Abstract
Inflammation is a potentially self-destructive process that needs tight control. We have identified a novel nuclear signaling mechanism through which the low-density lipoprotein receptor-related protein 1 (LRP1) limits transcription of lipopolysaccharide (LPS)-inducible inflammatory genes in vitro and in vivo. Stimulation with LPS increases proteolytic processing of the LRP1 ectodomain, resulting in the γ-secretase-dependent release of the LRP1 intracellular domain (ICD) from the plasma membrane and its translocation to the nucleus, where it binds to and represses the interferon-γ promoter. Basal transcription of LPS target genes and LPS-induced secretion of proinflammatory cytokines are increased in the absence of LRP1. Physical interaction of the LRP1 ICD with IRF-3 promotes its nuclear export and proteasomal degradation. Feedback inhibition of the inflammatory response through intramembranous processing of LRP1 thus defines a novel physiological role for γ-secretase.
Keywords: γ-secretase, inflammation, lipoprotein receptor, LPS, transcriptional regulation
Introduction
γ-secretase is an intramembraneous protease that processes a variety of transmembrane proteins resulting in the release of their intracellular domains (ICD) into the cytoplasm (1). Nuclear translocation and transcriptional regulation through the ICD was first shown for the members of the Notch family, where this process regulates gene expression during cell fate decisions and embryonic development (2). A similar γ-secretase-dependent cleavage event was recently described for the receptor tyrosine kinase ErbB4 (3), where the ICD functions as a transcriptional repressor of astrocytic genes thereby regulating the differentiation of neuronal precursor cells (4). Here we define a novel role for γ-secretase in the transcriptional regulation of inflammation through processing of the lipoprotein receptor LDL receptor-related protein 1 (LRP1).
LRP1 is a ubiquitously expressed, multifunctional member of the LDL receptor gene family (5) and serves as a modulator and integrator of several distinct signaling pathways in the vascular wall (6, 7), in the blood brain barrier (8), in neurons (9), and in macrophages from different tissues (10–12). We have shown earlier that, following PKC activation and metalloproteinase-induced shedding of the extracellular domain (ECD), the LRP1 ICD is released from the membrane by γ-secretase (13).
PKC activation induces the expression of matrix metalloproteinases and other inflammatory mediators (14) and this is an early event of the inflammatory response to infection and injury (15). Self-limiting mechanisms that keep the production of harmful inflammatory mediators in check are essential to avoid excessive cell damage and tissue destruction. We therefore hypothesized that the LRP1 ICD, which is released in the course of metalloproteinase activation, serves as a mediator of this negative feedback. To test our hypothesis we examined the role of LRP1 proteolytic processing in lipopolysaccharide-induced inflammatory signaling in vitro and in vivo.
Lipopolysaccharides (LPS) are components of the outer membrane of gram-negative bacteria that induce a strong host defense response in infected organisms including increased production of proinflammatory cytokines (16). LPS signal through a receptor complex that contains toll-like receptor 4 (TLR4). LPS binding to TLR4 induces two major branches of intracellular signaling cascades: MyD88-dependent signaling via the adapters MyD88 and MyD88-like leads to the early activation of NFκB. MyD88-independent signaling via the TIR-domain-containing adapter-inducing interferon-β (TRIF) and the TRIF-related adapter molecule (TRAM) results in the activation of IRF-3 (interferon regulatory factor 3) and late NFκB signaling (reviewed in (17), see scheme in Figure 2B).
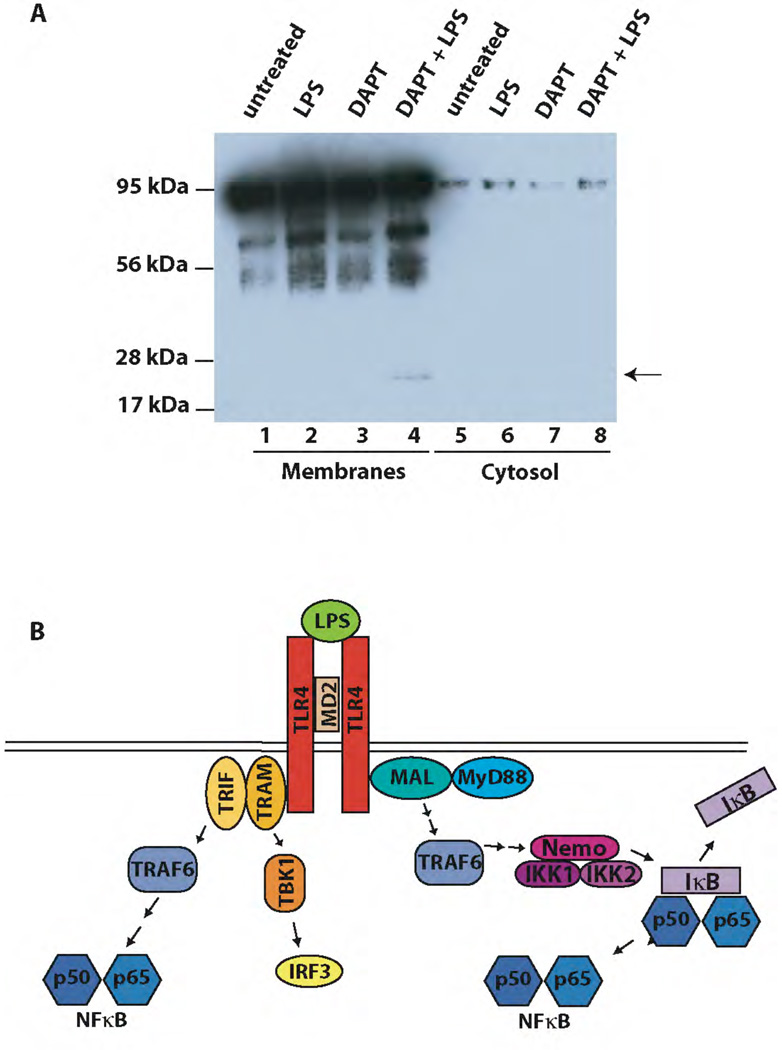
Figure 2. LPS enhances proteolytical processing of LRP1.
A) Wildtype primary macrophages were pretreated with the γ-secretase inhibitor DAPT at a final concentration of 10 µM for 2 h. Then cells were treated with 1 µg/ml LPS or left untreated for 9 h and cell membranes were prepared and analyzed by Western Blotting with the C-terminal LRP1 antibody. A representative experiment of n=4 is shown.
B) Schematic representation of LPS-induced signaling pathways.
Binding of LPS to its receptor complex leads to the activation of MyD88 (and Mal)-dependent signaling that results in the early activation of NFκB, and of MyD88-independent (TRIF and TRAM-dependent) pathways that lead to the activation of IRF-3 and late NFκB activation. TRIF – TIR-domain containing adaptor molecule, TRAM – TRIF-related adapter molecule, MyD88 – myeloid differentiation marker 88, MAL – MyD88-like, TRAF6 – tumor necrosis factor receptor-associated factor, TBK1 – TANK-binding protein kinase, NEMO – NFκB essential modulator, IKK – IκB kinase.
Here we report a novel role for γ-secretase in the self-restriction of inflammation through proteolytic processing of the lipoprotein receptor LRP1 and nuclear signal modulation by the LRP1 ICD. Exposure to LPS results in increased shedding of the LRP1 extracellular domain. Following γ-secretase-dependent cleavage the LRP1 ICD translocates to the nucleus where it binds to the transcription factor IRF-3 and facilitates its nuclear export. In the absence of LRP1 phosphorylated, active IRF-3 accumulates in the nucleus, resulting in increased transcription of LPS-inducible genes in lrp1-deficient fibroblasts and in vivo in peritoneal macrophages of conditional lrp1 knockout mice. LPS-induced release of proinflammatory cytokines is increased in LRP1-deficient macrophages. These findings reveal novel roles for γ-secretase and for the lipoprotein receptor LRP1 in the regulation of the inflammatory response, and as potential therapeutic targets for the modulation of inflammation.
Results
Nuclear localization of the free LRP1 ICD
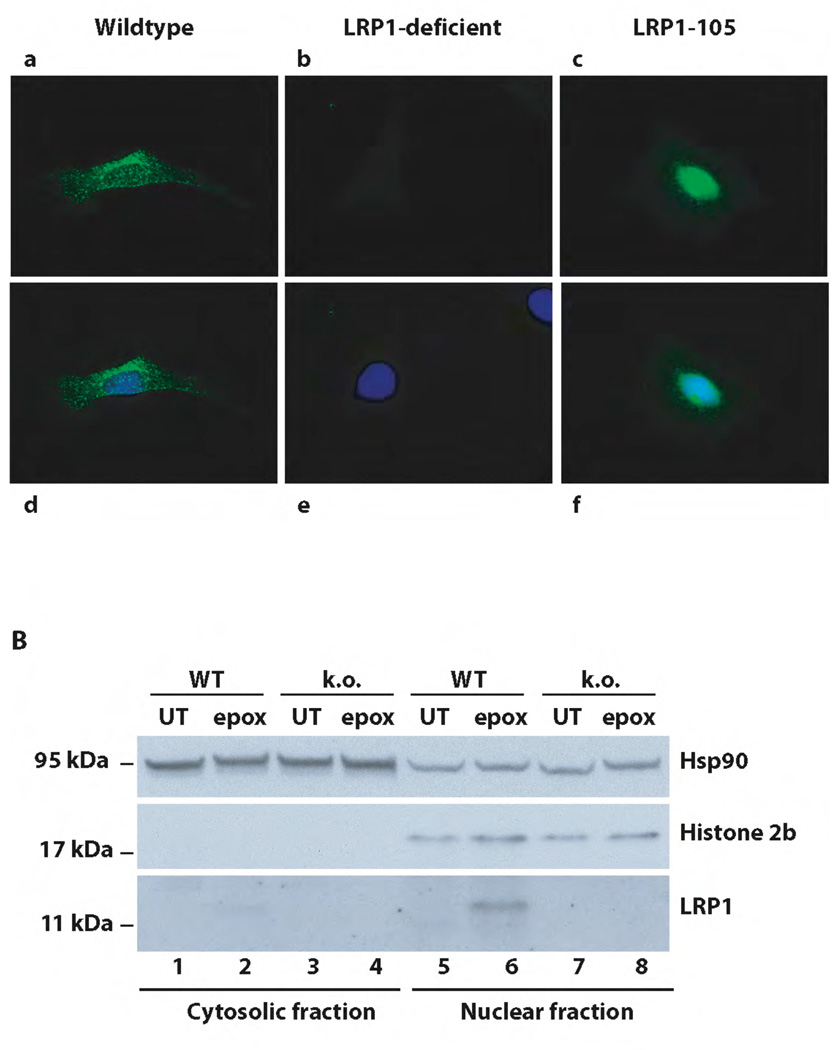
We recently demonstrated that LRP1 undergoes regulated proteolytic processing that culminates in the release of the receptor’s intracellular domain from the plasma membrane by the presenilin containing γ-secretase complex (13). To investigate the subsequent subcellular localization of the free LRP1 ICD, we stably transfected LRP1-deficient murine embryonic fibroblasts (MEF) with a cDNA coding for the LRP1 cytoplasmic domain (LRP1-105). As the free LRP1 ICD is subject to proteasomal degradation (13), the cells were treated with the proteasome inhibitor epoxomicin to allow the LRP1 ICD to accumulate. Immunocytochemistry revealed a predominantly nuclear fluorescent signal in the stably transfected cell line (Figure 1A, Panels c and f), whereas endogenous full length LRP1 in wildtype fibroblasts yielded a staining pattern compatible with its known endosomal localization (Panels a and d).
Figure 1. Nuclear localization of the free LRP1 ICD.
A) Wildtype (a and d), LRP1-deficient (b and e), and murine embryonic fibroblasts stably expressing the free LRP1 ICD (LRP1-105, c and f) were analyzed by immunocytochemistry with an antibody directed against the LRP1 C-terminus after treatment with 1 µM epoxomicin for 9h. Nuclear counterstaining was done by DAPI (d–f). A representative experiment of n=5 is shown. B) Nuclear and cytosolic extracts were prepared from wildtype (WT) and LRP1-deficient (k.o.) MEF after treatment with 1 µM epoxomicin or no treatment for 12h and were subsequently analyzed by Western Blotting with the C-terminal LRP1-antibody, a nuclear marker (α-Histone 2b), and a cytosolic marker (α-Hsp90). A representative experiment of n=5 is shown.
γ-secretase-dependent proteolytic processing of LRP1 takes place constitutively in mammalian cells (13 and supplementary figure 4). We therefore investigated the subcellular localization of the endogenous LRP1-ICD generated in wildtype fibroblasts. As immunocytochemistry was not sensitive enough for this purpose (Figure 1A, Panels a and d), we generated nuclear and cytosolic extracts from epoxomicin-treated wildtype (wt) and LRP1-deficient (ko) fibroblasts for immunoblotting. A carboxylterminal fragment of the size of the LRP1 ICD (approximately 12 kDa) accumulated predominantly in the nucleus of epoxomicin-treated wildtype (Figure 1B, lane 6) but not in LRP1-deficient cells (Figure 1B, lane 8) indicating that after separation from the cell membrane the free LRP1 cytoplasmic tail translocates to the nucleus.
LPS enhances the proteolytic processing of LRP1
The nuclear localization of the free LRP1 ICD indicates that it might function in transcriptional regulation. Increased γ-secretase-dependent production of the ICD occurs after PKC activation and metalloproteinase-induced shedding of the LRP1 extracellular domain (ECD) (13). As PKC activation and subsequent induction of metalloproteinases occurs in the course of the inflammatory response, we hypothesized that the LRP1 ICD might function in the transcriptional regulation of the inflammatory process. We therefore tested whether induction of the host defense response by LPS would modulate LRP1 processing.
Wildtype murine peritoneal macrophages were pretreated with the γ-secretase inhibitor DAPT and levels of the membrane-bound 25 kDa LRP1 fragment that is generated after shedding of the LRP1 ECD were compared in cells that had been treated with LPS or not. Membrane fractions were isolated and immunoblotted with an antibody directed against the LRP1 carboxylterminus. LPS-treatment led to increased extracellular cleavage of LRP1 and subsequent accumulation of the 25 kDa fragment (Figure 2A, lane 4) indicating that increased production of the soluble LRP1 ICD occurs in the course of the inflammatory response evoked by LPS (for an additional time course experiment see supplementary figure 5, for reproduction with highly purified rLPS see supplementary figure 6).
LRP1 modulates the activation of LPS-induced signaling pathways
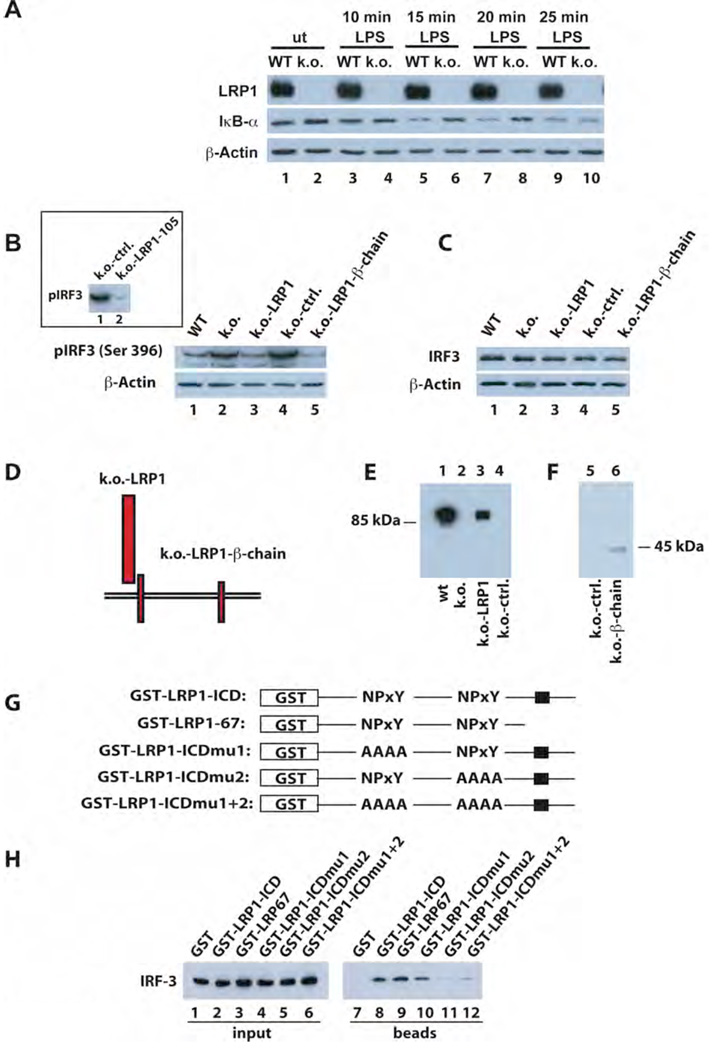
As proteolytic processing of LRP1 was increased in response to LPS, we next examined whether downstream LPS signaling events, including LPS target gene transcription, were modulated by the LRP1 ICD. Signaling and transcriptional regulation by LPS have been shown to involve the activation of transcription factors p65 NFκB and IRF-3 (Figure 2B). We therefore treated wildtype and LRP1-deficient fibroblasts with LPS and examined the levels of the NFκB inhibitory protein IκBα by immunoblotting. Basal levels of IκBα were slightly higher in the LRP-deficient cells and IκBα degradation in response to LPS was delayed compared to wildtype cells (Figure 3A, for quantification see supplementary Figure 1A).
Figure 3. LRP1 modulates LPS-activated signaling through direct interaction with IRF-3.
A) Whole cell lysates from wildtype and LRP1-deficient fibroblasts were prepared after treatment with 10 µg/ml LPS for the times indicated and from untreated controls. Lysates were analyzed by Western Blotting and staining with an anti-IκBα antibody. Anti-β-actin was used as a loading control. For quantification of Western Blotting results see supplementary Figure 1A.
B) Nuclear extracts were prepared from wildtype and LRP1-deficient fibroblasts as well as from LRP1-deficient cells stably transfected with the LRP1-cDNA (k.o.-LRP1), the empty plasmid vector (k.o.-ctrl.), or the LRP1-βchain (k.o.-LRP1-βchain). The extracts were analyzed by Western Blotting and staining with an anti-phospho-IRF-3 antibody. Staining for β-actin served as loading control. Inset: Analysis of nuclear extracts from LRP1-deficient cells stably transfected with the LRP1-ICD-cDNA (k.o.-LRP1-105) and the empty plasmid vector (k.o.-ctrl.) A representative experiment of n=5 is shown.
C) Total IRF-3 levels in the cell lines described under B) were identical as judged by analysis of whole cell lysates by Western Blotting and staining with an IRF-3 antibody. Staining with a β-actin antibody served as loading control.
D) Schematic representation of LRP1 and the LRP1 mutants retransfected into the LRP1-deficient fibroblasts.
E)/F) Wildtype (wt) and LRP1-deficient (k.o.) MEF and k.o. cells retransfected with LRP1, the LRP1-β-chain, or the empty plasmid vector were analyzed for LRP1 expression by Western Blotting with the C-terminal LRP1 antibody.
G) Schematic representation of the GST-LRP1 fusion proteins used in a pull-down assay to detect direct interaction of IRF-3 with LRP1. The LRP ICD was N-terminally fused to a GST tag. In addition a C-terminal LRP1 ICD deletion mutant and constructs with mutations in the first, second, or both NPxY motifs were employed. The black box indicates a putative casein kinase II phosphorylation site in the distal LRP1 ICD.
H) GST-LRP1 ICD fusion proteins (see G) were used to pull down IRF-3 from whole cell lysates of MEF. Equal input of fusion proteins was made visible by Ponceau staining of the transfer membrane (see supplementary figure 7). A representative experiment of n=3 is shown.
To examine activation of IRF-3, we prepared nuclear extracts of wildtype and LRP1-deficient fibroblasts and analyzed pIRF-3 levels by immunoblotting. Basal IRF-3 phosphorylation was increased in the absence of LRP1 (Figure 3B, lane 2 vs. 1). Retransfection of LRP1 (lane 3) or of the LRP1 ICD, either in the membrane-bound form (lane 5) or as free cytoplasmic domain (inset, lane 2), was sufficient to reduce pIRF-3 levels. Total IRF-3 levels were comparable in all cell lines (Figure 3C). For a schematic representation of transfected constructs and proof of expression see Figure 3D, 3E /F, and Figure 1A respectively.
To further investigate the dramatic effect of the LRP1 intracellular domain on IRF-3 activation, we tested if the ICD could directly interact with IRF-3 in a pull-down assay. Using full-length and mutant GST-tagged LRP1-ICD constructs (Figure 3G) we detected binding of the ICD to IRF-3 (Figure 3H, lane 8). This interaction was mediated by the second NPxY motif of the LRP1 ICD (Figure 3H, lanes 9–12).
The LRP1 ICD resides in the transcriptional complex on the interferon γ promoter and limits LPS-induced gene transcription in vivo
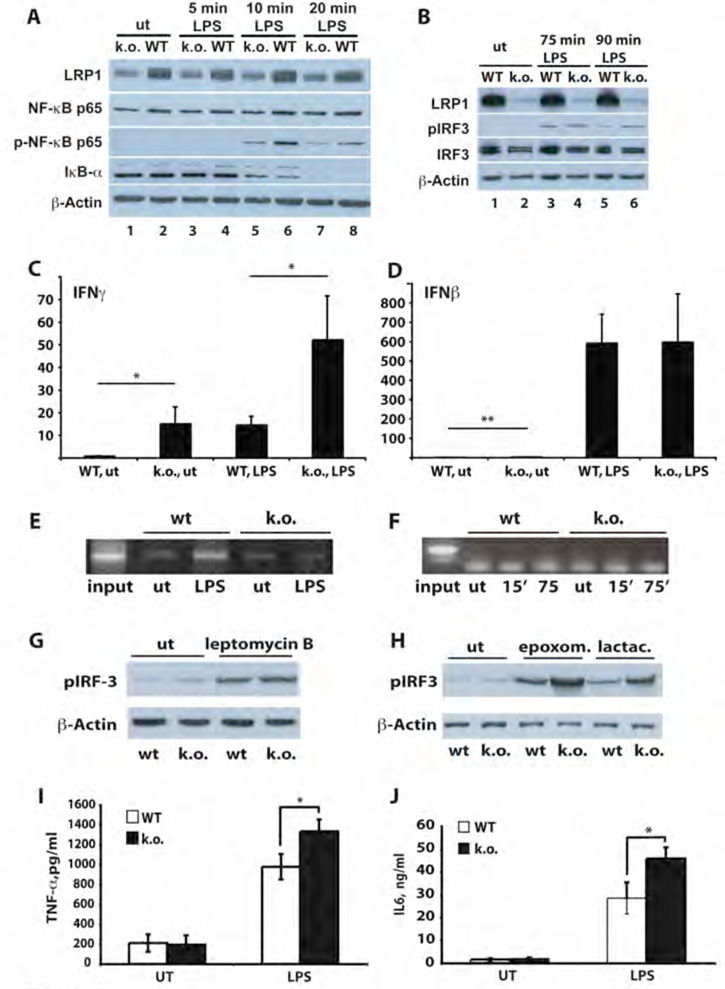
To investigate the role of LRP1 on NFκB-p65 and IRF-3 activation in vivo, primary peritoneal macrophages from mice with a conditional inactivation of the lrp1 gene in the myeloid lineage (LysCre;LRPlox/lox) were generated (see supplementary figure 8). After treatment with LPS, reduced p65 phosphorylation and IκBα degradation were observed in lrp1-deficient macrophages (Figure 4A, lane 5 vs lane 6 and lane 7 vs lane 8, respectively, for quantification see supplementary Figure 1B) compared to control cells (LRPlox/lox). LPS-induced IRF-3 phosphorylation, by contrast, was increased when LRP1 was lacking, consistent with our findings in fibroblasts (Figure 4B, lane 6 vs lane 5, for quantification see supplementary Figure 1C). Examination of expression levels of LPS-induced genes revealed that LRP1 repressed basal and LPS-induced transcription of interferon γ (Figure 4C). Chromatin precipitation with an antibody directed against the LRP1 carboxylterminus demonstrated the presence of the LRP1 ICD in the interferon γ transcriptional complex after LPS treatment (Figure 4E). Basal expression of interferon β, by contrast, was only minimally enhanced in the absence of LRP1 (Figure 4D) and no interaction of the LRP1 ICD with the interferon β promoter was detected (Figure 4F). These findings indicate that the LRP1 ICD represses a subset of, though not all, LPS-inducible genes. As LPS itself enhances the proteolytic processing of LRP1 (Figure 2A), negative transcriptional regulation by the LRP1 ICD generates a negative feedback loop of LPS-induced inflammatory signaling. This mechanism involves direct interaction with IRF-3, resulting on one hand in reduced activation of the protein, and repression of target gene promoters by the LRP1 ICD itself on the other.
Figure 4. The LRP1 ICD regulates the expression of a subset of LPS-induced genes by direct nuclear signaling and limiting nuclear pIRF-3.
A) Whole cell lysates were prepared from wildtype and LRP1-deficient macrophages after treatment with 1 µg/ml LPS for the times indicated and from untreated controls. The lysates were examined as described under 3A). Pooled macrophages from 4 k.o. and 4 wildtype mice were used for each repetition of the experiment (n=3). For quantification of Western Blotting results see supplementary Figure 1B.
B) Whole cell lysates were prepared from wildtype (WT) and LRP1-deficient macrophages (k.o.) treated with 1 µg/ml LPS for the times indicated. After Western Blotting antibodies directed against LRP1, pIRF-3, IRF-3 and β-actin were used for staining. Pooled macrophages from 3 k.o. and 3 wildtype mice were used for each repetition of the experiment (n=3). For quantification of Western Blotting results see supplementary Figure 1C.
C) Total RNA was prepared from wildtype and LRP1-deficient macrophages either treated with 1 µg/ml LPS or left untreated. Quantitative real-time PCR was used to assess expression levels of interferon γ. Bars represent the mean of 10 independent experiments, error bars depict SEM. Statistical analysis was done using the Student’s t-test. * p<0.05. For each of the 10 experiments one conditional k.o. and one control mouse were used.
D) Total RNA was prepared from wildtype and LRP1-deficient macrophages either treated with 1 µg/ml LPS or left untreated. Quantitative real-time PCR was used to assess expression levels of interferon β. Bars represent the mean of 6 independent experiments, error bars depict SEM. Statistical analysis was done using the Student’s t-test. * p<0.01. For each of the 6 experiments one conditional k.o. and one control mouse were used.
E) Wildtype and LRP1-deficient macrophages were treated with 1 µg/ml LPS for 15 min. or left untreated. Then DNA-binding proteins were cross-linked to the chromatin and cell lysates were prepared. After shearing of the DNA by sonification an immunoprecipitation with the C-terminal LRP1 antibody was carried out. The precipitate was used in a PCR reaction with primers binding to the interferon γ promoter. A representative experiment of n=3 is shown. For each of the 3 experiments one conditional k.o. and one control mouse were used.
F) Wildtype and LRP1-deficient macrophages were treated with 1 µg/ml LPS for the times indicated or left untreated. Chromatin immunoprecipitation was carried out as described above. Primers specific for the interferon β promoter were used in the PCR reaction. A representative experiment of n=3 is shown. For each of the 3 experiments one conditional k.o. and one control mouse were used.
G) Wildtype and LRP1-deficient murine embryonic fibroblasts were treated with 4 ng/ml leptomycin B for 19h or were left untreated. Nuclear extracts were prepared and analyzed by immunoblotting with a pIRF-3 antibody. β-actin served as a loading control. A representative experiment of n=3 is shown.
H) Wildtype and LRP1-deficient murine embryonic fibroblasts were treated with 1 µM epoxomicin or 10 µM lactacystin for 19h or were left untreated. Nuclear extracts were prepared and analyzed by immunoblotting with a pIRF-3 antibody. β-actin served as a loading control.
I) Wildtype and LRP1-deficient macrophages were treated with 10 ng/ml LPS for 4h or left untreated. Supernatants were then collected and TNF-α concentrations were determined by ELISA. Bars represent the mean of 6 independent experiments, error bars depict SEM. Statistical analysis was done using the Student’s t-test. * p<0.005. For each of the 6 experiments one conditional k.o. and one control mouse were used.
J) Wildtype and LRP1-deficient macrophages were treated with 1 µg/ml LPS for 24h or left untreated. Supernatants were then collected and IL-6 concentrations were determined by ELISA. Bars represent the mean of 6 independent experiments, error bars depict SEM. Statistical analysis was done using the Student’s t-test. * p<0.05. For each of the 6 experiments one conditional k.o. and one control mouse were used.
The opposing NFκB activation that we observed in lrp1-deficient cells thus likely reflects a counter-regulatory response evoked by deregulated inflammatory signaling, e.g. via increased IRF-3 activity. This may represent an adaptive mechanism similar to the one reported in tolerant macrophages that had been exposed to repeated or prolonged LPS stimulation (18).
LRP1 facilitates nuclear export of phosphorylated IRF-3
The LRP1 ICD interacts both with IRF-3 (Figure 3H) and with the promoter of at least one LRP1-regulated inflammatory gene (Figure 4E). We therefore hypothesized that the nuclear accumulation of pIRF-3 in the absence of the LRP1 ICD (Figure 3B) was the result of reduced inactivation of pIRF-3 rather than increased IRF-3 phosphorylation as phosphorylation of IRF-3 occurs in the cytosol. pIRF-3 activity is controlled by nuclear export through the shuttling receptor chromosome region maintenance/exportin 1 (CRM1) and proteasomal degradation (19, 20). To test whether LRP1 modulates either process we treated wildtype and LRP1-deficient fibroblasts with the CRM1 inhibitor leptomycin B or the proteasome inhibitors epoxomicin and lactacystin. Inhibition of nuclear export led to the accumulation of nuclear pIRF-3 in wildtype cells to the same increased level seen in LRP1-deficient cells (Figure 4G). Exposure to inhibitors of proteasomal degradation, by contrast, led to progressive accumulation of pIRF-3 in both cell lines. Together these results indicate that the LRP1 ICD reduces the levels of active pIRF-3 by facilitating its nuclear export and therefore its shuttling toward proteasomal degradation.
Increased secretion of proinflammatory cytokines by LRP1-deficient macrophages
To assess the physiological impact of the negative regulatory mechanism that we identified, we measured cytokine secretion by LPS-stimulated wildtype and LRP1-deficient peritoneal macrophages. Both LPS-induced TNF-α (Fig. 4I) and IL-6 (Fig. 4J) production were increased in the absence of LRP1 indicating that loss of the LRP1-dependent negative transcriptional control of LPS target genes translates into an exaggerated inflammatory phenotype of the affected cells.
Transcriptional profiling of LRP1-dependent LPS-inducible genes
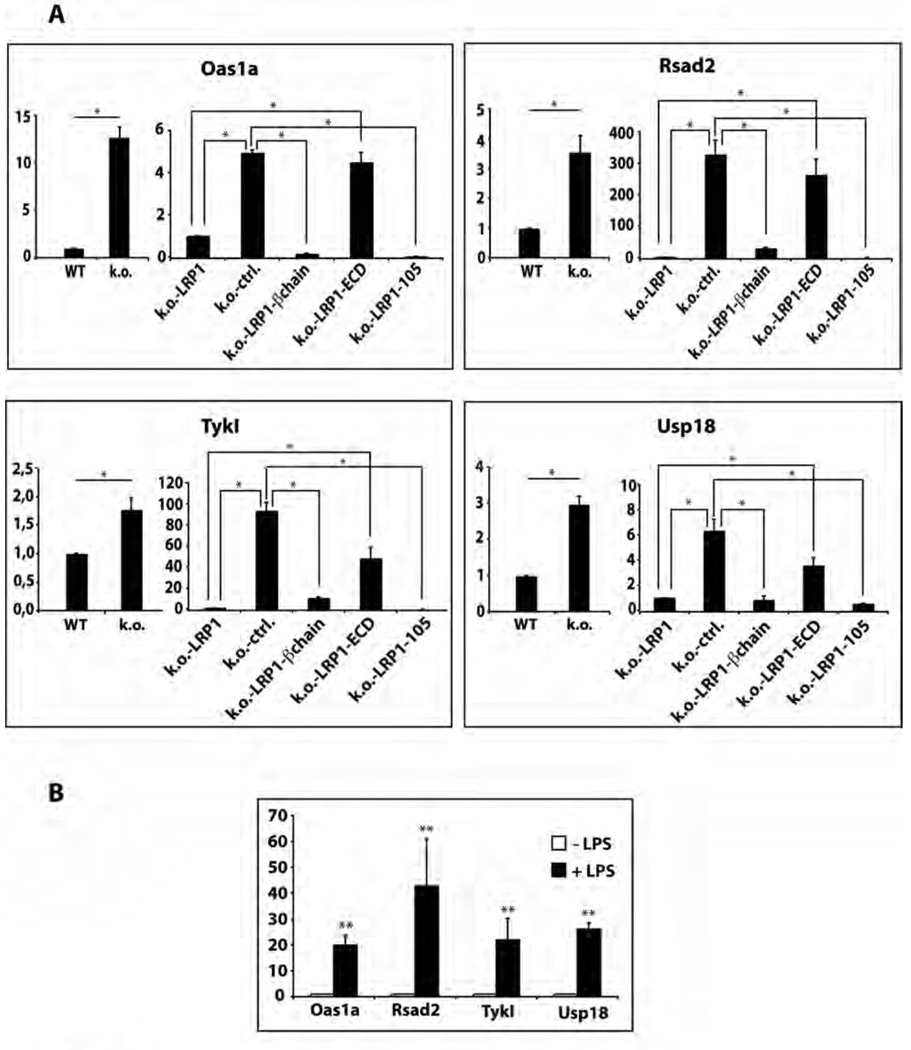
To identify additional LRP1 ICD target genes we performed a microarray screen on wildtype and LRP1-deficient fibroblasts, and on retransfected cells expressing different LRP1 mutants (see Figure 3D). Gene expression levels in the various cell lines were compared to those in wildtype fibroblasts and the data were stratified for genes whose expression levels were corrected both by retransfection of full-length LRP1 (k.o.-LRP1) and the truncated receptor construct containing the LRP1 ICD (k.o.-LRP1-β-chain), but not by expression of the LRP1 ECD (k.o.-LRP1-ECD) (suppl. Table 1). We confirmed the expression patterns of the thus identified potential target genes in the different cell lines by quantitative real-time PCR (Figure 5A). There was good concordance between the microarray and the RT-PCR results for all genes tested. Moreover, in the cell line that stably expressed only the free LRP1 ICD (k.o.-LRP1-105), mRNA expression of these genes (Rsad2, Usp18, Tyki, and Oas1a), which are all induced during inflammation (21–26) returned to wildtype-baseline levels or below supporting their LRP1-dependent transcriptional regulation (Figure 5A). We next tested the effect of LPS (2 µg/ml for 3 h) on these new candidate LRP1 target genes. All were responsive to the treatment (Figure 5B) further confirming that the LRP1 ICD controls the repression of LPS-inducible genes.
Figure 5. Identification of a subset of LPS-inducible genes that are repressed by the LRP1 ICD.
A) Total RNA from wildtype and LRP1-deficient MEF and from LRP1-deficient fibroblasts stably re-transfected with LRP1, the membrane-bound LRP1 ICD (LRP-β-chain), the LRP1 ECD, the free LRP1 ICD (LRP1-105) or the empty plasmid vector was analyzed by quantitative real-time PCR for expression levels of Usp18, Rsad2, Tyki, and Oas1. Bars represent the mean of 6 independent experiments, error bars depict SEM. Statistical analysis was done using the Student’s t-test. * p<0.001.
B) Wildtype fibroblasts were treated with 2 µg/ml LPS for 3h or were left untreated. RNA was prepared and examined by real-time PCR for expression of Usp18, Rsad2, Tyki, and Oas1. ** p<0.05, n=5.
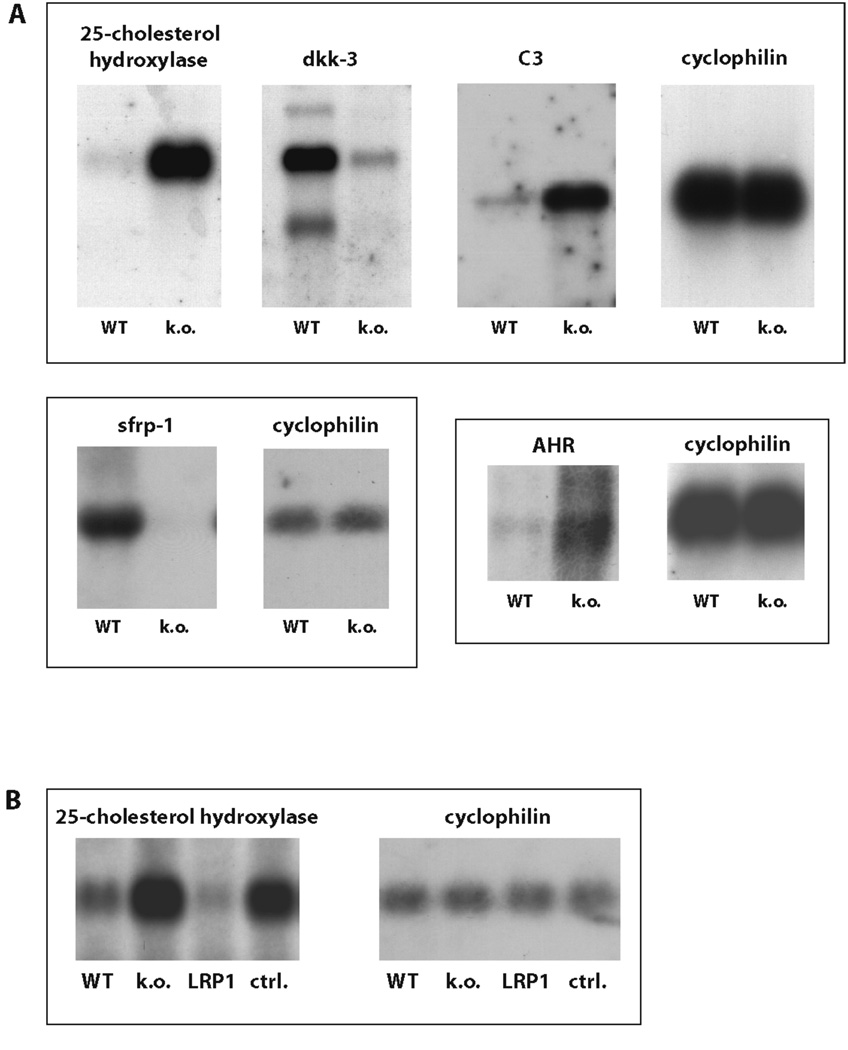
Further analysis of wildtype and LRP1-deficient fibroblasts by unbiased transcriptional profiling yielded a larger set of differentially expressed genes (suppl. Table 2) with functions in signal transduction, membrane trafficking and lipid metabolism. Several candidates were selected for Northern Blotting to confirm the result of the array experiments (Figure 6A). Specificity was verified by retransfection of the full-length LRP1-cDNA into the LRP1-deficient fibroblasts, which normalized gene expression levels to those of the wildtype (Figure 6B). Transcriptional regulation of these diverse genes likely involves differential mechanisms that reflect the multifunctionality of LRP1 and its importance in the regulation of diverse signal transduction pathways (7, 27–29).
Figure 6. LRP1 is involved in multiple signaling pathways.
A) RNA samples from wildtype and LRP1-deficient MEF were analyzed by Northern Blotting with cDNA probes for the genes indicated. Cyclophilin served as a loading control. A representative experiment of n=3 is shown.
Dkk-3 – dickkopf-3, C3 – complement factor 3, sfrp-1 – secreted frizzled-related protein-1, AHR – aryl hydrocarbon receptor.
B) RNA samples from wildtype and LRP1-deficient MEF and from LRP1-deficient fibroblasts stably re-transfected with an expression plasmid for LRP1 (control: empty plasmid vector) were examined for 25-cholesterol-hydroxylase expression. A representative experiment of n=3 is shown.
Discussion
We have uncovered a novel mechanism by which γ-secretase-dependent proteolytic processing of the lipoprotein receptor LRP1 limits the inflammatory response through nuclear signal modulation and repression of LPS-inducible genes (Figure 7). LPS treatment leads to increased shedding of the LRP1 extracellular domain followed by release of the LRP1 ICD from the plasma membrane. The soluble ICD translocates to the nucleus, where it participates in LPS-induced transcriptional complexes. It directly interacts with the LPS-activated transcription factor IRF-3 and promotes its nuclear export and proteasomal degradation, thereby limiting its transcriptional activity. As a result, a subset of LPS-inducible genes is repressed by the LRP1 ICD in a negative feedback loop.
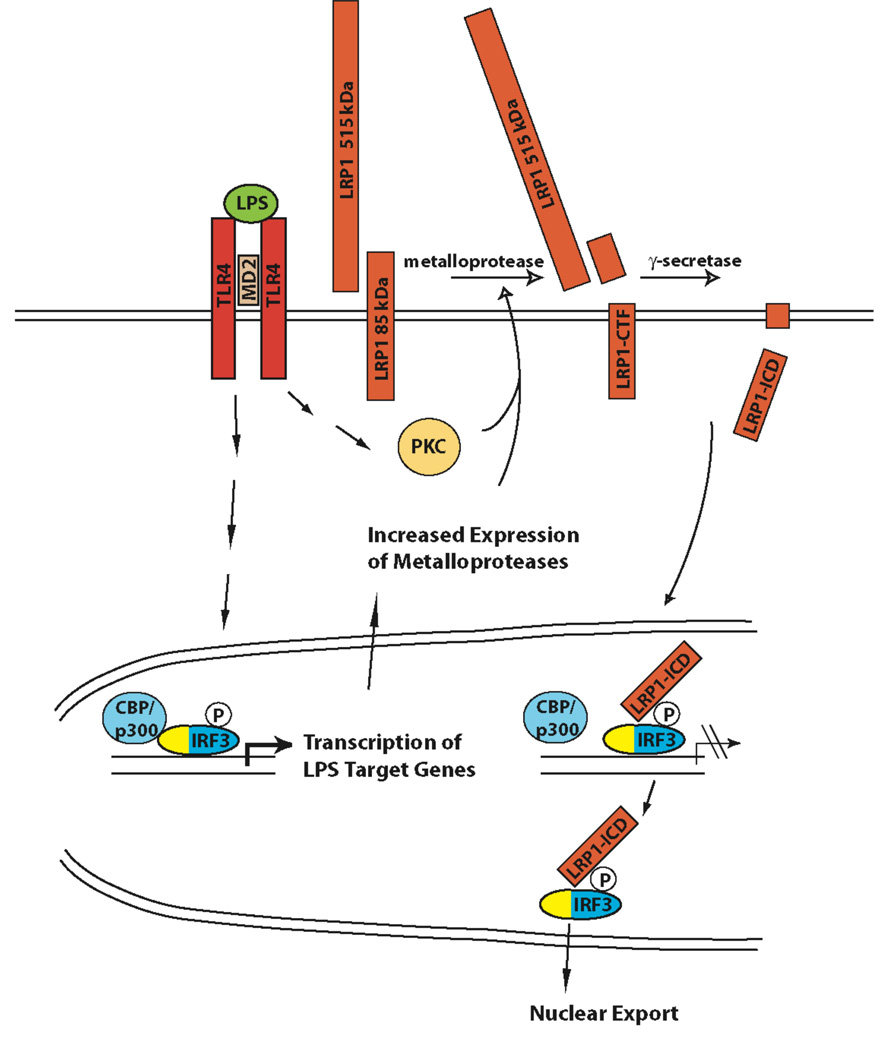
Figure 7. Proposed model for the negative feedback regulation of LPS-induced inflammatory signaling by γ-secretase-dependent LRP1 ICD generation.
Expression of a subset of LPS-induced genes is activated by IRF-3. Activation of IRF-3 and other LPS-induced signaling pathways leads to increased expression of metalloproteases. In addition, LPS-dependent PKC-activation also occurs (55). The proteolytical processing of LRP1 is therefore augmented. Increased shedding provides more substrate for the γ-secretase-mediated cleavage step that leads to LRP1 ICD release. The LRP1 ICD interacts with IRF-3 and displaces it from its binding to CBP/p300, thereby unmasking its nuclear export signal (yellow) and facilitating its nuclear export. Subsequently, IRF-3 target gene expression is reduced.
Nuclear translocation of the LRP1 ICD after γ-secretase-mediated release from the plasma membrane
We have shown by immunocytochemistry that the untagged recombinant soluble LRP1 ICD efficiently translocates to the nucleus (Figure 1 A). Cellular fractionation and immunoblotting also reveal nuclear localization of endogenous, physiologically produced LRP1 ICD (Figure 1B), suggesting a role in the regulation of gene transcription. Earlier work from our laboratory and by our colleagues had shown that a tagged LRP1 ICD construct translocates to the nucleus and modifies transcription from a heterologous reporter (13, 30, 31). However, the tags that were employed in these studies can themselves mediate nuclear translocation, and the physiological trafficking, localization and turn-over of the endogenous LRP1 ICD had remained unknown.
LPS treatment enhances the proteolytic processing of LRP1
Bacterial lipopolysaccharides (LPS) significantly enhanced the proteolytic processing of LRP1 (Figure 2A and supplementary Figures 5 and 6). After pretreatment with the γ-secretase inhibitor DAPT the 25 kDa carboxyl-terminal fragment of LRP1 (LRP1-CTF) that is produced by shedding of the extracellular domain accumulated in macrophages exposed to LPS indicating that LPS increases the extracellular cleavage of the receptor. We reported earlier that this is the rate-limiting step in LRP1 ICD production (13, 31) and is followed by further constitutive γ-secretase-dependent intramembranous processing of LRP1.
Shedding of the LRP1 extracelluar domain is mediated by a metalloprotease (32) and PKC activation enhances this extracellular cleavage of LRP1 (13). PKC activation, which occurs early in the inflammatory response, induces the expression of matrix metalloproteases and of other mediators of inflammation (14) suggesting a possible role for the LRP1 ICD in the inflammatory process. Treatment with LPS, which induces a strong host defense response and activates PKCε amongst other downstream mediators (33), shows that the LRP1 ICD is released in the course of the inflammatory reaction. This allows it to function as a feedback regulator that limits damage to normal tissue by unopposed inflammatory signaling. A physiological role for the LRP1 ICD during inflammation is further supported by the γ-secretase-dependent processing of LRP1 in phagosomes, where phagocytosis of foreign material and the inflammatory mediator interferon γ were shown to stimulate LRP1 ICD release (12).
The LRP1 ICD interacts with the LPS-activated transcription factor IRF-3 and limits its activity by facilitating its nuclear export
In the absence of LRP1 LPS-induced phosphorylation and activation of the transcription factor IRF-3 was prolonged in primary macrophages (Figure 4B) and pIRF-3 accumulated in the nucleus in murine embryonic fibroblasts (Figure 3B). Reexpression of the free LRP1 ICD in the fibroblasts was sufficient to reduce nuclear pIRF-3 levels to the baseline of wildtype cells (Figure 3B) indicating the ICD directly limits the availability of pIRF-3 in the nucleus independent of the LRP1 extracellular domain. Binding of the ICD to IRF-3 is mediated through the second NPxY motif (Figure 3H).
Activity of IRF-3 is regulated by its subcellular localization (19). IRF-3 contains both a nuclear import and a nuclear export signal and shuttles between cytoplasm and nucleus. Following its phosphorylation it binds to CBP or p300 leading to its nuclear sequestration (19). IRF-3 action is terminated through nuclear export by CRM1 and proteasomal degradation (20). Treatment of wildtype and LRP1-deficient fibroblasts with the CRM1 inhibitor leptomycin B equalized the levels of nuclear pIRF-3 in the two cell lines (Figure 4G). Inhibition of the proteasome, however, led to further accumulation of nuclear pIRF-3 both in wildtype and in LRP1-deficient cells (Figure 4H). Together these results indicate that the LRP1 ICD restricts transcriptional activation by IRF-3 by facilitating its nuclear export. It is conceivable that the direct interaction between the LRP1 ICD and IRF-3 interrupts IRF-3 binding to CBP/p300 and thus unmasks the nuclear export signal allowing IRF-3 to be shuttled to the cytoplasm by CRM1. Further studies will have to show whether this regulatory mechanism also applies when IRF-3 is activated by other signals than the LPS-TLR4 pathway. In a first analysis with polyinosinic polycytidylic acid (pIpC), a potent activator of IRF-3 that signals via TLR3, we found that treatment with this substance failed to induce proteolytical processing of LRP1 and thus to activate the first branch of the γ-secretase dependent negative feedback loop (supplementary figure 6). Activation of LRP1 ICD generation by LPS, on the other hand, allows the ICD to function as a negative feedback regulator of LPS signaling. This indicates some specificity in the regulatory pathway, but it is still possible, however, that there is LRP1 ICD production in other inflammatory conditions allowing for a more general role of the LRP1 ICD in determining IRF-3 activity.
Degradation of the NFκB inhibitory protein IκB is delayed in the constitutive absence of LRP1 (Figure 3A and Figure 4A). It was recently reported that LPS-induced transcriptional activity of IRF-3 is dependent upon NFκB (34). If the action of the LPS-induced transcription factors IRF-3 and NFκB is coordinated, decreased activation of NFκB in the absence of LRP1 might occur as a secondary counter-regulatory mechanism evoked by increased IRF-3 activity similar to the deficient NFκB signaling that has been observed under conditions of “LPS tolerance”, i.e. after repeated or prolonged LPS stimulation (18). In agreement with this model, transcriptional activation of NFκB targets by platelet factor 4 and interleukin 1 were also found to be dependent upon the presence of LRP1 (35, 36). An additional direct role for LRP1 in the activation of NFκB remains possible, too, but so far no molecular mechanism for such a LRP1 function has been elucidated. Further support for the indirect model comes from a recent study where modulation of NFκB activity through down-regulation of cell-surface TNFR1 by LRP1 was reported. Interestingly, this effect seemed to stem partly from a decrease in total TNFR1 and thus, too, might be mediated through transcriptional regulation by LRP1 (37).
The LRP1 ICD limits the transcription of inflammatory genes
Using chromatin immunoprecipitation (ChIP) we could show that the LRP1 ICD resides in the transcriptional complex that assembles on the interferon γ promoter (Figure 4E), where it represses LPS-induced gene transcription in vivo (Figure 4C) indicating that γ-secretase-dependent processing of LRP1 mediates transcriptional regulation. By contrast, ChIP failed to detect the LRP1 ICD on the interferon β promoter suggesting that only a defined subset of LPS-inducible genes is modulated by the ICD. Gene-specific modulation of LPS-induced transcriptional activation through interaction with differentially activated transcriptional co-factors has been described for nuclear receptors (38). Furthermore, chromatin modifications were shown to determine gene-specific control of transcription in inflammation (39). In addition, also the exact time course of gene activation in relation to that of LRP1 ICD release could determine whether an interaction can occur and might differentially favor direct or indirect IRF-3-dependent targets of the LPS signaling cascade. All mechanisms, alone or together could determine the ability of the LRP1 ICD to interact with the transcriptional complex on a given LPS-inducible gene.
Transcriptional profiling of LRP1 mutant cell lines identified several genes whose expression was modified specifically by the presence of the LRP1 intracellular domain (suppl. Table 1). All LRP1 ICD targets that we identified in this manner are LPS-inducible (23–25) and Figure 5B) indicating that expression of a substantial subset of LPS-regulated genes is modulated by the LRP1 ICD. Increased release of proinflammatory cytokines from LPS-treated LRP1-deficient macrophages demonstrated the physiological impact of the identified transcriptional control mechanism (Figure 4I and 4J).
During our search for LRP1-target genes by transcriptional profiling of wild type and LRP1-deficent cells, we identified a series of additional differentially expressed transcripts (suppl. Table 2). It is likely that expression levels of many of them are altered by indirect mechanisms reflecting the multifunctionality of the receptor (for review see (40)). For instance, the identification of TGFβ and Wnt signaling-related genes, such as schnurri, latent TGF β binding protein, sfrp1, and dkk-3 is consistent with the established role of LRP1 as a co-receptor for TGFβ (41) and with its interaction with frizzled proteins, the transmembrane receptors for Wnt signaling factors (42).
Physiological and pathophysiological implications
Regulation of LPS-induced signaling and gene expression defines a new role for γ-secretase and LRP1 as modulators of inflammation. Inflammation is an important pathogenetic factor of atherosclerosis and absence of feed-back suppression may be responsible in part for the increased atherosclerosis that develops in mice lacking LRP1 in macrophages and in vascular smooth muscle cells (6, 43, 44). Interestingly, atherosclerotic lesion development was mitigated in conditional knockout animals lacking lrp1 in smooth muscle cells by the PPARγ agonist rosiglitazone (7), which is also a negative regulator of LPS-inducible inflammatory genes (38, 45). Moreover, recent in vitro studies showed that the LRP1 ligand ApoE limits inflammatory signaling by IL-1 in smooth muscle cells in an LRP1-dependent manner (35), further supporting a mechanism where LRP1 protects the vascular wall by locally controlling the inflammatory response.
In addition, new evidence shows that LRP1 cleavage occurs in the penumbra of infarcted cerebral tissue, where it might modulate the occurrence of apoptotic cell death (46) suggesting that LRP1 has a more general role in the control of inflammation. This is potentially relevant to all conditions where inflammation contributes to pathogenesis. Examples include pulmonary inflammation in the context of acute lung injury, which is aggravated by TLR4 activation (47), and Alzheimer’s disease, where inflammation contributes to pathogenesis and polymorphisms of the LRP1 ligand apolipoprotein E constitute the most important known risk factor for sporadic disease (48). Moreover, control of inflammation by γ-secretase has to be taken into account when considering the therapeutic use of γ-secretase inhibitors, as loss of negative feedback may result in undesirable drug effects.
In summary, our findings define a novel mechanism by which lipoprotein receptors modulate nuclear signaling, demonstrate a role for γ-secretase in inflammation, and uncover an interdependence of lipoprotein receptor and inflammatory signaling. Modulation of the inflammatory response by γ-secretase and LRP1 thus offers a new potential target for therapeutic intervention and may allow conceptually novel treatment in a variety of inflammatory syndromes.
Materials and Methods
Chemicals
LPS (L4391) was purchased from Sigma Aldrich (Germany), rLPS was generously provided by M. Freudenberg and C. Galanos (MPI for Immunobiology, Freiburg, Germany), pIpC (P1530) was from Sigma Aldrich, DAPT and epoxomicin were obtained from Calbiochem (USA). Leptomycin B was from Biomol (Germany) and lactacystin from Merck (Germany).
Cell culture and stable transfection of murine embryonic fibroblasts (MEF)
MEF derived from wildtype or lrp1-deficient mouse embryos were maintained in DMEM with glucose 4.5g/l, 2 mM L-glutamine (Cambrex, Belgium), 100U/ml penicillin, 100µg/ml streptomycin sulfate (Cambrex), and 8% (v/v) FCS (Sigma Aldrich).
Cells were transfected by FuGene™ (Roche, Germany) and stable tranfectants were selected with zeocin at a concentration of 700µg/ml (Invitrogen, Germany) and subcloning of emerging colonies. For plasmids see supplement.
Immunocytochemistry
MEF or macrophages cultured on glass cover slips were fixed with 4 % paraformaldehyde, permeabilised with 0.2 % Triton X-100, quenched with 0.1 % NaBH4, and blocked with 10 % donkey serum and 1 % albumin in TBS. Incubation with the first antibody (affinity-purified rabbit polyclonal α-LRP1 (49) or 8G1 mouse monoclonal α-LRP1, Progen, Germany, or rabbit α-Iba1, Wako Chemicals, Germany) was done overnight at 4° C. Alexa 488-labelled donkey anti-rabbit or anti-mouse antibody (Invitrogen) was used 1:200 for the detection of bound primary antibody. Cover slips were mounted in aqueous mounting medium (Molecular Probes, USA) and examined with a Zeiss Axioplan 2 imaging microscope with apotome.
RNA preparation and Northern Blot analysis
Total RNA was prepared from MEF with RNA-STAT60 (Tel-Test, USA). 20 µg total RNA were used for Northern Blotting. Briefly, RNA samples were separated on a 1 % agarose / 5.5 % formaldehyde gel and transferred to a Hybond N+ membrane (Amersham, USA) by upward capillary transfer in 10x SSC. Nucleic acids were cross-linked to the membrane by UV-irradiation. RapidHyb buffer (Amersham) was used for prehybridisation and hybridisation. For hybridization, 1 ng probe was labelled with 32P-dCTP (Amersham) employing the Rediprime Labeling System (Amersham). Probe bound to the membrane was detected by autoradiography. For probes see supplement.
Microarray experiments
Total RNA from MEF was prepared with RNA-STAT60 (Tel-Test) or Trizol (Invitrogen). RNA was used for labelling as described in the Affymetrix technical bulletin. Hybridization, washing, scanning, and analysis of the Affymetrix GeneChip Murine Genome 430 A 2.0 arrays (Affymetrix, USA) were carried out as described (50) in the core facility of UTSW Medical Center or by Affymetrix service provider Kompetenzzentrum für Fluoreszente Bioanalytik (KFB), Regensburg Germany. Data obtained from the microarray hybridizations were processed with MICROARRAY SUITE 5.0 (Affymetrix) software.
Quantitative real-time PCR
RNA was extracted from MEF grown to 95–100 % confluence or mouse peritoneal macrophages with Trizol (Invitrogen) and was treated with RNase-free DNase I (Fermentas, Germany). For cDNA synthesis, random primers (Promega, Germany), M-MLV reverse transcriptase (Promega), RNase Inhibitor (Promega), and dNTPs (Genaxxon, Germany) were used.
The real-time PCR reaction was set up with 2x Absolute™ QPCR SYBR® Green Mix with Fluorescein (Abgene) on a single-colour real-time PCR detection system (BioRad MyiQ with MyIQ™ Optical System Software Vers. 1.0). For sequences of the primers used see supplement. To compare expression levels, the ΔΔct method was used. Ct values were standardised with respect to cyclophilin or gapdh. For all experiments, samples were assayed at least in duplicate and the mean of ct was used for further calculations. Experiments were repeated independently at least five times. Data were averaged and statistically analyzed as described in the figure legends.
Preparation of whole cell lysates and Western Blot analysis
Cells were harvested in ice cold PBS with 1 mM PMSF, 1 mM NaVO4, 25 mM β-Glycerolphosphate, and 10 mM NaF and RIPA Buffer (see supplement) was used for lysis. 30 µg of MEF cell lysate or 50 µg of macrophage lysate were subjected to SDS polyacrylamide gel electrophoresis and Western Blotting according to standard procedures. For primary antibodies used see supplement. After incubation with an HRP-conjugated secondary antibody, bound antibodies were visualized by enhanced chemiluminescence (ECL) using SuperSignal CL-HRP Substrate (Perbio, Germany).
Cell fractionation
Cells were harvested in ice cold PBS and were lysed in hypotonic Buffer A (buffer compositions see supplement). After centrifugation at 850 × g for 5 min at 4 °C, for membrane preparation, the supernatant was recentrifuged at 100,000 × g for 30 min. and the membrane pellet was resuspended in SDS buffer. For the preparation of nuclear extracts the pellet of the 850 × g centrifugation step was resuspended in buffer C, incubated at 4 °C for 60 min., and centrifuged at 100 000 g for 30 min. The supernatant was then used for further examination of nuclear proteins by Western Blotting.
Generation of GST Fusion Proteins
GST fusion plasmids of LRP1-ICD mutants were prepared by standard cloning procedures. For LRP1 sequences cloned into pGEX-4T1 (Amersham Pharmacia Biotech) see supplement. Fusion proteins were expressed in BL21 codon+ bacteria (Stratagene) following induction by 1 mM isopropyl-thio-D-galactopyranoside for 5 h. Proteins were recovered by Triton lysis (PBS with 1% Triton X-100 and Protease Inhibitors (Roche)) and purified using glutathione-agarose beads (Sigma Aldrich, Germany).
GST Pulldown Assay
MEF lysates were incubated with 50 µl of glutathione-agarose and 10 µg of the respective purified GST fusion protein for 6 h at 4 °C. Glutathione beads were washed rapidly three times in 150 mM NaCl, 10 mM Tris-HCl, pH 7.5, 2 mM MgCl2, 2 mM CaCl2, and 2 mM MnCl2 for 10 min. SDS sample buffer was added to the supernatant or beads. Proteins were separated by SDS-polyacrylamide gel electrophoresis and analyzed by immunoblotting with an IRF-3 antibody (Santa Cruz, sc 15991).
LysCreLRPlox/lox mice
Mice carrying a loxP-marked LRP1 allele were described previously (51). These mice were bred to animals transgenic for the viral Cre recombinase under the control of the lysozyme promoter (52). These mice were kindly provided by Irmgard Förster, Munich, Germany. For experiments, age-matched 3- to 6-month-old mice were used. Animals were kept under standard laboratory conditions. Experiments were carried out according to the principles of good laboratory animal care and were approved by local authorities.
For genotyping PCRs see supplement.
Preparation of thioglycollate-elicited peritoneal macrophages
Mice were injected intraperitoneally with 2 ml thioglycollate solution (3.85 g LB-thioglycollate in 100 ml ddH20, autoclaved for 30 min at 120 °C and 1 bar). After 4 days macrophages were collected in 2 % Pen-Strep in PBS. After centrifugation for 5 min at 300 × g and 4 °C cells were redissolved in 500 µl DMEM (1 g/l glucose, 2 mM glutamine; 8 % FCS; 1 % Pen-Strep). Macrophages were seeded as required and were washed three times with prewarmed PBS after 4 hours.
ChIP Assay
ChIP analysis was performed using the ChIP Assay Kit (Upstate Cell Signaling Solutions, UK). Briefly, mouse peritoneal macrophages were seeded at 3 × 106 cells per condition. Cells were either left untreated or stimulated for 15 min. with 1 µg/ml LPS. Then they were harvested and DNA was sheared by sonification with a Branson Digital Sonifier (Model 450) and a 3 mm diameter microtip probe (2 cycles of 10 s with an amplitude of 15 %). For the IP, 15 µl α-LRP1 serum was used. The following primers were employed for the PCR of the interferon gamma promoter (53): forward, 5'-ATCACCTCCATTGAAGGGCTTCCT-3'; reverse, 5'-AGTTTCCTTTCGACTCCTTGGGCT -3'.
PCR conditions were 94 °C for 2 min., 94 °C for 30 s, 55 °C for 30 s, 68 °C for 30 s (30 cycles) and 68 °C for 5 min. Primers for the interferon beta promoter were IFNb-forward: 5’-CCAGCAATTGGTGAAACTGTACAA–3’ and IFNb-reverse: 5’-CAGTGAGAATGATCTTCCTTCATGG–3’.
Cytokine measurements
Mouse TNF-alpha/TNFSF1A and Mouse IL-6 Quantikine ELISA Kits (R&D Systems) were used to measure the concentration of the respective cytokines in cell culture supernatants. Briefly, wildtype or LRP1-deficient peritoneal macrophages were seeded into 24-well plates at 3 × 105 per well. After stimulation with LPS supernatants were collected at 4h (TNF-α) or 24h (IL-6) for ELISAs. A FLUOstar Optima plate reader was used for absorbance measurements.
Supplementary Material
Abbreviations
- CRM1
chromosome region maintenance/exportin 1
- ECD
extracellular domain
- ICD
intracellular domain
- IRF-3
interferon regulatory factor 3
- GPI
glycosyl phosphatidyl inositol
- LDL
low-density lipoprotein
- LRP1
LDL receptor-related protein 1
- MD-2
Myeloid differentiation 2
- MyD88
myeloid differentiation 88
- NFκB
nuclear factor κ B
- TIR
Toll/interleukin-1 receptor
Footnotes
This manuscript has been accepted for publication in Science Signaling. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencesignaling.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References
- 1.Parks AL, Curtis D. Presenilin diversifies its portfolio. Trends Genet. 2007;23:140–150. doi: 10.1016/j.tig.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Ehebauer M, Hayward P, Martinez-Arias A. Notch signaling pathway. Sci STKE. 2006;2006:cm7. doi: 10.1126/stke.3642006cm7. [DOI] [PubMed] [Google Scholar]
- 3.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 4.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 5.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 7.Boucher P, Li WP, Matz RL, Takayama Y, Auwerx J, Anderson RG, Herz J. LRP1 functions as an atheroprotective integrator of TGFbeta and PDFG signals in the vascular wall: implications for Marfan syndrome. PLoS ONE. 2007;2:e448. doi: 10.1371/journal.pone.0000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, Schomburg ED, Noebels JL, Beffert U, Sweatt JD, Weeber EJ, Herz J. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol. 2004;24:8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 12.Jutras I, Laplante A, Boulais J, Brunet S, Thinakaran G, Desjardins M. Gamma-secretase is a functional component of phagosomes. J Biol Chem. 2005;280:36310–36317. doi: 10.1074/jbc.M504069200. [DOI] [PubMed] [Google Scholar]
- 13.May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002;277:18736–18743. doi: 10.1074/jbc.M201979200. Epub 12002 Mar 18720. [DOI] [PubMed] [Google Scholar]
- 14.Ogata Y, Pratta MA, Nagase H, Arner EC. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) is induced in rabbit articular chondrocytes by cotreatment with interleukin 1 beta and a protein kinase C activator. Exp Cell Res. 1992;201:245–249. doi: 10.1016/0014-4827(92)90271-9. [DOI] [PubMed] [Google Scholar]
- 15.Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2007;10:10. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 17.Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujihara M, Wakamoto S, Ito T, Muroi M, Suzuki T, Ikeda H, Ikebuchi K. Lipopolysaccharide-triggered desensitization of TNF-alpha mRNA expression involves lack of phosphorylation of IkappaBalpha in a murine macrophage-like cell line, P388D1. J Leukoc Biol. 2000;68:267–276. [PubMed] [Google Scholar]
- 19.Kumar KP, McBride KM, Weaver BK, Dingwall C, Reich NC. Regulated nuclear-cytoplasmic localization of interferon regulatory factor 3, a subunit of double-stranded RNA-activated factor 1. Mol Cell Biol. 2000;20:4159–4168. doi: 10.1128/mcb.20.11.4159-4168.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saitoh T, Tun-Kyi A, Ryo A, Yamamoto M, Finn G, Fujita T, Akira S, Yamamoto N, Lu KP, Yamaoka S. Negative regulation of interferon-regulatory factor 3-dependent innate antiviral response by the prolyl isomerase Pin1. Nat Immunol. 2006;7:598–605. doi: 10.1038/ni1347. [DOI] [PubMed] [Google Scholar]
- 21.Chin KC, Cresswell P. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc Natl Acad Sci U S A. 2001;98:15125–15130. doi: 10.1073/pnas.011593298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang D, Jiang H, Wu Q, Pestka S, Fisher PB. Cloning and characterization of human ubiquitin-processing protease-43 from terminally differentiated human melanoma cells using a rapid subtraction hybridization protocol RaSH. Gene. 2001;267:233–242. doi: 10.1016/s0378-1119(01)00384-5. [DOI] [PubMed] [Google Scholar]
- 23.Lee CG, O'Brien WE. A unique member of the thymidylate kinase family that is induced during macrophage activation. J Immunol. 1995;154:6094–6102. [PubMed] [Google Scholar]
- 24.Malakhova O, Malakhov M, Hetherington C, Zhang DE. Lipopolysaccharide activates the expression of ISG15-specific protease UBP43 via interferon regulatory factor 3. J Biol Chem. 2002;277:14703–14711. doi: 10.1074/jbc.M111527200. [DOI] [PubMed] [Google Scholar]
- 25.Olofsson PS, Jatta K, Wagsater D, Gredmark S, Hedin U, Paulsson-Berne G, Soderberg-Naucler C, Hansson GK, Sirsjo A. The antiviral cytomegalovirus inducible gene 5/viperin is expressed in atherosclerosis and regulated by proinflammatory agents. Arterioscler Thromb Vasc Biol. 2005;25:e113–e116. doi: 10.1161/01.ATV.0000170130.85334.38. [DOI] [PubMed] [Google Scholar]
- 26.Yan C, Sehgal PB, Tamm I. Signal transduction pathways in the induction of 2',5'-oligoadenylate synthetase gene expression by interferon alpha/beta. Proc Natl Acad Sci U S A. 1989;86:2243–2247. doi: 10.1073/pnas.86.7.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, Herz J. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low Density lipoprotein receptor-related protein in caveolae. J Biol Chem. 2002;277:15507–15513. doi: 10.1074/jbc.M200428200. Epub 12002 Feb 15519. [DOI] [PubMed] [Google Scholar]
- 28.Huang SS, Ling TY, Tseng WF, Huang YH, Tang FM, Leal SM, Huang JS. Cellular growth inhibition by IGFBP-3 and TGF-beta1 requires LRP-1. Faseb J. 2003;17:2068–2081. doi: 10.1096/fj.03-0256com. [DOI] [PubMed] [Google Scholar]
- 29.Loukinova E, Ranganathan S, Kuznetsov S, Gorlatova N, Migliorini MM, Loukinov D, Ulery PG, Mikhailenko I, Lawrence DA, Strickland DK. Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function betwenn LRP and the PDGF. J Biol Chem. 2002;277:15499–15506. doi: 10.1074/jbc.M200427200. Epub 12002 Feb 15419. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita A, Shah T, Tangredi MM, Strickland DK, Hyman BT. The intracellular domain of the low density lipoprotein receptor-related protein modulates transactivation mediated by amyloid precursor protein and Fe65. J Biol Chem. 2003;278:41182–41188. doi: 10.1074/jbc.M306403200. [DOI] [PubMed] [Google Scholar]
- 31.May P, Bock HH, Nimpf J, Herz J. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J Biol Chem. 2003;278:37386–37392. doi: 10.1074/jbc.M305858200. [DOI] [PubMed] [Google Scholar]
- 32.Quinn KA, Pye VJ, Dai YP, Chesterman CN, Owensby DA. Characterization of the soluble form of the low density lipoprotein receptor-related protein (LRP) Exp Cell Res. 1999;251:433–441. doi: 10.1006/excr.1999.4590. [DOI] [PubMed] [Google Scholar]
- 33.Castrillo A, Pennington DJ, Otto F, Parker PJ, Owen MJ, Bosca L. Protein kinase Cepsilon is required for macrophage activation and defense against bacterial infection. J Exp Med. 2001;194:1231–1242. doi: 10.1084/jem.194.9.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wietek C, Miggin SM, Jefferies CA, O'Neill LA. Interferon regulatory factor-3-mediated activation of the interferon-sensitive response element by Toll-like receptor (TLR) 4 but not TLR3 requires the p65 subunit of NF-kappa. J Biol Chem. 2003;278:50923–50931. doi: 10.1074/jbc.M308135200. [DOI] [PubMed] [Google Scholar]
- 35.Kawamura A, Baitsch D, Telgmann R, Feuerborn R, Weissen-Plenz G, Hagedorn C, Saku K, Brand-Herrmann SM, von Eckardstein A, Assmann G, Nofer JR. Apolipoprotein E interrupts interleukin-1beta signaling in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1610–1617. doi: 10.1161/ATVBAHA.106.129957. [DOI] [PubMed] [Google Scholar]
- 36.Yu G, Rux AH, Ma P, Bdeir K, Sachais BS. Endothelial expression of E-selectin is induced by the platelet-specific chemokine platelet factor 4 through LRP in an NF-kappaB-dependent manner. Blood. 2005;105:3545–3551. doi: 10.1182/blood-2004-07-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaultier A, Arandjelovic S, Niessen S, Overton CD, Linton MF, Fazio S, Campana WM, Cravatt BF, 3rd, Gonias SL. Regulation of tumor necrosis factor receptor-1 and the IKK-NF-kappaB pathway by LDL receptor-related protein explains the antiinflammatory activity of this receptor. Blood. 2008;111:5316–5325. doi: 10.1182/blood-2007-12-127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, Glass CK. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 40.May P, Herz J, Bock HH. Molecular mechanisms of lipoprotein receptor signalling. Cell Mol Life Sci. 2005;62:2325–2338. doi: 10.1007/s00018-005-5231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng WF, Huang SS, Huang JS. LRP-1/TbetaR-V mediates TGF-beta1-induced growth inhibition in CHO cells. FEBS Lett. 2004;562:71–78. doi: 10.1016/S0014-5793(04)00185-1. [DOI] [PubMed] [Google Scholar]
- 42.Zilberberg A, Yaniv A, Gazit A. The low density lipoprotein receptor-1, LRP1, interacts with the human frizzled-1 (HFz1) and down-regulates the canonical Wnt signaling pathway. J Biol Chem. 2004;279:17535–17542. doi: 10.1074/jbc.M311292200. [DOI] [PubMed] [Google Scholar]
- 43.Hu L, Boesten LS, May P, Herz J, Bovenschen N, Huisman MV, Berbee JF, Havekes LM, van Vlijmen BJ, Tamsma JT. Macrophage low-density lipoprotein receptor-related protein deficiency enhances atherosclerosis in ApoE/LDLR double knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2710–2715. doi: 10.1161/01.ATV.0000249641.96896.e6. [DOI] [PubMed] [Google Scholar]
- 44.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res. 2007;100:670–677. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 45.Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci U S A. 2003;100:6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polavarapu R, An J, Zhang C, Yepes M. Regulated intramembrane proteolysis of the low-density lipoprotein receptor-related protein mediates ischemic cell death. Am J Pathol. 2008;172:1355–1362. doi: 10.2353/ajpath.2008.070975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 49.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. Embo J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahadevappa M, Warrington JA. A high-density probe array sample preparation method using 10- to 100-fold fewer cells. Nat Biotechnol. 1999;17:1134–1136. doi: 10.1038/15124. [DOI] [PubMed] [Google Scholar]
- 51.Rohlmann A, Gotthardt M, Willnow TE, Hammer RE, Herz J. Sustained somatic gene inactivation by viral transfer of Cre recombinase. Nat Biotechnol. 1996;14:1562–1565. doi: 10.1038/nbt1196-1562. [DOI] [PubMed] [Google Scholar]
- 52.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 53.Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE, Sonenshein GE, Osborne BA. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. Embo J. 2006;25:129–138. doi: 10.1038/sj.emboj.7600902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.AcknowledgementsWe thank Simone Zenker and Jonathan Göldner for excellent technical assistance, Sebastian Goerke for help with the cloning of the GST fusion proteins, and Matthias Kirsch for scientific discussions.We are indebted to M. Frotscher and H.E. Blum for scientific advice and support.This work was supported by the DFG (Emmy Noether fellowship MA-2410/1-2 and 1-3 (P.M.), grant BO-1806/2-1 (H.H.B.)), by grants from the NIH (HL20948, HL63762, NS43408 (J.H.)),and by the Humboldt Foundation (Wolfgang Paul Award (J.H.)).
- 55.McGettrick AF, Brint EK, Palsson-McDermott EM, Rowe DC, Golenbock DT, Gay NJ, Fitzgerald KA, O'Neill LA. Trif-related adapter molecule is phosphorylated by PKC{epsilon} during Toll-like receptor 4 signaling. Proc Natl Acad Sci U S A. 2006;103:9196–9201. doi: 10.1073/pnas.0600462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulery PG, Beers J, Mikhailenko I, Tanzi RE, Rebeck GW, Hyman BT, Strickland DK. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer's disease. J Biol Chem. 2000;275:7410–7415. doi: 10.1074/jbc.275.10.7410. [DOI] [PubMed] [Google Scholar]
- 57.Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem. 2003;278:22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.