Abstract
BACKGROUND:
A number of case reports link the use of 5-aminosalicylic acid (5-ASA) to interstitial nephritis in patients with inflammatory bowel disease (IBD).
OBJECTIVE:
To investigate whether the long-term use of 5-ASA has harmful effects on renal function in patients with IBD.
METHODS:
A retrospective analysis of 171 consecutive outpatients with Crohn’s disease or ulcerative colitis was conducted. Serum creati-nine levels and body weight were measured before and after treatment to calculate the creatinine clearance (CrCl) rate.
RESULTS:
In 171 patients (93 women, 78 men), the mean (± SD) dose of 5-ASA was 3.65±0.85 g/day with a cumulative dose of 11±7.7 kg over an interval of 8.4±5.9 years. Serum creatinine concentrations increased from 76.8 μmol/L to 88.7 μmol/L (n=171; P<0.0001) and the CrCl rate fell significantly from 104.6 mL/min to 93.1 mL/min (n=81; P<0.0001). There was one case of interstitial nephritis reported. Treatment groups included mesalamine (74.3%), sulfasalazine (15.2%) and combination (sulfalsalazine/mesalamine [10.5%]) with treatment durations of 7.2±4.5, 12.3±8.7 and 11.2±6.7 years, respectively. The duration of treatment was the most important covariate for change in CrCl and when analyzed by treatment group, those treated with sulfasazine had a strong correlation (r=−0.54, P=0.0145), while nonsignificant in the mesalamine group (r=0.06, P=0.7017). The decline in CrCl was negatively correlated with the pretreatment CrCl rate (r=−0.34; P=0.0024) and positively correlated with the mean daily dose of 5-ASA (r=0.32; P=0.0034).
CONCLUSION:
The present study is the first to demonstrate a significant dose- and treatment duration-dependant decline in CrCl. The risks need to be further evaluated because 5-ASA is widely used for long-term maintenance therapy in patients with IBD.
Keywords: 5-ASA, Inflammatory bowel disease, Interstitial nephritis, Mesalamine, Sulfasalazine
Abstract
HISTORIQUE :
Un certain nombre de rapports de cas relient l’usage d’acide 5-aminosalicylique (5-ASA) à la néphrite interstitielle chez les personnes atteintes d’une maladie inflammatoire de l’intestin (MII).
OBJECTIF :
Examiner si l’utilisation à long terme du 5-ASA a des effets nuisibles sur la fonction rénale des personnes atteintes d’une MII.
MÉTHODOLOGIE :
Les auteurs ont procédé à une analyse rétrospective de 171 patients consécutifs atteints de la maladie de Crohn ou de la colite ulcéreuse. Ils ont mesuré les taux de créatinine sérique et le poids corporel avant et après le traitement pour calculer le taux de clairance de la créatinine (CrCl).
RÉSULTATS :
Chez 171 patients (93 femmes, 78 hommes), la dose moyenne de 5-ASA (±ÉT) était de 3,65±0,85 g/jour, la dose cumulative s’élevant à 11±7,7 kg sur un intervalle de 8,4±5,9 ans. Les concentrations de créatinine sérique ont passé de 76,8 μmol/L à 88,7 μmol/L (P<0,0001, n=171) et le taux de CrCl a chuté de manière significative, de 104,6 mL/min à 93,1 mL/min (n=81 ; P<0,0001). Un cas de néphrite interstitielle a été déclaré. Les groupes de traitement étaient la mésalamine (74,3 %), la sulfasalazine (15,2 %) et une polythérapie (sulfasalazine et mésalamine [10,5 %]) et la durée de traitement était de 7,2±4,5 ans, 12,3±8,7 ans et 11,2±6,7 ans, respectivement. La durée du traitement était la principale covariable de modification de la CrCl et, lorsqu’elle était analysée selon le groupe de traitement, les personnes traitées à la sulfasazine présentaient une solide corrélation (r=−0,54, P=0,0145), qui n’était toutefois pas significative dans le groupe traité à la mésalamine (r=0,06, P=0,7017). La diminution de la CrCl était corrélée de manière négative au taux de CrCl avant le traitement (r=−0,34, P=0,0024) et de manière positive à la dose quotidienne moyenne de 5-ASA (r=0,32, P=0,0034).
CONCLUSION :
La présente étude est la première à démontrer une diminution importante de la CrCl liée à la dose et à la durée du traitement. Il faut évaluer les risques de manière plus approfondie, car le 5-ASA est beaucoup utilisé pour le traitement d’entretien à long terme des personnes atteintes d’une MII.
Sulfasalazine has been widely used for the treatment of inflammatory bowel disease (IBD). In 1977, Azad et al (1) identified 5-aminosalicylic acid (5-ASA) as the active moiety of sulfasalazine, bound by a diazo bond to the sulpha drug sulfa-pyridine. The diazo reductase activity of bacteria in the colon cleaves the diazo bond to release the 5-ASA and the sulfa-pyridine (2). Sulfapyridine is mostly absorbed and is responsible for the majority of the adverse reactions attributed to sulfasalazine. Several studies have shown that 5-ASA and sulfasalazine have comparable anti-inflammatory benefits, while sulfapyridine does not achieve similar anti-inflammatory effects (3,4). Therefore, various formulations of 5-ASA have been developed, each attempting to prevent the inactivation of 5-ASA due to its instability in an acidic environment or from primary absorption in the proximal intestine (5). Mesalamine is the generic name for 5-ASA contained in a pH-sensitive or semipermeable coating that allows the 5-ASA to be released distally in the intestinal lumen (6–8). The introduction of mesalamine has facilitated effective treatment for IBD without the adverse effects attributed to the sulfapyridine moiety of sulfasalazine. However, the benefits are due to better tolerance rather than improved efficacy (9).
Mesalamine is the gold standard for treatment of ulcerative colitis (UC) and has shown to be effective in treating mild to moderate Crohn’s disease (CD) (10–15). Studies (16–22) demonstrate that both mesalamine and sulfasalazine are efficacious for inducing and maintaining remission, and are often used as long-term maintenance treatments. The improved side effect profile of mesalamine has enabled the use of doses that are much higher than doses possible with sulfasalazine. However, a number of cases of 5-ASA-induced nephrotoxicity have been reported in patients with IBD (23,24). Animal studies (25,26) demonstrated dose-related renal papillary necrosis; however, the doses were much higher than those typically used in humans. The use of 5-ASA is generally not associated with papillary necrosis in humans. Several case reports (27–30) have reported renal impairment in the forms of dose-dependent ‘analgesic nephropathy’, inhibition of cyclooxygenases or hypersensitivity leading to reversible interstitial nephritis in humans. The incidence of renal impairment in patients with IBD treated with 5-ASA is estimated to be one in 100 patients, and interstitial nephritis occurs in one in 500 patients (31,32). Mahmud et al (33) were unable to show nephrotoxicity after six months of mesalamine treatment in patients with UC using a low dose of 1.2 g/day. Similarly, Van Staa et al (34), in the British epidemiological study, were not able to demonstrate a relationship between the dose or type of 5-ASA and the incidence of renal disease among 19,025 IBD patients on 5-ASA. Based on their findings, interstitial nephritis related to 5-ASA use appears to be a rare event.
Although interstitial nephritis occurs infrequently, renal function can be compromised over time with the use of 5-ASA. In a recent report, de Jong et al (35) did not find a significant change in creatinine clearance (CrCl) over an 11-year interval in 200 patients with Crohn’s disease. However, the mean duration of treatment with 5-ASA was 8.6 years, much shorter than the interval that CrCl was measured. This may reflect why the decline in CrCl was reported to be within the expected physiological decline in renal function associated with aging. Fortunately, the kidneys have the capacity to regain function after an insult; thus, creatinine measurements need to be taken at the onset and end of treatment rather than time points outside of the actual treatment interval. Also, no case of interstitial nephritis was reported by de Jong et al (35).
Clinical experience continues to reveal that renal disease is relatively rare and is usually reversible with discontinuation of the drug (23,31). The aim of the present study was to investigate whether the long-term use of high-dose 5-ASAs has harmful effects on the renal function of IBD patients, and to assess a dose-related relationship and to compare the risks associated with the use of different 5-ASA preparations.
METHODS
Patients
The charts of 204 patients with confirmed CD or UC in the outpatient gastroenterology clinic at St Michael’s Hospital, Toronto, Ontario, were examined. Of these, the records of 171 patients were analyzed for changes in serum creatinine and urea levels during the time they were on 5-ASA. In 82 of the 171 patients, an in-depth analysis was undertaken. The number of patients in each data set is indicated in the text or in a table. The in-depth retrospective chart review collected the following data: sex, race, age at diagnosis, age at start of treatment, location of disease, complications of IBD, extraintestinal manifestations, surgeries and other comorbid illnesses. The type of 5-ASA, dose and duration of treatment were recorded. The cumulative doses of oral formulation of 5-ASA were calculated. Serum creatinine levels and body weight were measured just before the start of treatment and at the end of treatment or the current level if still on treatment. Factors known to effect renal function, specifically diseases such as hypertension, chronic renal impairment, heart failure and diabetes were documented. Potentially nephrotoxic drugs such as cyclosporine, bisphosphonate, nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 inhibitors and angiotensin-converting enzyme inhibitors are documented. Weight loss was recorded and defined as significant if an individual patient lost more than 20% of their total body weight or required intervention with total parenteral nutrition (TPN). The use of other immunosuppressive medications was also recorded. Patients were excluded from the study if insufficient data were available for analysis. The data were obtained and stored in an anonymous database that was approved by the medical ethics committee of St Michael’s Hospital, Toronto, Ontario.
Calculation of renal function
Renal function was monitored by measuring serum creatinine levels. The estimated serum CrCl rate was calculated using the Cockroft-Gault Formula:
The formula takes into account age, weight and sex, and because of differences in body composition, a correction factor of 0.85 for women (36) was applied. The CrCl was determined at two time points. The first was calculated at the start of treatment and the second at the end of treatment or the current level if still on treatment.
Statistical analysis
Data are presented with summary statistics for the study population and for the subpopulation of patients with complete data available before and after treatment. Categorical variables are summarized with counts and percentages, and the continuous variables are presented as means and SDs.
Comparisons related to change from baseline for continuous variables within each population of subjects was performed with the Wilcoxon signed rank test. Comparisons between groups with respect to continuous variables were performed with the Wilcoxon sum rank test. Comparisons between groups with respect to categorical variables were performed with the Pearson χ2 test or Fisher’s exact test for cases in which the number of observations was less than five per cell.
In addition, univariate analyses based on linear regression were performed to establish the relation between change in CrCl, age at start of treatment, mean daily dose, cumulative daily dose and pretreatment CrCl rates. Variables found significant were included in the multivariate linear regression analysis. All statistical tests were two-sided tests and considered significant at the 5% significance level.
RESULTS
The clinical characteristics and complications of the study population are presented in Table 1. There were 126 patients with CD and 54 with UC. Of 171 patients, the mean (± SD) age at the start of treatment was 36.2±14.7 years (range 13 to 88 years). There were 93 women (54.4%) and 78 men (45.6%). There was no significant difference in the characteristics between patients with CD and those with UC (data not shown).
TABLE 1.
Clinical characteristics of patients with inflammatory bowel disease treated with 5-aminosalicylic acid (n=171)
| Clinical characteristic | |
|---|---|
| Men | 78 (45.6) |
| Women | 93 (54.4) |
| Age at start of treatment, years (mean ± SD) | 36.2±14.7 |
| Serum creatinine at start of treatment, μmol/L (mean ± SD) | 77.2±17.5 |
| Duration of treatment, years (mean ± SD) | 8.4±5.9 |
| Inflammatory bowel disease | |
| Crohn’s disease | 126 (73.7) |
| Ulcerative colitis | 45 (26.3) |
| Comorbidities, n | 17 |
| Hypertension | 15 (8.8) |
| Diabetes | 2 (1.2) |
| Heart disease | 1 (0.6) |
| Chronic kidney disease | 0 (0.0) |
| Use of nephrotoxic medications, n | 43 |
| Bisphosphonates | 22 (12.9) |
| Angiotensin-converting enzyme inhibitors | 13 (7.6) |
| Nonsteroidal anti-inflammatory drugs | 8 (4.7) |
| Cyclooxygenase-2 inhibitors | 6 (3.5) |
| Cyclosporine | 2 (1.2) |
| Use of immunosuppressive medications, n | 100 |
| Corticosteroids | 53 (64.6) |
| Azathioprine | 27 (32.9) |
| Methotrexate | 8 (9.7) |
| Infliximab | 8 (9.7) |
| Cyclosporine | 2 (2.4) |
| 6-Mercaptopurine | 2 (2.4) |
| Use of enemas, n | 28 |
| 5-Aminosalicylic acid | 16 (9.4) |
| Steroids | 12 (7.0) |
Data presented as n (%) unless otherwise specified
Comorbidities with potentially significant renal complications were found in 17 patients (9.9%) with hypertension being the most predominant. None of the patients had clinically defined renal impairment at the start of treatment. However, 43 patients (25.2%) had received potentially nephrotoxic medications (Table 1). Of the study cohort, 12 patients (7.0%) had both potentially nephrotoxic comorbidities and received nephrotoxic medications, and 103 patients (60.2%) did not have either comorbidities or nephrotoxic medications.
Of the 82 patients for which a detailed analysis was performed, the mean duration of disease (CD or UC) was 19.8±10.9 years (Table 2). The predominant sites of disease were the ileum for CD and pancolitis for UC. Twenty-six patients (31.7%) had extraintestinal manifestations, including one with primary sclerosing cholangitis. Complications of IBD were documented in 49% of the patients; with fistulas in 20 (24.4%), bowel obstructions in 12 (14.6%), abscess in eight (9.8%), anal fissure in eight (9.8%), stricture in five (6.1%), thrombosis in two (2.4%) and anemia in one (1.2%).
TABLE 2.
Clinical characteristics of patients who underwent detailed analysis (n=82)
| Clinical characteristic | |
|---|---|
| Body weight at start of treatment, kg (mean ± SD) | 65.5±15.9 |
| Duration of disease, years (mean ± SD) | 19.8±10.9 |
| Crohn’s disease, n; location of disease | 57 |
| Ileum only | 24 (42.1) |
| Colon only | 14 (24.5) |
| Ileum and colon | 19 (33.3) |
| Ulcerative colitis, n; location of disease | 24 |
| Pancolitis | 15 (62.5) |
| Distal colitis | 4 (16.7) |
| Proctosigmoiditis | 5 (33.3) |
| Extraintestinal manifestations, n | 26 |
| Arthritis | 13 (15.9) |
| Nephrolithiasis | 5 (6.1) |
| Cholelithiasis | 4 (4.9) |
| Uveitis | 3 (3.7) |
| Erythema nodosum | 3 (3.7) |
| Pyoderma gangrenosum | 2 (2.4) |
| Primary sclerosing cholangitis | 1 (1.2) |
| Aphthous ulcers | 1 (1.2) |
| Sacroileitis | 1 (1.2) |
| Complications of inflammatory bowel disease, n | 40 |
| Fistula | 20 (24.4) |
| Bowel obstruction | 12 (14.6) |
| Abscess | 8 (9.8) |
| Anal fissure | 8 (9.8) |
| Stricture | 5 (6.1) |
| Thrombosis | 2 (2.4) |
| Anemia | 1 (1.2) |
| Total parenteral nutrition | 20 (11.7) |
Data presented as n (%) unless otherwise specified
During the observation period, a significant mean weight gain of 5.5±6.6 kg was documented for the 82 patients who underwent the detailed analysis (Table 2). Of these 82 patients, 56 experienced an overall weight gain during the treatment period and 14 of these 56 patients (25.0%) underwent a course of TPN. There were only eight patients who had an overall decline in weight, of which two required TPN.
Details of treatment
The mean dose of 5-ASA was 3.65±0.85 g/day (range 1 g/day to 8 g/day) and a mean cumulative dose of 11±7.7 kg (range 1.1 kg to 40.9 kg). The mean duration of treatment was 8.4±5.9 years (range one to 30 years). There was no difference in the dosage or treatment duration between patients with UC and CD (data not shown). The majority of the patients (74.3%) were treated with mesalamine, whereas 15.2% were treated with sulfasalazine and 10.5% were treated with a combination of mesalamine and sulfasalazine (Table 3). The duration of treatment was longest in those treated with sulfasalazine (13.8±9.0 years) with a mean cumulative dose of 17.1±11.9 kg. There was no statistically significant difference in the change in CrCl rates among the different types of 5-ASA.
TABLE 3.
Differential effects of the type of 5-aminosalicylic acid (5-ASA) on renal function
| Type of 5-ASA | Patients, n (%) | Cumulative dose, kg (mean ± SD) | Treatment duration, years (mean ± SD) | Change in creatinine clearance, % (mean ± SD) |
|---|---|---|---|---|
| Sulfasalazine | 26 (15.2) | 15.1±11.4 | 12.3 ±8.7 | −16.3 ±18.0 |
| Mesalamine | 127 (74.3) | 9.7±6.3 | 7.2 ±4.5 | −6.7 ±25.1 |
| Combination (sulfasalazine/mesalamine) | 18 (10.5) | 14.0±7.7 | 11.2 ±6.7 | −16.0 ±8.6 |
Renal function
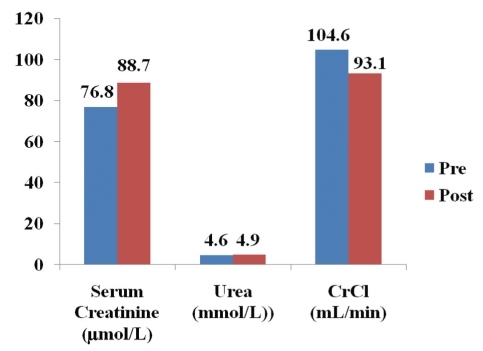
Serum creatinine levels remained within the normal range but increased significantly over the observation period from 76.8 μmol/L to 88.7 μmol/L (P<0.0001; n=171) (Figure 1). Urea levels remained within the normal range and increased from 4.6 mmol/L to 4.9 mmol/L (P=0.0056; n=113). The calculated CrCl rate fell significantly from 104.6 mL/min to 93.1 mL/min (P<0.0001; n=82). There was no significant difference in renal function between patients with UC and CD (data not shown). Interstitial nephritis was reported in only one patient within one year of treatment.
Figure 1).
Over the mean observation period of 8.4 years, the serum creatinine level increased from 76.8 μmol/L to 88.7 μmol/L (P<0.0001; n=171) and urea levels increased from 4.6 mmol/L to 4.9 mmol/L (P=0.0056; n=113) but both remained within the normal range. The calculated creatinine clearance (CrCl) rate fell significantly from 104.6 mL/min to 93.1 mL/min (P<0.0001; n=81). Post Post-treatment; Pre Pretreatment
Predictors of change in CrCl
Univariate correlation analysis showed that there was no effect of age at the start of treatment (r=−0.08; P=0.4523) or cumulative dose (r=−0.08; P=0.4712) on the change in CrCl but the mean dose of 5-ASA (r=0.32; P=0.0034) and the pretreatment CrCl rate (r=−0.34; P=0.0024) were significantly correlated with the rise in the CrCl rate of the whole study population (Table 4).
TABLE 4.
Univariate correlation analysis of creatinine clearance
| Predictors of creatinine clearance | r | P |
|---|---|---|
| Mean daily dose of 5-aminosalicylic acid | 0.32 | 0.0034 |
| Pretreatment creatinine clearance rate | −0.34 | 0.0024 |
| Age at start of treatment | −0.08 | 0.4523 |
| Cumulative dose | −0.08 | 0.4712 |
r Peason coefficient of correlation
In multivariate analysis, the decline in CrCl was negatively correlated with the pretreatment CrCl rate (β=−0.41; P<0.001) and positively correlated with mean daily dose of 5-ASA (β=14.01; P=0.0003) (Table 5).
TABLE 5.
Multivariate correlation analysis of creatinine clearance*
| Predictors of creatinine clearance | Beta coefficient | P |
|---|---|---|
| Pretreatment creatinine clearance rate | −0.41 | <0.0001 |
| Mean daily dose of 5-aminosalicylic acid | 14.01 | 0.0003 |
Overall R2=0.26
Effect of treatment on CrCl
Univariate analysis of patients who were treated with either mesalamine or sulfasalazine monotherapy demonstrate that treatment duration was significantly correlated with a change in the CrCl (r=−0.25; P=0.0487) whereas treatment effect (mesalamine versus sulfasalazine) was only marginally significant (P=0.07). The mean change in CrCl rate for the group treated with mesalamine was −7.5±24.7 mL/min, and −19.5±24.3 mL/min for the group treated with sulfasalazine. The cumulative dose and the diagnosis did not significantly affect CrCl (Table 6). Patients with CD had a change in CrCl of −10.3±25.5 mL/min and those with UC had a change in CrCl of −13.5±24.2 mL/min. The duration of treatment was the most important covariate for the whole population, and when analyzed by treatment group with change in CrCl, those treated with sulfasalazine had a strong correlation (r=−0.54, P=0.0154), while those treated with mesalamine had a nonsignificant duration of treatment (r=0.06; P=0.7017) (Table 7).
TABLE 6.
Univariate correlation analysis of creatinine clearance in patients treated with monotherapy
| Predictors of creatinine clearance | P |
|---|---|
| Sulfasalazine versus mesalamine | 0.0736 |
| Cumulative dose of 5-aminosalicylic acid | 0.2900 |
| Duration of treatment | 0.0487 |
| Diagnosis | 0.6429 |
TABLE 7.
Effect of treatment on univariate correlation analysis of creatinine clearance (CrCl)
| Predictors of CrCl in univariate analysis |
Whole population |
Patients on mesalamine |
Patients on sulfasalazine |
|||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Pretreatment CrCl rate | −0.34 | 0.0024 | −0.27 | 0.042 | −0.45 | 0.037 |
| Mean daily dose of 5-aminosalicylic acid | 0.32 | 0.0034 | 0.46 | 0.0002 | 0.029 | 0.899 |
| Duration of treatment | −0.21 | 0.0551 | 0.06 | 0.7017 | −0.54 | 0.0145 |
r Pearson correlation coefficient
Subgroup analysis with nephrotoxic comorbidities and/or medications
Subgroup analysis did not reveal a significant difference in age, weight, serum creatinine value before or after treatment, urea, CrCl, mean dose or cumulative dose in those with or without use of nephrotoxic medications, those with or without comorbidities and those with or without nephrotoxic medications and comorbidities (data not shown).
Of the 82 patients who underwent an in-depth analysis, a total of 41 patients (11.1%) experienced a decrease in CrCl of 10% or more. Of those 41 patients, 11 (26.8%) received a nephrotoxic medication, four (9.8%) had a comorbidity that could affect renal function and 11(26.8%) patients had both a comorbidity and received a nephrotoxic medication.
Univariate correlations for change in CrCl and average daily dose were significant in patients without either nephrotoxic comorbidities or medications (r=0.46; P=0.0002), without nephrotoxic comorbidities (r=0.36; P=0.0016) and without nephrotoxic medications (r=0.44; P=0.0004) (Table 8). Pretreatment CrCl correlated with change in CrCl in all groups except those who had comorbidities that are associated with risk for renal disease (r =−0.39; P=0.3855).
TABLE 8.
Univariate correlation analysis of creatinine clearance (CrCl) in patients with nephrotoxic comorbidities and/or medications
| Predictors of CrCl in univariate analysis |
Comorbidities or medications |
Nephrotoxic medications |
Comorbidities |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Without |
With |
Without |
With |
Without |
With |
|||||||
| r | P | r | P | r | P | r | P | r | P | r | P | |
| Mean daily dose of 5-aminosalicylic acid | 0.46 | 0.0002 | 0.029 | 0.8994 | 0.44 | 0.0004 | 0.059 | 0.8010 | 0.36 | 0.0016 | 0.24 | 0.6089 |
| Pretreatment CrCl rate | −0.27 | 0.0424 | − 0.45 | 0.0379 | −0.27 | 0.0383 | − 0.44 | 0.0433 | − 0.35 | 0.0029 | −0.39 | 0.3855 |
r Pearson correlation coefficient
DISCUSSION
The aim of the present study was to detect changes in renal function, as measured by the CrCl rate over time, in IBD patients treated with 5-ASA. The results show that subtle deterioration in renal function occurred in these patients over the observation period. Clinical disease due to interstitial nephritis occurred only in one of these patients.
The present study is the first to show that the risk of renal impairment was related to treatment duration and dose of 5-ASA. Patients were followed for a mean of 20 years and treated with 5-ASA for a mean of 8.4 years with variable degrees of cumulative exposure. Although the majority of patients were treated with mesalamine, which is a relatively newer formulation, the treatment duration was not sufficient to impact the change in CrCl that was found in the group treated with sulfasalazine. Thus, the effect of sulfasalazine on renal function is due to treatment duration rather than a drug effect. This has clinical implications because patients originally started and maintained on sulfasalazine will likely have an increased risk of decline in CrCl. Dose-related renal papillary necrosis has been documented in early animal studies (60 mg/kg to 100 mg/kg in dogs and 320 mg/kg in rats); however, this has not been seen in humans (25,26). The molecular structure of 5-ASA is similar to acetylsalicylic acid, phenacetin and aminophel, which are well documented to be nephrotoxic if taken in high doses for long periods of time (37–40). A similar study by de Jong et al (35) reported a mean 5-ASA dose of 2.8 g/day and cumulative doses of 6.6 kg over 8.6 years – much lower than the 3.6 g/day and cumulative dose of 11 kg over 8.4 years in the present study. The case-control study by Van Staa et al (34) followed patients for a mean of only six years, in which the dose was recorded as greater or less than 2 g/day. Unfortunately, this dose cut-off underestimates a true dose relationship because studies have shown that chronic high-dosing (ie, more than 3 g/day) can cause renal tubular dysfunction in patients with IBD (41). Schreiber et al (41) demonstrated an increased prevalence of tubular membrane protein excretion as a more sensitive index of renal function, which was related to 5-ASA dose. Zehnter et al (42) also showed that IBD patients treated with 5-ASA had elevated levels of tubular proteinuria but not in patients with IBD not receiving 5-ASA.
In addition, we found that renal dysfunction before the start of treatment correlated with a greater decline in CrCl. This suggests that although the dose of 5-ASA may be an independent predictor of nephrotoxicity, there may be a synergistic effect in patients with baseline pre-existing renal damage. Renal toxicity mediated by 5-ASA can have variable clinical presentations including acute renal failure (43–46), acute nephritis (46–51) or nephritic syndrome (52–55). Case reports of renal biopsies have shown evidence of active tubulointerstitial nephritis (49,50). In this study, the patients with the greatest decline in CrCl presented with asymptomatic azotemia and the low incidence of renal disease in this study may be an indication that measuring serum creatinine is not sensitive enough to detect early changes in renal function, rather than pathological changes consistent with interstitial nephritis. However, results from studies attempting to correlate urinary markers of renal damage and 5-ASA treatment have been mixed (41,42,56–59). The exact mechanism of renal impairment is not well understood but it is likely due to a delayed cell-mediated response (27,31,48). It has also been proposed that the toxicity is due to uncoupling of oxidative phosphorylation or inhibition of prostaglandin synthesis, thereby mediating renal hypoxia (32). This is similar to what has been shown with high-dose salicylate-induced nephrotoxicity (39,58). In addition, our patient with interstitial nephritis had recovery of renal function with discontinuation of 5-ASA, thus supporting a cause-effect relationship. Cases of challenge, dechallenge and rechallenge support the causal role of 5-ASA in renal impairment (22,31,32,46,49,60,61). Furthermore, withdrawal of 5-ASA treatment leads to recovery of renal function in 40% to 85% of cases in which the diagnosis was made within 10 months of initiating treatment (31,62). However, only one-third of patients show renal recovery if the time to diagnosis and thus, drug withdrawal is longer than 18 months (31), and end-stage renal disease is seen in 10% of patients (62). This underscores the importance of prompt diagnosis of renal impairment and discontinuation of 5-ASA treatment.
In the present study, 74% of patients were treated with mesalamine compared with 15% on sulfasalazine; however, no differences in the degree of renal impairment and the type of 5-ASA compound used were found. Although the peak serum concentrations for the various 5-ASA formulations differ theoretically, several studies have not been able to find a difference between oral preparations and renal toxicity (31,39,43,44,48).
The lack of correlation between CrCl and age suggests that the change was not likely to be an age-related phenomenon. The CrCl rate declined from 104.6 mL/min to 93.1 mL/min (P<0.0001) with a calculated annual decline of 1.37 mL/min/year, which is not consistent with the accepted decline in renal function attributed to aging (63). A significant change in patient weight was also observed in the present study. Patients with significant weight loss and/or severe disease were treated with TPN for nutritional support and achieved successful weight gain. The group of patients who experienced an overall weight gain had the largest proportion of patients who received TPN. Therefore, the significant change in weight may falsely increase the CrCl, which would mask a true decline in renal function. One limitation of our study is the lack of a control group. Ideally, the control group in the present study would be an age- and treatment duration-matched group of IBD patients who have never received 5-ASA. Data for such a group do not exist in our database because most patients with long-standing IBD from previous years were generally started and kept on 5-ASA. Hence, such a group was not feasible.
Our study suggests that, irrespective of clinically defined renal disease, chronic high-dose 5-ASA use has subtle nephro-toxic effects, particularly in patients with pre-existing compromised renal function. Patients with UC are successfully maintained on long-term 5-ASA, thereby continuously putting them at risk for renal toxicity. However, is the renal compromise worth the marginal benefit of 5-ASA on CD? Further studies examining the risks and benefits of 5-ASA in patients with UC and CD need to be undertaken. Also, caution needs to be exercised in patients with diabetes, hypertension, concurrent potentially nephrotoxic medications or those with previous exposure to 5-ASA leading to azotemia. Although there is a lack of evidence to guide monitoring, we suggest that the standard of care be that serum creatinine concentration be measured monthly for the first three months, every three months for the first year and annually thereafter. The time to development of renal impairment is varied and may be seen as late as several years. There is also a risk of developing irreversible renal disease if not recognized early, in which discontinuation of 5-ASA could result in recovery of renal function. Monitoring serum creatinine provides an easy and inexpensive way to prevent a detrimental side effect.
REFERENCES
- 1.Azad K, Piris J, Truelove SC. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet. 1977;2:892–5. doi: 10.1016/s0140-6736(77)90831-5. [DOI] [PubMed] [Google Scholar]
- 2.Peppercorn MA, Goldman P. The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. Lancet. 1977;2:892–5. [PubMed] [Google Scholar]
- 3.Rasmussen SN, Bondesen S, Hvidberg T, et al. 5-Aminosalicylic acid in a slow release preparation: Bioavailability, plasma level and excretion in humans. Gastroenterology. 1982;83:1062–70. [PubMed] [Google Scholar]
- 4.Christensen LA, Fallingborg J, Abildgaard K, et al. Topical and systemic availability of 5-aminosalicylate: Comparisons of three controlled release formulations in man. Aliment Pharmacol Ther. 1990;4:523–33. doi: 10.1111/j.1365-2036.1990.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 5.Klotz U, Maier KE. Pharmacology and pharmacokinetics of 5-aminosalicylic acid. Dig Dis Sci. 1987;32:46S–50S. doi: 10.1007/BF01312463. [DOI] [PubMed] [Google Scholar]
- 6.Rijk MC, van Schaik A, van Tongeren JH. Disposition of 5-aminosalicylic acid by 5-aminosalicylic acid-delivering compounds. Scand J Gastroenterol. 1988;23:107–12. doi: 10.3109/00365528809093858. [DOI] [PubMed] [Google Scholar]
- 7.Savartz N. Sulfasalazine: II. Some notes on the discovery and development of salazopyrin. Am J Gastroenterol. 1988;83:497–503. [PubMed] [Google Scholar]
- 8.Laursen S, Stockholm M, Bukhave K, Rask-Madsen J, Lauritsen K. Disposition of 5-aminosalicylic acid by olsalazine and three mesalazine preparations in patients with ulcerative colitis: Comparison of intraluminal colonic concentrations, serum values, and urinary excretion. Gut. 1990;31:1271–6. doi: 10.1136/gut.31.11.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chester AC, Diamond LH, Schreiner GE. Hypersensitivity to salicylazosulfapyridine. Renal and hepatic toxic reactions. Arch Intern Med. 1978;138:1138–9. doi: 10.1001/archinte.138.7.1138. [DOI] [PubMed] [Google Scholar]
- 10.Prakash A, Markham A. Oral delayed-release mesalazine: A review of its use in ulcerative colitis and Crohn’s disease. Drugs. 1999;57:383–408. doi: 10.2165/00003495-199957030-00013. [DOI] [PubMed] [Google Scholar]
- 11.Clemett D, Markham A. Prolonged-release mesalazine: A review of its therapeutic potential in ulcerative colitis and Crohn’s disease. Drugs. 2000;59:929–56. doi: 10.2165/00003495-200059040-00016. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland L, Roth D, Beck P, et al. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2002:CD000544. doi: 10.1002/14651858.CD000544. [DOI] [PubMed] [Google Scholar]
- 13.Schreder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: A randomized study. N Engl J Med. 1987;317:1625–9. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 14.Sninsky CA, Cort DH, Shanahan F, et al. Oral mesalamine (Asacol) for mildly to moderately active ulcerative colitis. A multicenter study. Ann Intern Med. 1991;115:350–5. doi: 10.7326/0003-4819-115-5-350. [DOI] [PubMed] [Google Scholar]
- 15.Hanauer S, Schwartz J, Robinson M, et al. Mesalamine capsules for treatment of active ulcerative colitis: Results of a controlled trial. Pentasa Study Group. Am J Gastroenterol. 1993;88:188–97. [PubMed] [Google Scholar]
- 16.Peppercorn MA. Sulfasalazine, pharmacology, clinical use, toxicity, and related new drug development. Ann Intern Med. 1984;101:377–86. doi: 10.7326/0003-4819-101-3-377. [DOI] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Hanauer SB. Systematic review: The pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Aliment Pharmacol Ther. 2003;17:29–42. doi: 10.1046/j.1365-2036.2003.01408.x. [DOI] [PubMed] [Google Scholar]
- 18.Rachmilewitz D. Coated mesalazine 5-aminosalicylic acid versus sulphasalazine in the treatment of active ulcerative colitis: A randomized trial. BMJ. 1989;298:82–6. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Hees PA, Bakker JH, van Tongeren JH. Effect of sulphapyridine, 5-aminosalicylic acid, and placebo in patients with idiopathic proctitis: A study to determine the active therapeutic moiety of sulphasalazine. Gut. 1980;21:632–35. doi: 10.1136/gut.21.7.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tremaine WJ, Schroeder KW, Harrison JM, Zinsmeister AR. A randomized, double-blind, placebo-controlled trial of the oral mesalamine (5-ASA) preparation, Asacol, in the treatment of symptomatic Crohn’s colitis and ileocolitis. J Clin Gastroenterol. 1994;19:278–82. doi: 10.1097/00004836-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Steinhart AH, Hemphill D, Greenberg GR. Sulfasalazine and mesalazine for the maintenance therapy of Crohn’s disease: A meta-analysis. Am J Gastroenterol. 1994;89:2116–124. [PubMed] [Google Scholar]
- 22.Fockens P, Mulder CJJ, Tytgat GNJ. Comparison of the efficacy and safety of 1.5 vs 3.0 g oral slow-release mesalazine (Pentasa) in the maintenance treatment of ulcerative colitis. Eur J Gastroenterol Hepatol. 1995;7:1025–30. doi: 10.1097/00042737-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Corrigan G, Stevens PE. Review article: Interstitial nephritis associated with the use of mesalazine in inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1–6. doi: 10.1046/j.1365-2036.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 24.Loftus EV, Jr, Kane SV, Bjorkman D. Systematic review: Short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2004;19:179–89. doi: 10.1111/j.0269-2813.2004.01827.x. [DOI] [PubMed] [Google Scholar]
- 25.Calder IC, Funder CC, Green CR, Ham KN, Tange JD. Nephrotoxic lesions from 5-aminosalicylic acid. Br Med J. 1972;1:152–4. doi: 10.1136/bmj.1.5793.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilyard KG, Joseph EC, Metcalf R. Mesalazine: an overview of key preclinical studies. Scand J Gastroenterol Suppl. 1990;172:52–55. doi: 10.3109/00365529009091911. [DOI] [PubMed] [Google Scholar]
- 27.Popoola J, Muller AF, Pollock L, O’Donnell P, Carmichael P, Stevens P. Late onset interstitial nephritis associated with mesalazine treatment. BMJ. 1998;317:795–97. doi: 10.1136/bmj.317.7161.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agharazii M, Marcotte J, Boucher D, Noël R, Lebel M. Chronic interstitial nephritis due to 5-aminosalicylic acid. Am J Nephrol. 1999;19:373–76. doi: 10.1159/000013480. [DOI] [PubMed] [Google Scholar]
- 29.Calvino J, Romero R, Pintos E, et al. Mesalazine-associated tubulointerstitial nephritis in inflammatory bowel disease. Clin Nephrol. 1998;49:265–267. [PubMed] [Google Scholar]
- 30.Brouillard M, Gheerbrant JD, Gheysens Y, et al. Chronic interstitial nephritis and mesalazine: 3 new cases? Gastroenterol Clin Biol. 1998;22:724–6. [PubMed] [Google Scholar]
- 31.World MJ, Stevens PE, Ashton MA, Rainford DJ. Mesalazine-associated interstitial nephritis. Nephrol Dial Transplant. 1996;11:614–21. doi: 10.1093/oxfordjournals.ndt.a027349. [DOI] [PubMed] [Google Scholar]
- 32.Thuluvath PJ, Ninkovic M, Calam J, Anderson M. Mesalazine induced interstitial nephritis. Gut. 1994;35:1493–6. doi: 10.1136/gut.35.10.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmud N, O’Toole D, O’Hare N, Freyne PJ, Weir DG, Kelleher D. Evaluation of renal function following treatment with 5-aminosalicylic acid derivatives in patients with ulcerative colitis. Aliment Pharmacol Ther. 2002;16:207–15. doi: 10.1046/j.1365-2036.2002.01155.x. [DOI] [PubMed] [Google Scholar]
- 34.Van Staa TP, Travis S, Leufkens HG, Logan RF. 5-aminosalicylic acids and the risk of renal disease: A large British epidemiologic study. Gastroenterology. 2004;126:1733–9. doi: 10.1053/j.gastro.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 35.de Jong DJ, Tielen J, Habraken CM, Wetzels JF, Naber AH. 5-Aminosalicylates and effects on renal function in patients with Crohn’s disease. Inflamm Bowel Dis. 2005;11:972–6. doi: 10.1097/01.mib.0000185402.65288.19. [DOI] [PubMed] [Google Scholar]
- 36.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 37.Eknoyan G. Analgesic nephropathy and renal papillary necrosis. Semin Neprol. 1984;4:65–76. [Google Scholar]
- 38.Dubach UC, Rosner B, Pfister E. Epidemiologic study of abuse of analgesic containing phenacetin. Renal morbidity and mortality (1976–79) N Engl J Med. 1983;308:357–62. doi: 10.1056/NEJM198302173080703. [DOI] [PubMed] [Google Scholar]
- 39.Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal anti-inflammatory drugs. N Engl J Med. 1984;310:563–72. doi: 10.1056/NEJM198403013100905. [DOI] [PubMed] [Google Scholar]
- 40.Bennett WH, Henrich WL, Stoff JS. The renal effects of nonsteroidal anti-inflammatory drugs: Summary and recommendations. Am J Kid Dis. 1996;28(Suppl 1):S56–S62. doi: 10.1016/s0272-6386(96)90570-3. [DOI] [PubMed] [Google Scholar]
- 41.Schreiber S, Hamling J, Zehnter E, et al. Renal tubular dysfunction in patients with inflammatory bowel disease treated with aminosalicylate. Gut. 1997;40:761–6. doi: 10.1136/gut.40.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zehnter E, Dorhofer H, Ziegenhagen DJ, Scheurlen C, Baldamus CA, Kruis W. Renal damage in patients with inflammatory bowel disease treated with 5-aminosalicylic acid and sulphasalazine. Gastroenterology. 1991;100:A264. (Abst) [Google Scholar]
- 43.Ransford RA, Langman MJ. Sulphasalazine and mesalazine: Serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002;51:536–9. doi: 10.1136/gut.51.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birketvedt GS, Berg KJ, Fausa O, Florholmen J. Glomerular and tubular renal functions after long-term medication of sulphasalazine, olsalazine, and mesalazine in patients with ulcerative colitis. Inflamm Bowel Dis. 2000;6:275–9. doi: 10.1002/ibd.3780060404. [DOI] [PubMed] [Google Scholar]
- 45.Riley SA, Mani V, Goodman MJ, Herd ME, Dutt S, Turnberg LA. Comparison of delayed release 5 aminosalicylic acid (mesalazine) and sulphasalazine in the treatment of mild to moderate ulcerative colitis relapse. Gut. 1988;29:669–74. doi: 10.1136/gut.29.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamling J, Raedler A, Helmchen U, Schreiber S. 5-ASA associated renal tubular acidosis with decreased renal function in Crohn’s disease. Digestion. 1997;58:304–7. doi: 10.1159/000201459. [DOI] [PubMed] [Google Scholar]
- 47.Witte T, Olbricht CJ, Koch KM. Interstitial nephritis associated with 5-aminosalicylic acid. Nephron. 1994;67:481–2. doi: 10.1159/000188024. [DOI] [PubMed] [Google Scholar]
- 48.Corrigan G, Stevens PE. Review article: Interstitial nephritis associated with the use of mesalazine in inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1–6. doi: 10.1046/j.1365-2036.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 49.Mehta RP. Acute interstitial nephritis. Can Med Assoc. 1990;143:1031–2. [PMC free article] [PubMed] [Google Scholar]
- 50.Izzedine H, Simon J, Piette AM, et al. Primary chronic interstitial nephritis in Crohn’s disease. Gastroenterology. 2002;123:1436–40. doi: 10.1053/gast.2002.36613. [DOI] [PubMed] [Google Scholar]
- 51.De Broe ME, Stolear JC, Nolwen EJ, Elseviers MM. 5-aminosalicylic acid (5-ASA) and chronic tubulointerstitial nephritis in patients with chronic inflammatory bowel disease: Is there a link? Nephrol Dial Transplant. 1997;12:1839–41. doi: 10.1093/ndt/12.9.1839. [DOI] [PubMed] [Google Scholar]
- 52.Moayyedi P, Fletcher S, Harnden P, Axon AT, Brownjohn A. Mesangiocapillary glomerulonephritis associated with ulcerative colitis: Case reports of two patients. Nephrol Dial Transplant. 1995;10:1923–4. [PubMed] [Google Scholar]
- 53.Wilcox GM, Aretz HT, Roy MA, Roche JK. Glomerulonephritis associated with inflammatory bowel disease. Report of a patient with chronic ulcerative colitis, sclerosing cholangitis, and acute glomerulonephritis. Gastroenterology. 1990;98:786–91. [PubMed] [Google Scholar]
- 54.Molina-Perez M, Gonzalez-Reimers E, Santolaria-Fernandez F, Maceira-Cruz B, Ravina-Cabrera M. Rapidly progressive glomerulonephritis and inflammatory bowel disease. Dis Colon Rectum. 1995;38:1006–7. doi: 10.1007/BF02049742. [DOI] [PubMed] [Google Scholar]
- 55.Hirsch DJ, Jindal KK, Trillo A, Cohen AD. Acute renal failure in Crohn’s disease due to IgA nephropathy. Am J Kid Dis. 1992;20:189–90. doi: 10.1016/s0272-6386(12)80550-6. [DOI] [PubMed] [Google Scholar]
- 56.Mahmud N, Stinson J, O’Connell MA, et al. Microalbuminuria in inflammatory bowel disease. Gut. 1994;35:1599–604. doi: 10.1136/gut.35.11.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sninsky C, Hanauer S, Powers B, et al. Sensitive markers of renal dysfunction are elevated in chronic ulcerative colitis CUC Gastroenterology 1995108A919(Abst). [Google Scholar]
- 58.Mahmud N, O’Connell MA, Stinson J, Goggins MG, Weir DG, Kelleher D. Tumour necrosis factor-a and microalbuminuria in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995;7:215–9. [PubMed] [Google Scholar]
- 59.Monteleone G, Cristina G, Parrello T, et al. Altered IgG (4) renal clearance in patients with inflammatory bowel diseases. Evidence for a subclinical impairment of protein charge renal selectivity. Nephrol Dial Transplant. 2000;15:498–501. doi: 10.1093/ndt/15.4.498. [DOI] [PubMed] [Google Scholar]
- 60.Manenti L, De Rosa A, Buzio C. Mesalazine-associated interstitial nephritis: Twice in the same patient. Nephrol Dial Transplant. 1997;12:2031. [PubMed] [Google Scholar]
- 61.Schroeder KW. Role of mesalazine in acute and long-term treatment of ulcerative colitis and its complications. Scand J Gastroenterol Suppl. 2002;236:42–7. doi: 10.1080/003655202320621445. [DOI] [PubMed] [Google Scholar]
- 62.Arend LJ, Springate JE. Interstitial nephritis from mesalazine: Case report and literature review. Pediatr Nephrol. 2004;19:550–3. doi: 10.1007/s00467-004-1411-6. [DOI] [PubMed] [Google Scholar]
- 63.Anderson S, Brenner BM. Effects of aging on the renal glomerulus. Am J Med. 1986;80:435–42. doi: 10.1016/0002-9343(86)90718-7. [DOI] [PubMed] [Google Scholar]