Abstract
Purpose of review
A number of microbicide candidates have failed to prevent HIV transmission in human clinical trials, and there is uncertainty as to how many additional trials can be supported by the field. Regardless, there are far too many microbicide candidates in development, and a logical and consistent method for screening and selecting candidates for human clinical trials is desperately needed. However, the unique host and cell specificity of HIV provides challenges for microbicide safety and efficacy screening, that can only be addressed by rigorous testing in relevant laboratory animal models.
Recent findings
A number of laboratory animal model systems ranging from rodents to nonhuman primates, and single versus multiple dose challenges have recently been developed to test microbicide candidates. These models have shed light on both the safety and efficacy of candidate microbicides as well as the early mechanisms involved in transmission. This article summarizes the major advantages and disadvantages of the relevant animal models for microbicide safety and efficacy testing.
Summary
Currently, nonhuman primates are the only relevant and effective laboratory model for screening microbicide candidates. Given the consistent failures of prior strategies, it is now clear that rigorous safety and efficacy testing in nonhuman primates should be a pre-requisite for advancing additional microbicide candidates to human clinical trials.
Keywords: HIV-1, microbicide, macaque, mouse, mucosal transmission
INTRODUCTION
It is now clear that laboratory animal safety and efficacy testing will be necessary for selecting microbicide candidates that will advance to human clinical trials. There are simply too many microbicide candidates in clinical development for all to be tested in human trials, and prior selection criteria (in vitro testing) have repeatedly proved misleading, and sometimes disastrous in predicting safety or efficacy in humans. With the failures of now several human clinical trials, it is obvious that prior selection criteria were inadequate for predicting efficacy, and more stringent tests of safety and efficacy are required to advance microbicide candidates into future trials. Unfortunately, there is no current consensus for screening microbicide candidates, yet most believe that rigorous tests of efficacy and safety in relevant animal model(s) should precede advancement of future microbicide candidates. This review highlights the advantages and disadvantages of current animal models for microbicide testing.
The need for animal testing for microbicides
Although the term “microbicide” implies microbe killing, it is increasingly apparent that substances that damage or destroy the outer envelope of HIV-1 are likely to damage mucosal surface and thus repeated use may actually increase the rate of HIV-1 transmission (1). The envelope of HIV and SIV (and other enveloped viruses) consists primarily of host cell membrane components (lipids). Therefore, microbicides that attack lipids in the viral envelope, such as surfactants, desiccants, cholesterol inhibitors, and detergents could also disrupt host epithelial cell membranes, resulting in a breach of the mucosal epithelium and/or the induction of inflammatory responses, both of which have the potential to increase the transmission rates of HIV-1. This in fact proved to be the case in human clinical trials of nonoxynol-9, a surfactant that increased rates of HIV-1 transmission when used repeatedly (2, 3). Studies performed in mice and macaques after the failed human clinical trial, revealed that nonoxynol-9 induced ulceration, sloughing, and inflammation in mucosal tissues (4, 5). Thus, rigorous preclinical testing of this compound in animals, including multiple dosing and challenge experiments, might have predicted the failure of this compound to prevent mucosal HIV transmission.
Although safety testing in cell culture and tissue explant models provide invaluable screening to assess basic toxicity of potential compounds, mild or cumulative inflammatory responses in tissues after prolonged or repetitive exposure to a compound may not be detected in cell cultures. In general, in vitro tests rely on cell lines (having a single cell origin) which lack the complex interplay of cells and mediators present in intact mucosal tissues which includes neutrophils, mast cells, antigen presenting cells, and others, all of which work in concert to mediate an inflammatory response. Even when mixed cell cultures such as primary peripheral blood mononuclear cells (PBMC) are used, these do not resemble mucosal lymphocytes in either their phenotype (i.e., chemokine receptor expression) or their state of activation. For example, PBMC contain very few CD4+CCR5+ memory cells, which are abundant in vaginal and rectal mucosal tissues, and are now known to be the major target for HIV replication and amplification (6–8). In summary, cell cultures simply may not detect potential allergenic or inflammatory responses that may occur following repetitive exposure to intact tissues.
Tissue culture explants such as vaginal or cervical tissues obtained from women represent a vast improvement over cell culture assays because they are fairly representative of intact human reproductive tissues, but even these have limitations in assessing safety or efficacy of microbicide candidates. First, tissue explants are usually derived from hysterectomy patients and the samples obtained may be diseased, inflamed or have abnormal hormone profiles, all of which may potentially affect responses to microbicides. Second, explants lack a blood supply, thus local inflammatory mediators released in response to stimuli cannot recruit cells to the site. Third, explants deteriorate rapidly, particularly the epithelium, making it difficult to interpret studies involving penetration of microbicides or challenge viruses. Finally, repetitive exposures cannot be performed in cell or tissue cultures. Although numerous compounds can effectively kill HIV in vitro, only repetitive applications to an intact mucosal surface in a living animal can definitively reveal whether repeated application of a microbicide candidate will induce inflammation or damage mucosal architecture, which we now know will increase rates of HIV transmission.
Native rodent and rabbit models
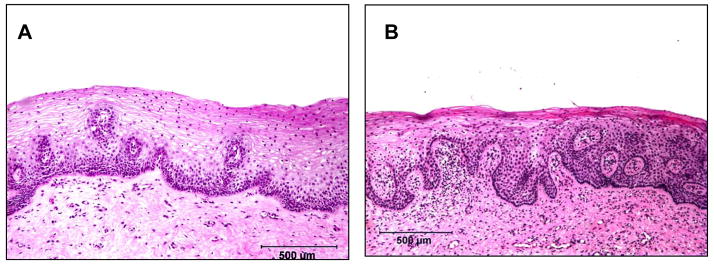
Although rodents and lagomorphs are relatively inexpensive and easy to handle, neither native mice, rats, nor rabbits are permissible to HIV infection and thus cannot be used for microbicide efficacy testing (9). Severe combined immunodeficient (SCID) mice engrafted with human PBMC and/or thymic tissues have been used for testing antiviral drugs (10, 11), but the engrafted human cells (which again are of limited phenotypes) do not adequately repopulate mucosal tissue sites, and thus these models are of little use for mucosal challenge experiments (9). Furthermore, rodents and lagomorphs have significant differences in their reproductive cycles and vaginal/cervical anatomy compared to humans. For example, rodents have estrous, rather than menstrual cycles, and unlike the human vaginal epithelium, during certain phases of the estrous cycle of mice, columnar epithelial cells appear in the vaginal epithelium (12). Similarly, portions of the rabbit vagina are lined by columnar epithelium (13), which differs from the vagina of primates, which is entirely lined by stratified squamous epithelium (Figures 1 and 2).
Figure 1.
Comparative histology of the vaginal mucosa of a human (A) and rhesus macaque (B). Note the similarities in the thickness and the arrangement of the the squamous epithelium. Vaginal biopsies were obtained from a normal woman in the luteal phase of the menstrual cycle prior to initiating a study to examine the effects of progestins on the vaginal epithelium (45) and from a normal rhesus macaque in the luteal phase.
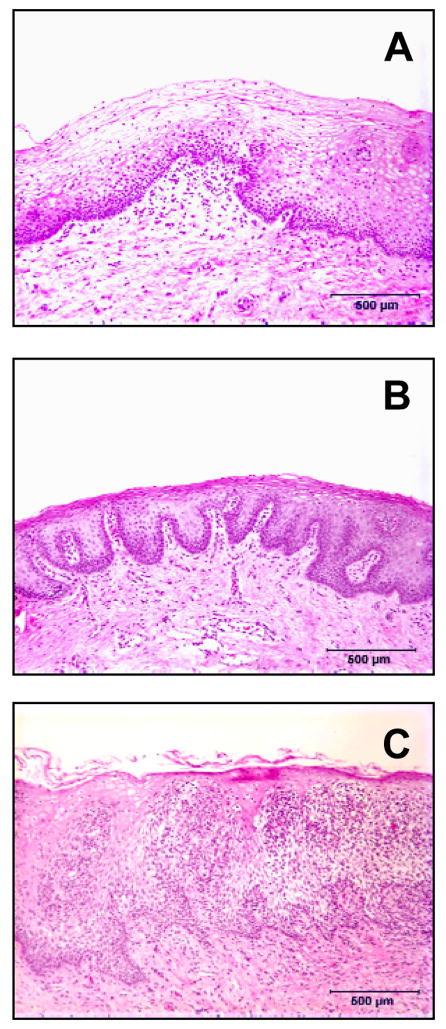
Figure 2.
Comparative histology of the vaginal mucosa from the same woman in the luteal (A) and follicular (B) stages of the menstrual cycle. Note that during the luteal phase (menses) there are areas of thinning in the vaginal mucosa where he epithelium is only a few cell layers thick. Photomicrograph C is from a woman with subclinical (asymptomatic) inflammation characterized by numerous infiltrates of inflammatory cells penetrating the squamous epithelium. Such conditions (thinning or inflammation of the vaginal epithelium) may increase vaginal HIV transmission rates. All biopsies were obtained from normal asymptomatic women prior to initiating a study to examine the effects of progestins on the vaginal epithelium (45).
Despite these limitations, small animal models are clearly useful for initial safety screening of microbicide candidates, particularly in repetitive or multiple dosing experiments. Both mice and rabbits have been used in pre-clinical safety testing of microbicide candidates (5, 14–18) but the anatomic, physiologic, and immunologic differences in these species must be carefully considered when interpreting results. However, since there are far too many candidate microbicides even for screening in nonhuman primates, rodent models may be useful for initial safety screening.
Humanized mouse model
Recently, a new humanized mouse model has been developed which shows promise for microbicide safety and efficacy testing. Dr. Garcia and colleagues have demonstrated that transplantation of autologous human fetal liver CD34+ stem cells into NOD/SCID mice previously implanted with human fetal thymic and liver tissues results in establishment and maintenance of human T and B cells, monocytes and macrophages (19). Importantly for microbicide testing, they have also demonstrated that human immune cells reconstitute intestinal/rectal (20) and vaginal (21) mucosal tissues. Although these mice still retain murine MHC antigens, which may limit interpretation of immune responses, the fact that human CD4+ T cells repopulate mucosal sites makes this a promising model for efficacy screening of topical (rectal or vaginal) microbicides against HIV. In fact, Denton et al., recently demonstrated that vaginal transmission of HIV could be prevented in this model using pre-exposure prophylaxis treatment with a common combination of reverse transcriptase inhibitors (21). Although this is still a mouse model, and many of the disadvantages discussed above persist, but this model may prove to be an invaluable and relatively inexpensive model for safety and efficacy screening of microbicide candidates, at least as long as human fetal tissues remain available. However, comparative studies between nonhuman primates, human tissue explants, and the humanized mouse model are needed to better assess the utility of the latter.
Nonhuman primate models
Undoubtedly, nonhuman primates (NHP) remain the premier laboratory animal model for studying the transmission, immunology, and pathogenesis of HIV (7), and are essential for the pre-clinical safety and efficacy testing of candidate microbicides. Only NHP have the anatomy, immunology, and reproductive physiology comparable to humans (Figure 1). Moreover, NHP have similar susceptibility to highly relevant viruses including the simian immunodeficiency virus (SIV) and several human/simian immunodeficiency virus chimeras (SHIVs), which have been engineered specifically for testing various aspects of HIV transmission, pathogenesis, and immune responses. Although most NHP do not support active viral replication of HIV-1, similarities between the cellular receptors involved for HIV-1 attachment and entry between NHP permit infection of most NHP cells with HIV-1, and several laboratories inoculate NHP cells and tissues with HIV-1 to examine the early events in infection/transmission. Depending on the mechanism of action of the microbicide tested, a variety of different challenge viruses may be used in NHP. For example, wild-type SIV may be suitable for testing most microbicides, as it has similarities to HIV in genetic sequence, target cells, and pathogenesis. However, there are some differences in the genetic sequences between SIV and HIV where a SHIV may be preferable for testing anti-HIV-1 drugs. For example, efficacy testing of antiviral compounds that target HIV reverse transcriptase (RT) may be tested against a SHIV encoding the HIV RT (RT-SHIV). Similarly, investigators may elect to test fusion inhibitors that specifically target viral CD4 or CCR5 binding sites against SHIVs that encode HIV-1 envelope sequences such as SHIV162P3 (a CCR5 utilizing SHIV) or SHIV-KU-1 (a CXCR4 utilizing SHIV). Regardless, there are a number of wild-type and genetically engineered viruses that are easily vaginally and rectally transmissible that are useful to test the efficacy of topical (or systemic) microbicides in NHP models. Furthermore, these models may be used in repetitive challenge experiments to demonstrate the duration of microbicide efficacy, and continued safety after repetitive exposure.
NHP models also provide options for assessing resistance to high and low dose vaginal and rectal transmission in both single and multiple low dose exposure models. One “disadvantage” of NHP intravaginal transmission models is that their susceptibility to vaginal transmission may be “too similar” to that of humans, in that the frequency of vaginal SIV transmission to macaques is very low when challenged with relatively small doses of virus approximating those found in HIV-infected semen. Usually multiple exposures (see below), or very high doses of SIV are required to infect sufficient numbers of rhesus macaques intravaginally with SIV (22). To overcome this, most investigators have used progestins to synchronize the menstrual cycles and thin the vaginal epithelium, which dramatically increases vaginal transmission rates in NHP (23, 24). It has been proposed that this is because the target cells that are susceptible to HIV infection and viral replication reside deep within the vaginal mucosa (lamina propria) rather than the superficial epithelium, and anything that thins the epithelium or brings inflammatory cells into the epithelium increases transmission rates to macaques (25). Although controversial, increasing evidence suggests that hormonal induced thinning, breaks (trauma) and/or inflammation in the vaginal epithelium may also be associated with increased rates of HIV transmission in women (7, 26, 27) and see Figure 2. However, the early events involved in mucosal HIV transmission are unknown, and with the assistance of NHP models, are currently under intense investigation.
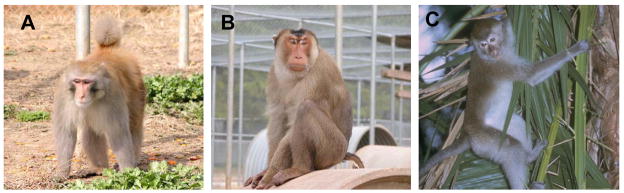
In summary, NHP models permit much flexibility in the route, dose, frequency and viral challenge selection for that may be exploited to answer specific questions regarding microbicide safety, efficacy, and the mechanisms of viral entry and/or blockade. The following sections will highlight the 3 most widely utilized species of NHP used in microbicide research, namely the rhesus, pigtailed, and cynomolgus macaque (Figure 3).
Figure 3.
Macaque species most commonly used in microbicides research including the rhesus macaque (A), pigtailed macaque (B) and cynomolgus macaque (C).
Rhesus macaques
Due to their availability and relevance to HIV infection in humans, rhesus macaques (Macaca mulatta) are the most widely used NHP species for AIDS research. In addition, the vaginal anatomy of rhesus macaques closely resembles that of humans, particularly during certain phases of the menstrual cycle (Figure 1). Moreover, rhesus have been used in a number of microbicide studies including examinations of topical and systemic distribution, safety (18, 28) and potential for efficacy (24, 29–33). In addition, by vaginally applying specific fusion inhibitors of CD4 or CCR5 binding, it has also been demonstrated that both CD4 and CCR5 attachment/binding are key events in vaginal transmission of CCR5-utilizing SHIVs (24)(30, 32, 33).
Although macaques do not naturally harbor SIV in the wild, rhesus macaques inoculated with SIV develop similar clinical signs, CD4+ T cell declines, and eventually develop AIDS closely resembling the course of HIV infection in humans. Furthermore, SIV utilizes the same receptors for attachment and entry into cells as HIV, making this and the related SHIVs invaluable for microbicide testing.
Rhesus macaques originate from Asia, primarily India and China, and even though they have subtle differences, they readily interbreed and share a common genus and species (Macaca mulatta). Chinese rhesus macaques control plasma viremia and disease progression slightly better than Indian rhesus (34), yet it is currently unclear as to whether this is a due to inherent (genetic) differences between these hosts, or due to the fact that most SIV/SHIVs were originally passaged/generated in Indian rhesus macaques/cells.
Regardless, India no longer exports rhesus macaques, so their current availability is limited to existing breeding colonies in the USA and a few other locales. Most macaques in the National Primate Research Centers (NPRC’s) in the USA are of Indian origin, but since macaques do not become sexually mature for 3–4 years, and since breeding facilities require abundant females to sustain populations, female rhesus macaques are increasingly being imported from China for large scale vaginal microbicide testing. Although Chinese macaques have lower “set point” viremia following infection with SIV/SHIV, studies indicate that they are equally as susceptible to vaginal transmission with SIV and SHIV, and equally suitable for testing the safety and efficacy of microbicide candidates (unpublished observations). Thus, their current availability, combined with the extensive knowledge generated from decades of SIV research in this model makes the rhesus macaque (of either Chinese or Indian origin) a suitable model for large scale microbicide testing.
Pigtail macaques
Pigtail macaques (Macaca nemestrina) are another species that has been widely used for microbicide safety and efficacy testing (35, 36) (4, 37–39). In fact, some investigators prefer pigtails for vaginal studies because like humans, they give birth year round, whereas rhesus are primarily seasonal breeders. In addition, the vaginal and rectal flora of pigtails has also been show to be similar to humans, and they are susceptible to some of the same sexually transmitted diseases (36, 40).
Compared to rhesus macaques, pigtails seem more susceptible to SIV transmission, and once infected, they have higher viral loads, and usually a more rapid progression to disease. In addition, there is evidence that pigtails support HIV replication better than other NHP species. Although HIV-1 still replicates poorly in this model, the fact that these animals support HIV replication at all (41) is considered important by some investigators, who are trying to identify or engineer HIV strains that may eventually demonstrate sustained viral replication in this species, in attempts to improve the macaque model of HIV infection (42, 43).
Until such a model exists, it is still debatable as to whether the SIV/SHIV/pigtail model is “better” than the SIV/SHIV/rhesus model for studying HIV infection of humans. The rhesus model has long been criticized as a model for HIV infection because SIV-infected macaques have higher viral loads and more rapid progression to disease than HIV-infected humans. Since pigtails have even higher viral loads and faster progression than rhesus, most investigators have elected to use the latter for immunology and pathogenesis studies. However, the increased susceptibility of pigtails to SIV/SHIV vaginal transmission has also been exploited to develop a multiple low-dose challenge model for vaginal microbicide testing. As mentioned above, prior studies have required either high doses of virus and/or thinning of the vaginal epithelium using progestin-based hormones to vaginally infect rhesus macaques. Using pigtails, Ron Otten and other investigators at the CDC developed a multiple low dose challenge model using weekly vaginal exposures to concentrations of virus more closely resembling those found in semen of HIV-infected patients (38). By applying 10 tissue culture infectious doses (TCID50) intravaginally of SHIV162P3 weekly, they have shown that most pigtails can be infected within a few weeks, and that microbicides may prevent transmission in this model when applied prior to each exposure (38). Thus, this model is clearly useful for testing the efficacy and safety of microbicide candidates. However, unpublished studies in our lab have failed to infect rhesus macaques using this same route, dose and strain of SHIV, even after 8 weekly exposures (unpublished observations), which we believe reflects the greater susceptibility of the pigtailed macaque to SIV/SHIV transmission. Regardless, the susceptibility of pigtails to vaginal SIV/SHIV transmission, as well as to other human STDs, combined with the similarities of their reproductive tracts make them an excellent model for testing the safety and efficacy of topical microbicides (36, 40).
The major disadvantage of the pigtailed macaque model is their limited availability, ironically for some of the same reasons mentioned above. First, the demand for pigtails for pathogenesis, immunology, or vaccine studies has never been as high as for rhesus, because viral loads and disease progression in the latter more closely resemble those of HIV-infected humans. Second, the fact that they do breed year round adds levels of complexity to their colony management. Third, it has become increasingly difficult to internationally transport nonhuman primates, largely due to restrictions imposed on/by airlines and currently, no airline imports primates from areas where pigtails are available directly to the USA. Finally, there is increased pressure on the NPCR’s to become “specific pathogen free” meaning they are negative for selected viruses, and there is little desire to import pigtails from areas where little viral testing is available. In summary, the NPRC’s currently have a very restricted supply of female pigtails, and they simply are not available in sufficient numbers to support any significant level of microbicide testing in the foreseeable future.
Cynomolgus macaques
Cynomolgus macaques (Macaca fasicularis), also known as long-tailed or crab-eating macaques are a smaller species of macaque that has been fairly widely used for HIV research. Since cynomolgus macaques are smaller and less expensive than rhesus, and currently widely available, comparative efficacy studies of this species are warranted to assess their utility as a model for microbicide testing. Studies by Dorothy Patton and Sharon Hillier’s groups have shown that the smaller size of the vaginal vault of cynomolgus macaques make colposcopy and vaginal biopsies more difficult than in larger macaques, so their utility for vaginal safety assessment may be limited (1). Regardless, the basic anatomy, vaginal pH and vaginal and rectal flora of cynomolgus macaques and humans are similar, suggesting this model may be suitable for microbicide efficacy testing (1). To our knowledge, vaginal microbicide efficacy studies have not been performed in cynomolgus macaques, but rectal microbicide testing has demonstrated the utility of this model for at least intrarectal microbicide testing (44). Regardless, comparative studies between this species and rhesus are currently needed to validate this model for both vaginal and rectal microbicide testing.
CONCLUSIONS
Partly due to the recent failures in human microbicide trials, there is an emerging consensus that pre-clinical safety and efficacy testing of microbicide candidates in nonhuman primates should precede advancing additional candidates to human trials. Although there is still some resistance to animal testing for both ethical and financial concerns, the fact remains that for a host specific virus like HIV, there is currently no logical alternative to testing in nonhuman primates. Some human clinical trials have resulted in increased transmission rates to recipients, and additional failures have the potential to do irreparable harm to the field. Thus, future candidates should be selected using the most rational approach for selecting only the most safe and potent candidates. Even though nonhuman primate testing is relatively expensive, it pales in comparison to both the financial and ethical costs of another failed human clinical trial. Furthermore, there are over 400 microbicide candidates in various phases of development, and it is unlikely the field will support more than a few more trials at best. Thus, a logical and consistent method for screening compounds in vitro followed by rigorous testing in relevant laboratory animals should be adopted so that only the safest and most effective compounds advance to clinical trials. Some may argue that data in any laboratory animal model may not necessarily correlate with efficacy in humans, and until we have an effective human microbicide, this will remain debatable. However, even if we discover (in hindsight) that laboratory animals did not accurately predict efficacy in humans, at least history will record that we selected candidates based on the most rational and logical approach, using all of the available knowledge and resources currently available.
References
- 1.Moore JP, Shattock RJ. Preventing HIV-1 sexual transmission--not sexy enough science, or no benefit to the bottom line? J Antimicrob Chemother. 2003 Dec;52(6):890–2. doi: 10.1093/jac/dkh011. [DOI] [PubMed] [Google Scholar]
- 2.Turpin JA. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin Investig Drugs. 2002;11(8):1077–97. doi: 10.1517/13543784.11.8.1077. [DOI] [PubMed] [Google Scholar]
- 3.Hillier SL, Moench T, Shattock R, Black R, Reichelderfer P, Veronese F. In vitro and in vivo: the story of nonoxynol 9. J Acquir Immune Defic Syndr. 2005 May 1;39(1):1–8. doi: 10.1097/01.qai.0000159671.25950.74. [DOI] [PubMed] [Google Scholar]
- 4.Patton DL, Cosgrove Sweeney YT, Rabe LK, Hillier SL. Rectal applications of nonoxynol-9 cause tissue disruption in a monkey model. Sex Transm Dis. 2002 Oct;29(10):581–7. doi: 10.1097/00007435-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Galen BT, Martin AP, Hazrati E, Garin A, Guzman E, Wilson SS, et al. A comprehensive murine model to evaluate topical vaginal microbicides: mucosal inflammation and susceptibility to genital herpes as surrogate markers of safety. J Infect Dis. 2007 May 1;195(9):1332–9. doi: 10.1086/513279. [DOI] [PubMed] [Google Scholar]
- 6.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004 Sep 20;200(6):749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lackner AA, Veazey RS. Current Concepts in AIDS Pathogenesis: Insights from the SIV/Macaque Model. Annu Rev Med. 2007 Feb 18;58:461–76. doi: 10.1146/annurev.med.58.082405.094316. [DOI] [PubMed] [Google Scholar]
- 8.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004 Sep 20;200(6):761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 9.Shacklett BL. Can the new humanized mouse model give HIV research a boost. PLoS Med. 2008 Jan 15;5(1):e13. doi: 10.1371/journal.pmed.0050013. This commentary further highlights the advantages and disadvantages of the SIV/macaque and humanized mouse model for studying HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCune JM. Development and applications of the SCID-hu mouse model. Semin Immunol. 1996 Aug;8(4):187–96. doi: 10.1006/smim.1996.0024. [DOI] [PubMed] [Google Scholar]
- 11.Mosier DE, Gulizia RJ, Baird SM, Wilson DB, Spector DH, Spector SA. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991 Feb 15;251(4995):791–4. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 12.Lamb JCt, Newbold RR, Stumpf WE, McLachlan JA. Transitional changes in the surface epithelium of the cycling mouse vagina, cervix and uterus: scanning electron microscopic studies. Biol Reprod. 1978 Nov;19(4):701–11. doi: 10.1095/biolreprod19.4.701. [DOI] [PubMed] [Google Scholar]
- 13.Barberini F, Correr S, De Santis F, Motta PM. The epithelium of the rabbit vagina: a microtopographical study by light, transmission and scanning electron microscopy. Arch Histol Cytol. 1991 Oct;54(4):365–78. doi: 10.1679/aohc.54.365. [DOI] [PubMed] [Google Scholar]
- 14.Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, et al. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006 Jan 5;439(7072):89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 15.Doncel GF, Chandra N, Fichorova RN. Preclinical assessment of the proinflammatory potential of microbicide candidates. J Acquir Immune Defic Syndr. 2004 Oct;37 Suppl 3:S174–80. [PubMed] [Google Scholar]
- 16.D’Cruz OJ, Uckun FM. Mucosal safety of PHI-443 and stampidine as a combination microbicide to prevent genital transmission of HIV-1. Fertil Steril. 2007 Oct;88(4 Suppl):1197–206. doi: 10.1016/j.fertnstert.2007.01.131. [DOI] [PubMed] [Google Scholar]
- 17.Kish-Catalone TM, Lu W, Gallo RC, DeVico AL. Preclinical evaluation of synthetic -2 RANTES as a candidate vaginal microbicide to target CCR5. Antimicrob Agents Chemother. 2006 Apr;50(4):1497–509. doi: 10.1128/AAC.50.4.1497-1509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuttall JP, Thake DC, Lewis MG, Ferkany JW, Romano JW, Mitchnick MA. Concentrations of Dapivirine in the Rhesus Macaque and Rabbit following Once Daily Intravaginal Administration of a Gel Formulation of [14C] Dapivirine for 7 Days. Antimicrob Agents Chemother. 2008 Mar;52(3):909–14. doi: 10.1128/AAC.00330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006 Nov;12(11):1316–22. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 20.Sun Z, Denton PW, Estes JD, Othieno FA, Wei BL, Wege AK, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007 Apr 16;204(4):705–14. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 21.Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008 Jan 15;5(1):e16. doi: 10.1371/journal.pmed.0050016. This paper demonstrates that human cells repopulate mucosal tissues in the humanized mouse model after stem cell transplantation, and demonstrates that the model may be useful for topical microbicide efficacy screening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller CJ, Alexander NJ, Sutjipto S, Lackner AA, Gettie A, Hendrickx AG, et al. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989;63:4277–84. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2(10):1084–9. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 24.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV gp120. Nature Med. 2003;9:343–6. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 25.Poonia B, Wang X, Veazey RS. Distribution of simian immunodeficiency virus target cells in vaginal tissues of normal rhesus macaques: implications for virus transmission. J Reprod Immunol. 2006 Dec;72(1–2):74–84. doi: 10.1016/j.jri.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science. 2005 Jun 10;308(5728):1582–3. doi: 10.1126/science.1112489. [DOI] [PubMed] [Google Scholar]
- 27.Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005 Oct 1;366(9492):1182–8. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 28.Ratterree M, Gettie A, Williams V, Malenbaum S, Neurath AR, Cheng-Mayer C, et al. Safety and distribution of cellulose acetate 1,2-benzenedicarboxylate (CAP), a candidate anti-HIV microbicide in rhesus macaques. Aids. 2005 Oct 14;19(15):1595–9. doi: 10.1097/01.aids.0000185990.16477.47. [DOI] [PubMed] [Google Scholar]
- 29.Boadi T, Schneider E, Chung S, Tsai L, Gettie A, Ratterree M, et al. Cellulose acetate 1,2-benzenedicarboxylate protects against challenge with pathogenic X4 and R5 simian/human immunodeficiency virus. Aids. 2005 Oct 14;19(15):1587–94. doi: 10.1097/01.aids.0000186020.24426.62. [DOI] [PubMed] [Google Scholar]
- 30.Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005 Nov 3;438(7064):99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 31.Veazey RS, Klasse PJ, Ketas TJ, Reeves JD, Piatak M, Jr, Kunstman K, et al. Use of a Small Molecule CCR5 Inhibitor in Macaques to Treat Simian Immunodeficiency Virus Infection or Prevent Simian-Human Immunodeficiency Virus Infection. J Exp Med. 2003 Nov 17;198(10):1551–62. doi: 10.1084/jem.20031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veazey RS, Springer MS, Marx PA, Dufour J, Klasse PJ, Moore JP. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat Med. 2005 Dec;11(12):1293–4. doi: 10.1038/nm1321. [DOI] [PubMed] [Google Scholar]
- 33.Lederman MM, Veazey RS, Offord R, Mosier DE, Dufour J, Mefford M, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004 Oct 15;306(5695):485–7. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 34.Ling B, Veazey RS, Luckay A, Penedo C, Xu K, Lifson JD, et al. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. Aids. 2002 Jul 26;16(11):1489–96. doi: 10.1097/00002030-200207260-00005. [DOI] [PubMed] [Google Scholar]
- 35.Patton DL, Sweeney YT, Balkus JE, Hillier SL. Vaginal and rectal topical microbicide development: safety and efficacy of 1.0% Savvy (C31G) in the pigtailed macaque. Sex Transm Dis. 2006 Nov;33(11):691–5. doi: 10.1097/01.olq.0000216022.18321.d3. [DOI] [PubMed] [Google Scholar]
- 36.Patton DL, Cosgrove-Sweeney YT, Rabe LK, Hillier SL. The pig-tailed macaque rectal model: microflora and chlamydial infection. Sex Transm Dis. 2001 Jul;28(7):363–6. doi: 10.1097/00007435-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Patton DL, Sweeney YC, Tsai CC, Hillier SL. Macaca fascicularis vs. Macaca nemestrina as a model for topical microbicide safety studies. J Med Primatol. 2004 Apr;33(2):105–8. doi: 10.1111/j.1600-0684.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 38.Otten RA, Adams DR, Kim CN, Jackson E, Pullium JK, Lee K, et al. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis. 2005 Jan 15;191(2):164–73. doi: 10.1086/426452. [DOI] [PubMed] [Google Scholar]
- 39.Patton DL, Cosgrove Sweeney YT, McCarthy TD, Hillier SL. Preclinical safety and efficacy assessments of dendrimer-based (SPL7013) microbicide gel formulations in a nonhuman primate model. Antimicrob Agents Chemother. 2006 May;50(5):1696–700. doi: 10.1128/AAC.50.5.1696-1700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patton DL, Sweeney YT, Agnew KJ, Balkus JE, Rabe LK, Hillier SL. Development of a nonhuman primate model for Trichomonas vaginalis infection. Sex Transm Dis. 2006 Dec;33(12):743–6. doi: 10.1097/01.olq.0000218871.89901.61. [DOI] [PubMed] [Google Scholar]
- 41.Batten CJ, De Rose R, Wilson KM, Agy MB, Chea S, Stratov I, et al. Comparative evaluation of simian, simian-human, and human immunodeficiency virus infections in the pigtail macaque (Macaca nemestrina) model. AIDS Res Hum Retroviruses. 2006 Jun;22(6):580–8. doi: 10.1089/aid.2006.22.580. [DOI] [PubMed] [Google Scholar]
- 42.Liao CH, Kuang YQ, Liu HL, Zheng YT, Su B. A novel fusion gene, TRIM5-Cyclophilin A in the pig-tailed macaque determines its susceptibility to HIV-1 infection. Aids. 2007 Dec;21 Suppl 8:S19–26. doi: 10.1097/01.aids.0000304692.09143.1b. [DOI] [PubMed] [Google Scholar]
- 43.Igarashi T, Iyengar R, Byrum RA, Buckler-White A, Dewar RL, Buckler CE, et al. Human immunodeficiency virus type 1 derivative with 7% simian immunodeficiency virus genetic content is able to establish infections in pig-tailed macaques. J Virol. 2007 Oct;81(20):11549–52. doi: 10.1128/JVI.00960-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai CC, Emau P, Jiang Y, Tian B, Morton WR, Gustafson KR, et al. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2003 Jul;19(7):535–41. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- 45.Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60(1):15–24. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]