NeuroRx. 2005;2:44–53. doi: 10.1602/neurorx.2.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Andaloussi S, Holm T, Langel U. Cell-penetrating peptides: mechanisms and applications. Curr Pharm Des. 2005;11:3597–3611. doi: 10.2174/138161205774580796. [DOI] [PubMed] [Google Scholar]
- 34.Kroger N, Deutzmann R, Sumper M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science. 1999;286:1129–1132. doi: 10.1126/science.286.5442.1129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.